Abstract
TRPV4 is a Ca2+- and Mg2+-permeable cation channel within the vanilloid receptor subgroup of the transient receptor potential (TRP) family, and it has been implicated in Ca2+-dependent signal transduction in several tissues, including brain and vascular endothelium. TRPV4-activating stimuli include osmotic cell swelling, heat, phorbol ester compounds, and 5′,6′-epoxyeicosatrienoic acid, a cytochrome P450 epoxygenase metabolite of arachidonic acid (AA). It is presently unknown how these distinct activators converge on opening of the channel. Here, we demonstrate that blockers of phospholipase A2 (PLA2) and cytochrome P450 epoxygenase inhibit activation of TRPV4 by osmotic cell swelling but not by heat and 4α-phorbol 12,13-didecanoate. Mutating a tyrosine residue (Tyr-555) in the N-terminal part of the third transmembrane domain to an alanine strongly impairs activation of TRPV4 by 4α-phorbol 12,13-didecanoate and heat but has no effect on activation by cell swelling or AA. We conclude that TRPV4-activating stimuli promote channel opening by means of distinct pathways. Cell swelling activates TRPV4 by means of the PLA2-dependent formation of AA, and its subsequent metabolization to 5′,6′-epoxyeicosatrienoic acid by means of a cytochrome P450 epoxygenase-dependent pathway. Phorbol esters and heat operate by means of a distinct, PLA2- and cytochrome P450 epoxygenase-independent pathway, which critically depends on an aromatic residue at the N terminus of the third transmembrane domain.
The TRPV subfamily of the transient receptor potential (TRP) family of cation channels consists of at least six mammalian channels homologous to the vanilloid receptor (for a unifying nomenclature, see ref. 1). The TRPV channels are activated by a variety of signals, including chemical and thermal stimuli, cell swelling, low intracellular Ca2+, and endogenous or synthetic ligands (2–10). Members of this subfamily contain a hydrophobic core region comprising six putative transmembrane segments (TM1–TM6), a pore-loop region between TM5 and TM6, a cytoplasmic N terminus with three to six ankyrin repeats, and a cytoplasmic C terminus (1, 3). The TRPV subfamily can be subdivided into two groups. One group is formed by TRPV1–TRPV4, which display a moderate Ca2+ selectivity (PCa/PNa < 10, in which P is permeability), a weak field-strength monovalent cation permeability sequence, and steep temperature dependence (5, 6, 11–16). The second group is formed by TRPV5 and TRPV6, which are highly Ca2+ selective (PCa/PNa > 100) and display a permeability sequence for monovalent cations consistent with a strong field-strength binding site but show little temperature dependence (10, 17, 18).
TRPV4 was identified originally as a channel activated by hypotonic cell swelling (11, 13, 19), but later reports show that it can be activated also by synthetic agonists, such as the phorbol ester 4α-phorbol 12,13-didecanoate (4α-PDD) (5), temperatures >27°C (6, 20), and acidic pH and citrate (ref. 21; see also ref. 5). Moreover, recent findings from our group indicate that the endocannabinoid anandamide and its hydrolysis product arachidonic acid (AA) are potent endogenous agonists for TRPV4 channels acting through the cytochrome P450 epoxygenase-dependent formation of 5′,6′-epoxyeicosatrienoic acid (5′,6′-EET) (22). Recent in vivo studies in mouse and rat suggest a role for TRPV4 as an osmosensor and mechanosensor in sensory nerves and brain (21, 23, 24). Although TRPV4 has the potential to act as an integrator of diverse physical and chemical signals, little is known about its gating mechanism. In particular, it is not known whether these different stimuli activate the channel by means of a common pathway or separate pathways.
Studies addressing the gating mechanism of TRPV4 by cell swelling exclude potential pathways such as membrane stretch (11), intracellular ionic strength, and G proteins (12). In a recent study, evidence was presented that activation of TRPV4 by hypotonicity involves its phosphorylation by Lyn, a member of the Src family of tyrosine kinases (25). Here, we present data that contradict the conclusions of this study on the role of tyrosine phosphorylation (25), and we present evidence for an alternative activation pathway. Given that cell swelling activates phospholipase A2 (PLA2) and PLA2-dependent AA release in several cell types (26–28), we explored whether this mechanism might be involved in TRPV4 activation. Our data suggest that cell swelling couples to TRPV4 by means of the PLA2-dependent formation of AA and its subsequent cytochrome P450 epoxygenase-dependent metabolization to 5′,6′-EET. Activation of TRPV4 by 4α-PDD and heat is fully independent of PLA2 and cytochrome P450 epoxygenase but requires an aromatic residue on position 555 near the N terminus of the third transmembrane domain (TM3). These data demonstrate that physical and chemical stimuli use at least two distinct pathways for activating TRPV4.
Materials and Methods
Cell Culture and Transfection. Human embryonic kidney (HEK)-293 cells were grown in DMEM containing 10% (vol/vol) human serum, 2 mM l-glutamine, 2 units/ml penicillin, and 2 mg/ml streptomycin at 37°C in a humidity-controlled incubator with 10% CO2/90% air atmosphere. HEK-293 cells were transiently transfected with the murine TRPV4 (mTRP12; European Molecular Biology Laboratory database accession no. CAC20703) vector, cloned as a BamHI fragment into the BclI site of the pCAGGS/IRES-GFP vector, which allows detection of transfected cells based on their green fluorescence when illuminated at 475 nm. For transfection, we used l-alanyl-l-glutamine (VWR International, Leuven, Belgium) and Mirus TransIT-293 (Mirus, Madison, WI). GFP-negative cells from the same batch were used as controls.
Site-Directed Mutagenesis. All mutants were obtained by means of the standard PCR overlap extension technique. The plasmid encoding the mTRPV4 receptor was denatured and annealed to a mutagenic primer. The resulting fragments were connected in a final overlap PCR. The chimerical overlap PCR fragments were inserted into the vector by using BsaI as the restriction enzyme. The sequence of all mutants was verified by sequence analysis.
