Abstract
Contact inhibition of cell growth is essential for embryonic development and maintenance of tissue architecture in adult organisms, and the growth of tumors is characterized by a loss of contact inhibition of proliferation. The recently identified Hippo signaling pathway has been implicated in contact inhibition of proliferation as well as organ size control. The modulation of the phosphorylation and nuclear localization of Yes-associated protein (YAP) by the highly conserved kinase cascade of the Hippo signaling pathway has been intensively studied. However, cell-surface receptors regulating the Hippo signaling pathway in mammals are not well understood. In this study, we show that Hippo signaling pathway components are required for E-cadherin–dependent contact inhibition of proliferation. Knockdown of the Hippo signaling components or overexpression of YAP inhibits the decrease in cell proliferation caused by E-cadherin homophilic binding at the cell surface, independent of other cell–cell interactions. We also demonstrate that the E-cadherin/catenin complex functions as an upstream regulator of the Hippo signaling pathway in mammalian cells. Expression of E-cadherin in MDA-MB-231 cells restores the density-dependent regulation of YAP nuclear exclusion. Knockdown of β-catenin in densely cultured MCF10A cells, which mainly depletes E-cadherin–bound β-catenin, induces a decrease in the phosphorylation of S127 residue of YAP and its nuclear accumulation. Moreover, E-cadherin homophilic binding independent of other cell interactions is sufficient to control the subcellular localization of YAP. Therefore, Our results indicate that, in addition to its role in cell–cell adhesion, E-cadherin-mediated cell–cell contact directly regulates the Hippo signaling pathway to control cell proliferation.
Keywords: α-catenin, cell density, Merlin/Nf2, NHERF
In unicellular organisms, cell growth and division are unlimited and mainly controlled by nutrients in the environment. In contrast, metazoans restrain cell growth and division through an interplay between growth factor signaling and contact inhibition. Regardless of external growth factor-containing medium and active internal cellular metabolism, human cells restrict proliferation and cell division when the culture becomes confluent (1). This so-called contact inhibition of proliferation is a well-known property of normal differentiated tissues and needs to be tightly regulated for proper tissue morphogenesis (2). Contact inhibition is overcome in rapidly growing tissues during embryonic development, tissue regeneration, and wound healing. Furthermore, uncontrolled growth because of the loss of contact inhibition of proliferation is a hallmark of solid tumors (3, 4). Despite these insights, the underlying regulatory mechanisms of the contact inhibition of proliferation remain poorly understood, although cadherin-mediated cell–cell adhesion is thought to play an important role (2).
Cadherins are key regulators of embryonic development and adult tissue homeostasis (5). Cadherins mediate Ca2+-dependent cell adhesion and cell junction formation, and their cytoplasmic domains are associated with various catenins that mediate cytoskeletal association and signaling (6). E-cadherin is expressed in epithelial cells and intercellular homophilic binding of E-cadherin leads to the formation of the epithelial junctional complex and a tight polarized cell layer (7). Loss of E-cadherin expression through genetic or epigenetic alterations promotes tumor progression and metastasis (8, 9). On the other hand, overexpression of E-cadherin in cancer cells impedes tumor progression and invasion (10–12), not only because of E-cadherin’s adhesive function at the cell surface, which physically blocks the movement of cells and facilitates other cell–cell interactions, but also because of its inhibition of β-catenin signaling and other growth signaling pathways (12, 13). Independent of other cell–cell interactions, homophilic binding of E-cadherin directly transduces growth inhibitory signals through modulation of growth factor receptor tyrosine kinase (RTK) and Src family kinase signaling pathways (13).
Recent studies suggest that Nf2 tumor-suppressor Merlin plays a role in contact inhibition of proliferation by modulating RTK signaling through interaction with cadherins and catenins (14–16). Merlin directly associates with α-catenin to promote maturation of the adherens junction (17) and links it to the junctional polarity complex. Merlin is also known to regulate the Hippo signaling pathway through its interaction with Kibra and Expanded (18). The Hippo signaling pathway controls organ size by inhibiting cell proliferation and promoting apoptosis. The protein kinase cascade of the Hippo signaling pathway stimulates the nuclear exclusion and inactivation of transcriptional coactivator Yes-associated protein (YAP) and its paralog TAZ (transcriptional activator with PDZ binding motif) (19). The core kinase cascade of the pathway in mammals consists of the Ste20-like protein kinase Mst1/2, the WW domain containing protein WW45, the adaptor protein Mob, and nuclear Dbf2-related (NDR) family protein kinase large tumor suppressor (Lats)1/2. YAP has also been shown to be involved in contact inhibition, as its phosphorylation and nuclear localization are regulated by cell density through the Hippo signaling pathway in a Merlin-dependent manner (20, 21).
