Abstract
The effects of the cellular environment on innate immunity remain poorly characterized. Here, we show that in Drosophila ATP-sensitive potassium channels (KATP) mediate resistance to a cardiotropic RNA virus, Flock House virus (FHV). FHV viral load in the heart rapidly increases in KATP mutant flies, leading to increased viremia and accelerated death. The effect of KATP channels is dependent on the RNA interference genes Dcr-2, AGO2, and r2d2, indicating that an activity associated with this potassium channel participates in this antiviral pathway in Drosophila. Flies treated with the KATP agonist drug pinacidil are protected against FHV infection, thus demonstrating the importance of this regulation of innate immunity by the cellular environment in the heart. In mice, the Coxsackievirus B3 replicates to higher titers in the hearts of mayday mutant animals, which are deficient in the Kir6.1 subunit of KATP channels, than in controls. Together, our data suggest that KATP channel deregulation can have a critical impact on innate antiviral immunity in the heart.
Keywords: ion channel, myocarditis, potassium efflux, aging, tolbutamide
Viral infections represent a major threat for living organisms, from prokaryotes to complex eukaryotes, including humans. Therefore, the characterization of the cellular and molecular mechanisms determining the outcome of viral infections is a focus of intense investigation.
A primary determinant of survival to virus infection in animals is the innate immune response, which allows the host to interfere with viral replication and to control the viral load (1). Considerable efforts on different experimental models have led to the realization that dsRNAs, which are produced in the course of viral replication, are a major trigger of innate antiviral responses. In vertebrates, foreign dsRNA molecules are recognized by proteins of the Toll-like receptor (TLR) and RIG-I–like receptor (RLR) families, leading to the production of type I and III interferons, which in turn mediate induction of antiviral molecules (2). In both plants and invertebrates, dsRNAs are recognized by RNase III enzymes of the Dicer (Dcr) family, and trigger antiviral RNAi (3). Interestingly, the cytosolic RLRs and the Dicer enzymes share an evolutionary conserved DExD/H box helicase domain, revealing some similarities in the sensing of viral nucleic acids by plants, invertebrates, and vertebrates (4). In the fruit fly Drosophila melanogaster, Dcr-2 accounts for the production of siRNAs of viral origin, which are loaded on the Argonaute (AGO) protein family member AGO2, with the help of the dsRNA binding protein R2D2. These siRNAs guide the slicer enzyme AGO2 toward cRNA sequences, thus achieving antiviral silencing. In agreement with the essential roles of Dcr-2 and AGO2 in the siRNA pathway, flies mutant for these genes are highly susceptible to viral infections (5–7). Though our information on the biochemistry of RNA interference is rapidly expanding, a central and as yet poorly explored area is the control of RNA interference by the cellular environment.
In addition to the immune response, which controls the viral load, it is becoming apparent that homeostatic mechanisms enable the host to control the cellular or tissue damage caused by a given dose of virus (8, 9). In the course of a collaborative effort aimed at understanding the genetic basis of antiviral defenses, one of our groups recently identified an N-ethyl-N-nitrosourea (ENU)-induced strain of mutant mice, mayday, which show an increased frequency of sudden death upon infection with the mouse cytomegalovirus (MCMV). These mice produce normal levels of cytokines upon infection, and die with viral titers in the spleen similar to wild-type controls, indicating that their death does not result from a failure to control the viral burden (9). The mayday mutation is an allele of the gene Kcnj8, which encodes the inwardly rectifying ATP-sensitive potassium (KATP) channel Kir6.1. KATP channels are octameric complexes composed of four pore-forming Kir subunits and four sulfonylurea receptor (SUR) subunits that regulate the opening of the channel (10) (Fig. 1A). In mice, these channels allow smooth muscle cells in coronary arteries to adapt to the vasoconstriction triggered by the inflammatory cytokines produced by the innate immune system in response to infection (9, 11). KATP channels are evolutionarily ancient, and we previously reported that silencing of the SUR ortholog (dSUR) in the Drosophila heart reduces the survival following infection by the RNA virus Flock House virus (FHV) (9). This observation suggests that KATP channels play an evolutionarily conserved role in host–virus interactions, and raises the question of the exact mechanism by which they regulate survival to viral infection in organisms with physiologies as different as flies and mammals.