Solutions. The standard extracellular solution for electrophysiological measurements contained 150 mM NaCl, 6 mM CsCl, 1 mM MgCl2, 5 mM CaCl2, 10 mM glucose, and 10 mM Hepes, pH 7.4 with NaOH. The osmolarity of this solution, as measured with a vapor pressure Wescor 5500 osmometer (Schlag, Gladbach, Germany), was 320 ± 5 milliosmolar. For measuring swelling-activated currents, we used an isotonic solution containing 105 mM NaCl, 6 mM CsCl, 5 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, 90 mM d-mannitol, and 10 mM glucose, pH 7.4 with NaOH (320 ± 5 milliosmolar). Cell swelling was induced by omitting mannitol from this solution (giving 240 milliosmolar, a 25% reduction of osmolarity).
The pipette solution was composed of 20 mM CsCl, 100 mM cesium aspartate, 1 mM MgCl2, 10 mM Hepes, 4 mM Na2ATP, 10 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA), and 0.08 mM CaCl2. The free Ca2+ concentration of this solution is ≈1 nM, which reliably prevented the activation of the endogenous VRAC in HEK-293 cells (29). In preliminary experiments using a 200 nM Ca2+ pipette solution, we observed that cell swelling activated large currents by means of VRAC, as described (30). The non-PKC-activating phorbol ester 4α-PDD (Sigma) was applied at a concentration of 1 μM from a 10 mM stock solution in ethanol. AA (Sigma) was used at a concentration of 10 μM from a 10 mM stock solution in water. In some experiments, the cells were incubated with 4-bromophenacyl bromide (pBPB; 100 μM; Sigma), 3-[(4-octadecyl)benzoyl]acrylic acid (OBAA; 100 μM; Calbiochem), arachidonyl trifluoromethyl ketone (AACOCF3; Calbiochem; 20 μM), N-(p-amylcinnamoyl)anthranilic acid (ACA; Calbiochem; 20 μM), miconazole (10 μM; Sigma), or 17-octadecynoic acid (17-ODYA; 10 μM; ICN) for 15 min before the addition of the various activation stimuli. The tyrosine kinase inhibitors PP1 (10 μM; Biomol, Plymouth Meeting, PA) and genistein (100 μM; Biomol) were applied to the extracellular medium for 30 min before the application of the various activating stimuli. In the case of activation by heat, the perfusate was warmed by using a water jacket device. We changed the temperature in the bath from 22°Cto43°C in 50 s. Other experiments were performed at room temperature (22–25°C).
Electrophysiological Recordings. Whole-cell membrane currents were measured with an EPC-9 amplifier (HEKA Electronics, Lambrecht, Germany) by using ruptured patches. Patch electrodes had a dc resistance of between 2 and 4 MΩ when filled with intracellular solution. An Ag–AgCl wire was used as a reference electrode. Capacitance and access resistance were monitored continuously. To minimize voltage errors, 50–70% of the series resistance was electronically compensated. We applied a ramp protocol, consisting of a voltage step from the holding potential of 0 mV to -100 mV, followed by a 400-ms linear ramp to 100 mV. This protocol was repeated every 5 s. Cell membrane capacitance values were used to calculate current densities.
Measurement of Intracellular Ca2+ Concentration ([Ca2+]i). [Ca2+]i was measured in parallel experiments with a monochromator-based imaging system consisting of a Polychrome IV monochromator (TILL Photonics, Planegg, Germany) and a charge-coupled device camera (Roper Scientific, Ottobrunn, Germany) connected to an Axiovert 200M inverted microscope (Zeiss). The monochromator and camera were controlled by the METAFLUOR (Version 4.65; Universal Imaging, Downingtown, PA) software running on a PC with a Pentium III processor. Cells were loaded with Fura-2 by incubation for 20 min at 37°C in a standard extracellular solution containing 2 μM Fura-2 acetoxymethyl ester. For [Ca2+]i measurements, fluorescence was measured during excitation at 340 nm and 380 nm. After correction for the individual background fluorescence signals, the ratio of the fluorescence at both excitation wavelengths (F340/F380) was monitored simultaneously in up to 30 transfected cells and nontransfected control cells from within a single view field. For every condition, at least 20 cells in at least three independent experiments were assayed.
To convert the fluorescence ratio R into absolute Ca2+ concentration, we used the equation [Ca2+] = Keff(R - Ro)/(R1 - R), where Keff, Ro, and R1 are calibration constants, which have to be determined for a given set-up. Ro was determined as the fluorescence ratio in cells incubated for 20 min with 10 μM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate acetoxymethyl ester and perfused with Ca2+-free medium. R1 was estimated from the maximal fluorescence ratio on stimulation with 1 μM ionomyocin in a solution containing 30 mM Ca2+. The three constants are related to Kd, the dissociation constant of the indicator dye, and α, the isocoefficient, by the equation Keff = Kd(R1 + α)/(Ro + α). The isocoefficient α was determined as described by Zhou and Neher (31). Kd values for Fura-2 were taken from Paltauf-Dobuzynska and Graier (32), taking into account the temperature-dependent changes in Fura-2 affinity.
There was some inherent variability in basal [Ca2+]i and in the amplitude of TRPV4-dependent Ca2+ signals in different batches of transfected cells. Therefore, the test conditions were always compared with suitable controls from the same batches of cells.
Data Analysis. Electrophysiological data were analyzed by using WINASCD software (Guy Droogmans, available at ftp://ftp.cc.kuleuven.ac.be/pub/droogmans/winascd.zip). The ORIGIN 6.1 software package (OriginLab, Northampton, MA) was used for statistical analysis and data display. Data are expressed as mean ± SEM. Significant difference between individual groups was tested by using Student's t test or ANOVA.