The upstream mechanisms regulating Hippo pathway activation have not been studied as intensively as the kinase cascade and regulation of YAP (22). In Drosophila, but not in mammals, genetic studies have identified the Fat atypical cadherin as the transmembrane protein acting upstream of the core Hippo kinase cascade (23–25). Recently, in Drosophila, polarity proteins including the apical transmembrane protein Crumbs and the membrane-associated signaling proteins Lgl and aPKC have been found to regulate the Hippo signaling pathway through Expanded and Hippo (Hpo) (26, 27). However, transmembrane receptors that deliver the contact-dependent growth inhibitory signals to the Hippo signaling pathway in mammals have not yet been identified. In this study, we show that E-cadherin directly mediates contact inhibition of proliferation via Hippo signaling pathway components and the regulation of the subcellular localization of YAP.
Results
Hippo Pathway Components Are Required for E-Cadherin–Dependent Contact Inhibition of Proliferation.
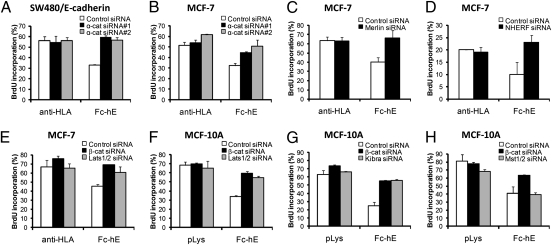
E-cadherin homophilic ligation directly regulates cell proliferation independent of other cell–cell interactions (13). This mechanism is dependent on cadherin-associated β-catenin, as depletion of β-catenin eliminates proliferation inhibition by E-cadherin ligation (13) (Fig. 1). Similar to depletion of β-catenin, knockdown of α-catenin in MCF-7 and SW480/E-cadherin cells blocked the inhibition of cell proliferation by E-cadherin (Fig. 1 A and B). E-cadherin ligation also partially inhibits EGFR-mediated growth signaling by inhibiting the transphosphorylation of Tyr-845 of EGFR by Src family kinases (13).
Fig. 1.
Hippo pathway components are required for E-cadherin–dependent contact inhibition of proliferation. At 12 to 24 h posttransfection of siRNA, cells were harvested and seeded at very low density on fibronectin-coated coverslips. Fc-hE–coated microspheres were presented to create pure E-cadherin homophilic binding. Anti-HLA or polylysine-coated microspheres were used as controls for bead binding to MCF-7 or MCF10A cells, respectively. Control and Fc-hE beads were bound for 24 h and cells were treated with 50 μM of BrdU for the last 6 h. BrdU incorporation was calculated by counting the number of positive BrdU immunofluorescence staining cells from the population of completely isolated, DAPI stained cells. (A and B) Depletion of α-catenin blocks E-cadherin–dependent contact inhibition of proliferation in SW480/E-cadherin (A) or MCF-7 (B) cells. (C and D) Depletion of Merlin (C) or NHERF (D) eliminates E-cadherin–dependent contact inhibition of proliferation in MCF-7 cells. (E and F) Depletion of β-catenin or Lats1/2 inhibits the E-cadherin bead-induced inhibition of proliferation in MCF-7 (E) and MCF10A (F) cells. Decrease of endogenous β-catenin or Lats1 protein levels by siRNA transfection in MCF-7 cells is shown in Fig. S1A. (G) Depletion of Kibra leads to the elimination of the E-cadherin bead-induced inhibition of proliferation in MCF10A cells. (H) Compared with β-catenin or Lats1/2 depletion, knockdown of Mst1/2 does not inhibit E-cadherin–dependent contact inhibition of proliferation in MCF10A cells. Decrease of endogenous Mst1/2 by siRNA transfection in MCF-7 cells is shown in Fig. S1B.
Recent studies suggest that the Nf2 tumor-suppressor Merlin plays an important role in the contact inhibition of proliferation (14, 15, 28). Upon cell-cell contact, together with the adaptor protein NHERF (Na+/H+ exchanger regulatory factor), Merlin interacts with the cadherin/catenin complex and attenuates downstream signaling from the EGFR (15). We thus wished to determine whether the proliferation inhibitory role of E-cadherin, regardless of other cell–cell interactions, depends on Merlin or NHERF. To address this question, we performed proliferation assays using E-cadherin–coated beads to create pure cadherin contacts (13). Binding of extracellular domain of E-cadherin-IgG Fc domain chimera (Fc-hE)-coated protein-A microspheres caused a decrease in proliferation of control MCF-7 cells (control siRNA), as shown previously (13), whereas depletion of Merlin or NHERF using specific siRNA reversed the proliferation inhibitory signal from E-cadherin bead homophilic ligation (Fig. 1 C and D). This finding suggests that Merlin and NHERF are required for proliferation inhibition mediated by E-cadherin ligation at the cell surface.