Fig. 1.
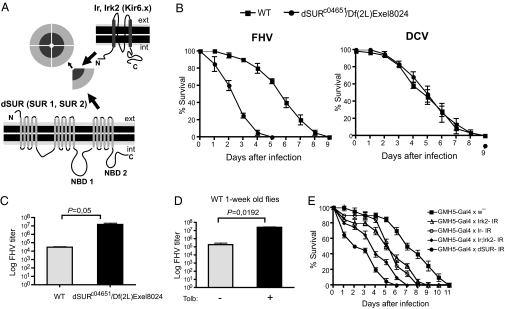
dSUR, Ir, and Irk2 are important for resistance to FHV infection in Drosophila. (A) Structural organization of the cardiac KATP channel. Kir and SUR are the constitutive subunits of the cardiac KATP channel. Four subunits of the inwardly rectifying K+ channel, encoded by the genes Ir or Irk2, associate with the ABC regulatory protein encoded by dSUR, to form a functional KATP channel octamer. SUR possesses two cytoplasmic nucleotide binding domains (NBD1 and NBD2). The names of the mammalian orthologs are given in parentheses. (B) Survival curves of dSUR mutant and control flies after infection with 2 × 106 pfu/mL FHV or 2 × 106 DCV particles of a dose lethal to 50% of flies tested, per milliliter. (C) FHV titers in dSUR mutant and control flies at day 3 following virus infection. (D) FHV titers in wild-type (WT) 1-wk-old flies fed on the KATP antagonist tolbutamide and control flies fed on sucrose solution only, at day 3 following virus infection. (E) Survival curves of flies obtained from crosses between UAS-dSUR RNAi, UAS-Ir RNAi, UAS-Irk2 RNAi, and UAS-Ir;Irk2 RNAi lines (IR, inverted repeat) with the heart-specific GMH5-Gal4 driver and control flies [GMH5-Gal4 line crossed with white (w−) flies] after infection with FHV. Data represent the mean ± SDs of three (C and D) or two (B and E) independent experiments each involving two groups of 10 flies.
Here we have characterized the role of KATP channels in FHV-infected Drosophila. Using both genetics and pharmacology, we show that KATP channels are involved in an antiviral resistance mechanism, as impairment of their expression or function leads to a strong increase of the viral titer and accelerated death of the FHV-infected flies. We further show that FHV is a cardiotropic virus and that KATP channels control the FHV viral load in the heart. Potassium efflux does not affect an intrinsic property of the viral replication cycle, but rather modulates antiviral RNA interference in the heart. These results indicate that tissue-specific regulation of resistance mechanisms by the cellular environment can have dramatic consequences on the outcome of an infection. We illustrate this point by showing that the KATP agonist drug pinacidil exerts a spectacular protective effect against FHV infection in Drosophila.
Results
Drosophila KATP Channels Are Involved in the Resistance to FHV Infection.
We previously reported that knockdown of the expression of the gene dSUR, which encodes the regulatory subunit of KATP potassium channels (Fig. 1A), increases the lethality of Drosophila after infection with FHV (9). Flies mutant for dSUR [c04651/Df(2L)Exel8024] are viable, indicating that dSUR does not carry essential developmental functions. When infected with FHV, dSUR mutant flies succumb rapidly, 4–5 d before control flies. By contrast, these mutant flies do not show increased lethality compared with controls when challenged with Drosophila C virus (DCV), the entomopathogenic bacteria Enterococcus faecalis, Enterobacter cloacae, or the fungus Beauveria bassiana (Fig. 1B) (9). To see if the rapid death of FHV-infected dSUR mutant flies reflects a defect in homeostasis or resistance, we monitored the viral load in dSUR mutant and control flies. dSUR mutant flies show increased accumulation of viral RNAs, and contain up to two logs more infectious viral particles than control flies 3 d after infection, indicating that dSUR regulates the resistance to FHV infection (Fig. 1C). Consistent with the survival data, DCV-infected dSUR mutant flies contain similar viral titers as control flies.
