Abstract
Understanding the molecular principles of synaptic vesicle fusion is a long-sought goal. It requires the development of a synthetic system that allows manipulations and observations not possible in vivo. Here, we report an in vitro system with reconstituted synaptic proteins that meets the long-sought goal to produce fast content release in the millisecond time regime upon Ca2+ triggering. Our system simultaneously monitors both content and lipid exchange, and it starts from stable interacting pairs of donor and acceptor vesicles, mimicking the readily releasable pool of synaptic vesicles prior to an action potential. It differentiates between single-vesicle interaction, hemifusion, and complete fusion, the latter mimicking quantized neurotransmitter release upon exocytosis of synaptic vesicles. Prior to Ca2+ injection, the system is in a state in which spontaneous fusion events between donor and acceptor vesicles are rare. Upon Ca2+ injection, a rapid burst of complete fusion events emerges, followed by a biphasic decay. The present study focuses on neuronal SNAREs, the Ca2+ sensor synaptotagmin 1, and the modulator complexin. However, other synaptic proteins could be added and their function examined. Ca2+ triggering is cooperative, requiring the presence of synaptotagmin, whereas SNAREs alone do not produce a fast fusion burst. Manipulations of the system mimic effects observed in vivo. These results also show that neuronal SNAREs alone do not efficiently produce complete fusion, that the combination of SNAREs with synaptotagmin lowers the activation barriers to full fusion, and that complexin enhances this kinetic control.
Keywords: fast content mixing, single-vesicle fusion assay, membrane fusion, lipid mixing
Neuronal communication is made possible by the release of neurotransmitters, which in turn depends on the fusion of neurotransmitter-containing vesicles with the active zone in axonal terminals. Synaptic vesicle fusion is triggered by an influx of Ca2+ ions into the neuron upon depolarization. Neurotransmitter release is quantized (1); that is, it involves a few to tens of individual synaptic fusion events. The process of individual synaptic vesicle fusion is in turn controlled by a set of relatively few proteins, such as the SNARE proteins (2–5), the Ca2+ sensor for fast synchronous release synaptotagmin 1 (6–8), and the modulator complexin (9–11). Thus, neurotransmitter release is a macroscopic biological phenomenon that is ultimately controlled by a few individual molecules. The understanding of the underlying molecular mechanisms thus requires methods that are inherently capable of observing single vesicles and single molecules (12, 13).
Ideally, observations of single vesicles and single molecules would be performed in live neurons. Although progress for such studies has been made (14), they currently only provide limited information because the necessary genetic manipulations or labeling techniques may not provide the spatial and time resolution required for studying the dynamics of neurotransmitter release. Thus, there is an urgent need to develop minimal synthetic systems that, at least qualitatively, have the neurotransmitter release characteristics observed in neurons and that allow manipulations and observations not possible in vivo.
Previous attempts of in vitro reconstitutions of neurotransmitter release had fundamental limitations. Most of these previous systems did not directly probe the release of synaptic vesicle content, but rather the exchange of lipids between membranes. These experiments were based on the misconception that membrane lipid exchange (also referred to as “lipid mixing”) is indicative of content release (also referred to as “content mixing”). Lipid mixing (which causes partial fusion of membranes) is necessary, but not sufficient for pore formation between membranes and subsequent content release. For example, lipid mixing can occur without content mixing (15, 16), a phenomenon that has been observed in viral fusion where lipid mixing occurs several seconds before content mixing (17, 18), and vacuolar fusion (19). Furthermore, even inner leaflet mixing can occur without content mixing (16). There are other limitations of these earlier in vitro studies: Often only a subset of some of the key constituents were used, and all previous assays utilized a constant Ca2+ concentration, rather than a stepwise increase. It addition, synaptic vesicle fusion processes are inherently heterogeneous, so it is important to use single-particle and single-molecule techniques (12, 13) because observation of the average of many such vesicles may obscure an accurate view of the underlying processes.
Here, we describe a synthetic single-vesicle system with reconstituted synaptic proteins that overcomes the limitations of the previous studies. Our system addresses the long-standing challenge to produce a millisecond content release burst upon Ca2+ triggering, as monitored by a simultaneous content and lipid-mixing assay with single vesicles. It starts from stable interacting pairs of donor and acceptor vesicles, mimicking the so-called readily releasable pool of synaptic vesicles prior to Ca2+ injection (20). The system differentiates between single-vesicle interaction, hemifusion (or membrane lipid exchange), and complete fusion (i.e., pore formation), the latter mimicking quantized neurotransmitter release upon exocytosis of synaptic vesicles. Because single vesicles are observed and the time course of individual fusion events are monitored, the observations are analogous to quantized Ca2+-triggered neurotransmitter release of one or more synaptic vesicles. We limited this study to neuronal SNAREs [specifically, synaptobrevin-2, syntaxin-1A, and synaptosomal-associated protein 25A (SNAP-25A)], synaptotagmin 1, and complexin 1. However, other synaptic proteins could be added to the system and their function examined.
Early in vitro ensemble liposome studies showed that SNARE complex formation catalyzes lipid mixing (21). However, unlike neurotransmitter release, this process is constitutive and relatively slow (22, 23), even when preconditioning the system with a small C-terminal fragment (residues 49–96) of synaptobrevin (24), and, as mentioned above, these experiments did not measure content release, but rather lipid mixing. Likewise, a more recent single-vesicle experiment with SNAREs and full-length synaptotagmin 1 only monitored lipid mixing, and vesicles were observed in the presence of a constant Ca2+ concentration (25), so conclusions about Ca2+-triggered content release cannot be drawn from this study. An ex vivo cell-based assay with “flipped” SNAREs and other factors demonstrated that SNAREs are capable of promoting content exchange (26), but fusion kinetics is much slower (approximate 10 min timescale) than that of neurotransmitter release, even in the presence of synaptotagmin and complexin (11). Only very few liposome-based experiments included a content-mixing indicator in studies of neuronal SNARE-dependent fusion (23, 27–29). In these studies, fusion was relatively slow (23, 27), very rare (28), or only vesicle leakage instead of fusion was observed (29). Moreover, these early content-mixing studies did not investigate Ca2+-triggered fusion because synaptotagmin 1 and complexin were not present. Our results suggest that previous experiments that only used lipid-mixing indicators have to be repeated because lipid-mixing kinetics can be profoundly different from content-mixing kinetics.
The studies presented in this paper reveal insights in the molecular mechanism of Ca2+-triggered synaptic vesicle fusion. We find that neuronal SNAREs alone do not efficiently overcome the activation barriers to achieve full fusion between membranes. These activation barriers are lowered upon Ca2+ triggering when synaptotagmin 1 and SNAREs are combined, with further enhancement by complexin. Furthermore, the ability to observe single-vesicle fusion kinetics in our system revealed considerable heterogeneity of individual fusion events; such information would be lost in an ensemble experiment.
Results
Experimental Design.
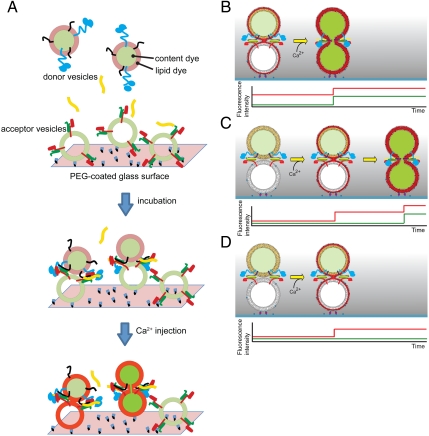
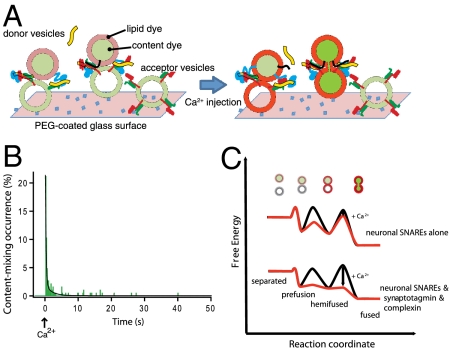
To study the kinetics of single-vesicle fusion, we developed a triple-fluorescence-based single-vesicle assay that can distinguish between vesicle–vesicle interaction, hemifusion, and complete fusion. The system starts from a metastable state of interacting donor and acceptor vesicles that are formed during an incubation period (Fig. 1A). Ca2+ buffer is then injected in order to rapidly reach a defined Ca2+ concentration in the sample chamber. We monitor the instance of Ca2+ injection by detecting the fluorescence intensity of the cascade-blue dye that is part of the injected Ca2+ buffer. For observing instances of vesicle interaction, hemifusion, and complete fusion, two spectrally distinct fluorescent dyes, sulforhodamin B and 1,1'-dioctadecyl-3,3,3',3' tetramethylindodicarbocyanine (DiD), are used as content and lipid markers, respectively, which are incorporated into donor vesicles in a self-quenched state. Donor vesicles that are bound to acceptor vesicles via SNARE protein–protein interactions are identified by the appearance of weak lipid dye fluorescence at a particular spot within the evanescent wave of the total internal reflection (TIR) microscope (Fig. S1). Lipid- or content-mixing events between single donor and acceptor vesicles that occur upon Ca2+ injection will produce stepwise fluorescence intensity increases for single donor vesicles due to dilution and concurrent dequenching of the dyes. The simultaneous observation of lipid and content fluorescent dyes differentiates among characteristic events involving single interacting pairs of donor and acceptor vesicles, such as complete fusion (Fig. 1 B and C, last stages), hemifusion (outer leaflet mixing, Fig. 1D, stage after Ca2+ injection), and interaction of vesicles without lipid mixing (Fig. 1 C and D, stages prior to Ca2+ injection). Furthermore, instances of vesicle leakage are differentiated from fusion events (Fig. S2); such leakage events occurred very infrequently in our system, with less than approximately 0.01% probability—this low leakage rate was accomplished in part by using the content dye sulforhodamine B instead of calcein used in our earlier work (30).