Results
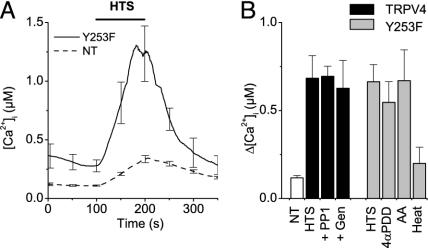
Hypotonicity-Induced Activation of TRPV4 Is Not Mediated by Tyrosine Phosphorylation. Recently, Xu et al. (25) reported that hypotonic stress results in phosphorylation of TRPV4 by Lyn, a member of the Src family of tyrosine kinases, and that this phosphorylation is essential for hypotonicity-induced channel activation. In particular, they described that hypotonic stress resulted in the PP1- and genistein-sensitive phosphorylation of TRPV4 at residue Tyr-253 and that cells expressing the Y253F mutant no longer responded to hypotonic cell swelling. However, we were unable to reproduce this crucial finding. As shown in Fig. 1A, application of hypotonic solution to HEK-293 cells expressing the Y253F mutant induced a robust increase in [Ca2+]i to a level that was virtually identical to the level in cells expressing WT TRPV4 (Fig. 1B). This hypotonicity-induced Ca2+ increase was abolished in the absence of extracellular Ca2+ and blocked by 10 μM ruthenium red (data not shown), as it was in cells expressing WT TRPV4. Moreover, pretreatment of TRPV4-transfected cells with the tyrosine kinase inhibitors PP1 and genistein at concentrations that strongly inhibit phosphorylation of Tyr-253 (25) did not affect the hypotonicity-induced changes in [Ca2+]i (Fig. 1B). Cells expressing the Y253F mutant also displayed a robust increase in [Ca2+]i in response to other TRPV4-activating stimuli (Fig. 1B). Taken together, these results indicate that phosphorylation of Tyr-253 is not essential for activation of TRPV4, and they prompted us to investigate alternative activation pathways for the hypotonicity-induced activation of the channel.
Fig. 1.
Tyr-253 is not required for the hypotonicity-induced activation of TRPV4. (A) Effect of stimulation with 25% hypotonic solution on [Ca2+]i in nontransfected (NT) HEK cells (dashed line) and cells transfected with the Y253F mutant (solid line). (B, left) Average [Ca2+]i increases induced by hypotonic stimulation in NT cells (n = 31), WT TRPV4-transfected cells (HTS, n = 32), and TRPV4-transfected cells treated with the tyrosine kinase inhibitors PP1 (10 μM; n = 22) or genistein (Gen; 100 μM; n = 23). (B, right) Average [Ca2+]i increases in Y253F-transfected cells induced by hypotonic cell swelling (HTS; n = 22), 1 μM4α-PDD (4αPDD; n = 18), 10 μMAA(n = 21), and heating to 42°C (Heat; n = 22). For every condition, data from at least three independent experiments were pooled.
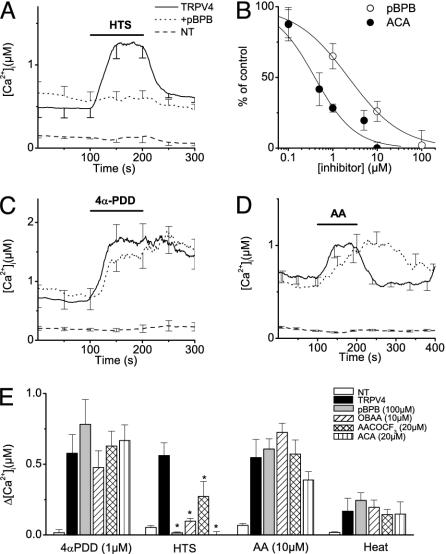
Swelling-Induced Activation of TRPV4 Requires PLA2 Activity. Cell swelling has been demonstrated to activate PLA2 in several cell types (26–28). Given that PLA2 activity causes release of fatty acids from membrane phospholipids, we asked whether swelling-induced activation of TRPV4 might occur by means of PLA2-dependent release of AA. Therefore, we tested the effect of four structurally unrelated PLA2 inhibitors (pBPB, ACA, OBAA, and AACOCF3) on changes in [Ca2+]i on hypotonic cell swelling. In agreement with the findings described in refs. 11, 13, 19, and 22, TRPV4-expressing cells had significantly higher basal [Ca2+]i than nontransfected controls (Fig. 2 A, C, and D; see also Fig. 7A, which is published as supporting information on the PNAS web site). Note that this finding was also the case for the Y253F mutant (Figs. 1A and 7A). This higher basal [Ca2+]i can be attributed to the basal TRPV4 channel activity in the absence of stimuli (11, 12, 33). The finding that [Ca2+]i in nonstimulated cells is not significantly affected by preincubation with the various PLA2 blockers (Fig. 2 A, C, and D; see also Fig. 7B) indicates that this basal activity of TRPV4 does not depend on PLA2. As shown in Fig. 2A, the TRPV4-dependent increase in [Ca2+]i, induced by extracellular hypotonicity, was prevented in the presence of 100 μM pBPB. The concentration of pBPB for half-maximal inhibition of the [Ca2+]i increase was 2.3 μM (Fig. 2B). ACA and OBAA were even more potent inhibitors of the hypotonicity-induced increase in [Ca2+]i, with half-maximal inhibition at concentrations of 400 nM and 1.3 μM, respectively (Fig. 2 B and E and data not shown). AACOCF3 was less potent, reducing the hypotonicity-induced Ca2+ increase by ≈50% at 20 μM (Fig. 2E).
Fig. 2.
Effect of the PLA2-inhibitor pBPB on [Ca2+]i in HEK cells expressing WT TRPV4. (A) Effect of stimulation with 25% hypotonic solution on [Ca2+]i in nontransfected (NT) HEK cells (dashed line), TRPV4-transfected cells (solid line), and TRPV4-transfected cells treated with 100 μM pBPB (dotted line). Note the significantly higher basal [Ca2+]i levels in TRPV4-transfected cells (see Fig. 7). (B) Dose–response curve for the inhibition of the hypotonicity-induced [Ca2+]i increase by pBPB (○) and ACA (•). (C and D) Same as A, except that stimulation was with 1 μM 4α-PDD (C) or 10 μM AA (D). (E) Average [Ca2+]i increases in response to 1 μM4α-PDD, 25% hypotonic solution, 10 μM AA, and heating to 42°C in NT cells and TRPV4-transfected cells treated with four different PLA2 inhibitors (100 μM pBPB, 10 μM OBAA, 20 μM AACOCF3, and 20 μM ACA, respectively). For every condition, n ≥ 16 in at least three independent experiments. *, P < 0.05, compared with nontreated TRPV4-expressing cells.