As an independent test of the requirement for KATP channels during infection, we investigated the effect of the drug tolbutamide, which acts as an antagonist of KATP channels. We previously reported that flies fed on a tolbutamide solution died more rapidly than control flies after infection with FHV (9). The tolbutamide treatment leads to increased accumulation of viral RNAs and infectious particles in infected flies, with a two-log increase in viral load 3 d after infection (Fig. 1D).
SUR molecules function as the regulatory subunit of Kir6.x channels in mammals. The D. melanogaster genome contains three genes encoding channels related to mammalian Kir genes, known as Ir, Irk2, and Irk3 (Fig. S1A). Among these, Ir and Irk2 are closely related to one another, and belong to the same clade as mammalian Kir2, Kir3, Kir5, and Kir6, whereas Irk3 is more distantly related to these molecules. Based on their expression in the hindgut and the Malpighian tubules, these genes have been proposed to function in the osmoregulation of the fly (12). We monitored expression of Ir and Irk2 by RT-PCR, and found that these genes are indeed expressed in the excretory system of Drosophila, but that they are mainly expressed, like dSUR, in the heart (Fig. S1B). We knocked down expression of these genes in the heart using the heart-specific GMH5-Gal4 driver (13), and observed that both Ir and Irk2 are required to slow infection by FHV (Fig. 1E). Knockdown of both Ir and Irk2 caused a more severe phenotype than single knockdowns, but not as severe as the knockdown of dSUR. By contrast, silencing of Irk3 expression did not affect resistance to FHV infection (Fig. S1C).
Overall, both genetic and pharmacological data point to an essential role for KATP channels in the resistance to FHV infection, raising the question of the mechanism involved.
FHV Is a Cardiotropic Virus.
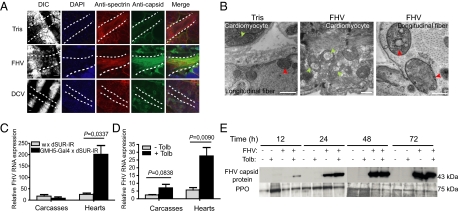
We monitored the presence of virus in the heart of infected wild-type flies by immunofluorescence, and observed that FHV efficiently infects heart muscle cells. By contrast, we did not detect the presence of DCV in the heart, even though the virus can readily be observed in surrounding fat body cells (Fig. 2A). Typical FHV-induced changes in the morphology of mitochondria (14) were observed in longitudinal fibers and cardiomyocytes (Fig. 2B). We noted a more rapid accumulation of FHV capsid proteins in the heart of dSUR-silenced flies compared with controls (Fig. S2A). Similar results were obtained when the amount of viral RNAs in the heart or the rest of the body was monitored by quantitative RT-PCR (Fig. 2C). Finally, tolbutamide treatment of wild-type flies led to an increase of FHV viral RNAs mostly in the heart (Fig. 2D) and to increased viremia at the early time points of infection (Fig. 2E). Together, these data indicate that FHV is a cardiotropic virus and that dSUR participates in a tissue-autonomous manner in the control of viral load in the heart, thus limiting viremia and spreading to other tissues.
Fig. 2.
