Abstract
Mechanisms underlying experience-dependent refinement of cortical connections, especially GABAergic inhibitory circuits, are unknown. By using a line of mutant mice that lack activity-dependent BDNF expression (bdnf-KIV), we show that experience regulation of cortical GABAergic network is mediated by activity-driven BDNF expression. Levels of endogenous BDNF protein in the barrel cortex are strongly regulated by sensory inputs from whiskers. There is a severe alteration of excitation and inhibition balance in the barrel cortex of bdnf-KIV mice as a result of reduced inhibitory but not excitatory conductance. Within the inhibitory circuits, the mutant barrel cortex exhibits significantly reduced levels of GABA release only from the parvalbumin-expressing fast-spiking (FS) interneurons, but not other interneuron subtypes. Postnatal deprivation of sensory inputs markedly decreased perisomatic inhibition selectively from FS cells in wild-type but not bdnf-KIV mice. These results suggest that postnatal experience, through activity-driven BDNF expression, controls cortical development by regulating FS cell-mediated perisomatic inhibition in vivo.
Keywords: neurotrophin, plasticity, brain derived neurotrophic factor
Neuronal activity plays an important role in neural circuit development and function. Sensory experience drives the refinement of cortical sensory maps during critical periods (1, 2) and in adulthood (3). Such a process may involve a fine balance between experience-dependent strengthening of excitatory synaptic connections and concomitant maturation of inhibitory networks (4). Recently, we have demonstrated that inhibitory circuits, but not excitatory circuits, exhibit a massive postnatal maturation process that is regulated by sensory experiences (5, 6). Brain-derived neurotrophic factor (BDNF), a secretory neurotrophin known to regulate excitatory synaptic transmission and plasticity, has also been implicated in the development and function of GABAergic interneurons (7–10). However, evidence linking endogenous BDNF to sensory activity-dependent plasticity of inhibitory circuits in vivo is lacking. Moreover, given that many aspects of BDNF cell biology is regulated by neuronal activity (11), how BDNF and sensory inputs interact to control cortical circuit development is an important question begging for answers.
The transcription of bdnf gene is mediated by eight discrete promoters (I–VIII); each drives a unique 5′ exon (exons I–VIII) that is spliced onto the common 3′ coding exon (exon IX) to synthesize the same prepro-BDNF protein (12). Some promoters maintain basal levels of BDNF expression necessary for neuronal survival and differentiation, whereas others drive activity-dependent BDNF expression (11). In the neocortex, experiments that used exon-specific probes have demonstrated that transcription from promoter IV is the most sensitive to the changes in neuronal activity, including sensory inputs to the cortex (13, 14). It will be interesting to determine whether activity-dependent BDNF protein expression, driven by promoters I, IV, or others, elicits functions different from basal BDNF expression. By using a strain of genetically modified mice (KIV−/−) that exhibit relatively normal basal expression but severely reduced activity-dependent BDNF expression in the cortex, two groups have recently demonstrated a critical role of activity-dependent BDNF expression in GABAergic transmission (15, 16). However, it is unclear how activity-dependent BDNF expression regulates inhibitory circuits. Moreover, what is the functional role of activity-driven BDNF expression in the experience-dependent circuit maturation in vivo?
To address these questions, we used the mouse whisker somatosensory system, in which we have previously demonstrated a set of well defined changes in the inhibitory circuit by whisker trimming (5). To overcome the technical difficulty of studying GABAergic interneurons in layer IV, we crossed KIV−/− with glutamate acid decarboxylase 67–GFP mice (GAD67-GFP) (17) to generate bdnf-KIV−/− GAD67-GFP+/− mice (called KIV−/−-GFP+/− mice here for simplicity). These mice have two important properties: reduced activity-dependent BDNF expression and GFP labeling of the majority of GABAergic neurons. Our studies demonstrated that activity-dependent BDNF expression controls excitatory/inhibitory (E/I) balance by selectively regulating inhibitory networks in the barrel cortex in vivo. These results provide unique insights into sensory-dependent plasticity of neocortical networks.
Results
Activity-Dependent BDNF Expression in the Whisker-Barrel Cortex System.
The KIV−/− mice were generated to specifically eliminate activity-dependent bdnf transcription through promoter IV (16). Further characterization revealed that the activities of promoters I and III, which are also involved in activity-dependent bdnf transcription, were inhibited in the KIV−/− line as well (18). Western blot and immunohistochemistry studies have indicated that, although the basal level of BDNF protein in the cortex remains largely unchanged, the activity-induced increase in BDNF protein expression is essentially absent (16, 18). Thus, the KIV−/− line could serve as a tool to study the functional consequences of lacking activity-dependent expression of BDNF protein, rather than promoter IV specifically. The GAD67-GFP+/− mice are the heterozygotes of a knock-in line in which GABAergic interneurons were selectively labeled by GFP (17). Previous studies showed that GAD67-GFP+/−, with GFP replacing GAD67 on one of the chromosomes, did not alter the function of brain GABAergic interneurons (19). We crossed the KIV−/− mice with GAD67-GFP mice to generate KIV+/+-GFP+/− and KIV−/−-GFP+/− mice, which were used as control and experimental groups, respectively. There was no significant deficit in gross brain anatomy and cytoarchitectonic organization in the KIV−/−-GFP+/− mice (Fig. S1C). Cytochrome oxidase (CO) histochemistry and Vglut2 immunohistochemistry showed that, in tangential as well as thalamocortical (TC) sections, the barrel cortices exhibited patterns in rows A through E of barrel field in both groups, suggesting that the formation of barrels and the posterior medial barrel subfield were present in the mutant, KIV−/−-GFP+/− mice (Fig. 1A).