In contrast, preincubation with pBPB (Fig. 2C) or the other PLA2 inhibitors did not affect the increase in [Ca2+]i in response to 1 μM 4α-PDD (Fig. 2E), the most potent synthetic TRPV4 agonist known so far. Likewise, these PLA2 inhibitors did not significantly affect the amplitude of the TRPV4-dependent increase in [Ca2+]i induced by AA or heat (Fig. 2 D and E). We noted, however, that the response to AA was slower and that the Ca2+ transient was prolonged in the presence of all four PLA2 inhibitors (Fig. 2D and data not shown). A possible explanation for this effect might be that PLA2 inhibition results in lower basal AA levels and/or in altered activity of AA-metabolizing enzymes. The response to 5′,6′-EET in TRPV4-expressing cells was unaffected also by PLA2 blockade: a low dose (500 nM) of 5′,6′-EET increased [Ca2+]i by 161 ± 38 nM (n = 17) in cells preincubated with 20 μM ACA, compared with 127 ± 29 nM (n = 24) in nontreated cells.
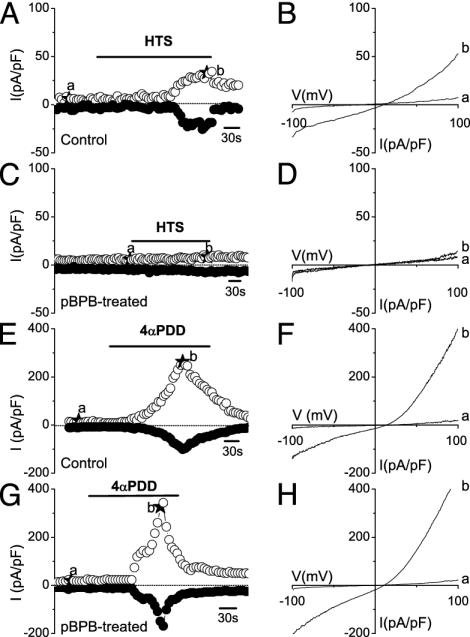
To exclude the possibility that PLA2 inhibitors affected the Ca2+ metabolism of TRPV4-expressing cells rather than the regulation of the channel itself, we measured TRPV4 channel activation directly by using the whole-cell patch-clamp technique. Currents through TRPV4 are outwardly rectifying cation currents characterized by a relative Ca2+ permeability ratio (PCa/PNa)of ≈6 and an Eisenman IV permeation sequence for monovalent cations (5, 16). Note that under the patch-clamp conditions used in this study (ruptured patches and strong Ca2+ buffering), little basal-current activity is detected in TRPV4-expressing cells (33). Application of a 25% hypotonic solution in the absence of PLA2 inhibitors caused activation of typical outwardly rectifying currents (Fig. 3 A and B). In agreement with the [Ca2+]i measurements, these currents were absent in pBPB-treated cells (Fig. 3 C and D). In contrast, application of 4α-PDD evoked robust outwardly rectifying currents in TRPV4-expressing cells (Fig. 3 E and F), which were unaffected by the pBPB pretreatment (Fig. 3 G and H). Note that WT TRPV4 current responses to osmotic swelling were always 5–10 times smaller than current responses to stimulation with 4α-PDD (Fig. 3), whereas the [Ca2+]i responses to these stimuli were of comparable magnitude (e.g., compare with Fig. 2 A and C). We attribute this apparent discrepancy to the rapid desensitization of TRPV4 in the continued presence of 4α-PDD, which limits the amount of Ca2+ influx and, thus, the amplitude of the Ca2+ signal. Additionally, some cellular compounds required for swelling-induced activation of TRPV4 may get lost in the whole-cell configuration and, hence, reduce the amplitude of the swelling-induced current (33).
Fig. 3.
Effect of PLA2 inhibitor pBPB on whole-cell currents in HEK cells expressing WT TRPV4. (A) Time course of whole-cell currents at -80 and 80 mV in a TRPV4-transfected HEK cell, showing current activation on stimulation with hypotonic solution (HTS). (B) Current–voltage relations obtained at the time points indicated in A. (C and D) Same as A and B, except that treatment was with 100 μM pBPB. Note that no current activation was observed. (E and F) Same as in A and B, except that TRPV4-transfected HEK cells were stimulated with 1 μM4α-PDD. (G and H) Same as in E and F, showing the lack of effect of pBPB on 4α-PDD-induced TRPV4 activation.
Taken together, the above-described [Ca2+]i measurements and current recordings indicate that activation of TRPV4 by cell swelling requires PLA2 activity, whereas 4α-PDD, AA, and heat activate TRPV4 in a PLA2-independent manner. Moreover, the finding that activation of TRPV4 by three stimuli is unaffected by all four PLA2 inhibitors argues against a direct effect of these compounds on TRPV4.
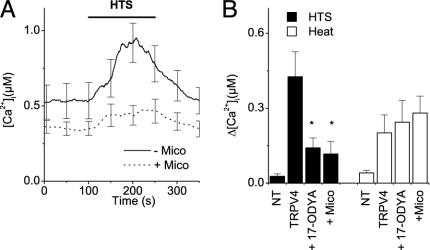
Blockers of Cytochrome P450 Epoxygenase Inhibit Swelling-Induced Activation of TRPV4. We have shown recently that AA activates TRPV4 in an indirect manner involving the cytochrome P450 epoxygenase-dependent formation of 5′,6′-EET, which opens the channel in a membrane-delimited manner (22). As shown in Fig. 4, miconazole, an inhibitor of cytochrome P450 epoxygenase, and 17-ODYA, an inhibitor of the cytochrome P450 epoxygenase and ω and ω-1 hydroxylases (22), strongly reduced the swelling-induced rise in [Ca2+]i in TRPV4-expressing cells. Taken together, the effects of the inhibitors of PLA2 and cytochrome P450 epoxygenase are suggestive of a model in which cell swelling activates TRPV4 by means of the PLA2-dependent production of AA and its subsequent metabolization to 5′,6′-EET (22). In contrast, activation of TRPV4 by heat (Fig. 4B) and 4α-PDD (22) was insensitive to miconazole and 17-ODYA, indicating that these stimuli couple to the channel by means of separate pathways.