FHV, but not DCV, infects the Drosophila heart. (A) Confocal microscopy of the colocalization of spectrin and FHV or DCV in the hearts of virus-infected WT 1-wk-old flies double-labeled with anti-spectrin (red; Alexa Fluor 546) and antibody to viral capsid protein (green; Alexa Fluor 488) at 3 d following virus challenge. Injections with Tris buffer were used as negative controls. Data are representative of three experiments with at least 10 flies for each treatment. (Scale bar: 35 μm.) (B) Electron micrographs showing FHV-induced spherular invaginations of the outer mitochondrial membrane (arrowheads) of infected, but not control (Tris) cardiomyocytes (green arrowheads) and longitudinal fibers (red arrowheads) that are associated ventrally with the Drosophila heart (33). (Scale bar: 500 nm.) (C) Quantitative RT-PCR of FHV RNA levels in the hearts and carcasses from dSUR-silenced flies and control flies at 3 d after virus infection. (D) Quantitative RT-PCR of FHV RNA levels in the hearts and carcasses from WT 1-wk-old flies fed on the KATP blocker tolbutamide and control flies fed on sucrose solution only, at day 3 following infection with FHV. For C and D, data represent the mean ± SD of three independent experiments involving at least 30 flies per replicate. (E) Immunoblot for expression of FHV capsid protein in the hemolymph of 1-wk-old WT flies treated with tolbutamide and control flies kept on sucrose solution only at different times after virus infection. Prophenoloxidase (PPO) protein levels in the hemolymph of flies served as a loading control. Data are representative of two independent experiments involving at least 30 flies for each treatment.
KATP Channels Regulate Antiviral Innate Immunity in the Heart.
To test whether potassium efflux regulates FHV infection at the level of viral replication, as opposed to other steps of the viral cycle, such as viral entry, viral uncoating, or capsid synthesis and assembly, we used a transgenic viral replicon. In this system, sequences corresponding to RNA1 from FHV and encoding the viral RNA-dependent RNA polymerase (RdRP) are placed under the control of the UASGal4 system in transgenic flies (Fig. S2B). We previously reported that RNA1 produced from this transgene, even at low levels (in the absence of a Gal4 driver), is rapidly amplified due to the expression of the viral RdRP (5). We monitored RNA1 expression by quantitative RT-PCR in UAS-FHV RNA1 transgenic flies treated with tolbutamide and untreated controls, and observed a strong increase in the levels of RNA1 in the treated flies. The majority of the increase of RNA1 in flies treated with the drug was attributed to the heart (Fig. S2C). Thus, KATP channels act at the level of the accumulation of viral RNAs in infected cells.
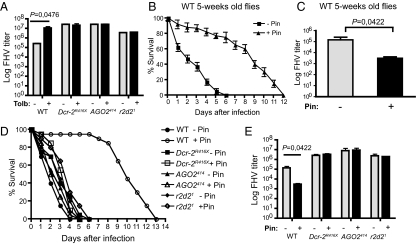
To test whether KATP channels have a direct effect on the viral polymerase or affect the host resistance to infection, we analyzed the effect of tolbutamide on FHV-infected flies carrying mutations in essential genes of the antiviral RNAi pathway. Whereas addition of tolbutamide resulted in an increase by two orders of magnitude in the FHV titer in wild-type flies, it had no effect in Dcr-2, AGO2, or r2d2 mutant flies (Fig. 3A). Consistent with these data, tolbutamide treatment did not aggravate the survival of Dcr-2, AGO2, or r2d2 mutant flies infected by FHV (Fig. S3A). We then tested for a genetic interaction between AGO2 and dSUR. For this, we compared the resistance to FHV infection (survival and viral load) of flies heterozygous for AGO2 and dSUR, as well as flies double heterozygous for both AGO2 and dSUR. Flies heterozygous for both AGO2 and dSUR die more rapidly (Fig. S3B) and contain higher viral loads than flies heterozygous for one of the mutants (Fig. S3C), pointing to a genetic interaction between AGO2 and dSUR. Similar results were observed when Dcr-2 was used instead of AGO2 (Fig. S3 D and E). These data reveal that cardiac potassium channels do not affect an intrinsic property of the viral RdRP, but rather affect the antiviral RNAi pathway.