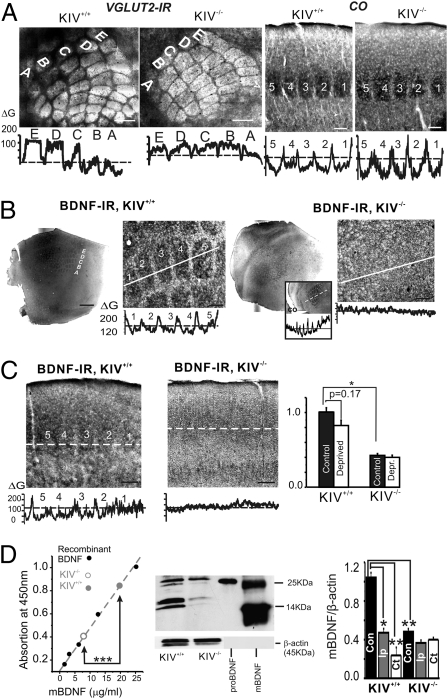
Fig. 1.
Intact barrel structure with reduced expression of BDNF in the KIV−/− mice. (A) Vglut2 IR in a tangential section (Left) and CO histochemistry in a TC section (Right) show all barrels (rows A–E) intact in KIV−/− mice. (Scale bar: 150 μm.) Bottom: Line profile of grayscale intensity of vglut2-IR and CO across different rows shows the existence of barrels (marked with numbers 1–5 or letters A–E) and septum. (B) Photograph of BDNF IR and line profile of the photo in layer IV tangential section of barrel cortex (1× and 10× magnification, respectively). The intensity of BDNF IR was measured along the white lines across the barrel field and presented in the grayscale line profiles below. The grayscale line profile clearly shows existence of the barrel pattern in the KIV+/+ but not KIV−/− mice. Inset: Barrel field in KIV−/− mice was identified via similar region in an adjacent section stained with CO, which clearly indicates the barrel field, with a line profile below. (C) Photograph of BDNF IR and line profile of the photo (Lower) in TC sections across the barrel cortex. Bar graph (Right) shows the effect of all-row whisker trimming (n = 6 mice in each group) on the differences in grayscale intensities (ΔG) of BDNF IR between barrel and septum. (D) Regulation of BDNF levels by sensory inputs. Left: BDNF absorption levels measured with BDNF Emax ImmunoAssay System (Promega). Center: Example of Western blot showing proBDNF and mBDNF in lysate from KIV+/+ and KIV−/− mice, respectively. Purified proBDNF and mBDNF are shown in right lanes for comparison. Right: Quantification of the Western blots. Lysate was obtained from freshly cut barrel cortex from KIV+/+ and KIV−/− mice (n = 3 and n = 6 mice in each group, respectively). In this and all figures, one-way ANOVA was used (*P < 0.05, **P < 0.01, and ***P < 0.001).
By using an anti-BDNF antibody, we next showed that BDNF immunoreactivity (IR) exhibited a barrel shape and distribution (Fig. 1B, Left) in the control KIV+/+ -GFP+/− mice, similar to the classical barrel pattern seen with Vglut2 (Fig. 1A, Left) or CO (Fig. 1A, Right) staining in a tangential section of flattened barrel cortex. In contrast, the barrel pattern of the BDNF IR, but not CO, was completely abolished in the mutant mice (Fig. 2B, Right). A similar situation also exists in the TC slices (Fig. 1C). The differences in BDNF IR between the barrel (Fig. 1 B and C, 1–5) and septum (weaker staining between barrels) were measured and denoted ΔG. A statistical analysis is shown in Fig. 1C. Sensory deprivation by all-row trimming of whiskers significantly reduced the ΔG in the control cortex. Interestingly, the BDNF IR was already reduced by 59 ± 6% in KIV−/−-GFP+/− cortex compared with KIV+/+ -GFP+/− cortex, possibly because of already-reduced activity-driven BDNF expression in the mutant mice. Moreover, sensory deprivation did not further reduce ΔG (Fig. 1C, bar graph). Taken together, these results suggest that BDNF protein expressed in the barrels is up-regulated by sensory input under normal circumstances (i.e., standard housing conditions), and whisker trimming-induced regulation has disappeared in the mutant mice. It should be noted that thalamic and subcortical BDNF IR did not reveal BDNR IR in the TC axons (Fig. S1 A and B). Thus, BDNF in the barrel cortex is produced locally in the cortex, rather than derived from subcortical afferent inputs.