Fig. 1.
Experimental design of the single-vesicle content and lipid-mixing system. (A) Acceptor vesicles containing syntaxin (red) complexed with SNAP-25 (green) are tethered to a PEG-coated glass surface via biotin-neutravidin interactions. Protein-free tethered vesicles are added to fill the voids between the acceptor vesicles (not shown for clarity). Donor vesicles containing synaptobrevin (black) and synaptotagmin 1 (blue) are labeled with content indicator fluorescent dyes (light and dark green), and lipid analog fluorescent dyes (gray and red). Both fluorescent dyes are initially self-quenched. Donor vesicles are added to the sample chamber (the incubation period actually consists of two stages, see Materials and Methods) in the presence or absence of complexin (yellow). Donor vesicles that are interacting with acceptor vesicles are identified by the appearance of weak lipid dye fluorescence spots in the evanescent wave. After the incubation period, a Ca2+ solution at a specified concentration is rapidly injected into the sample chamber, effectively replacing the sample volume 20 times (Materials and Methods). The arrival of the Ca2+ solution in the evanescent wave is monitored by the fluorescence intensity of the cascade-blue dye that is part of the injected Ca2+ solution. (B) Expected lipid dye fluorescence intensity (red) and content dye fluorescence intensity (green) time traces for an immediate content-mixing event starting from a hemifused state—i.e., with the outer leaflets merged—prior to Ca2+ injection. Upon Ca2+ injection, the inner leaflets merge and complete fusion occurs. (C) Expected fluorescence intensity time traces for a delayed content-mixing event. Upon Ca2+ injection, the outer leaflets of interacting donor and acceptor vesicles merge (Upper). After a time delay, the inner leaflets are also merging, and complete fusion occurs, resulting in the expected fluorescence intensity time traces shown in the lower panel. (D) Expected fluorescence intensity time traces for a hemifusion event. Upon Ca2+ injection, the outer leaflets of interacting donor and acceptor vesicles merge without undergoing complete fusion during the observation time period (Upper), resulting in the expected time trace shown in the lower panel.
Characterization of Reconstituted Vesicles and Starting State of the System.
We performed a series of experiments to characterize the reconstituted donor (synaptobrevin, synaptotagmin 1) and acceptor (syntaxin/SNAP-25) vesicles. We used physiological compositions of donor (i.e., synaptic vesicle; ref. 31) and acceptor (i.e., active zone) membranes and physiological densities of the reconstituted proteins. The reconstituted vesicles exhibited a monodisperse size distribution as assessed by cryoelectron microscopy (cryo-EM) and dynamic light scattering (Figs. S3 and S4). The cryo-EM experiments also verified the integrity of the vesicles before and after fusion, and they indicate that the vesicles are unilamellar (Fig. S3 A–D).
We next determined the distribution of the number of protein molecules reconstituted into single vesicles by counting sequential photobleaching steps of dyes conjugated with proteins where only a fraction of the proteins were fluorescently labeled (Fig. S5). After correcting for the dilution factor and labeling efficiency, the average numbers of incorporated syntaxin, synaptobrevin, and synaptotagmin 1 molecules were 129 ± 11, 179 ± 14, and 28 ± 2 (for our 78-nm diameter vesicles), respectively. Thus, the reconstituted proteins have physiological densities as compared to observed distributions in synaptic vesicles with an average diameter of approximately 42 nm (32). More than 90% of syntaxin and synaptobrevin and 100% of synaptotagmin 1 molecules were properly reconstituted—that is, they point their cytosolic domains to the outside of the vesicles (Fig. S6). Purified syntaxin/SNAP-25 acceptor vesicles exhibited a 1∶1 protein ratio (Fig. S6A, left-most gel bands) suggesting that the majority of syntaxin molecules form productive 1∶1 binary acceptor complexes with SNAP-25 (33).
Acceptor vesicles were then tethered to a pacified PEG-coated surface (34, 35) (Materials and Methods). Donor vesicles with content and lipid dyes were added and allowed to bind to acceptor vesicles via protein–protein interactions. After a defined incubation period, excess donor vesicles were removed by extensive washing and, depending on the experiment, including complexin in the buffer.
To characterize the starting state of our system, we performed a single-vesicle lipid-mixing assay with SNAREs alone, starting data acquisition right after addition of donor vesicles to the sample chamber (Fig. S7). Donor vesicles that are bound to acceptor vesicles underwent both fast and slow lipid-mixing processes (i.e., hemifusion) with ca. 20% probability during the 500-s observation period of this experiment (without Ca2+). We interpret the fast process as instances of donor–acceptor vesicle pairs where a small number of trans SNARE complexes spontaneously form and trigger lipid mixing. The slower process could be related to diffusion of SNARE proteins in the vesicle membranes to form an encounter complex, followed by protein folding of a trans SNARE complex which is on the same order of magnitude as the slow lipid-mixing process that we observe (24, 36). The remaining ca. 80% of vesicles are simply interacting via trans SNARE complexes, but without lipid exchange. In all subsequent experiments, we used a sufficiently long second incubation period (30 min) after removing excess donor vesicles to ensure that such folding processes have completed and the amount of hemifusion (approximately 20% of the vesicle pairs) has reached a plateau. We generally observe only one donor vesicle bound to a single acceptor vesicle based on the measured subsequent lipid and content-mixing events; very rare instances of multiple content-mixing events were excluded from the analysis. Thus, our system has a well-defined starting state of donor vesicles that are bound to acceptor vesicles, akin to the readily releasable pool of primed synaptic vesicles at the active zone of a synapse (20).
Observation of Fast Complete Fusion upon Ca2+ Injection.
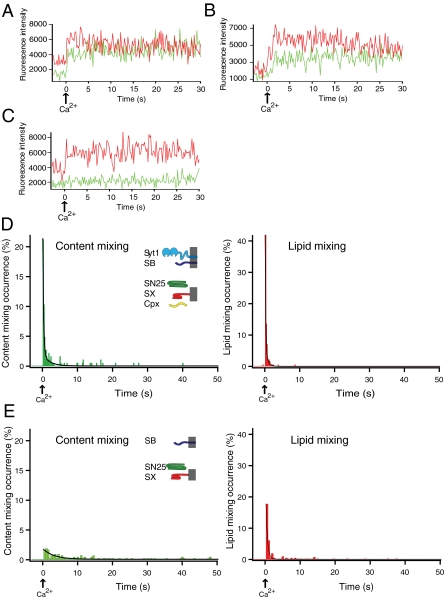
We injected Ca2+ into the sample chamber, starting from a set of single donor vesicles (containing both synaptobrevin and synaptotagmin 1) that are interacting with single acceptor vesicles (containing syntaxin/SNAP-25 acceptor complexes) in the presence of complexin (referred to as the “full system” in the following, although other factors are also important for synaptic vesicle fusion). The content and lipid dye fluorescence intensities rapidly increased for many vesicle pair spots upon Ca2+ injection (Movie S1), caused by dequenching of the dyes due to their respective mixing processes. Representative fluorescence intensity traces from single vesicles are shown in Fig. 2 A–C, revealing considerable heterogeneity of individual fusion pathways upon Ca2+ triggering. Content-mixing histogram analysis of all these individual traces revealed a rapid transition from approximately zero fusion to maximum fusion within one time bin (200 ms) upon injection of Ca2+—i.e., with a rise time (i.e., the characteristic time from zero fusion to maximum fusion) that is faster than the time resolution of our setup (Fig. 2D, Left) (in fact, the time to reach 50% of the peak has to be considerably less than 0.1 s because one would otherwise observe two time bins to reach the maximum value). This rapid onset of fusion is followed by a biphasic decay that could be fitted to a double exponential function (Fig. 2D, Left, black line). The overwhelming contribution is from a fast exponential decay with a time constant of 0.25 ± 0.04 s (SD), whereas the remaining, smaller contribution is from a slower decay with a time constant of 1.6 ± 0.9 s (SD). Over the course of the observation period, a majority population (61 ± 4%) underwent complete fusion, and minority populations exhibited hemifusion only (14 ± 4%) or no change in fluorescence intensity (25 ± 9%).
Fig. 2.