Fig. 4.
Blockers of cytochrome P450 epoxygenase inhibit the hypotonicity-induced activation of TRPV4. (A) Effect of stimulation with 25% hypotonic solution on [Ca2+]i in nontreated TRPV4-transfected HEK cells (solid line) and TRPV4-transfected HEK cells treated with miconazole (10 μM; dashed line). (B) Effect of miconazole (10 μM) and 17-ODYA (10 μM) on the elevation of [Ca2+]i induced by hypotonic stimuli (filled bars) and heating to 42°C (open bars). For every condition, n ≥ 22 in at least three independent experiments. *, Significant differences, compared with nontreated TRPV4-expressing cells.
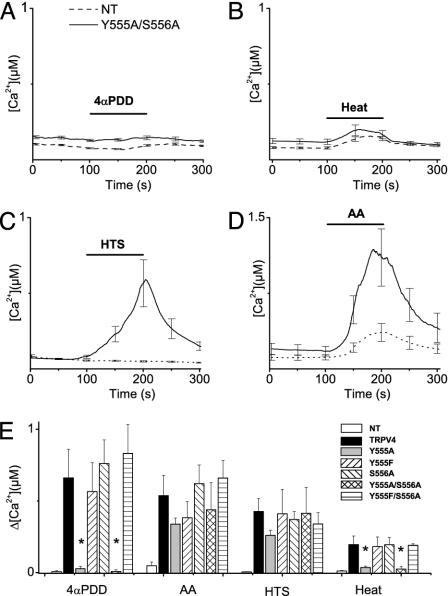
A Tyrosine Near the N Terminus of TM3 Is Crucial for Activation by Heat and 4α-PDD. In a recent report, Jordt and Julius (34) showed that a YS motif in the TM2–TM3 linker domain of TRPV1 is involved in the binding of the TRPV1 ligand capsaicin. Although this YS motif of TRPV1 is not conserved in TRPV4, a YS sequence (Tyr-555 and Ser-556) is found at the putative N terminus of TM3, a few amino acids closer to the C terminus of TRPV4. To investigate the role of these residues in channel activation, we generated a double mutant (Y555A/S556A) in which both residues were replaced by alanine.
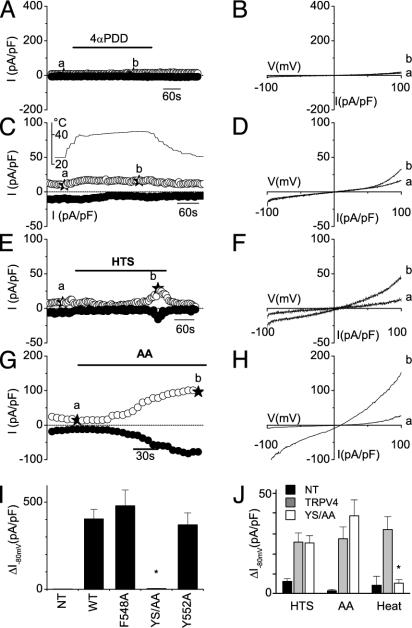
Basal [Ca2+]i levels in HEK cells expressing the Y555A/S556A mutant were significantly lower than they were in cells expressing WT TRPV4, and they were comparable with those in nontransfected cells (Fig. 5; see also Fig. 7). Moreover, HEK cells expressing the Y555A/S556A mutant did not show any [Ca2+]i increase in response to 1 μM 4α-PDD (Fig. 5A) or heating to 42°C (Fig. 5B). However, the Y555A/S556A mutant still formed a functional channel because stimulation by 10 μM AA (Fig. 5C), hyposmotic cell swelling (Fig. 5D), and 500 nM 5′,6′-EET (a [Ca2+]i increase of 116 ± 5 nM, n = 15) caused increases in [Ca2+]i that were comparable with the response of WT TRPV4 (see Fig. 2). In agreement with the [Ca2+]i measurements, stimulation with 1 μM 4α-PDD (Fig. 6 A, B, and I) or heating to 42°C (Fig. 6 C, D, and J) failed to induce a significant current increase in cells expressing the Y555A/S556A mutant, whereas typical TRPV4 currents could be induced by hyposmotic cell swelling (Fig. 6 E, F, and J) and 10 μM AA (Fig. 6 G, H, and J). To test whether the Y555A/S556A modifies the shape of the current–voltage relation of TRPV4, we quantified the degree of outward rectification by measuring the ratio of outward to inward current at +80 and -80 mV. This calculation yielded a value of 1.28 ± 0.09 for the Y555S/S556A mutant, which is not significantly different from the value for WT TRPV4 (1.22 ± 0.05).
Fig. 5.
Effect of various activation stimuli on [Ca2+]i in HEK cells expressing the TRPV4 YS mutant (Y555A/S556A). (A–D) Time course of Ca2+ concentration in nontransfected cells (dashed line) and cells expressing the YS mutant (solid line) on stimulation with 1 μM4α-PDD (n = 65) (A), heating to 42°C(n = 34) (B), 25% hypotonic solution (n = 27) (C), or 10 μM AA (n = 24) (D). (E) Average [Ca2+]i increases in nontransfected HEK cells and HEK cells transfected with TRPV4 WT, Y555A, Y555F, S556A, Y555A/S556A, and Y555F/S556A in response to 1 μM 4α-PDD, 10 μM AA, 25% hypotonic solution, and heating to 42°C, respectively. For every condition, n ≥ 17 in at least three independent experiments. *, Significant differences, compared with cells expressing WT TRPV4.
Fig. 6.