Importantly, Merlin is known to be an upstream regulator of the Hippo signaling pathway, which has been implicated in organ size control, as well as contact inhibition of growth (18, 20, 21). We therefore examined whether E-cadherin mediates contact inhibition through the Hippo signaling pathway in MCF-7 and MCF10A cells. siRNA-mediated depletion of the Lats1/2 kinases, which phosphorylate and regulate the activity of YAP, inhibited the E-cadherin bead-induced decrease in cell proliferation, similar to depletion of β-catenin (Fig. 1 E and F, and Fig. S1A). Depletion of endogenous Kibra, which is known to bind Merlin and activate the Hippo signaling pathway, reversed the E-cadherin bead-induced proliferation inhibition (Fig. 1G). These data show that upstream and downstream components of the Hippo signaling pathway are required for E-cadherin ligation-mediated contact inhibition of proliferation. Knockdown of the Mst1/2 kinase, however, showed no significant effect on the cell proliferation inhibition induced by E-cadherin ligation at the surface of MCF10A cells (Fig. 1H and Fig. S1B), suggesting that it may not be involved, similar to other recent findings for mouse embryonic fibroblasts (MEFs) using knockout mice (29, 30). As proposed, another kinase may mediate phosphorylation of Lats kinases in these cells.
E-Cadherin Is Required for Cell Density-Dependent YAP Subcellular Localization.
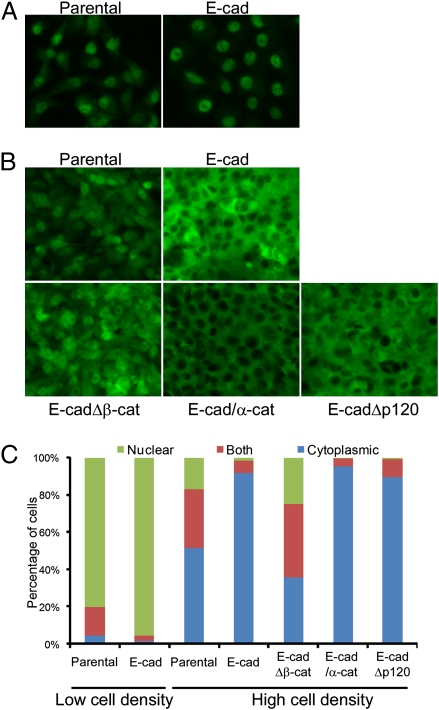
The phosphorylation and localization of YAP is regulated by cell density via the Hippo signaling pathway (21). In sparse cell cultures, YAP is predominantly localized in the nucleus, but in dense cell cultures it is phosphorylated by Lats kinase and translocated to the cytoplasm. Because depletion of Hippo signaling components (Merlin, Lats1/2, and Kibra) resulted in the loss of proliferation inhibition by E-cadherin ligation, we hypothesized that E-cadherin is an upstream regulator of the Hippo signaling pathway. We therefore investigated whether YAP localization is controlled by the E-cadherin using MDA-MB-231 cell lines, which express different types of doxycycline-inducible E-cadherin (11). The parental MDA-MB-231 cell line expresses no E-cadherin (11). In sparse cell cultures, most YAP protein was localized in the nucleus of all MDA-MB-231 cell lines, whether expressing E-cadherin or the E-cadherin–negative parental MDA-MB-231 cells (Fig. 2 A and C). The YAP protein remained localized in the nucleus of parental MDA-MB-231 cells, even under high cell density (Fig. 2 B and C). Interestingly, doxycycline-induced expression of full-length E-cadherin caused the redistribution of YAP from nucleus to cytoplasm in dense cell cultures (Fig. 2 B and C), suggesting that E-cadherin is required for the density-dependent regulation of YAP localization. Induced expression of E-cadherin Δp120 mutant (which is incapable of binding to p120) in MDA-MB-231 cells showed density-dependent YAP subcellular localization, indicating that direct binding of p120 to E-cadherin is not necessary for the E-cadherin–dependent YAP localization control. However, expression of an E-cadherin Δβ-catenin mutant, which binds neither endogenous β-catenin nor α-catenin but can still mediate some degree of physical cell adhesion (11), showed no density-dependent YAP subcellular localization regulation (Fig. 2 B and C). A majority of these cells exhibited YAP in the nucleus, even at high cell density. Expression of an E-cadherin/α-catenin fusion, which bypasses the need for β-catenin to mediate strong cell adhesion (11), also mediated density-dependent redistribution of YAP to the cytoplasm. Taken together, these results show that E-cadherin regulates YAP localization in response to cell density and that E-cadherin associated β-catenin and α-catenin, but not p120, are involved in regulating the Hippo signaling pathway.
Fig. 2.