Fig. 3.
Potassium channels regulate antiviral RNA interference in the Drosophila heart. (A) FHV titers in 1-wk-old Dcr-2, Argonaute-2 (AGO2), r2d2 mutant, and WT control flies at day 3 following feeding on tolbutamide or control sucrose solution alone and virus infection. (B) Survival curves of WT 5-wk-old flies fed on the KATP agonist pinacidil and control flies fed on sucrose solution alone after infection with FHV. (C) FHV titers in WT 5-wk-old flies fed on pinacidil and control flies fed on sucrose solution alone at 3 d following virus infection. (D) Survival curves of 5-wk-old Dcr-2, AGO2, r2d2 mutant, and WT control flies following treatment with pinacidil and infection with FHV. (E) FHV titers in 5-wk-old Dcr-2, AGO2, r2d2 mutant, and WT control flies at day 3 after feeding on pinacidil or sucrose solution and virus infection. Data represent the mean ± SD of three independent experiments each involving at least 30 flies (viral load) or two groups of 10 flies (survival) per treatment.
The KATP Agonist Drug Pinacidil Protects Flies Against FHV infection.
Based on our findings, we reasoned that drugs activating potassium channels in the Drosophila heart may exert protective effects upon FHV infection. We investigated the effect of the KATP agonist drug pinacidil, and did not observe an effect in our standard assay conditions with young flies (1 wk old; Fig. S4A). Previous work has shown that dSUR expression decreases with aging, and that this decrease in dSUR expression is associated with increased pacing-induced heart failure (15). We monitored resistance to FHV infection in aging flies, and observed a strong effect of aging on resistance to FHV infection (Fig. S4B). In particular, 5-wk-old wild-type flies were highly susceptible to FHV infection, with 50% of the flies dying within the first 24 h of the infection, reaching 100% mortality in 5–6 d. We treated 5-wk-old flies with pinacidil 3 h before infection with FHV, and observed a dramatic increase in their survival (Fig. 3B). Pinacidil treatment of dSUR knockdown old flies did not protect them against FHV infection, confirming that the drug was acting on KATP channels and not on other targets (Fig. S4C). Pinacidil-treated old, wild-type flies, but not dSUR knockdown old flies, exhibited a significantly reduced viral load compared with old control flies, indicating that the drug strongly increased the resistance to infection (Fig. 3C and Fig. S4D). Five-wk-old flies also died more rapidly than young flies when challenged with DCV or bacteria. In these cases, however, pinacidil treatment had only weak or no effect on the survival of the flies (Fig. S5 A–C).
Pinacidil treatment did not improve the resistance to FHV infection of Dcr-2, AGO2, and r2d2 null mutant flies, indicating that a functional antiviral RNAi pathway is required for the effect of the drug (Fig. 3 D and E). These results confirm that potassium channels regulate innate immunity in the heart, and reveal that the KATP agonist drug pinacidil can be used to protect flies against a cardiotropic virus.
Mammalian KATP Channels Are Involved in the Control of Coxsackievirus B3 (CVB3) Infection in the Heart.