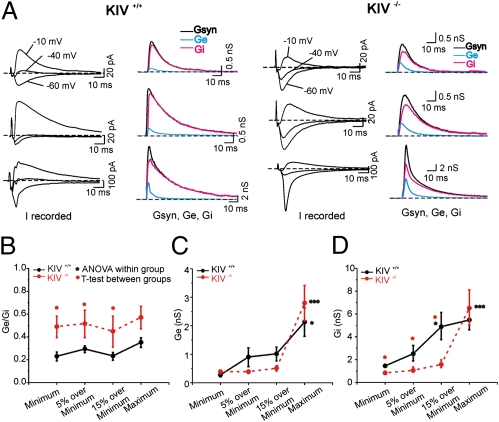
Fig. 2.
Altered E/I balance in the KIV−/− mice. (A) Actual recording of postsynaptic current (Irecorded) for each genotype recorded under voltage-clamp recording with three holding potentials (−10, −40, −60 mV; left graph for each genotype). The responses were evoked by local stimulation under three stimulation intensities: the minimum (Top), 15% over the minimum (Middle), and the maximum (Bottom). Traces were averaged from 10 consecutive sweeps. The right graph for each genotype shows continuous plot of Gsyn (black), Ge (blue), and Gi (magenta) from layer IV spiny neurons at three different stimulus conditions in the KIV+/+ and KIV−/− mice, respectively. (B–D) Mean E/I ratio (Ge/Gi) Ge, and Gi values induced by a range of stimulus conditions in KIV+/+ (black) and KIV−/− (red) mice, respectively (n = 10 neurons in each group).
Next, we measured BDNF expression in the normal and mutant mice. Quantitative analyses using ELISA (Fig. 1D, Left) and Western blot (Fig. 1D, Right) revealed a greater than twofold decrease in BDNF protein levels in the brain homogenate from the barrel cortex of naive (i.e., untreated) KIV−/− -GFP+/− mice compared with controls. More importantly, whisker trimming resulted in a marked reduction of BDNF expression in the control mice, but not in the mutant mice. Quantification of mature BDNF (mBDNF) after normalization to β-actin on Western blot revealed a significant reduction in mBDNF in the contralateral and ipsilateral cortex after all-row trimming in control animals (Fig. 1D, Right; KIV+/+ group). In the mutant mice, however, the level of mBDNF protein was markedly reduced without whisker trimming (Fig. 1D, “Con” in KIV+/+ vs. KIV−/− groups), and all-row whisker trimming failed to further decrease mBDNF in either contralateral cortex or ipsilateral barrel cortex (Fig. 1D, Right; KIV−/− group). Thus, sensory deprivation via whisker trimming can significantly alter local cortical BDNF expression in the barrel cortex.
Altered E/I Balance in Single Principal Neurons.
The E/I ratio was measured directly in the barrel cortex to determine the impact of lack of activity-dependent BDNF expression. The method we used here was based on the continuous measurement of conductance dynamics during stimulus-evoked synaptic responses previously described using cat cortex in vivo (20). The excitatory and inhibitory conductances underlying mixed synaptic responses were extracted by using the equations by Cruikshank et al. (21). In the present study, synaptic currents were determined for each holding potential (three potentials were used for the analysis). We plotted a synaptic current/voltage curve at each time point and fitted these plots with linear regression. Based on the slopes and voltage intercepts, synaptic conductance (Gsyn) and reversal potential (Esyn) were calculated, respectively. Gsyn was then separated into an excitatory Gsyn (Ge) and an inhibitory Gsyn (Gi), and values were plotted as continuous waveforms (Fig. 2 and Fig. S2). A detailed description of this method was published earlier (22), in which we showed that the E/I ratio is developmentally regulated and is very stable across a variety of stimulus conditions in four different cortical layers at the same postnatal age. In this study, we also provide pharmacological validation of extracted conductance (Fig. S2). By using a minimum stimulus intensity, as well as other intensities (5% over minimum, 15% over minimum, and maximum; Fig. 2), we measured the E/I ratio in postnatal day 30 (P30) spiny neurons from KIV+/+ -GFP+/− and KIV−/−-GFP+/− mice. With all stimulus intensities, we found that there were significantly larger E/I ratios (i.e., Ge/Gi) in spiny neurons from the KIV−/−-GFP+/− mice (Fig. 2B). The differences in E/I ratio were entirely caused by differences in Gi but not Ge (with the only exception at maximum stimulation; Fig. 2C vs. Fig. 2D), suggesting that the KIV mutant selectively affects maturation of inhibitory but not excitatory circuits. In subsequent experiments, therefore, we focused on inhibitory circuits and their activity-dependent plasticity.
Impairments of Experience-Dependent Structural Plasticity of Inhibitory Circuits.