Fast Ca2+-triggered fusion kinetics. (A–C) Representative observed fluorescence intensity time traces for the full system [i.e., synaptobrevin (SB), syntaxin (SX), SNAP-25 (SN25), synaptotagmin 1 (Syt1), and complexin (Cpx)] for (A) immediate content mixing, (B) delayed content mixing, and (C) hemifusion only. Time t = 0 corresponds to the instance of 5 mM Ca2+ injection as determined by the appearance of cascade-blue fluorescence. A video showing the fluorescence of the content and lipid-mixing dyes in a field of view for one of multiple experiments is shown in Movie S1. (D) Representative histograms of the occurrence of Ca2+-triggered content- (Left) and lipid-mixing (Right) events for the full system using 200 ms time binning for one of multiple experiments (histograms were generated from n = 2357 individual traces). Events during a 3-s period before Ca2+ injection are also shown (if any). Time t = 0 corresponds to the instance of 5 mM Ca2+ injection as determined by the appearance of cascade-blue fluorescence. Histograms are normalized with respect to the number of interacting vesicles. The black lines are fits to the histograms [for content mixing, f(t) = 0.03 + 30.3 e-4.4t + 2.0 e-0.5t, and for lipid mixing, f(t) = 0.003 + 69.4 e-6.7t + 7.5 e-1.5t]. (E) Representative histograms of the occurrence of Ca2+-triggered content- (Left) and lipid-mixing (Right) events for neuronal SNAREs alone (500 ms time binning) for one of multiple experiments (histograms were generated from a total of 1,292 individual traces; n = 1292). The black lines are fits to the histograms [for content mixing, f(t) = 0.09 + 1.80 e-0.32t, and for lipid mixing, f(t) = 0.08 + 31.6 e-2.32t]. A video showing the fluorescence of the content and lipid-mixing dyes in a field of view is shown in Movie S2.
In contrast to the full system, there is only little complete fusion upon Ca2+ injection with neuronal SNAREs alone (i.e., without synaptotagmin 1 and complexin), followed by a single exponential decay of fusion with a slow time constant of 3.2 ± 1.4 s (SD) (Fig. 2E, Left and Movie S2). Only 20 ± 2% of the interacting vesicles exhibit complete fusion over the course of the observation period of 50 s and an additional 18 ± 4% of the interacting vesicles underwent hemifusion, but no complete fusion, during the observation period. The content-mixing histogram also provides evidence for a steady background fusion rate independent of Ca2+ after Ca2+ injection (approximately 0.2% of interacting vesicles per second, based on the limiting value that the fitted exponential function reaches for large times). The peak of the lipid-mixing histogram is about 10-fold higher than that of the content-mixing histogram (Fig. 2E, Right). Thus, most of this lipid-mixing peak relates to hemifusion-only events, and about half of these hemifusion events are followed by delayed complete fusion. The Ca2+-triggered fusion and lipid-mixing events that we observe for neuronal SNAREs alone are likely due to interactions between Ca2+ and phosphatidylinositol 4,5-bisphosphate (PIP2)-containing membranes (37) of the acceptor vesicles.
Cooperativity of Ca2+ -Triggered Fusion.
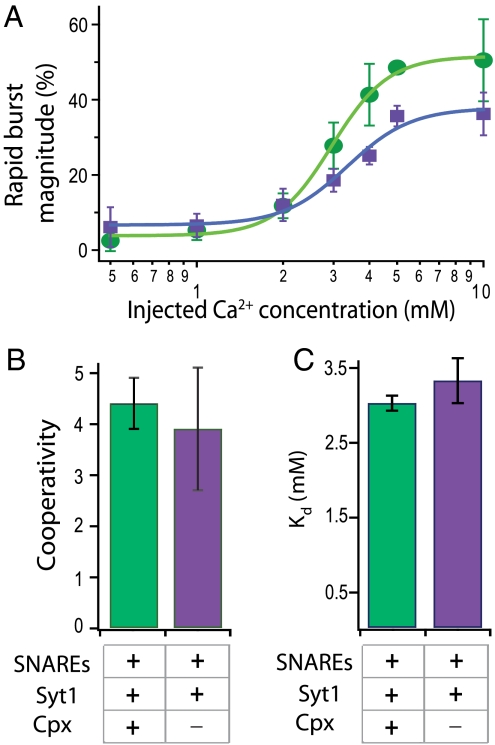
To examine the Ca2+ dependence of triggered release, we performed a series of single-vesicle fusion assays as a function of concentration of the injected Ca2+ buffer. Fig. 3A (green dots) shows the rapid burst magnitude (as defined in Materials and Methods) vs. injected Ca2+ concentration with neuronal SNAREs, synaptotagmin 1, and complexin. The curve can be well fit to a Hill function (Fig. 3 B and C). To study the effect of complexin on cooperativity, we carried out the same series of experiments with only SNAREs and synaptotagmin 1 (Fig. 3A, purple dots). Both cooperativity (Hill coefficient ca. 4) and Ca2+ affinity (Kd ∼ 3 mM) are statistically similar to those of the full system. Thus, synaptotagmin 1 molecules and their interactions with SNAREs and membranes produce a cooperative behavior as a function of Ca2+ concentration. Complexin is therefore not required for the cooperativity of Ca2+ triggering, consistent with the apparent lack of a Ca2+ binding site for complexin.
Fig. 3.
Cooperativity of Ca2+-triggered fusion. (A) Shown are the rapid burst magnitude (as defined in Materials and Methods) for the full system (SNAREs, synaptotagmin 1, and complexin) (green) and with all components except complexin (purple) as a function of Ca2+ concentration; mean values with error bars (standard deviations) are computed for multiple experiments. (B and C) Mean values of the cooperativity and Kd obtained by fitting a Hill function to the Ca2+ titration curves for the full system and the system without complexin (error bars indicate the goodness of the fit).
There are a total of five possible Ca2+ binding sites in the soluble C2 domains of synaptotagmin 1 that produce four intrinsic Ca2+ binding affinities of 50, 140, 490 μM, and 3.1 mM as assessed by isothermal titration calorimetry at 25 °C (38). Although synaptotagmin 1 binds to anionic membranes with micromolar affinity, and PIP2 further enhances this binding (38), membrane binding itself may not be sufficient to trigger fusion. The millimolar Ca2+ concentration required in our system implies that all Ca2+ binding sites are occupied. We conclude that other factors missing in our assay must act in concert with SNAREs, synaptotagmin, and complexin in order to explain the different Ca2+ range that is observed for action potential induced neurotransmitter release, starting at a few micromolar and saturating at 100 μM (39, 40).
Role of Synaptotagmin-1.
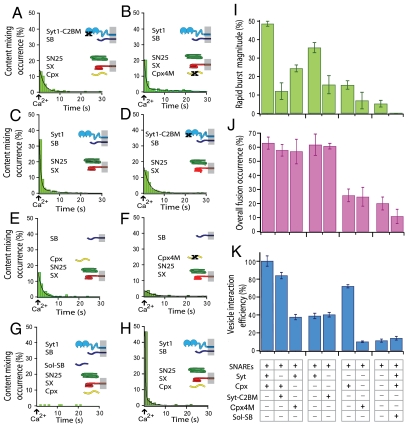
We substituted synaptotagmin 1 with a mutant that disrupts the Ca2+ binding site of the C2B domain of synaptotagmin 1 (D309A, D363A, and D365A, referred to as Syt1-C2BM); this mutant decreases the Ca2+-dependent interaction of synaptotagmin 1 with anionic membranes and abolishes synchronous excitatory and inhibitory postsynaptic responses (41). For the Syt1-C2BM mutant, the rapid burst magnitude is reduced by a factor of approximately 5 (Fig. 4 A and I) as compared with the rate of the system containing wild-type synaptotagmin 1. We obtained a good fit to the content-mixing histogram with a single exponential decay function, suggesting that the underlying slow fusion process is similar to neuronal SNAREs alone. In contrast, both the total fusion occurrence over the entire observation period (Fig. 4J) and the vesicle interaction efficiency (Fig. 4K) were similar to those of the system with wild-type synaptotagmin 1. Thus, the Syt1-C2BM mutation has primarily kinetic consequences: It greatly reduces the rapid burst by delaying fusion events upon Ca2+ injection.
Fig. 4.
Effects on fast Ca2+-triggered fusion by manipulations of synaptotagmin 1, complexin, and SNAREs. (A) Content-mixing histogram (representative from multiple experiments, total number of traces analyzed n = 314) with SNAREs (SB, synaptobrevin; SX, syntaxin; SN25, SNAP-25), the mutant of synaptotagmin 1 (Syt1) that disrupts Ca2+ binding to the C2B domain (Syt1-C2BM), and complexin (Cpx). The black line is a fit to the histogram [f(t) = 0.37 + 17.3 e-0.49t]. (B) Representative content-mixing histogram (n = 480) with SNAREs, Syt1, and the mutant of complexin that disrupts SNARE complex binding (Cpx4M). The black line is a fit to the histogram [f(t) = 0.12 + 36.9 e-1.40t + 2.0 e-0.10t]. (C) Representative content-mixing histogram (n = 1109) with SNAREs and Syt1. The black line is a fit to the histogram [f(t) = 0.17 + 77.71 e-2.02t + 5.76 e-0.26t]. (D) Representative content-mixing histogram (n = 629) with SNAREs and the Syt1-C2BM mutant. The black lines is a fit to the histogram [f(t) = 0.21 + 19.59 e-0.42t]. (E) Representative content-mixing histogram (n = 1910) with SNAREs and Cpx. The black line is a fit to the histogram [f(t) = 0.05 + 10.50 e-0.90t + 2.27 e-0.20t]. (F) Representative content-mixing histogram (n = 508) with SNAREs and mutant Cpx4M. The black line is a fit to the histogram [f(t) = 0.15 + 3.95 e-0.28t]. (G) Content-mixing histogram (n = 168) with SNAREs, the cytoplasmic domain of synaptobrevin (Sol-SB), Syt1, and Cpx. The cytoplasmic domain of synaptobrevin was incubated with acceptor vesicles prior to adding donor vesicles, greatly reducing trans SNARE complex formation. (H) Representative content-mixing diagram (n = 778) for the full system with wild-type proteins, similar to Fig. 2D, Left, except for a 0.5-s exposure time for each image and 1-s binning for calculating the content-mixing histogram. The black line is a fit to the histogram [f(t) = 0.07 + 294.34 e-3.94t + 7.03 e-0.41t]. The time constants obtained from this fit are close to those for the fit to the histogram with 200-ms binning (Fig. 2D, Left) (they are within experimental error obtained from multiple experiments). All content-mixing histograms were normalized with respect to the number of interacting vesicles. Histograms are only shown for the first 30 s because few events occurred after this period; very few, if any events occurred in the few seconds before Ca2+ triggering. Time t = 0 corresponds to the instance of Ca2+ injection as determined by the appearance of cascade-blue fluorescence. The time bin of the histograms is 1 s. Corresponding lipid-mixing histograms are shown in Fig. S8. (I) Bar graph of the rapid burst magnitude for the experiments (time bin = 1 s). (J) Bar graph of the overall fusion occurrence. (K) Bar graph of the vesicle interaction efficiency (see Materials and Methods for definition of the rapid burst magnitude, overall fusion occurrence, and vesicle interaction efficiency). Means and error bars (standard deviations) were obtained from multiple experiments.