E-cadherin expression regulates cell density-dependent redistribution of YAP from nucleus to cytoplasm. (A and B) Parental MDA-MB-231 cells and stable MDA-MB-231 clones, which contain doxycycline-inducible full-length E-cadherin, E-cadherin Δβ-catenin, E-cadherin–α-catenin fusion, or E-cadherin Δp120 transgene were seeded on fibronectin-coated coverslips in 24-well plates sparsely (1 × 104 cells) or densely (2 × 105 cells). Wild- or mutant-type E-cadherin expression was induced by the treatment of 2 μg/mL of doxycycline for 2 d. Cells were cultured under sparse (A) or dense (B) conditions, and endogenous YAP was stained by anti-YAP antibody. (Magnification, 200×.) (C) Subcellular localization of YAP in A and B was quantified using Blobfinder.
Depletion of β-Catenin Induces the Nuclear Accumulation of YAP in Dense Cell Cultures.
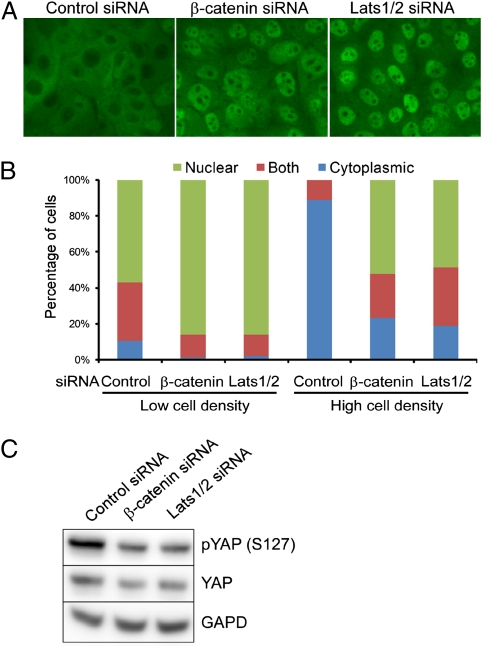
Induced expression of E-cadherin in MDA-MB-231 cells demonstrates its involvement in the regulation of YAP sublocalization in response to cell density. In addition, E-cadherin–blocking antibody was found to increase nuclear YAP in internal cells of mouse preimplantation embryos (31). However, either cadherin expression or treatment with E-cadherin–blocking antibody could potentially influence other cell interactions indirectly through its role in cell–cell adhesion. Nonetheless, our findings suggest that E-cadherin–associated β-catenin and α-catenin specifically are involved in regulating the Hippo signaling pathway. To test whether cadherin-associated catenins regulate contact-dependent Hippo signaling independent of the cell-adhesion function of E-cadherin, we depleted endogenous β-catenin in MCF10A cells through siRNA transfection and examined the localization of YAP at different cell densities. We first confirmed previous observations that YAP is excluded from the nuclei of MCF10A cells when they reach high density and that siRNA-mediated depletion of Lats1/2 leads to nuclear accumulation of YAP in dense cell cultures (Fig. 3 A and B). Similar to results in previous studies (13), depletion of β-catenin did not lead to the loss of adhesion, presumably because of functional compensation by plakoglobin (γ-catenin), a related cadherin-associated junctional protein (32, 33). β-Catenin depletion also did not change the expression level and localization pattern of E-cadherin expression (13). Depletion of β-catenin in densely cultured MCF10A cells resulted in increased nuclear accumulation of YAP (Fig. 3 A and B) and decreased YAP phosphorylation on the S127 residue (Fig. 3C). Similar results were observed for A431 cells and MCF-7 cells, supporting the generality of the phenomena (Fig. S2). In sparse cell cultures, depletion of β-catenin or Lats1/2 did not significantly change the nuclear localization of YAP protein (Fig. 3B). Because almost all of the β-catenin proteins in dense epithelial cells lacking Wnt signaling are E-cadherin–bound (13), these data suggest that E-cadherin–bound β-catenin can influence the downstream activity of the Hippo signaling pathway.
Fig. 3.
Depletion of β-catenin in MCF10A cells induces the nuclear accumulation of YAP and decreases the phosphorylation of the YAP S127 residue in dense cell cultures. (A and B) MCF10A cells transfected with control, β-catenin, or Lats1/2 siRNA (positive control) were harvested and 2 × 105 cells were seeded on fibronectin-coated coverslips in 24-well plates for 2 d more. (Magnification, 400×.) Localization of endogenous YAP was identified by immunofluorescence staining (A) and quantified (B). (C) Knockdown of β-catenin or Lats1/2 decreases the phosphorylation of YAP S127 residue in densely cultured MCF10A cells.
E-Cadherin Directly Controls the Localization of YAP, and YAP Is Involved in Growth Inhibition by E-Cadherin.