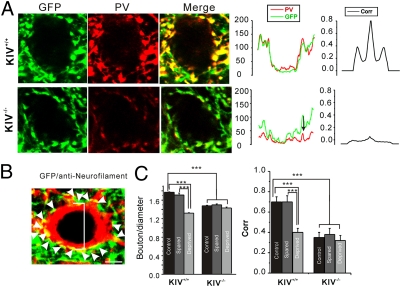
In layer IV of barrel cortex, axon terminals of the parvalbumin (PV)-expressing basket cells form a characteristically dense plexus of perisomatic varicosities on the soma of spiny cells (Fig. 3A). These presumed basket synapses were found on the somata of spiny and star pyramidal neurons of barrel cortex layer IV (Fig. 3B). Robust decreases in PV expression, GAD expression, and the number of perisomatic inhibitory synaptic boutons were observed in P30 barrel cortex after 3-wk whisker trimming (5). Here, we found that the perisomatic boutons (yellow spots) were significantly decreased at baseline level without any manipulation in KIV−/−-GFP+/− mice (Fig. 3A). In KIV+/+ -GFP+/− mice, whisker trimming of rows D and E caused a significant decrease in the number of perisomatic boutons in the corresponding sensory-deprived (i.e., rows D and E) barrels, but not in neighboring spared barrels (Fig. 3C; Fig. S3 includes photos of experimental conditions). In contrast, the down-regulation of the number of perisomatic GABAergic boutons by whisker trimming was not observed in the KIV−/− -GFP+/− mice (Fig. 3C). In addition, there was a significant decrease in PV expression from the remaining GAD67-GFP+ boutons, as evaluated via cross-correlation analysis of PV and GFP signals in these boutons (Fig. 3 A and C and Fig. S3). These data suggest that activity-dependent structural plasticity of inhibitory networks, characterized by reduction in the number of perisomatic boutons per spiny neuron and reduction in PV expression in both soma and GABAergic terminals, was abolished in the KIV−/− -GFP+/− mice. In addition, the KIV−/−-GFP+/− mice themselves have less PV and smaller perisomatic boutons, presumably because of a developmental delay in the maturation of PV+ GABAergic circuits (23). Thus, activity-dependent expression of BDNF plays a critical role in the experience-dependent structural plasticity of the PV-expressing FS GABAergic network in barrel cortex.
Fig. 3.
Impairment in activity-dependent regulation of inhibitory perisomatic boutons. (A) Confocal micrographs of GFP and PV IR in layer IV barrel cortex. (Scale bars: 2 μm.) For each merged image, grayscale line profile of the entire image for PV (red) and GFP (green) and the cross-correlation (Corr) plot for the two line profile curves are shown on the right. Note that the expression of PV and GFP were highly overlapping (i.e., higher cross-correlation) in the KIV+/+ but not KIV−/− mice. (B) A neurofilament-positive (i.e., glutamatergic; red) neuron innervated by perisomatic GFP-positive (i.e., GABAergic; green) boutons. The number of GFP-positive perisomatic varicosities was normalized against the diameter of each presumed spiny neuron (Middle). (C) Bar graph shows the plot of mean number of perisomatic boutons per cell (normalized cell diameter), and cross-correlation (Right) in KIV+/+ and KIV−/− mice with whisker trimming manipulations (spared or deprived) and without (control) whisker trimming manipulations (n = 12 slices from four brains in each group).
Impairment of Experience-Dependent Perisomatic Inhibition from FS but Not RSNP Cells.
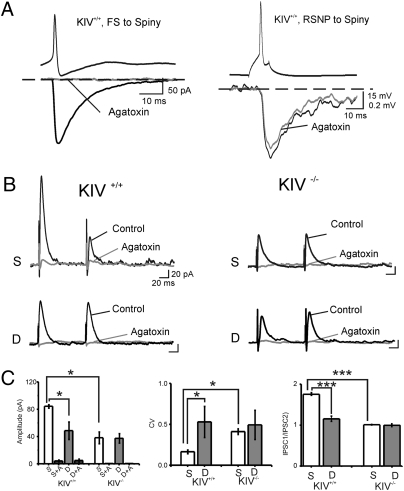
We next examined evoked inhibitory postsynaptic synaptic currents (IPSCs; eIPSCs) in spiny neurons and their modulation by whisker trimming. The eIPSCs were evoked at threshold (i.e., minimal) intensity by an extracellular electrode placed next to the spiny cells. Under these conditions, the eIPSCs showed all-or-none responses, suggesting a single fiber response (i.e., unitary IPSCs), presumably from a single basket cell. It has been reported that P/Q channels were predominantly expressed at GABAergic terminals of FS basket cells, whereas N-type calcium channels were predominantly expressed at GABAergic terminals of non-FS [or regular spiking nonpyramidal (RSNP)] GABAergic cells in hippocampus or neocortex (24, 25). Indeed, with paired recordings, we found that FS but not RSNP-mediated unitary IPSCs was sensitive to the P/Q channel blocker ω-agatoxin-IVA in barrel cortex layer IV (Fig. 4A). Next, we found that the eIPSCs in all conditions were nearly eliminated by bath application of ω-agatoxin-IVA (2 μM, n = 6 in each group; Fig. 4 B and C). These results suggest that eIPSCs recorded on spiny neurons were produced largely by FS cells, and there were no abnormal substitution of P/Q channels in terminals of FS cells in the KIV−/− mice.
Fig. 4.
Lack of effects of whisker trimming on ω-agatoxin–sensitive eIPSCs in KIV−/− mice. (A) FS-, but not RSNP neuron-, mediated IPSCs are selectively blocked by ω-agatoxin-IVA (2 μM). Left: Example of unitary IPSCs derived from a presynaptic FS cell in the absence (black trace) and presence (gray trace) of the toxin. Right: Example of unitary IPSPs derived from a presynaptic RSNP cell in the absence (black trace) and presence (gray trace) of the toxin (n = 4 pairs in each group). (B) Examples of recordings of eIPSCs induced with minimal stimulus in sensory spared (S) and deprived (D) area at a holding potential (Vhold) of 0 mV. (C) Mean amplitudes of eIPSCs, CV, and PPD in KIV+/+ versus KIV−/− mice, respectively (n = 15 in spared vs. n = 7 in deprived cortices).