Complete removal of synaptotagmin has an even more pronounced effect by essentially abolishing the rapid burst (Fig. 4 E and I), but also reducing the overall fusion occurrence (Fig. 4J), while only slightly lowering the interaction efficiency (Fig. 4K). These observed effects on fusion kinetics correlate well with the abolishment of fast synchronous release upon synaptotagmin 1 knockout and the poor rescue with the Syt1-C2BM mutant (41). Likewise, the lack of an effect on the interaction efficiency between donor and acceptor vesicles is reminiscent of the lack of an effect on the readily releasable pool of primed synaptic vesicles in cortical neurons in synaptotagmin 1 knockout mice (41).
Role of Complexin.
We investigated the role of complexin by replacing it with the mutant Cpx4M (R48A, R59A, K69A, Y70A) that reduces binding to the SNARE complex (10). As with wild-type complexin, there is a rapid fusion burst upon Ca2+ triggering, but the magnitude is greatly reduced compared to that of the full system (Fig. 4 B and I). In contrast, the total fusion occurrence during the entire observation period with Cpx4M is statistically the same as that of the full system with wild-type complexin (Fig. 4J). Interestingly, the vesicle interaction efficiency is also reduced (by a factor of approximately 3) compared to the full system with wild-type complexin (Fig. 4K).
We next tested the effect of removal of complexin on fast Ca2+-triggered fusion (i.e., only SNAREs and synaptotagmin 1 are present in the system). Despite the absence of complexin, there is a rapid burst upon Ca2+ injection (Fig. 4C), although the rapid burst magnitude is significantly reduced compared to that of the full system (Fig. 4 H and I). As with the full system, the substitution of wild-type synaptotagmin 1 with the Syt1-C2BM mutant essentially eliminates the rapid burst (Fig. 4 D and I), resulting in behavior similar to with neuronal SNAREs alone. The total fusion occurrence over the entire observation period is similar to that of full system when complexin is absent (Fig. 4J), whereas the vesicle interaction efficiency is greatly reduced (Fig. 4K). As with the Cpx4M mutant, the absence of complexin slows Ca2+-triggered fusion kinetics and lowers the interaction efficiency between donor and acceptor vesicles. Thus, our system mimics the reduction of fast synchronous release in complexin knockdown and rescue studies of cortical rat neurons (10). Furthermore, the reduced vesicle interaction efficiency in the absence of complexin correlates with the reduction of the readily releasable pool of primed synaptic vesicles in complexin 1 knockout studies in rat cortical neurons (42). The molecular basis of complexin’s function in enhancing the interaction between donor and acceptor vesicles may be explained by previous single-molecule studies: Interaction between complexin and syntaxin/SNAP-25 promotes folding of the flexible syntaxin/SNAP-25 complex, thereby making it more likely to interact efficiently with synaptobrevin (43).
Dependence on SNARE Complex Formation.
As control, we disrupted trans SNARE complex formation by using the soluble cytoplasmic fragment of synaptobrevin, residues 1–96. When acceptor vesicles are incubated with excess of this fragment prior to the injection of donor vesicles into the sample chamber, it siphons away most available acceptor (syntaxin/SNAP-25) complexes. As a consequence, the vesicle interaction efficiency was reduced to approximately 14 ± 2 (SD)% (Fig. 4K, right-most bar). For those vesicles that did interact, the total fusion occurrence over the entire observation period was dramatically reduced (Fig. 4J), and no rapid fusion burst was observed (Fig. 4 G and I), compared to the full system. Thus, as expected, disruption of the ability to form trans SNARE complex greatly reduces vesicle interaction and essentially abolishes fusion.
Discussion
Single-Vesicle Content-Mixing System with Fast Ca2+-Triggered Fusion Kinetics.
Our single-vesicle content-mixing system exhibits fast complete fusion and cooperativity upon Ca2+ injection (Figs. 2D and 3A). Starting from interacting single donor and acceptor vesicle pairs, we monitored instances of content and lipid mixing. SNAREs, synaptotagmin 1, and complexin are all essential to obtain the most pronounced rapid burst of content mixing (Fig. 4). The burst has a rise time that is so fast that it cannot be resolved with our system, followed by a slower decay with a time constant of 250 ms. In analogy to our Ca2+ delivery method, in vivo experiments that employ photolysis of caged Ca2+ also produce a stepwise increase of Ca2+ concentration in the axonal terminal (44, 45). These in vivo experiments revealed a very rapid rise for presynaptic release and postsynaptic currents (ca. 1 ms), followed by a slower decay (< 10 ms) at ambient temperature. It is gratifying that the Ca2+-triggered content release in our system shows a similar characteristic shape (i.e., a rapid rise followed by a slower decay). The Ca2+ range for our system is different from that observed for action potential induced neurotransmitter release (approximately 1–100 μM) (39, 40), although the cooperativity (Hill coefficient ca. 4) of our system is physiological. It should be noted that the lipid-mixing-only assay reported in ref. 25 did not produce cooperative behavior (paradoxically, less fusion was observed at higher Ca2+ concentration).
The system used in our present study, most probably does not include all of the factors needed to reproduce the Ca2+ dose response and faster decay kinetics observed in vivo. For example, the proteins Munc18 (46), RIM (47), Munc13 (48), and Ca2+-dependent activator protein for secretion (CAPS) (49) play important roles in exocytosis, and their molecular function could be assessed with our single-vesicle content-mixing system. It is remarkable, however, that the minimal system of SNAREs, synaptotagmin, and complexin qualitatively mimics effects on Ca2+-triggered fast synchronous release (correlated with the observation of content mixing in our system) and the readily releasable pool of synaptic vesicles (correlated with the vesicle interaction efficiency in our system) for key mutants of synaptotagmin 1 and complexin observed by in vivo studies in cortical neurons (10, 41) (Fig. 4).
Content Mixing Vs. Lipid Mixing.
Measuring just lipid mixing can be misleading: Although there is a burst of lipid mixing for neuronal SNAREs alone upon Ca2+injection, the kinetics of content mixing (the quantity that is relevant for neurotransmitter release) is actually much slower for neuronal SNAREs alone compared to the full system (compare Fig. 2 D and E). Thus, our results indicate that an increase in lipid mixing cannot be reliably interpreted as complete fusion, in agreement with other studies on a variety of biological fusion processes (15–19). Even at a single-vesicle level, it is often not possible to detect two clearly defined increases in lipid mixing, resulting in the inability to distinguish between inner and outer leaflet mixing for such a single step instance. Although there is a more pronounced lipid-mixing peak for the full system compared to SNAREs alone, consistent with previous lipid-mixing liposome experiments (50, 51), this difference in lipid mixing does not reveal the dramatic difference in content-mixing kinetics (compare Fig. 2 D and E, Left). In addition, our system can distinguish individual instances of vesicle leakage from that of content release (Fig. S2); this is very difficult to discern by ensemble assays, especially because only a few leakage events could have a significant effect on the average fluorescence intensity. In any case, our system has a very low probability of vesicle leakage events (less than 0.01%). Thus, our single-vesicle content-mixing system is a major advance compared to previous in vitro approaches to study synaptic vesicle fusion. Many lipid-mixing-only experiments carried out over the past decade may need to be repeated with content-mixing indicators.
Ca2+-Triggered Catalysis of SNARE-Dependent Fusion by Synaptotagmin.