Effect of the YS mutation on activation of TRPV4 currents by agonist stimulation and hypotonic swelling. (A–H) Time course of whole-cell currents at -80 and +80 mV (A, C, E, and G) and current–voltage relations obtained at the indicated time points (B, D, F, and H) in HEK cells expressing the Y555A/S556A mutant, showing the lack of response to 4α-PDD (A and B) and heating to 42°C (C and D) and current activation on stimulation with 25% hypotonic solution (E and F) and 10 μMAA(G and H). (I) Maximal increases in inward-current density at -80 mV on stimulation with 1 μM 4α-PDD in nontransfected cells (n = 7), cells expressing WT TRPV4 (n = 6), and the F548A (n = 5), Y555A/S556A (YS/AA; n = 11), and Y552A (n = 6) mutants. (J) Comparison of the average inward current activated by 25% hypotonicity, 10 μM AA, and heating to 42°C in nontransfected cells and cells expressing WT and mutant TRPV4 (n = 5–13). *, Significant differences, compared with cells expressing WT TRPV4.
Next, we tested the molecular requirements for 4α-PDD and heat activation in more detail by substituting different amino acids for Tyr-555 and Ser-556. Mutating Tyr-555 to alanine (Y555A mutant) strongly reduced activation by 1 μM 4α-PDD and heating to 42°C but did not impair activation by 10 μM AA or hyposmotic cell swelling (Fig. 5E). Moreover, basal Ca2+ levels in cells expressing Y555A were significantly lower than they were in cells expressing WT TRPV4, and they were comparable with the basal Ca2+ levels of nontransfected cells (see Fig. 7). In contrast, mutating Ser-556 to alanine (S556A mutant) had no effect on basal Ca2+ or on the ability of the channel to respond to 4α-PDD, heating, AA, or hyposmotic cell swelling (Figs. 5E and 7). Thus, Tyr-555, rather than the full YS motif, is important for activation by 4α-PDD and heat. Next, we tested whether the hydroxyl moiety on residue 555 is an important determinant of TRPV4 activation by substituting a phenylalanine for Tyr-555. As shown in Fig. 5E, mutants Y555F and Y555F/S556A displayed Ca2+ responses to 4α-PDD, heating, AA, or hyposmotic cell swelling that were not significantly different from that of WT TRPV4. Taken together, these data indicate that the presence of an aromatic residue at position 555 is required for activation of TRPV4 by 4α-PDD and heat but is dispensable for activation by AA or cell swelling.
As a final test of the specificity of mutating Tyr-555, we made two additional mutants (F548A and Y552A) in which the two nearby aromatic residues (Phe-548 and Tyr-552) were replaced by alanine. Both mutants displayed robust current activation by 1 μM4α-PDD (Fig. 6I), indicating that these aromatic residues are not critically involved in channel activation.
Discussion
Mammalian homologues of the Drosophila TRP channel gene encode a family of at least 21 ion channel proteins. They are distributed widely in mammalian tissues, but their specific physiological functions and activation mechanisms are often unclear. TRPV4, a member of the TRPV subfamily, was originally considered as a Ca2+-permeable sensor of cell volume (11–13, 19), but later reports demonstrated that it can also be activated by phorbol esters (5), moderate heat (6, 20), and AA metabolites (22). It is presently unknown how these completely different stimuli converge on a single molecular event: the opening of the TRPV4 conduction pathway.
Recently, Xu et al. (25) suggested that activation of TRPV4 in response to hypotonicity was mediated by Lyn kinase-dependent phosphorylation of residue Tyr-253. However, our present results do not support such a mechanism. First, we found no inhibitory effect of tyrosine kinase inhibitors on the activation of TRPV4. Second and most important, hypotonic cell swelling fully activated the Y253F mutant. Thus, although we cannot exclude the possibility that phosphorylation of Tyr-253 has a modulatory effect on TRPV4 activation, we conclude that activation on hypotonic cell swelling occurs by means of a different pathway.
Two observations prompted us to investigate a possible involvement of a PLA2-dependent pathway in the hypotonicity-induced activation of TRPV4. First, it has been demonstrated in a number of cell types that cell swelling leads to the activation of PLA2, resulting in the release of polyunsaturated fatty acids, including AA, from membrane phospholipids (26–28). Second, we found recently that AA acts as a potent activator of TRPV4 (22). Our present results demonstrate that PLA2 activity is indeed necessary for TRPV4 activation by cell swelling. Four structurally unrelated PLA2 blockers inhibited swelling-induced Ca2+ signals and whole-cell currents in TRPV4-expressing cells. The PLA2 blockers had no effect on channel activation by 4α-PDD, AA, or heat, excluding the possibility of a direct effect of these substances on TRPV4. Moreover, we found that blockers of cytochrome P450 epoxygenase prevent activation of TRPV4 by hypotonicity. These blockers prevent metabolization of AA to 5′,6′-EET, which acts as a membrane-delimited activator of TRPV4 (22). Based on these data, we suggest that cell swelling is coupled to TRPV4 activation by means of a sequence of events consisting of (i) swelling-induced activation of PLA2, (ii) PLA2-dependent release of AA from membrane phospholipids, (iii) cytochrome P450 epoxygenase-dependent metabolization of AA to 5′,6′-EET, and (iv) activation of TRPV4 by 5′,6′-EET.
Also, our present results provide evidence that the molecular requirements for gating of TRPV4 by 4α-PDD and heat are different from the molecular requirements for gating by cell swelling and AA. First, activation by 4α-PDD and heat is insensitive to inhibition of PLA2 and cytochrome P450 epoxygenase. Moreover, we demonstrated that Tyr-555 at the N terminus of TM3 is crucial for the activation by 4α-PDD and heat but not for activation by hypotonic cell swelling, AA, or 5′,6′-EET. Interestingly, responses to 4α-PDD and heat were not affected by replacement of Tyr-555 with phenylalanine, but they were strongly impaired when Tyr-555 was replaced with alanine. Thus, the presence of an aromatic residue, rather than the tyrosine hydroxyl moiety at position 555, seems to be the important determinant for activation.