Paired-pulse depression (PPD) is associated with a decrease in release probability and is a characteristic property for intracortical inhibitory synapses formed between FS cells and spiny neurons (5). In the control mice, PPD of eIPSCs in spiny neurons was reliably observed in the spared cortical area, but was completely eliminated in sensory input-deprived cortices (Fig. 4B, Left). In the KIV−/− mice, the amplitude of the first eIPSC of the pair was reduced, PPD disappeared in the spared area, and whisker trimming had no effect on the amplitudes or the PPD of the eIPSCs (Fig. 4B, Right). Fig. 4C shows quantitative analysis of the data on eIPSC amplitude (Fig. 4C, Left) and PPD (Fig. 4C, Right). Another important effect of whisker trimming is an increase in the variability of the eIPSC amplitude (i.e., coefficient of variance) (5). In the KIV−/− mice, the coefficient of variance in the spared area increased, but whisker trimming (row D) failed to induce further change in the coefficient of variance (Fig. 4C). Thus, the whisker trimming-induced robust changes in eIPSC properties were completely abolished in the KIV−/− mice. Taken together, these results reveal the importance of activity-dependent BDNF expression in the experience-dependent reorganization of the local inhibitory circuits in the barrel cortex.
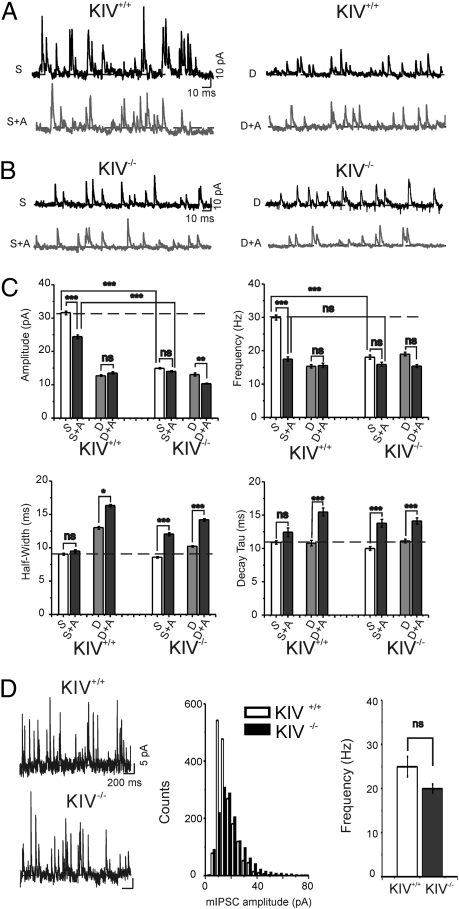
A number of control experiments were performed. First, all eIPSCs were virtually eliminated by ω-agatoxin-IVA (Fig. 4B), suggesting that sensory deprivation and activity-dependent BDNF expression affect the same type of neurons: the FS cells. Second, sensory deprivation also induced decreases in the amplitude and frequency, but increases in the half-width and decay time, of spontaneous IPSCs [miniature IPSCs (mIPSCs) recorded in the presence of the sodium channel blocker tetrodotoxin] in control, but not in mutant, mice (Fig. 5 A–C). Last, the amplitude distribution of mIPSCs in KIV−/− animals was similar to that in control mice (Fig. 5D). Thus, activity-dependent BDNF expression affects evoked (i.e., calcium-dependent) but not basal GABA release. Finally, there were no differences between any groups in the presence of ω-agatoxin-IVA, indicating that defects in KIV mutant are exclusively related to activity-mediated GABA release in FS cells (Figs. 4C and 5C, 1 and 2). These data suggest that the abnormal GABAergic transmission in KIV−/− but not KIV+/+ mice was caused at least in part by presynaptic changes in FS cells, including a reduced number and size of presynaptic boutons (e.g., Fig. 3 and Fig. S3).
Fig. 5.
Lack of sensory deprivation induced decrease sIPSCs in KIV−/−-GFP+/− mice. (A and B) Sample sIPSCs recorded in neurons from the spared and deprived region in KIV+/+ and KIV−/− mice at a Vhold of 0 mV. (C) Statistical graph for amplitudes, frequency, half-width, and decay time constant (τdecay). (A, ω-agatoxin-IVA 2 μM; dashed lines, controls.) (D) mIPSCs recorded in untreated control KIV+/+ and KIV−/− mice in the presence of TTX (Left). Histogram (Middle) of mIPSC amplitudes in representative cells and mean frequency of mIPSCs (Right; n = 10 cells in each group; P > 0.5, Kolmogorov–Smirnov test and one-way ANOVA, respectively).