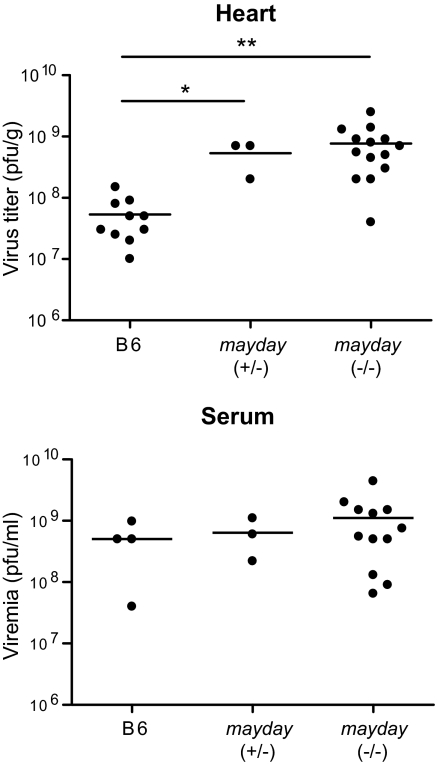
Because KATP channels limit a cardiotropic infection in flies, we decided to test the importance of mammalian Kcnj8 in the containment of a cardiotropic infection in mice. We assessed virus loads in the heart and serum of mayday mutants compared with WT C57BL/6J mice. Mice were infected with 500 pfu of CVB3 i.p., and virus loads were compared at 3 d postinfection (dpi). Though there was no significant difference in viremia between mayday and control mice, a significant increase in virus load in heart tissue was observed in both homozygous and heterozygous mayday mutants, compared with the control mice (Fig. 4). We note that exaggerated CVB3 proliferation is observed even in heterozygous mayday mutant mice, whereas the deleterious effect of the mayday mutation (hypersensitivity to MCMV infection or LPS administration, leading to acute lethality) is strictly recessive (9). The cellular mechanism of the antiviral effect may therefore be quite different from the cellular mechanism of protection against coronary artery vasoconstriction, despite the likelihood that the same heterooctameric channel complex mediates both phenomena.
Fig. 4.
Higher virus load in the heart of Kcnj8 (mayday) mutant mice. Eight-wk-old mayday and C57BL/J control mice were infected with 500 pfu of CVB3 i.p. At 3 dpi, mice were bled for sera, and hearts were collected after perfusion. Virus titers were determined by the plaque assay on HeLa cells. *P = 0.0001, **P = 0.0020.
Discussion
Our findings reveal that the evolutionarily conserved cardiac KATP channels play an essential role in the resistance to infection by the cardiotropic RNA virus FHV in Drosophila, through modulation of RNA interference. RNAi plays a major role in the regulation of several biological processes, including the control of infections. As such, it does not come as a surprise that RNA silencing must be regulated. Indeed, it was recently reported that molecules from the RNAi pathway, such as TRBP, AGO, or PIWI, can be regulated by phosphorylation, hydroxylation, or methylation (16). We now show that ion channels can also affect antiviral RNAi. It will be interesting in future studies to investigate whether potassium channels also regulate other RNAi pathways in the heart, such as the miRNA or the endosiRNA pathway, which may regulate heart physiology in the absence of viral infection (17).
KATP channels were previously shown to play a role in the survival to viral infection in mice, but through a different mechanism—namely, the modulation of the response to cytokines in coronary arteries (9, 11). This discrepancy may reflect the differences existing between insects and mammals both at the level of antiviral innate immunity (e.g., the prominent role of RNAi in flies vs. IFN-mediated inducible response in mammals) and host physiology (e.g., the important effect of inflammatory cytokines on the vasculature in mammals). An alternative hypothesis is that KATP channels may play a previously unnoticed dual role in virus–host interactions, modulating the effects of cytokines and also participating in the regulation of innate immunity mechanisms.