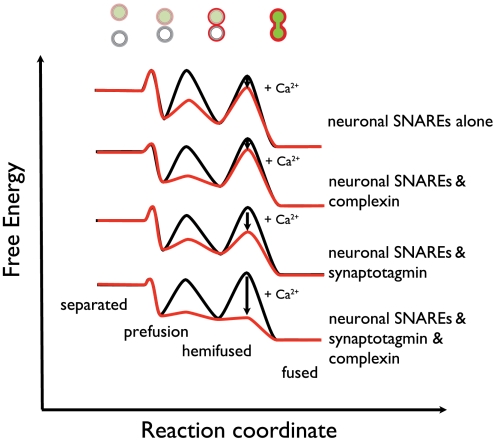
Fig. 5 is a qualitative summary of our findings in a framework of states and transitions between states in analogy to theories about the steps involved in biological membrane fusion (52). Neuronal SNAREs alone (i.e., without synaptotagmin and complexin) readily promote donor–acceptor vesicle interaction, and some lipid mixing, consistent with previous results (53). Yet, neuronal SNAREs alone do not efficiently produce fast Ca2+-triggered complete fusion (Fig. 2E, Left). Using a different content-mixing assay, spontaneous complete fusion activity of neuronal SNAREs alone in the absence of Ca2+ was also found to be slow (23); in fact, endogenous fusion activity appears to be slower for neuronal SNAREs than for yeast SNAREs (54, 55). Thus, one can speculate that neuronal SNAREs have evolved to generate less power than yeast SNAREs to overcome the transition barriers for complete fusion between membranes.
Fig. 5.
Free energy profiles of Ca2+-triggered fusion. Free energy profiles for Ca2+-triggered fusion in the presence of neuronal SNAREs, synaptotagmin 1, and complexin, and subsets of these factors. The initially separated, prefusion, hemifused, and (completely) fused states of the donor and acceptor vesicles are assigned certain free energies with transition barriers between the states. The heights of the minima and transition barriers are for illustration only and not meant to be quantitative. Likewise, the relative positions of the separated state for the four free energy profiles are arbitrary. Whereas SNARE-mediated membrane interactions readily occur (prefused state), large kinetic barriers exist toward complete fusion in the absence of Ca2+ (black lines). Upon Ca2+ injection (red lines), these barriers are dramatically lowered for the system containing SNAREs, synaptotagmin 1, and complexin, triggering a fast fusion response. In contrast, little fusion is achieved with neuronal SNAREs alone.
The combination of synaptotagmin 1 and SNAREs effectively lowers the transition barriers for complete fusion between membranes upon Ca2+ injection (Fig. 5). Ca2+ binding to synaptotagmin is essential for this process because mutation of the Ca2+ binding site of the C2B domain of synaptotagmin 1 greatly reduces the rapid burst magnitude (Fig. 4A), consistent with its effect on fast synchronous neurotransmitter release (41). How could synaptotagmin 1 in concert with SNAREs drive fusion upon Ca2+ injection starting from a metastable state of interacting membranes? First, synaptotagmin has a profound effect on membranes and membrane interactions (56, 57), possibly destabilizing the membrane near a hemifusion stalk. Second, it might trigger protein conformational changes in SNAREs upon Ca2+ binding, as suggested by single-molecule studies of the interaction between synaptotagmins and SNARE complex (58, 59). Third, it might induce formation of additional trans SNARE complexes upon Ca2+ triggering that would provide the energy to drive the system to full fusion. Future developments of the single-vesicle fusion system should allow time-resolved studies of the molecular mechanism involved in this process.
Enhancement of Ca2+ Control by Complexin.
Complexin increases the rapid fusion burst as well as vesicle interaction efficiency consistent with its activating function (Fig. 4 I and K). However, in the absence of complexin, some Ca2+-triggered rapid fusion still occurs at higher Ca2+ concentrations (compare the green and purple lines in Fig. 3A). In contrast, at lower Ca2+, complexin had little effect. These results suggest that complexin’s activating characteristic (10, 11) is a manifestation of two separate effects: enhancement of the fast fusion burst (accompanied by reduction of “slow” or spontaneous fusion events) at higher Ca2+ concentration, and an enhancement of the membrane interaction efficiency. Moreover, complexin is not absolutely required to observe cooperative Ca2+-triggered fusion—SNAREs and synaptotagmin 1 are sufficient and constitute a most minimal Ca2+-triggered fusion system, albeit less efficiently than in the presence of complexin. This conclusion could not be drawn in previous studies that only used lipid-mixing indicators. Such work concluded that neuronal SNAREs alone produce significant lipid-mixing activity on their own (also reproduced in our lipid-mixing studies, Figs. S7 and S8). Because lipid mixing was interpreted at the time as complete fusion, it was thought that some sort of fusion clamp must always exist to prevent full fusion in the absence of Ca2+. Our results now show that such a clamp is not absolutely required for neuronal SNAREs: Very little complete fusion is observed in the starting state of interacting vesicles until Ca2+ arrives, even in the absence of complexin (see Fig. 2 D and E, Left, before Ca2+ injection). Along similar lines, the conclusions drawn from other lipid-mixing studies implying an arresting role of synaptotagmin before Ca2+ triggering (60) have to be revisited.
Outlook.
Our single-vesicle content-mixing system provides a platform to study the role of various factors in Ca2+-triggered synaptic vesicle fusion. The results presented in this paper demonstrate the power of the synthetic system: One can deliberately omit or manipulate components to determine which parts are essential and monitor what happens to the system when essential parts are missing. By directly observing individual protein–protein interactions in conjunction with single-vesicle content and lipid-mixing indicators, this system will provide a foundation to investigate the molecular mechanism of synaptic neurotransmitter release. We predict that a molecular understanding of this fundamental biological process is within reach.
Materials and Methods
Protein Expression and Purification.
Expression of complexin and SNAP-25 in Escherichia coli.
Recombinant full-length rat neuronal complexin 1 (simply referred to as complexin), the mutant Cpx4M of complexin (R48A, R59A, K69A, Y70A) (10), and the mutant of SNAP-25A (simply referred to as SNAP-25) (61) with all endogenous cysteine residues substituted with serine were subcloned into pET-28b (Novagen, EMD chemicals) and expressed as N-terminal His6-tagged fusion proteins in BL21 (DE3) E. coli cells (Novagen, EMD chemicals). Cells were grown with 35 mg/mL kanamycin in terrific broth media. Cell induction was performed with 1 mM IPTG at an optical density A600 = 1.0 for 3 h at 37 °C.
Expression of SNAP-25 in Spodoptera frugiperda (Sf9) cells.
By virtue of the removal of the endogenous cysteine residues and expression in E. coli, SNAP-25 is not palmitoylated. We therefore also expressed SNAP-25 in Sf9 cells using a similar protocol as for full-length synaptotagmin 1, which resulted in palmitoylation of the endogenous SNAP-25 residues as assessed by mass spectroscopy. We observed similar rapid bursts and decay time constants with and without SNAP-25 palmitoylation (compare the content-mixing histograms in Fig. 2D and Fig. S9). However, due to significantly lower yield of SNAP-25 expressed in Sf9 cells, we therefore used SNAP-25 expressed in E. coli for most experiments.
Expression of full-length syntaxin and synaptobrevin in E. Coli.
We modified the full-length rat neuronal syntaxin-1A (S193C, C271S, C272S) expression construct used in previous work (61) by replacing the N-terminal His6-tag thrombin cleavage site with a tobacco etch virus (TEV) protease site to prevent secondary cleavage of syntaxin by thrombin (this modified construct is simply referred to as syntaxin). This mutant of full-length syntaxin-1A, and full-length wild-type (and the S28C mutant of) rat neuronal synaptobrevin-2 were expressed in E. Coli as described in previous work (61).
Expression of full-length synaptotagmin in Sf9 cells.
A construct of rat neuronal full-length synaptotagmin 1 (residues 1–421) with a C-terminal His10 tag was cloned into the pENTR/TEV/D-TOPO entry vector (Invitrogen), using PCR primers synaptotagmin 1 TOPO forward 5′caccatggtgagtgccagtcat3′ and synaptotagmin 1 TOPO reverse 5′tcaatgatgatggtgatgatggtggtg 3′ for directional cloning into the TOPO entry vector (based on pENTR directional TOPO cloning kit). The recombinant entry vector was used to perform the LR Clonase™ II reaction to transfer the synaptotagmin 1 full-length gene in the baculodirect C-term destination vector (Invitrogen). The TOPO constructs for the Syt1-C2BM mutant of synaptotagmin 1 (D309A, D363A, D365A) (41) and the cysteine mutant of synaptotagmin 1 (C74S, C75S, C77S, C79S, C82S) were synthesized by GENEART AG.
Recombinant full-length rat neuronal synaptotagmin 1, the Syt1-C2BM mutant, and the cysteine mutant were expressed as C-terminal His10-tagged fusion proteins in Sf9 insect cells (Invitrogen). Sf9 cells were grown in tetranitromethane-fumarate hydratase (TNM-FH) media (GIBCO, Invitrogen) supplemented with 5% FBS (GIBCO, Invitrogen), 2 mM glutamine (GEMINI Bio-Products) and 50 μg/mL antifungal gentamycin (GEMINI Bio-Products) at 27 °C. Cells were infected at a density of 2.5 × 106/mL with virus at multiplicity of infection 5. Virus infected cells were harvested 72 h after infection. By virtue of expression in Sf9 cell, synaptotagmin 1 is deemed to be palmitoylated as observed in vivo (62).
Purification of complexin and SNAP-25.
His6-tagged fusion proteins were purified by Ni2+-nitrilotriacetic acid (NTA) sepharose (Qiagen) affinity chromatography from crude cell lysate. The bound proteins were washed with 50 mM imidazole concentration in a buffer containing 20 mM Hepes (pH 7.4), 300 mM NaCl, and 2 mM DTT, and then eluted in 400 mM imidazole. Eluted proteins were further purified by size-exclusion chromatography (AKTA, GE Healthcare) using a Superdex 200 10/300 column (GE Healthcare) in buffer containing 20 mM Hepes (pH 7.4), 100 mM NaCl, and 4 mM DTT; the elution profiles consisted of a dominant, symmetric peak for each of the proteins. Protein fractions pooled from the major peak are 95% pure as determined by SDS gel electrophoresis. The His6 tags were cleaved by thrombin (Haematologic Technologies) treatment overnight at 4 °C. After His6-tag cleavage, the proteins were purified by size-exclusion chromatography (SEC).