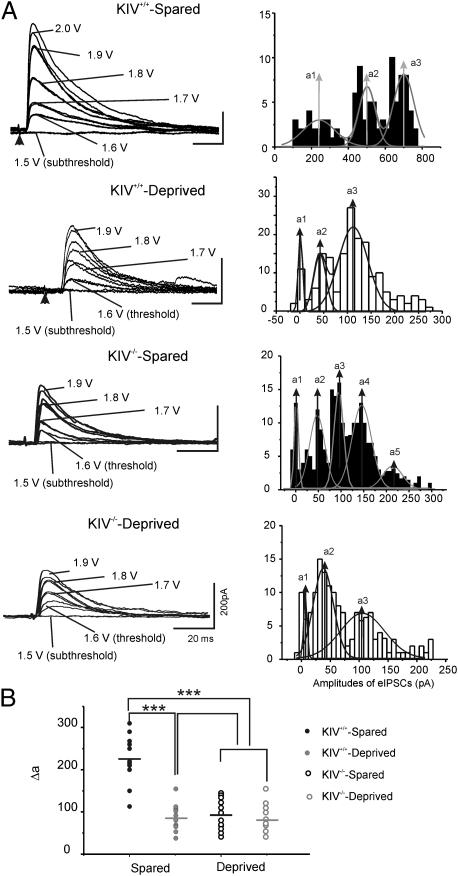
To further examine potential synaptic mechanisms underlying the effects of whisker trimming on eIPSCs, we incrementally increased the stimulus intensity and plotted the amplitude distribution histograms of the evoked events. They were then fitted by multipeak Gaussian curves to provide objective evaluation of the quantal content of transmitter release. Typical eIPSC amplitude distribution showed two or three evoked peaks in Gaussian fitted curves (Fig. 6A, Right). This approach was previously used to estimate up-regulation of inhibitory quanta size in epileptic regions (26) and whisker trimming-induced down-regulation of quanta size in layer IV cells (5). We found that the delta values between these peaks (i.e., estimated quantal value) were similar to the amplitudes of unitary IPSCs onto spiny cells provided by basket cells (27). These data are consistent with the idea that the evoked inhibitory responses were largely perisomatic and mediated by FS cells. Moreover, there was a significant reduction in the quantal value in deprived neurons, and these effects were totally abolished in KIV−/− mice (Fig. 6B). Because a major increase in the strength of GABAergic transmission occurs during the second and third postnatal weeks in an activity-dependent manner (5), these results suggest that postnatal sensory activities in vivo promote the transformation of functional inhibitory transmission in FS cells (but not RSNP cells) from an immature to a mature phenotype and that activity-dependent BDNF expression plays a critical role in this process.
Fig. 6.
eIPSCs induced with graded stimulus intensities. (A) Left: Example of eIPSCs evoked by extracellular stimuli with graded intensity (from subthreshold to suprathreshold) in cells located in spared barrels and deprived barrels in layer IV at a Vhold of 0 mV. Right: Histogram of amplitudes of eIPSCs in respective recordings on the left. The distributions of amplitudes were fitted with multipeak Gaussian distribution. [a1–a4, values of center of the peak; solid lines, multipeak Gaussian fit.] (B) Scatter graph shows the value of mean Gaussian multi-peak distance (δa) in spared (filled circles) and deprived (open circles) cells.
Discussion
Two recent studies selectively isolated the activity-dependent components of BDNF expression and tested some of its functional significance. In one study (15), point mutations in the CaRE3/CRE (CREm) site of promoter IV were made in mice. The CREm mice exhibit reduced inhibitory synaptic currents and GABAergic markers in cortical neurons (15). In another study, activity-dependent BDNF expression was largely disrupted by knock-in of GFP gene into the BDNF exon IV (16, 18). The KIV−/− mice exhibit impaired PV-expressing in FS GABAergic interneurons in the prefrontal cortex, and an altered spike time-dependent synaptic potentiation (16). These studies demonstrate the specific requirement for activity-dependent BDNF expression in the development of cortical inhibitory synapses. However, the functional consequences of the disruption of the activity-dependent BDNF expression in neuronal circuit maturation in vivo are unknown. By using the KIV−/−-GFP+/− line, which allows the visualization of GABAergic interneurons, we have now addressed two important questions. First, how does activity manipulation affect the endogenous BDNF expression in specific neural circuits in vivo? Second, how does activity-dependent BDNF expression contribute to experience-dependent plasticity of GABAergic network in vivo?
Our first major finding is that whisker sensory activity drives BDNF expression in the barrel cortex in an input-specific manner (i.e., whisker to barrel), based on several lines of evidence: (i) BDNF IR exhibited a barrel pattern in KIV+/+ but not KIV−/− mice (Fig. 1 B and C). This result suggests that natural sensory inputs to the barrels in the somatosensory cortex control BDNF expression. (ii) Whisker trimming, which reduces sensory inputs to the barrels, resulted in a selective reduction in BDNF IR in KIV+/+ but not KIV−/− mice (Fig. 1C), suggesting an input-dependent modulation of BDNF expression. (iii) Western blot analysis revealed that whisker trimming led to a reduction in BDNF protein levels in barrel cortex in KIV+/+ mice but not KIV−/− mice (Fig. 1D). Combining immunohistochemistry with Western blot data, our results indicate that approximately two thirds of endogenous BDNF synthesis in the barrel cortex in vivo is synthesized in activity-dependent manner and is driven by sensory inputs.