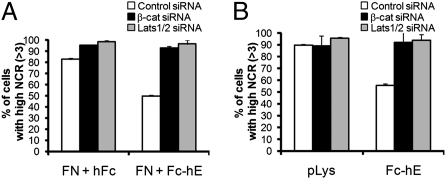
To further test whether E-cadherin homophilic binding independent of other cell interactions directly controls the Hippo signaling pathway, we examined whether homophilic E-cadherin ligation alone could change the subcellular localization of YAP. In sparse cell cultures, most YAP proteins were localized in the nucleus of MCF10A cells (Fig. 3B). Interestingly, either plating MCF10A cells sparsely on E-cadherin protein-coated coverglass (Fig. 4A) or attachment of E-cadherin beads to the surface of isolated MCF10A cells (Fig. 4B and Fig. S3) led to a decrease in nuclear YAP relative to cytoplasmic YAP. Moreover, siRNA-mediated depletion of either β-catenin or Lats1/2 inhibited the effect of E-cadherin ligation, resulting in increased nuclear YAP (Fig. 4 A and B, and Fig. S3), suggesting that regulation of YAP activity by E-cadherin depends on E-cadherin–bound β-catenin and the Hippo signaling kinase cascade.
Fig. 4.
Homophilic ligation of E-cadherin controls the localization of endogenous YAP protein. MCF10A cells transfected with control, β-catenin, or Lats1/2 siRNA were plated at very low density. (A) Cells were plated on either E-cadherin protein and fibronectin-coated coverslips (FN + Fc-hE), or fibronectin and Fc domain alone as control (FN + hFc). (B) Fc-hE coated or polylysine coated control microspheres were applied to the surface of MCF10A cells. After 24 h, endogenous YAP was stained and the nuclear-to-cytoplasmic YAP ratio (NCR) was quantified.
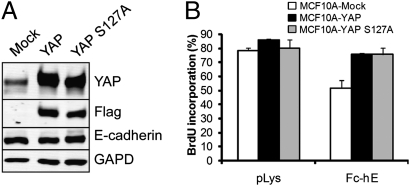
The involvement of E-cadherin in the regulation of the Hippo signaling pathway led us to test the effect of increased YAP activity on E-cadherin–dependent contact inhibition of proliferation. The Hippo signaling pathway regulates YAP through either of two mechanisms: phosphorylation of S127 residue and nuclear exclusion (20), or control of YAP levels through phosphorylation by CK1, and subsequent SCFβ-TRCP-mediated ubiquitination, and degradation (34). We therefore generated MCF10A cell lines stably overexpressing Flag-tagged YAP or phosphorylation deficient mutant Flag-tagged YAP S127A (Fig. 5A) and performed proliferation assays using E-cadherin bead to stimulate contact inhibition. In contrast to control MCF10A cells, overexpression of YAP or YAP S127A in MCF10A cells blocked the proliferation inhibition induced by E-cadherin bead ligation (Fig. 5B). Taken together, these findings suggest that E-cadherin homophilic ligation mediates contact inhibition of proliferation through regulation of YAP activity.
Fig. 5.
YAP overexpression blocks the proliferation inhibitory effect of E-cadherin. (A) MCF10A cells stably expressing Flag-YAP or Flag-YAP S127A were generated. Endogenous E-cadherin protein level and overexpression of exogenous protein was verified by Western blot. (B) Overexpression of YAP or YAP S127A inhibits the E-cadherin–dependent contact inhibition of proliferation.
Discussion
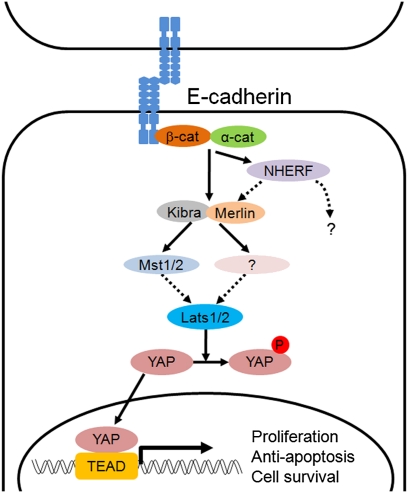
In this study, we demonstrate that Hippo signaling-pathway components are required for E-cadherin–mediated contact inhibition of proliferation. Depletion of Hippo signaling-pathway components (Merlin, Lats, and Kibra) or overexpression of YAP blocks the E-cadherin–mediated contact inhibition of proliferation. Because our experiments examine the role of E-cadherin homophilic ligation in contact inhibition, independent of other cell interactions, these findings also suggest that E-cadherin directly stimulates the Hippo tumor-suppressor pathway to trigger growth-inhibitory signaling. Indeed, we find that the E-cadherin–catenin complex regulates the nuclear localization of YAP, the transcriptional effector of the Hippo pathway. Re-expression of E-cadherin in MDA-MB-231 cells reconstitutes the density-dependent control of YAP subcellular localization; knockdown of β-catenin in densely cultured MCF10A cells induces the nuclear accumulation and a decrease in the phosphorylation of S127 residue of YAP; and homophilic ligation of E-cadherin alone directly decreased the level of nuclear YAP relative to the cytoplasmic YAP in a process dependent on β-catenin and Lats1/2. These results provide the evidence that E-cadherin homophilic binding, independent of other cell–cell interactions, directly regulates the Hippo signaling pathway. Therefore, we identify E-cadherin as an upstream cell-surface receptor that regulates Hippo signaling in mammalian cells (Fig. 6).