K+ ions have previously been shown to regulate the induction of the NRLP3 inflammasome, which converts procaspase-1 into active caspase-1, an important aspect of the innate immune response (18). The inflammasome processes prointerleukin (IL)-1β into the active 17-kDa IL-1β inflammatory cytokine. One signal activating the NRLP3 inflammasome is the extracellular release of ATP, which activates the purinergic receptor P2X7R, leading to K+ efflux and NRLP3 inflammasome activation. Treatment of cells with the K+ ionophore toxin nigericin is sufficient to trigger the NRLP3 inflammasome, pointing to the important role of potassium ions in the regulation of the production of the essential inflammatory cytokine IL-1β. Interestingly, the sulfonyl urea drug glyburide (also known as glibenclamide), which functions like tolbutamide as an inhibitor of KATP channels, was recently reported to inhibit the NRLP3 inflammasome in bone marrow-derived macrophages (19). In a more direct connection to the context of viral infections, RIG-I appears to play a dual role upon recognition of RNA virus infection, activating on one hand the transcription factors of the NF-κB and IRF families through the signaling adaptor MAVS, and triggering inflammasome activation through the adaptor ASC on the other. Treatment of bone marrow-derived dendritic cells with glyburide completely abrogates vesicular stomatitis virus-induced secretion of IL-1β, providing further support for a role of K+ ions in the regulation of antiviral innate immunity in mammals (20). In addition to the inflammasome, antiviral apoptosis can also be triggered by the voltage-dependent K+ channels Kv2.1 in mammals (21). We now add RNA interference to the list of innate immunity mechanisms regulated by K+ ions (Fig. S6). An intriguing question in all these cases pertains to the mechanism by which K+ ions regulate these targets, and whether this function is directly regulated by K+ ions, or indirectly through modified concentrations of other ions, such as Ca2+ or Na+. Of note, inhibition of the sodium-potassium pump by cardiac glycosides was recently shown to block induction of IFN-β gene expression (22). This effect is mediated by the direct inhibition by Na+ ions of the ATPase activity of RIG-I, an observation that is particularly interesting in light of the conservation of the DExD/H box helicase domains of RIG-I and Dcr-2 (23).
Potassium channels play an important role in cardiac repolarization both in flies and mammals (24, 25). An unexpected finding of our study is that FHV is a cardiotropic virus in Drosophila, providing a useful model to study virus–cardiomyocyte interaction in vivo in this genetically tractable model. Cardiotropic viruses play an important role in the onset of myocarditis in humans, which can lead to sudden death by cardiac arrest (26). However, the mechanisms leading to cardiomyopathy following viral infection in mammals are still poorly understood. One striking observation is that the cardiotropic viruses causing myocarditis are common and will be encountered by the vast majority of the population in their lifetime. However, only some 10% of the infected subjects will develop myocarditis (27, 28), which points to an important role for the genetic background and/or environmental factors in the onset of this disease. For example, defects in an antiviral immunity pathway may be associated with increased viral replication and tissue damage. Curiously, not much is known at this stage about antiviral host defense in cardiomyocytes, and the available data point to complex regulation (29–31). The fact that patients suffering from myocarditis do not appear to be particularly prone to other types of viral infections suggest that either heart-specific mechanisms of antiviral host defense are affected in these patients, or that certain heart-specific conditions regulate the interaction of the cardiomyocyte with the virus. Our findings in Drosophila, in conjunction with the established role of K+ ions in the regulation of the inflammasome discussed above, suggest that control of K+ ion efflux could be one such condition. Moreover, we observed a higher virus load in the hearts of both homozygous and heterozygous mayday mutant mice after CVB3 infection, although they did not show exaggerated viremia compared with control mice. These data are consistent with an ancestral antiviral function served by KATP channels, now retained in the mammalian heart, but not necessarily other tissues. Moreover, KATP channels appear to have been adapted to other functions, including the preservation of cardiovascular homeostasis during infection.
In summary, our data highlight a unique layer of complexity in the regulation of antiviral immunity. In addition to the general (e.g., type I IFN in mammals, or RNAi in invertebrates) and the tissue-specific [e.g., TLR-mediated gene induction in plasmacytoid dendritic cells in mammals (32) or Vago induction in the Drosophila fat body (23)] mechanisms of antiviral host defense, we describe here a tissue-specific regulation by the cellular environment of antiviral innate immunity. Our data indicate that genetic defects or drugs acting on this cellular environment, as opposed to direct effects on the virus or the immune system, can have major effects on the outcome of the infection. Therefore, the characterization of tissue-specific regulatory mechanisms of innate immunity represents an original and promising avenue of research for the development of novel therapeutics against infectious diseases.