Several mechanisms could explain the lack of stimulation by 4α-PDD and heat in the TRPV4 Y555A and Y555A/S556A mutants. First, there could be a defect in trafficking of these mutant channels to the surface membrane. However, this possibility seems unlikely given that they can still be activated by hypotonic cell swelling, AA, and 5′-6′-EET. Second, Tyr-555 could be involved in the binding of 4α-PDD, either directly or by means of accessory proteins. Finally, Tyr-555 may not be involved directly in 4α-PDD binding, but, rather, it may be a structural element necessary for the transduction of the signal of ligand binding to the opening of the channel. At present, we cannot discriminate between the latter two possibilities. This determination would require the direct measurement of 4α-PDD binding to membranes containing WT and mutant TRPV4, similar to the demonstration of the capsaicin binding site in TRPV1 (34). In contrast to TRPV1, which reportedly is activated directly by heat, recent findings indicate that heat activates TRPV4 in cell-attached patches but not in cell-free inside-out patches (6, 35). This finding might indicate that heat activation of TRPV4 requires production of a soluble TRPV4-activating molecule. Our finding that Tyr-555 is essential for activation of TRPV4 not only by the direct TRPV4 ligand 4α-PDD but also by heat could point to the existence of an endogenous phorbol ester-like TRPV4 activator produced by heating.
TRPV4 displays some basal activity in the absence of a clear stimulus, which can be appreciated from the spontaneous single-channel openings in cell-attached patches (11), the increased basal whole-cell currents shortly after establishment of the whole-cell configuration (12, 33), and the higher basal Ca2+ levels in TRPV4-transfected cells (11, 13, 19, 22). Interestingly, we found that cells expressing mutants Y555A and Y555A/S556A had significantly lower basal Ca2+ levels compared with WT TRPV4, suggesting that basal activity depends also on Tyr-555. Possibly, basal activity represents channel activity at room temperature, which is no longer seen in the less heat-sensitive Y555A and Y555A/S556A mutants.
Previous studies have shown an interaction between heat- and swelling-evoked activation of TRPV4: responses to hypotonic cell swelling are potentiated at elevated temperatures (13), whereas heat-evoked responses are diminished in hypertonic conditions (20). Our finding that heat-evoked responses are abolished in the Y555A and Y555A/S556A mutants, whereas activation by cell swelling is preserved, implies that some degree of independence exists between these two physical TRPV4 stimuli. Normal heat activation in the presence of cytochrome P450 epoxygenase inhibitors confirms this assertion. An analogous situation has been observed for TRPV1, in which protons, heat, and capsaicin show positive cooperativity in activating the channel, although they act on the channel by means of mechanistically distinguishable pathways (36, 34). Apparently, TRPV channels are endowed with multiple activating and regulatory sites that allow them to integrate distinct physical and chemical stimuli from the environment.
In conclusion, our data indicate that cell swelling activates TRPV4 by means of the PLA2-dependent formation of AA, which is then metabolized further by cytochrome P450 epoxygenase to form the TRPV4-activating messenger 5′-6′EET. Activation by 4α-PDD and heat occurs by means of a distinct PLA2- and cytochrome P450 epoxygenase-independent pathway and involves a Tyr-555 at the N-terminal part of TM3. Our results give insight into the structure–function relation of TRPV4 gating and the mechanisms whereby TRPV4 integrates thermal, chemical, and mechanical stimuli in the opening of a single Ca2+-permeable pore.
Supplementary Material
Acknowledgments
We thank Prof. Veit Flockerzi and Dr. Ulrich Wissenbach (Universität des Saarlandes, Homburg, Germany) for providing the mTRP12 clone. We acknowledge the help of Prof. Minne Casteels (Katholieke Universiteit Leuven) for critical evaluation of our data on cell swelling. T.V. is a Postdoctoral Fellow of the Fund for Scientific Research–Flanders (FWO–Vlaanderen). This work was supported by the Belgian Federal Government; the Flemish Government; Onderzoeksraad Katholieke Universiteit Leuven (Leuven, Belgium) Grants GOA 99/07, FWO G.0237.95, FWO G.0214.99, FWO G.0136.00, and FWO G.0172.03; and the Belgian Prime Minister's Office Interuniversity Poles of Attraction Program.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TRP, transient receptor potential; 5′,6′-EET, 5′,6′-epoxyeicosatrienoic acid; AA, arachidonic acid; PLA2, phospholipase A2; 4α-PDD, 4α-phorbol 12,13-didecanoate; TM3, third transmembrane domain; pBPB, 4-bromophenacyl bromide; OBAA, 3-[(4-octadecyl)benzoyl]acrylic acid; AACOCF3, arachidonyl trifluoromethyl ketone; ACA, N-(p-amylcinnamoyl)anthranilic acid; 17-ODYA, 17-octadecynoic acid; HEK, human embryonic kidney; [Ca2+]i, intracellular Ca2+ concentration.