Fig. 6.
A model for an E-cadherin–mediated Hippo signaling pathway. Homophilic binding of E-cadherin between two cells stimulates the Hippo signaling pathway, which control proliferation by inhibiting the activity of YAP in the nucleus. Broken arrows indicate steps unresolved by the present study (see Discussion for more details).
Although the signal transduction cascade of the core kinases in the Hippo signaling pathway has been intensively studied, the upstream cell-surface regulators have not been well understood (22). In Drosophila, the Fat atypical cadherin has been identified as a transmembrane protein regulating the Hippo signaling pathway within epithelial cells (23). However, in mouse, the homolog,Fat4 is predominantly expressed in mesenchymal cells and Fat4 mutant mice exhibited none of the phenotypes associated with defects in the Hippo signaling pathway nor any effects on Yap or Lats1 (24, 25). These results suggest that Fat4 may not be an important cell-surface receptor for the Hippo signaling pathway in mammals; and we propose instead that classic cadherins, especially E-cadherin, play this role.
We also identify NHERF as a potentially unique upstream membrane-associated regulator of Hippo signaling, because depletion of NHERF blocks the E-cadherin ligation-dependent inhibition of proliferation. NHERF is an adaptor protein that is known to bind numerous proteins, including Merlin, β-catenin, EGFR, TAZ, and YAP (35). In fact, YAP had been previously identified as a c-Yes–associated protein interacting with c-Yes and NHERF at the apical plasma membrane (36). Further work will be needed to determine whether NHERF mediates contact inhibition directly through the Hippo pathway and whether it is an important component of the Hippo signaling pathway in other tissues and developmental contexts.
Although we found that several Hippo pathway components, including the YAP protein kinase Lats1/2, are required for E-cadherin–mediated contact inhibition, the mammalian Hpo kinase orthologs Mst1/2 did not appear to be required. Mst1 and Mst2 serine/threonine kinases, are known to associate with adaptor protein WW45 to phosphorylate and activate Lats kinase (18). We cannot rule out the possibility that Mst1/2 remaining after siRNA transfection (<∼10%) (Fig. S1B) are sufficient to mediate Hippo signaling. However, there is precedence for Mst1/2 independent Hippo pathway signaling. Mst1/2 are not required for cell density-stimulated YAP cytoplasmic translocation in MEFs lacking both genes (29). The inducible removal of Mst1/2 in newborn mice shows tissue-specific organ size control (30). It has been suggested that kinases distinct from Mst1/2 control Lats phosphorylation and Hippo signaling in MEF cells (29, 37). Furthermore, it has been found that FAT functions to regulate the levels of Warts/Lats independent of Hippo/Mst in Drosophila (38). However, we see no changes in Lats1/2 levels as result of β-catenin knockdown (Fig. S1A).
The detailed molecular mechanism by which E-cadherin homophilic ligation stimulates the Hippo pathway is not yet clear. Importantly, we find that cadherin-associated α- and β- catenins are involved in Hippo signaling. Both catenins are required for E-cadherin–mediated contact inhibition. Unlike β-catenin, knockdown of α-catenin in MCF-7 cells disrupts cell–cell adhesion (Fig. S1 C and D), making it hard to distinguish from indirect effects because of the loss of other cell–cell interactions. Nonetheless, expression of an E-cadherin–α-catenin fusion in MDA-MB-231 cells stimulates the redistribution of YAP from nucleus to cytoplasm in a density-dependent manner, suggesting that E-cadherin–bound α-catenin contributes to the regulation of the Hippo signaling pathway. A recent study revealed that α-catenin acts as a negative regulator of Yap through binding to 14-3-3–bound YAP (39). In contrast to our findings, the regulation of YAP phosphorylation and activity was shown to be independent of the Hippo pathway core kinases Lats1/2, cadherin proteins, and β-catenin. This study either reveals a completely distinct role for α-catenin in YAP regulation from the cadherin-catenin–mediated contact inhibition we are reporting, or somehow it missed an important role for these components.