Purification of full-length syntaxin and synaptobrevin.
In contrast to previous work (61), cell membranes were collected and solubilized in buffer containing 1% n-dodecyl-β-d-maltopyranoside (DDM), 20 mM Hepes (pH 7.4), 500 mM NaCl, and 2 mM DTT. His6-tagged full-length syntaxin and synaptobrevin were purified using Ni2+-NTA agarose (Qiagen). DDM was exchanged into 110 mM octyl-β-d-glucoside (OG) while proteins are bound to Ni2+-NTA beads. The protein samples were further purified by SEC using a Superdex 200 10/300 column (GE Healthcare) in 110 mM OG, 20 mM Hepes (pH 7.4), 300 mM NaCl, and 1 mM DTT. His6-tag removal from purified syntaxin and synaptobrevin was performed by adding TEV protease (Invitrogen) and thrombin (Haematologic Technologies), respectively, at room temperature for 4–5 h while dialyzing against Vesicle Buffer [20 mM Hepes (pH 7.4), 90 mM NaCl, 20 μM EGTA, and 1% 2-mercaptoethanol] supplemented with 110 mM OG. Proteins were 95% pure as determined by SDS gel electrophoresis. Right after His6-tag cleavage, the proteins were reconstituted into vesicles as described below.
Purification of full-length synaptotagmin.
Cell membranes were prepared by disrupting the cells by ultrasonication at 10/20 on/off pulse for 3 min. Sodium phosphate buffer [containing 20 mM sodium phosphate (pH 7.4), 600 mM NaCl, 2 mM DTT, 1 g/l BSA, EDTA free complete protease inhibitor 2 tablets, and 1 mM PMSF) used for cell breakage was supplemented with 5 mg DNase. Membrane solubilization was performed in 20 mM of sodium phosphate buffer (pH 7.4), 600 mM NaCl, 1% DDM (ANATRACE), and 2 mM DTT.
Solubilized proteins were loaded onto 1–2 mL of Ni2+-NTA sepharose resin and incubated overnight at 4 °C. DDM was exchanged to 150 mM OG (ANATRACE, Affymetrix) while proteins were bound to Ni2+-NTA beads. Ni2+-NTA elution was followed by SEC as a second step of purification using a Superdex 200 10/300 column (GE Healthcare) in the buffer containing 20 mM sodium phosphate pH 7.4, 300 mM NaCl, 110 mM OG and 4 mM DTT. Final protein concentration obtained after SEC varied from 25 ± 40 μM. His6 tags were cleaved by PreScission protease (GE Healthcare) overnight at 4 °C. Cleaved His6 tags were removed by dialysis against the same buffer as for the SEC. The elution profiles consisted of a dominant, symmetric peak for each of the proteins. The proteins were then subjected to Mono S ion exchange chromatography (GE Healthcare) after reducing the salt concentration to 100 mM NaCl. Bound protein was eluted with a NaCl salt gradient in 20 mM sodium phosphate buffer (pH 7.4), 4 mM DTT, and 110 mM OG. Proteins were 95% pure as determined by SDS gel electrophoresis. Both glycosylated and nonglycosylated proteins were present and used in all experiments. We tested the glycosylation state by using a tunicamycin assay (addition of tunicamycin during Sf9 cell expression resulting in nonglycosylated protein, Fig. S6).
The cytoplasmic domain of rat synaptobrevin-2 (1–96) was expressed and purified as previously described (61).
Vesicle Composition and Reconstitution of Syntaxin, Synaptobrevin, and Synaptotagmin 1.
Proteins were reconstituted into vesicles with a detergent depletion method (63). Acceptor (syntaxin/SNAP-25) vesicles were prepared from Brain Total Lipid Extract, supplemented with 20 mol% Cholesterol, 3.5 mol% PIP2, and 1 mol % biotinylated phosphatidylethanolamine (PE) (all lipids from Avanti Polar Lipids). Donor (synaptobrevin or synaptobrevin/synaptotagmin 1) vesicles were prepared from a mixture of phosphatidylcholine∶PE∶phosphatidylserine∶cholesterol∶DiD (44.5∶20∶12∶20∶3.5) (DiD from Invitrogen and other lipids from Avanti Polar Lipids) according to the observed composition of synaptic vesicles (31). Lipid mixtures in chloroform were dried in a clean glass tube with nitrogen stream and stored in a desiccator overnight. Lipid films were dissolved in 110 mM OG buffer and protein (synaptobrevin and syntaxin for donor and acceptor vesicles, respectively) was added at a protein to lipid ratio of 1∶200. For syntaxin/SNAP-25 vesicles, SNAP-25 solution (five times the concentration of syntaxin) was added to the protein-lipid mixture in order to prevent formation of dead-end 2∶1 syntaxin/SNAP-25 complexes (33). Protein concentrations were determined using UV-visible absorption at 280 nm. Detergent free buffer [20 mM Hepes, (pH 7.4), 90 mM NaCl, 1 % 2-mercaptoethanol] was added to the protein-lipid mixture until the detergent concentration was below the critical micelle concentration; the solutions were purified with a CL4B column and dialyzed overnight with Bio-beads SM2 (Bio-Rad) in protein-free Vesicle Buffer [20 mM Hepes (pH 7.4), 90 mM NaCl, 20 μM EGTA, 1% 2-mercaptoethanol]. Donor (synaptobrevin or synaptobrevin/synaptotagmin 1) vesicles were formed in the presence of 50 mM sulforhodamine B (Invitrogen), prior to SEC and dialysis. Size distributions of reconstituted vesicles were characterized with quasi-elastic dynamic light scattering (Nano-ZS90, Malvern Instruments) and cryoelectron microscopy (Figs. S3 and S4), revealing an average diameter of 78 nm. The effect of two different vesicle diameters (30 and 100 nm) on vesicle interaction (“vesicle–vesicle binding”) and lipid-mixing kinetics was found to be relatively small (64), suggesting that much larger acceptor vesicle diameters (as mimicking the active zone geometry) would not significantly affect our results. The proper insertion of proteins into vesicles was assessed by partial proteolysis (Fig. S6).
Surface Preparation for Single-Vesicle Experiments.
Cover and slide glasses were thoroughly rinsed and sonicated in acetone and methanol repeatedly more than 10 times over several days. 3% vol/vol solution of 3-aminopropyltriethoxysilane (Sigma-Aldrich) in acetone was applied to cover glasses for 30 min. Subsequently, surfaces were coated with a PEG/biotinPEG mixture consisting of PEG-SCM 5000 and Bio-PEG-SCM 5000 (both Laysan Bio) at a 9∶1 ratio, sulfate buffer (0.5 M potassium sulfate, 50 mM sodium phosphate), and incubated for 2 h. Residual PEG solution on the surfaces was removed with a large excess of deionized water (Millipore).
Protein Number Distributions in Single Vesicles.
The single-cysteine mutants of full-length syntaxin, synaptobrevin, and synaptotagmin 1 were labeled with Cy3-maleimide (Amersham Biosciences). Ni-NTA bound single-cysteine containing mutants of full-length synaptotagmin 1, synaptobrevin, and syntaxin were washed with 300 mM NaCl, 100 μM tris(2-carboxyethyl)phosphine, 110 mM OG, and 20 mM sodium phosphate (pH 7.4) or 20 mM Hepes (pH 7.4) and incubated with Cy3-maleimid (Amersham Biosciences) for 2 h at room temperature, followed by overnight incubation at 4 °C. Excessive washing of the bound protein was performed to remove free dye, and labeled protein was then eluted in 500 mM NaCl, 2 mM DTT, 110 mM OG, and 20 mM sodium phosphate (pH 7.4) or 20 mM Hepes (pH 7.4). Eluted proteins were further purified by SEC (AKTA, GE Healthcare) using a Superdex 200 10/300 column (GE Healthcare) in buffer containing 20 mM sodium phosphate or Hepes (pH 7.4), 100 mM NaCl, and 4 mM DTT. The labeling efficiency was 80% for the S193C mutant of full-length syntaxin, 100% for the S28C mutant of full-length synaptobrevin, and 12% for the single-cysteine mutant of synaptotagmin 1 as determined by UV-visible absorption of the dyes at 553 and 653 nm and the protein at 280 nm (A280), and their respective extinction coefficients. The A280 value stayed within 3% error upon denaturation of the samples in 6 M GuHCL.
Labeled proteins were serially diluted with unlabeled proteins and reconstituted into vesicles. For the donor (synaptobrevin, synaptotagmin 1) vesicles, 1 mol % biotinylated-PE was added into the original lipid mixture to enable surface immobilization. The protein-reconstituted vesicles were immobilized on PEG-coated glass surfaces similar to previously described methods (34) and imaged by single-vesicle TIR fluorescence microscopy (see below) until the signal disappeared by photobleaching. Among several ratios of labeled/unlabeled protein mixtures, we selected one that showed distinct fluorescence intensity photobleaching steps for most of the vesicles and determined the distribution of the number of labeled proteins in single vesicles (Fig. S5). The average number and distribution of labeled proteins in single vesicles was obtained by fitting the distribution with a lognormal function. Using the known dilution factors of labeled and unlabeled proteins, and labeling efficiencies, the distribution of the total number of reconstituted proteins was calculated from the measured number of labeled proteins in single vesicles. The reconstitution efficiencies were 54.3%, 75.4%, and 53.2%, respectively, calculated by comparison of the measured protein to lipid ratio to that in the initial mixture. The protein number (Fig. S5) and vesicle size distributions (Figs. S3 and S4) are relatively homogenous, an important prerequisite for in vitro fusion studies (65).