References
- 1.Montell, C., Birnbaumer, L., Flockerzi, V., Bindels, R. J., Bruford, E. A., Caterina, M. J., Clapham, D., Harteneck, C., Heller, S., Julius, D., et al. (2002) Mol. Cell 9, 229-231. [DOI] [PubMed] [Google Scholar]
- 2.Clapham, D. E., Runnels, L. W. & Strubing, C. (2001) Nat. Rev. Neurosci. 2, 387-396. [DOI] [PubMed] [Google Scholar]
- 3.Harteneck, C., Plant, T. D. & Schultz, G. (2000) Trends Neurosci. 23, 159-166. [DOI] [PubMed] [Google Scholar]
- 4.Montell, C., Birnbaumer, L. & Flockerzi, V. (2002) Cell 108, 595-598. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe, H., Davis, J. B., Smart, D., Jerman, J. C., Smith, G. D., Hayes, P., Vriens, J., Cairns, W., Wissenbach, U., Prenen, J., et al. (2002) J. Biol. Chem. 277, 13569-13577. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe, H., Vriens, J., Suh, S. H., Benham, C. D., Droogmans, G. & Nilius, B. (2002) J. Biol. Chem. 277, 47044-47051. [DOI] [PubMed] [Google Scholar]
- 7.Hoenderop, J. G., Vennekens, R., Muller, D., Prenen, J., Droogmans, G., Bindels, R. J. & Nilius, B. (2001) J. Physiol. (London) 537, 747-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoenderop, J. G., Nilius, B. & Bindels, R. J. (2002) Annu. Rev. Physiol. 64, 529-549. [DOI] [PubMed] [Google Scholar]
- 9.Vennekens, R., Voets, T., Bindels, R. J., Droogmans, G. & Nilius, B. (2002) Cell Calcium 31, 253-264. [DOI] [PubMed] [Google Scholar]
- 10.Yue, L., Peng, J. B., Hediger, M. A. & Clapham, D. E. (2001) Nature 410, 705-709. [DOI] [PubMed] [Google Scholar]
- 11.Strotmann, R., Harteneck, C., Nunnenmacher, K., Schultz, G. & Plant, T. D. (2000) Nat. Cell Biol. 2, 695-702. [DOI] [PubMed] [Google Scholar]
- 12.Nilius, B., Prenen, J., Wissenbach, U., Bodding, M. & Droogmans, G. (2001) Pflügers Arch. 443, 227-233. [DOI] [PubMed] [Google Scholar]
- 13.Liedtke, W., Choe, Y., Marti-Renom, M. A., Bell, A. M., Denis, C. S., Sali, A., Hudspeth, A. J., Friedman, J. M. & Heller, S. (2000) Cell 103, 525-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caterina, M. J., Rosen, T. A., Tominaga, M., Brake, A. J. & Julius, D. (1999) Nature 398, 436-441. [DOI] [PubMed] [Google Scholar]
- 15.Caterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D. & Julius, D. (1997) Nature 389, 816-824. [DOI] [PubMed] [Google Scholar]
- 16.Voets, T., Prenen, J., Vriens, J., Watanabe, H., Janssens, A., Wissenbach, U., Bodding, M., Droogmans, G. & Nilius, B. (2002) J. Biol. Chem. 277, 33704-33710. [DOI] [PubMed] [Google Scholar]
- 17.Voets, T., Prenen, J., Fleig, A., Vennekens, R., Watanabe, H., Hoenderop, J. G. J., Bindels, R. J. M., Droogmans, G., Penner, R. & Nilius, B. (2001) J. Biol. Chem. 276, 47767-47770. [DOI] [PubMed] [Google Scholar]
- 18.Vennekens, R., Hoenderop, J. G., Prenen, J., Stuiver, M., Willems, P. H., Droogmans, G., Nilius, B. & Bindels, R. J. (2000) J. Biol. Chem. 275, 3963-3969. [DOI] [PubMed] [Google Scholar]
- 19.Wissenbach, U., Bodding, M., Freichel, M. & Flockerzi, V. (2000) FEBS Lett. 485, 127-134. [DOI] [PubMed] [Google Scholar]
- 20.Güler, A., Lee, H., Shimizu, I. & Caterina, M. (2002) J. Neurosci. 22, 6408-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki, M., Mizuno, A., Kodaira, K. & Imai, M. (2003) J. Biol. Chem. 278, 22664-22668. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe, H., Vriens, J., Prenen, J., Droogmans, G., Voets, T. & Nilius, B. (2003) Nature 424, 434-438. [DOI] [PubMed] [Google Scholar]
- 23.Alessandri-Haber, N., Yeh, J. J., Boyd, A. E., Parada, C. A., Chen, X., Reichling, D. B. & Levine, J. D. (2003) Neuron 39, 497-511. [DOI] [PubMed] [Google Scholar]
- 24.Mizuno, A., Matsumoto, N., Imai, M. & Suzuki, M. (2003) Am. J. Physiol. 285, C96-C101. [DOI] [PubMed] [Google Scholar]
- 25.Xu, H., Zhao, H., Tian, W., Yoshida, K., Roullet, J. B. & Cohen, D. M. (2003) J. Biol. Chem. 278, 11520-11527. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen, S., Lambert, I. H., Thoroed, S. M. & Hoffmann, E. K. (2000) Eur. J. Biochem. 267, 5531-5539. [DOI] [PubMed] [Google Scholar]
- 27.Basavappa, S., Pedersen, S. F., Jorgensen, N. K., Ellory, J. C. & Hoffmann, E. K. (1998) J. Neurophysiol. 79, 1441-1449. [DOI] [PubMed] [Google Scholar]
- 28.Thoroed, S. M., Lauritzen, L., Lambert, I. H., Hansen, H. S. & Hoffmann, E. K. (1997) J. Membr. Biol. 160, 47-58. [DOI] [PubMed] [Google Scholar]
- 29.Szucs, G., Heinke, S., Droogmans, G. & Nilius, B. (1996) Pflügers Arch. 431, 467-469. [DOI] [PubMed] [Google Scholar]
- 30.Nilius, B., Eggermont, J., Voets, T., Buyse, G., Manolopoulos, V. & Droogmans, G. (1997) Prog. Biophys. Mol. Biol. 68, 69-119. [DOI] [PubMed] [Google Scholar]
- 31.Zhou, Z. & Neher, E. (1993) J. Physiol. (London) 469, 245-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paltauf-Doburzynska, J. & Graier, W. F. (1997) Cell Calcium 21, 43-51. [DOI] [PubMed] [Google Scholar]
- 33.Strotmann, R., Schultz, G. & Plant, T. D. (2003) J. Biol. Chem. 278, 26541-26549. [DOI] [PubMed] [Google Scholar]
- 34.Jordt, S. E. & Julius, D. (2002) Cell 108, 421-430. [DOI] [PubMed] [Google Scholar]
- 35.Chung, M. K., Lee, H. & Caterina, M. J. (2003) J. Biol. Chem. 278, 32037-32046. [DOI] [PubMed] [Google Scholar]
- 36.Jordt, S. E., Tominaga, M. & Julius, D. (2000) Proc. Natl. Acad. Sci. USA 97, 8134-8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.