Our second major conclusion is that activity-dependent BDNF expression is required for experience-dependent modulation of cortical inhibitory networks in vivo. (i) Whisker trimming induced down-regulation of PV expression in wild-type, but not KIV−/− mice (Fig. 3). (ii) Deprivation of sensory inputs to the barrel cortex also decreased the number of perisomatic boutons in KIV+/+ but not KIV−/− mice (Fig. 3). (iii) Disruption of activity-dependent BDNF expression prevented sensory deprivation induced barrel-specific attenuation of GABAergic transmission (Figs. 4 and 5). (iv) Whisker trimming induced plasticity in inhibitory synaptic transmission was also entirely abolished in the KIV−/− mice (Figs. 4–6). It is remarkable that a relatively mild manipulation on activity-dependent but not basal BDNF expression machinery could completely abolish whisker trimming-induced plasticity of GABAergic network in the barrel cortex in vivo. Our results also support the notion that neuronal activity and BDNF works together to promote the maturation of GABAergic interneurons and their synapses (28, 29).
Our third, and perhaps the most surprising, conclusion regards the synaptic mechanism underlying experience-dependent modulation of cortical inhibitory networks: cell type and synapse specificity of the effect of activity-dependent BDNF expression. (i) Inhibitory circuits, but not excitatory circuits, are affected in the KIV−/−-GFP+/− mice (Fig. 2). (ii) Within inhibitory networks, the effects of whisker trimming selectively affect the synaptic transmission mediated by FS but not RSNP cells (Figs. 4 and 5). (iii) The effects were largely limited to the presynaptic properties that involve P/Q-type calcium channels, as well as reduced number of presynaptic boutons (Fig. 3), but not postsynaptic properties (Figs. 4 and 5). Although BDNF has been postulated to play critical roles in neuronal circuit development and plasticity (7, 9, 30), we demonstrated the selectivity of BDNF-mediated effects on inhibitory networks in vivo. Previous studies on BDNF regulation of GABAergic function mostly used neuronal cultures in which the neural circuits were disrupted (28, 29). Cortical GABAergic interneurons do not express BDNF (8, 31), and the release of BDNF is closely related to the active state of pyramidal neurons (32, 33). Therefore, it is critical to study BDNF-mediated signaling locally in an intact neural circuit with different activity levels. In the KIV−/−-GFP+/− mice, all the previously documented structural plasticity (i.e., expression of PV and number of perisomatic GABAergic boutons; Fig. 3) and functional plasticity [PPD, coefficient of variance (CV), quantal amplitude of IPSCs; Figs. 4–6] were abolished, suggesting that activity-dependent BDNF expression mediates the sensory-dependent plasticity in barrel cortex layer IV. These findings call for continued investigation of underlying mechanisms involved in regulation of the activity-dependent expression of BDNF, and for the extension of these studies to allow understanding of similar programs involved in translating neuronal activities into structural and functional circuit plasticity.
Materials and Methods
All experiments that involved mice were approved by animal care and use committee of the University of Wyoming.
Sensory Deprivation.
Mice were divided into three groups: (i) row D and E (in the left mystacial pad) removed, (ii) all whiskers (in the left mystacial pad) removed, and (iii) sham-treated normal mice (i.e., control group) (34). Animals in the experimental groups began to have whiskers (only from left mystacial pad) removed at P7, and this process lasted until the mice were 28 to 30 d old, based on methods described earlier (5).
Mouse Crossing and Genotyping.
The KIV+/− mice were crossed with GAD67-GFP+/−, and the resulting F1 KIV+/−-GFP+/− mice were crossed again to generate F2 homozygous KIV−/−-GFP+/− mice. Mouse genotyping methods were described earlier by Sakata et al. (16).
Statistics.
Upon group division, data were compared across groups with single-factor ANOVA, followed by the Tukey honestly significant difference test to determine intergroup significance. P < 0.05 was considered to represent a statistically significant difference. In some experiments, a t test or Kolmogorov-Smirnov test was used as well.
Supplementary Material
Acknowledgments
We thank Dr. Yuchio Yanagawa at the Department of Genetic and Behavioral Neuroscience, Gunma University Graduate School of Medicine (Maebashi, Japan) for the gift of the GAD67-GFP mouse. Confocal microscopy was performed in the University of Wyoming's Microscopy Core Facility, which is supported by Wyoming Neuroscience Centers of Biomedical Research Excellence (National Institutes of Health) Grant RR015553. This research is supported by National Institutes of Health Grants NS057415 (to Q.-Q.S.) and by the Intramural Research Programs of the National Institute of Mental Health (B.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105296108/-/DCSupplemental.