Combined Single-Vesicle Interaction, Content, and Lipid-Mixing Assay.
A PEG-coated glass surface was incubated with neutravidin solution (50 μg/mL, 20 mM, 90 mM NaCl) for 15 min. Acceptor (syntaxin/SNAP-25) vesicles were immobilized on the resulting surface similar to previously described methods (34), and excess vesicles were removed by thoroughly washing with Vesicle Buffer. Protein-free vesicles with the same lipid composition as donor vesicles with biotinylated-PE, were added to cover the entire surface; excess vesicles were removed by washing with Vesicle Buffer. Donor (synaptobrevin + /-synaptotagmin 1) vesicles encapsulating sulforhodamine and labeled with DiD either with or without 20 μM complexin were introduced into the sample chamber. After incubation (typically 10 s, in some cases up to 30 min), excess vesicles were removed by extensive washing with Vesicle Buffer with or without 20 μM complexin. Note that this starting condition implies that all Ca2+ binding sites of the synaptotagmin 1 molecules are unoccupied because the Vesicle Buffer contains 20 μM EGTA and the Ca2+ binding affinities for synaptotagmin 1 are below this concentration (38). Imaging experiments with Ca2+ injection were started after an additional 30 min incubation period. Note that in most experiments (except Fig. S7) we could not differentiate between interacting (but not hemifused) and hemifused vesicles for the starting state due to current technical limitations, so we analyzed state changes of the system by changes in fluorescence intensities upon Ca2+ injection as illustrated in Fig. 1.
Ca2+ buffer at the specified concentration was injected to the chamber at 20 μL/s velocity, effectively replacing the sample volume 20 times. Injection of Ca2+ buffer was performed with a motorized syringe pump (EW-74900-20 dual-syringe infusion/withdrawal pump, Cole-Parmer) except when otherwise noted. The injected Ca2+ buffer also contained 2 μM cascade-blue dye (Invitrogen).
After injection, it took less than a second for the fluorescence intensity of the cascade-blue dye to reach saturation (Movies S1 and S2). Because the fluorescence signal reaches its maximum value, it means that the dyes equilibrate within the evanescent wave excitation volume within less than a second. As the diffusion coefficient of Ca2+ is more than an order of magnitude faster than the diffusion coefficient for the 0.7 kD dye, it follows that the Ca2+ ions equilibrate at the specified concentration no later the equilibrium time of the dye. The specified Ca2+ concentration should be accurate to more than 5% because the sample volume is exchanged 20 times. Thus, the dye fluorescence signal is an accurate representation of the specified Ca2+ concentration in the sample volume penetrated by the evanescent wave used to illuminate the dye and provides an upper limit to the delivery time of Ca2+. Furthermore, if there were any significant delay of the delivery of Ca2+ in our system, one would expect a systematic lowering of the first time bin in the histograms in comparison to the fitted exponential decay functions (compare fitted functions and histograms in Figs. 2 D and E and 4). No such systematic effect is observed, supporting the accuracy of our Ca2+ indicator.
Stepwise fluorescence intensity increases for single donor vesicles upon Ca2+ injection are due to dilution and concurrent dequenching of the dyes. We can rule out a hypothetical scenario where some donor vesicles would simply move closer to the surface within the evanescent wave because we ensured that there is no nonspecific interaction between donor vesicles and the surface.
Analysis of Single-Vesicle Fluorescence Intensity Time Traces.
The spots corresponding to interacted vesicles were identified and background-corrected with a customized program written for IDL graphic system (ITT Visual Information Solutions) (66). For each pixel, the mean value of the neighboring background pixels was subtracted from the pixel. The fluorescence intensity for each vesicle was calculated by summing up all the background-corrected pixels inside a circular binary mask centered at the centroid of the vesicle. The fluorescence time traces were classified similar to the representative examples shown in Fig. 2 A–C. Time differences between Ca2+ injection and instances of lipid and content mixing were determined by inspection of the time traces. Histograms of these time differences were generated for lipid- and content-mixing instances, respectively.
Consecutive fluorescence images were acquired with exposure times of 200 and 500 ms unless noted otherwise. Vesicle scattering prevented data acquisition at shorter exposure times due to background noise. Content- and lipid-mixing histograms were calculated with 200 ms, 500 ms, or 1 s binning; for 1-s binning, consecutive 500-ms bins were combined into one bin. We used 1-s binning with 500-ms exposure times for some of the comparisons in order to reduce noise. At least three independent sets of experiments were performed, each with freshly prepared proteins and vesicles, and representative histograms are taken from one of the corresponding experiments unless noted otherwise. All histograms were normalized with respect to the number of interacting vesicles for the particular experiment.
Exponential decay functions were fitted to the histograms using IGOR Pro (WaveMetrics). The goodness of fit was evaluated with χ2 in order to determine how many fitting parameters are needed. The time constants of the exponential fits to histograms calculated with 200-ms and 1-s binning are similar (Figs. 2D and 4H), so for some experiments, we choose a histogram binning of 500 ms s or 1 s. In agreement with our observations, computer simulations of an exponential decay with greatly exaggerated noise showed that, for a given time bin, time constants of one-third of the time bin can be reliably determined.
For quantitative comparison of the content-mixing histograms for different experiments, we calculated the following four quantities. The vesicle interaction number is the number of donor vesicles that are interacting with acceptor vesicles, as determined by the number of fluorescent lipid dye spots (many of them are clearly visible before Ca2+ injection, but some of them are weak, so we confirmed these weak spots as interacting vesicles by a subsequent increase in fluorescence after Ca2+ injection). This quantity can be compared among different experiments in Fig. 4 because the concentration of acceptor vesicles on the surface and the incubation times are identical in all experiments. Furthermore, we normalized the vesicle interaction number relative to the full system (we refer to this as the vesicle interaction efficiency). The overall fusion occurrence is the number of donor vesicles that completely fuse with acceptor vesicles during the entire observation period (50 s) divided by the vesicle interaction number. The rapid burst amplitude is the number of donor vesicles that completely fuse with acceptor vesicles during the first 1 s after Ca2+ injection divided by the vesicle interaction number. Standard deviations for these quantities were obtained from multiple sets (at least three) of experiments, each with freshly prepared proteins and vesicles.
Supplementary Material
Acknowledgments.
We thank Drs. Keith Weninger and Mark Bowen for initial tethered liposome experiments to study single-vesicle fusion, Drs. Thomas Südhof and William Weis for stimulating discussions, Ankita Shah for help with protein purification, and the National Institutes of Health for support (R01-MH63105 to A.T.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 11729.
See Author Summary on page 11737.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107900108/-/DCSupplemental.