Importantly, knockdown of β-catenin resulted in increased nuclear accumulation of YAP and decreased YAP phosphorylation on S127 in dense cell cultures. As shown in our previous study (13), proliferation inhibition mediated by E-cadherin ligation depends on E-cadherin–bound β-catenin, but not on the transcriptional activity of nuclear β-catenin. Depletion of β-catenin in dense cell cultures in the absence of Wnt signaling mainly decreases E-cadherin–β-catenin interaction because the cytoplasmic and nuclear levels of β-catenin are kept low by degradation (40). In contrast to α-catenin, depletion of β-catenin in mammalian cells does not disrupt cell–cell adhesion (ref. 13 and present study), presumably because of functional compensation by plakoglobin (32, 33). Therefore, β-catenin knockdown in this context selectively reveals its role in Hippo signaling independent of its role in cell adhesion or Wnt signaling. Note also that that this context, β-catenin bound to E-cadherin may serve a tumor suppressor-like function by controlling the Hippo signaling pathway, in contrast to its more well-known function as an oncogene in the context of the Wnt signaling pathway. This finding is not really surprising as E-cadherin, which functions in association with catenins, is well known to be a tumor suppressor. Recent studies have also shown that the Hippo signaling pathway restricts Wnt signaling either by TAZ interaction with DVL2 (41) or by inhibiting the interaction of YAP with β-catenin on target genes in the nucleus (42). These interactions of TAZ/YAP with the Wnt pathway are distinct from our observations of a Wnt pathway-independent role for β-catenin upstream of the Hippo signaling pathway.
Molecular components and interactions are known that can potentially explain the link between cadherins and the Hippo signaling pathway. Merlin interacts with Hippo pathway components Expanded and Kibra, which interacts with the Hpo kinase in Drosophila (18). Merlin also interacts with the cadherin-associated catenins, which we have implicated in the pathway (14–17, 28). NHERF is another potential candidate that may provide part of a molecular link, and there may be other components as well. Ultimately, it will be important to understand how E-cadherin receptor activation (i.e., homophilic binding by another E-cadherin protein) alters these protein interactions or their posttranslational modifications so as to activate downstream signaling events in the Hippo pathway.
E-cadherin can effect the growth of cells in tissues in a number of ways. Its adhesive function at the cell surface leads to a junctional barrier, limiting the accessibility of growth factors to their receptors, inhibition cell movement out of the epithelium, and the establishment of many cell–cell interactions that indirectly inhibit cell growth, including tight junction, gap junctions, and juxtacrine ligand-receptor interaction (43). Furthermore, E-cadherin also directly interacts with other effector proteins, including β-catenin, to inhibit its nuclear transport during Wnt signaling, and receptor tyrosine kinases to regulate their signaling activities (9). E-cadherin is also known to modulate the activity of Rho/Rac family GTPases (9) and is involved in the establishment and maintenance of polarity (44). Linking E-cadherin to the Hippo signaling pathway adds an important new aspect of cadherin function and may help explain how its adhesive functions and other signaling interactions are integrated to regulate cell growth in various developmental processes or adult tissue homeostasis.
Methods
Preparation of Protein-Coated Microspheres and Protein-Coated Glass Coverslips.
Protein A-coated beads were prepared as described in ref. 13, with minor modifications. Twenty-five microliters of protein A-coated polystyrene microspheres (Bangs Laboratories) were washed in 1 mM sodium acetate, pH 3.9, followed by washing two times with beads buffer (20 mM Hepes, 50 mM NaCl, and 2 mM CaCl2, pH 8.0). Then, 5 μg Fc-hE recombinant protein was bound to the microspheres, suspended in 50 μL of beads buffer, and incubated for 2 h at 4 °C with shaking. Anti-HLA antibody (for MCF-7 cells) or polylysine (for MCF10A cells) was used as control. The coated beads were washed two times with beads buffer and incubated with 1% BSA and 5 μg human Fc protein in 50 μL of beads buffer for 1 h to block nonspecific protein binding. After being washed three times in beads buffer, beads were resuspended in 250 μL of 2 mM CaCl2 and 1 mM MgCl2 containing HBSS++. After brief sonication in the cup-horn sonicator to dissociate bead aggregates, 15 μL of beads were transferred to coat the surface of the cells. The experiments were also done using protein-coated glass coverslips, in which coverslips were coated overnight at 4 °C with 10 μg of fibronectin along with either 10 μg of Fc-hE or 10 μg of anti-HLA antibody. Coverslips were washed in PBS and coated with 1% BSA in PBS for 1 h before plating cells.
E-Cadherin Bead-Based Proliferation Assay.