References
- 1.Stryker MP. Postnatal development of ocular dominance columns in layer IV of the cat's visual cortex and the effects of monocular deprivation. Arch Ital Biol. 1978;116:420–426. [PubMed] [Google Scholar]
- 2.Wiesel TN, Hubel DH. Ordered arrangement of orientation columns in monkeys lacking visual experience. J Comp Neurol. 1974;158:307–318. doi: 10.1002/cne.901580306. [DOI] [PubMed] [Google Scholar]
- 3.Polley DB, Kvasnák E, Frostig RD. Naturalistic experience transforms sensory maps in the adult cortex of caged animals. Nature. 2004;429:67–71. doi: 10.1038/nature02469. [DOI] [PubMed] [Google Scholar]
- 4.Hensch TK, et al. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiao Y, Zhang C, Yanagawa Y, Sun QQ. Major effects of sensory experiences on the neocortical inhibitory circuits. J Neurosci. 2006;26:8691–8701. doi: 10.1523/JNEUROSCI.2478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun QQ. The missing piece in the ‘use it or lose it’ puzzle: Is inhibition regulated by activity or does it act on its own accord? Rev Neurosci. 2007;18:295–310. doi: 10.1515/revneuro.2007.18.3-4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang ZJ, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 8.Marty S, MdaP Berzaghi, Berninger B. Neurotrophins and activity-dependent plasticity of cortical interneurons. Trends Neurosci. 1997;20:198–202. doi: 10.1016/s0166-2236(96)01026-0. [DOI] [PubMed] [Google Scholar]
- 9.McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 10.Woo NH, Lu B. Regulation of cortical interneurons by neurotrophins: From development to cognitive disorders. Neuroscientist. 2006;12:43–56. doi: 10.1177/1073858405284360. [DOI] [PubMed] [Google Scholar]
- 11.Lu B. Pro-region of neurotrophins: role in synaptic modulation. Neuron. 2003;39:735–738. doi: 10.1016/s0896-6273(03)00538-5. [DOI] [PubMed] [Google Scholar]
- 12.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanda S, Mack KJ. Multiple promoters direct stimulus and temporal specific expression of brain-derived neurotrophic factor in the somatosensory cortex. Brain Res Mol Brain Res. 1998;62:216–219. doi: 10.1016/s0169-328x(98)00242-3. [DOI] [PubMed] [Google Scholar]
- 14.Timmusk T, et al. Identification of brain-derived neurotrophic factor promoter regions mediating tissue-specific, axotomy-, and neuronal activity-induced expression in transgenic mice. J Cell Biol. 1995;128:185–199. doi: 10.1083/jcb.128.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakata K, et al. Critical role of promoter IV-driven BDNF transcription in GABAergic transmission and synaptic plasticity in the prefrontal cortex. Proc Natl Acad Sci USA. 2009;106:5942–5947. doi: 10.1073/pnas.0811431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamamaki N, et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 18.Martinowich K, Schloesser RJ, Jimenez DV, Weinberger DR, Lu B. Activity-dependent brain-derived neurotrophic factor expression regulates cortistatin-interneurons and sleep behavior. Mol Brain. 2011;4:11. doi: 10.1186/1756-6606-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchida T, Oki Y, Yanagawa Y, Fukuda A. A heterozygous deletion in the glutamate decarboxylase 67 gene enhances maternal and fetal stress vulnerability. Neurosci Res. 2011;69:276–282. doi: 10.1016/j.neures.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Borg-Graham LJ, Monier C, Frégnac Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature. 1998;393:369–373. doi: 10.1038/30735. [DOI] [PubMed] [Google Scholar]
- 21.Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Jiao YY, Sun QQ. Developmental maturation of excitation and inhibition balance in principal neurons across four layers of somatosensory cortex. Neuroscience. 2011;174:10–25. doi: 10.1016/j.neuroscience.2010.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chattopadhyaya B, et al. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali AB, Nelson C. Distinct Ca2+ channels mediate transmitter release at excitatory synapses displaying different dynamic properties in rat neocortex. Cereb Cortex. 2006;16:386–393. doi: 10.1093/cercor/bhi117. [DOI] [PubMed] [Google Scholar]
- 25.Poncer JC, McKinney RA, Gähwiler BH, Thompson SM. Either N- or P-type calcium channels mediate GABA release at distinct hippocampal inhibitory synapses. Neuron. 1997;18:463–472. doi: 10.1016/s0896-6273(00)81246-5. [DOI] [PubMed] [Google Scholar]
- 26.Nusser Z, Hájos N, Somogyi P, Mody I. Increased number of synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- 27.Sun QQ, Huguenard JR, Prince DA. Barrel cortex microcircuits: Thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci. 2006;26:1219–1230. doi: 10.1523/JNEUROSCI.4727-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin X, Hu H, Mathers PH, Agmon A. Brain-derived neurotrophic factor mediates activity-dependent dendritic growth in nonpyramidal neocortical interneurons in developing organotypic cultures. J Neurosci. 2003;23:5662–5673. doi: 10.1523/JNEUROSCI.23-13-05662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marty S, Wehrlé R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci. 2000;20:8087–8095. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 31.Gorba T, Wahle P. Expression of TrkB and TrkC but not BDNF mRNA in neurochemically identified interneurons in rat visual cortex in vivo and in organotypic cultures. Eur J Neurosci. 1999;11:1179–1190. doi: 10.1046/j.1460-9568.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- 32.Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- 33.Leslie KR, Nelson SB, Turrigiano GG. Postsynaptic depolarization scales quantal amplitude in cortical pyramidal neurons. J Neurosci. 2001;21:RC170. doi: 10.1523/JNEUROSCI.21-19-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.