References
- 1.Fatt P, Katz B. The electric activity of the motor end-plate. Proc R Soc Lond B. 1952;140:183–186. doi: 10.1098/rspb.1952.0055. [DOI] [PubMed] [Google Scholar]
- 2.Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 3.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 4.Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 5.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 6.Tang J, et al. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Chacon R, et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 8.Pang ZP, Shin OH, Meyer AC, Rosenmund C, Sudhof TC. A gain-of-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex binding in synaptic exocytosis. J Neurosci. 2006;26:12556–12565. doi: 10.1523/JNEUROSCI.3804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue M, et al. Binding of the complexin N terminus to the SNARE complex potentiates synaptic-vesicle fusogenicity. Nat Struct Mol Biol. 2010;17:568–575. doi: 10.1038/nsmb.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maximov A, Tang J, Yang X, Pang ZP, Sudhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giraudo CG, et al. Alternative zippering as an on-off switch for SNARE-mediated fusion. Science. 2009;323:512–516. doi: 10.1126/science.1166500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joo C, Balci H, Ishitsuka Y, Buranachai C, Ha T. Advances in single-molecule fluorescence methods for molecular biology. Annu Rev Biochem. 2008;77:51–76. doi: 10.1146/annurev.biochem.77.070606.101543. [DOI] [PubMed] [Google Scholar]
- 13.Brunger AT, Weninger K, Bowen M, Chu S. Single-molecule studies of the neuronal SNARE fusion machinery. Annu Rev Biochem. 2009;78:903–958. doi: 10.1146/annurev.biochem.77.070306.103621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westphal V, et al. Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science. 2008;320:246–249. doi: 10.1126/science.1154228. [DOI] [PubMed] [Google Scholar]
- 15.Dennison SM, Bowen ME, Brunger AT, Lentz BR. Neuronal SNAREs do not trigger fusion between synthetic membranes but do promote PEG-mediated membrane fusion. Biophys J. 2006;90:1661–1675. doi: 10.1529/biophysj.105.069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan YH, van Lengerich B, Boxer SG. Effects of linker sequences on vesicle fusion mediated by lipid-anchored DNA oligonucleotides. Proc Natl Acad Sci USA. 2009;106:979–984. doi: 10.1073/pnas.0812356106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemble GW, Danieli T, White JM. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994;76:383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 18.Floyd DL, Ragains JR, Skehel JJ, Harrison SC, van Oijen AM. Single-particle kinetics of influenza virus membrane fusion. Proc Natl Acad Sci USA. 2008;105:15382–15387. doi: 10.1073/pnas.0807771105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jun Y, Wickner W. Assays of vacuole fusion resolve the stages of docking, lipid mixing, and content mixing. Proc Natl Acad Sci USA. 2007;104:13010–13015. doi: 10.1073/pnas.0700970104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rizzoli SO, Betz WJ. The structural organization of the readily releasable pool of synaptic vesicles. Science. 2004;303:2037–2039. doi: 10.1126/science.1094682. [DOI] [PubMed] [Google Scholar]
- 21.Weber T, et al. SNAREpins: Minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 22.Holt M, Riedel D, Stein A, Schuette C, Jahn R. Synaptic vesicles are constitutively active fusion machines that function independently of Ca2+ Curr Biol. 2008;18:715–722. doi: 10.1016/j.cub.2008.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Bogaart G, et al. One SNARE complex is sufficient for membrane fusion. Nat Struct Mol Biol. 2010;17:358–365. doi: 10.1038/nsmb.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pobbati AV, Stein A, Fasshauer D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science. 2006;313:673–676. doi: 10.1126/science.1129486. [DOI] [PubMed] [Google Scholar]
- 25.Lee HK, et al. Dynamic Ca2+-dependent stimulation of vesicle fusion by membrane-anchored synaptotagmin 1. Science. 2010;328:760–763. doi: 10.1126/science.1187722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu C, et al. Fusion of cells by flipped SNAREs. Science. 2003;300:1745–1749. doi: 10.1126/science.1084909. [DOI] [PubMed] [Google Scholar]
- 27.Nickel W, et al. Content mixing and membrane integrity during membrane fusion driven by pairing of isolated v-SNAREs and t-SNAREs. Proc Natl Acad Sci USA. 1999;96:12571–12576. doi: 10.1073/pnas.96.22.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowen M, Weninger K, Ernst J, Chu S, Brunger AT. Single-molecule studies of synaptotagmin and complexin binding to the SNARE complex. Biophys J. 2005;89:690–702. doi: 10.1529/biophysj.104.054064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T, Smith EA, Chapman ER, Weisshaar JC. Lipid mixing and content release in single-vesicle, SNARE-driven fusion assay with 1–5 ms resolution. Biophys J. 2009;96:4122–4131. doi: 10.1016/j.bpj.2009.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowen ME, Weninger K, Brunger AT, Chu S. Single molecule observation of liposome-bilayer fusion thermally induced by soluble N-ethyl maleimide sensitive-factor attachment protein receptors (SNAREs) Biophys J. 2004;87:3569–3584. doi: 10.1529/biophysj.104.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 32.Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Fasshauer D, Margittai M. A transient N-terminal interaction of SNAP-25 and syntaxin nucleates SNARE assembly. J Biol Chem. 2004;279:7613–7621. doi: 10.1074/jbc.M312064200. [DOI] [PubMed] [Google Scholar]
- 34.Yoon TY, Okumus B, Zhang F, Shin YK, Ha T. Multiple intermediates in SNARE-induced membrane fusion. Proc Natl Acad Sci USA. 2006;103:19731–19736. doi: 10.1073/pnas.0606032103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pertsinidis A, Zhang Y, Chu S. Subnanometre single-molecule localization, registration and distance measurements. Nature. 2010;466:647–651. doi: 10.1038/nature09163. [DOI] [PubMed] [Google Scholar]
- 36.Bar-On D, et al. Imaging the assembly and disassembly kinetics of cis-SNARE complexes on native plasma membranes. FEBS Lett. 2008;582:3563–3568. doi: 10.1016/j.febslet.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 37.Levental I, et al. Calcium-dependent lateral organization in phosphatidylinositol 4,5-bisphosphate (PIP2)- and cholesterol-containing monolayers. Biochemistry. 2009;48:8241–8248. doi: 10.1021/bi9007879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radhakrishnan A, Stein A, Jahn R, Fasshauer D. The Ca2+ affinity of synaptotagmin 1 is markedly increased by a specific interaction of its C2B domain with phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2009;284:25749–25760. doi: 10.1074/jbc.M109.042499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneggenburger R, Neher E. Presynaptic calcium and control of vesicle fusion. Curr Opin Neurobiol. 2005;15:266–274. doi: 10.1016/j.conb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Meinrenken CJ, Borst JG, Sakmann B. Local routes revisited: The space and time dependence of the Ca2+ signal for phasic transmitter release at the rat calyx of Held. J Physiol. 2003;547:665–689. doi: 10.1113/jphysiol.2002.032714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Pang ZP, Shin O-H, Südhof TC. Synaptotagmin-1 functions as the Ca2+-sensor for spontaneous release. Nat Neurosci. 2009;12:759–766. doi: 10.1038/nn.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Kaeser-Woo YJ, Pang ZP, Xu W, Südhof TC. Complexin clamps asynchronous release by blocking a secondary Ca2+ sensor via its accessory alpha-helix. Neuron. 2010;68:907–920. doi: 10.1016/j.neuron.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weninger K, Bowen ME, Choi UB, Chu S, Brunger AT. Accessory proteins stabilize the acceptor complex for synaptobrevin, the 1∶1 syntaxin/SNAP-25 complex. Structure. 2008;16:308–320. doi: 10.1016/j.str.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wölfel M, Schneggenburger R. Presynaptic capacitance measurements and Ca2+ uncaging reveal submillisecond exocytosis kinetics and characterize the Ca2+ sensitivity of vesicle pool depletion at a fast CNS synapse. J Neurosci. 2003;23:7059–7068. doi: 10.1523/JNEUROSCI.23-18-07059.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun J, et al. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerber SH, et al. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science. 2008;321:1507–1510. doi: 10.1126/science.1163174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaeser PS, et al. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin OH, et al. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat Struct Mol Biol. 2010;17:280–288. doi: 10.1038/nsmb.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jockusch WJ, et al. CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell. 2007;131:796–808. doi: 10.1016/j.cell.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Tucker WC, Weber T, Chapman ER. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science. 2004;304:435–438. doi: 10.1126/science.1097196. [DOI] [PubMed] [Google Scholar]
- 51.Stein A, Radhakrishnan A, Riedel D, Fasshauer D, Jahn R. Synaptotagmin activates membrane fusion through a Ca2+-dependent trans interaction with phospholipids. Nat Struct Mol Biol. 2007;14:904–911. doi: 10.1038/nsmb1305. [DOI] [PubMed] [Google Scholar]
- 52.Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- 53.Xu Y, Zhang F, Su Z, McNew JA, Shin Y-K. Hemifusion in SNARE-mediated membrane fusion. Nat Struct Mol Biol. 2005;12:417–422. doi: 10.1038/nsmb921. [DOI] [PubMed] [Google Scholar]
- 54.Diao J, et al. A single-vesicle content mixing assay for SNARE-mediated membrane fusion. Nat Commun. 2010;1:54. doi: 10.1038/ncomms1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diao J. Urbana-Champaign, IL: University of Illinois; 2010. Single-molecule fluorescence resonance energy transfer study of SNARE-mediated membrane fusion. PhD thesis. [Google Scholar]
- 56.Arac D, et al. Close membrane-membrane proximity induced by Ca2+-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat Struct Mol Biol. 2006;13:209–217. doi: 10.1038/nsmb1056. [DOI] [PubMed] [Google Scholar]
- 57.Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- 58.Choi UB, et al. Single-molecule FRET-derived model of the synaptotagmin 1-SNARE fusion complex. Nat Struct Mol Biol. 2010;17:318–324. doi: 10.1038/nsmb.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vrljic M, et al. Molecular mechanism of the synaptotagmin-SNARE interaction in Ca2+-triggered vesicle fusion. Nat Struct Mol Biol. 2010;17:325–331. doi: 10.1038/nsmb.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chicka MC, Hui E, Liu H, Chapman ER. Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca2+ Nat Struct Mol Biol. 2008;15:827–835. doi: 10.1038/nsmb.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weninger K, Bowen ME, Chu S, Brunger AT. Single-molecule studies of SNARE complex assembly reveal parallel and antiparallel configurations. Proc Natl Acad Sci USA. 2003;100:14800–14805. doi: 10.1073/pnas.2036428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veit M, Sollner TH, Rothman JE. Multiple palmitoylation of synaptotagmin and the t-SNARE SNAP-25. FEBS Lett. 1996;385:119–123. doi: 10.1016/0014-5793(96)00362-6. [DOI] [PubMed] [Google Scholar]
- 63.Lasic DD. The mechanism of vesicle formation. Biochem J. 1988;256:1–11. doi: 10.1042/bj2560001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cypionka A, et al. Discrimination between docking and fusion of liposomes reconstituted with neuronal SNARE-proteins using FCS. Proc Natl Acad Sci USA. 2009;106:18575–18580. doi: 10.1073/pnas.0906677106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen X, et al. SNARE-mediated lipid mixing depends on the physical state of the vesicles. Biophys J. 2006;90:2062–2074. doi: 10.1529/biophysj.105.071415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y. Palo Alto, CA: Stanford University; 2009. Resolving cadherin interactions at the single molecule level. PhD thesis. [DOI] [PMC free article] [PubMed] [Google Scholar]