Cell monolayers were washed twice in HBSS++ and incubated with 0.02% trypsin in HBSS++ for 30 min. The cells were spun down and resuspended in complete medium. Next, 1,500 cells per well were transferred to 24-well plates containing 10 μg of fibronectin-coated coverslips and incubated for 4 h to allow cell attachment. After adding 15 μL of antibody or protein-coated beads, cells were incubated for 24 at 37 °C and 5% CO2. Six hours before fixing cells, 50 μM BrdU was added. Coverslips were washed and BrdU incorporation was detected by immunofluorescence staining using an anti-BrdU monoclonal antibody, whereas nuclei were detected by staining with DAPI. The BrdU-labeled cells were counted from the population of completely isolated cells present on the coverslips, and the percentage of BrdU incorporation in this population was calculated. To examine the effect of E-cadherin bead on localization of YAP, MCF10A cells were cultured in DMEM/F12 medium supplemented with 5% of horse serum, 0.5 μg/mL hydrocortisone, 100 ng/mL cholera toxin, and 10 μg/mL insulin. E-cadherin beads were attached as described above and endogenous YAP protein was determined by indirect immunofluorescence staining.
Supplementary Material
Acknowledgments
We thank Yuliya Petrova and Martha Spano for generous technical help in purifying E-cadherin-IgG Fc domain chimera protein. The work was supported by National Institutes of Health Grants R37 GM037432 and GM037432S (to B.M.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103345108/-/DCSupplemental.
References
- 1.Levine EM, Becker Y, Boone CW, Eagle H. Contact inhibition, macromolecular synthesis, and polyribosomes in cultured human diploid fibroblasts. Proc Natl Acad Sci USA. 1965;53:350–356. doi: 10.1073/pnas.53.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fagotto F, Gumbiner BM. Cell contact-dependent signaling. Dev Biol. 1996;180:445–454. doi: 10.1006/dbio.1996.0318. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Mayor R, Carmona-Fontaine C. Keeping in touch with contact inhibition of locomotion. Trends Cell Biol. 2010;20:319–328. doi: 10.1016/j.tcb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 6.Harris TJ, Tepass U. Adherens junctions: From molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 7.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajra KM, Fearon ER. Cadherin and catenin alterations in human cancer. Genes Chromosomes Cancer. 2002;34:255–268. doi: 10.1002/gcc.10083. [DOI] [PubMed] [Google Scholar]
- 9.Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: How does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 11.Wong AS, Gumbiner BM. Adhesion-independent mechanism for suppression of tumor cell invasion by E-cadherin. J Cell Biol. 2003;161:1191–1203. doi: 10.1083/jcb.200212033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottardi CJ, Wong E, Gumbiner BM. E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J Cell Biol. 2001;153:1049–1060. doi: 10.1083/jcb.153.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrais M, Chen X, Perez-Moreno M, Gumbiner BM. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell. 2007;18:2013–2025. doi: 10.1091/mbc.E06-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003;17:1090–1100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClatchey AI, Fehon RG. Merlin and the ERM proteins—Regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 2009;19:198–206. doi: 10.1016/j.tcb.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell. 2010;19:727–739. doi: 10.1016/j.devcel.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halder G, Johnson RL. Hippo signaling: Growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: New connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20:638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grusche FA, Richardson HE, Harvey KF. Upstream regulation of the hippo size control pathway. Curr Biol. 2010;20:R574–R582. doi: 10.1016/j.cub.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 23.Reddy BV, Irvine KD. The Fat and Warts signaling pathways: New insights into their regulation, mechanism and conservation. Development. 2008;135:2827–2838. doi: 10.1242/dev.020974. [DOI] [PubMed] [Google Scholar]
- 24.Saburi S, et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 25.Mao Y, et al. Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development. 2011;138:947–957. doi: 10.1242/dev.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr Biol. 2010;20:582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol. 2010;20:573–581. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 28.Okada T, You L, Giancotti FG. Shedding light on Merlin’s wizardry. Trends Cell Biol. 2007;17:222–229. doi: 10.1016/j.tcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Zhou D, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song H, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci USA. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishioka N, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Huelsken J, et al. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: Conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shenolikar S, Voltz JW, Cunningham R, Weinman EJ. Regulation of ion transport by the NHERF family of PDZ proteins. Physiology (Bethesda) 2004;19:362–369. doi: 10.1152/physiol.00020.2004. [DOI] [PubMed] [Google Scholar]
- 36.Mohler PJ, et al. Yes-associated protein 65 localizes p62(c-Yes) to the apical compartment of airway epithelia by association with EBP50. J Cell Biol. 1999;147:879–890. doi: 10.1083/jcb.147.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao B, Lei Q, Guan KL. Mst out and HCC in. Cancer Cell. 2009;16:363–364. doi: 10.1016/j.ccr.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Cho E, et al. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 39.Schlegelmilch K, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varelas X, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Heallen T, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: Orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 44.Orlando K, Guo W. Membrane organization and dynamics in cell polarity. Cold Spring Harb Perspect Biol. 2009;1:a001321. doi: 10.1101/cshperspect.a001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.