Materials and Methods
Drosophila Strains and Infections.
w− and yw flies were used as wild-type controls. Most mutant lines used in the experiments (UAS-dSUR RNAi lines; GMH5-Gal4 driver; UAS-RNA1 FHV; Dcr2R416x, AGO2414, and r2d21 mutants) have been previously described. SURc04651 mutant flies and the deficiency Df(2L)Exel8024 were obtained from the Bloomington Stock Centre and the Exelixis Collection (Harvard Medical School), respectively. UAS-Ir (v28430 and v28431), UAS-Irk2 (v4341), and UAS-Irk3 (v3886) RNAi lines were purchased from the Vienna Drosophila RNAi Center (VDRC). All fly lines were tested for Wolbachia infection and cured whenever necessary. Raising of fly stocks and infections were done as previously described (5, 9). Two replicates of 10 flies were used for each treatment, and every assay was replicated three times.
Mice and Virus.
Kcnj8mayday/mayday and Kcnj8+/mayday mice (C57BL/6J background) were used for Coxsackievirus B3 (CVB3) infection. Age-matched C57B/6J mice were used as controls. Mice were i.p. injected with 500 pfu of CVB3 (Woodruff strain), which was generously provided by L. Whitton (The Scripps Research Institute). Heart and serum were harvested at 3 dpi and subjected to virus titration by the conventional plaque assay on HeLa cells. All animal protocols were approved by The Scripps Research Institute Department of Animal Resources in compliance with Institutional Animal Care and Use Committee guidelines.
Drug Treatment.
Stock solutions (1 M) of tolbutamide and pinacidil (Sigma) were initially prepared in dimethylsulfoxyde. Adult flies were fed on a 1% sucrose solution supplemented with tolbutamide or pinacidil (2 mM final concentration) for 3 h before infection with FHV.
RNA and Protein Analysis.
Analysis of RNA expression was performed by real-time quantitative RT-PCR as previously described (23). For hemolymph protein analysis, hemolymph was collected from flies at various time points following infection using thin glass capillaries adjusted to the Nanoject II apparatus. Electrophoresis and immunoblot analysis, as well as immunostaining, were performed as described (23).
Median Tissue Culture Infective Dose Assays.
Infected flies were homogenized in Schneider's Drosophila media (BioWest) and following centrifugation of fly parts, supernatants were filtered and dilutions of virus suspensions were used to infect D. melanogaster Kc167 cells in a 96-well plate format. The presence of virus was scored 24 h later by immunofluorescence.
Statistical Analysis.
Data were analyzed using GraphPad Prism software. The mean values were compared by nonparametric unpaired t test. In all tests, P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank E. Santiago and M. Yamba for excellent technical help; J. Kalschmidt for assistance with some experiments; N. Matt for advice and help for the confocal microscopy; M. Miehe for technical help for the electron microscopy; A. Schneeman for the anti-FHV antiserum; and D. Ferrandon, J. M. Reichhart, and P. Fornes for helpful discussions. Confocal microscopy was done at the Strasbourg Esplanade Cellular Imaging Facility (funded by Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Université de Strasbourg, and Region Alsace). Electron microscopy was done at the Technical Platform of Imaging Facility at the Center of Neurochemistry (Strasbourg, France). This work was supported by National Institute of Health Grants PO1 AI070167 (to B.B., J.-L.I., and J.A.H.) and RO1 HL54732 (to R.B.), the Balzan Foundation (J.A.H.), and the Centre National de la Recherche Scientifique. K.O. was supported by a Scientist Development Award from the American Heart Association. S.C. acknowledges financial support from the Fondation pour la Recherche Médicale.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108926108/-/DCSupplemental.
References
- 1.Beutler B, et al. Genetic analysis of resistance to viral infection. Nat Rev Immunol. 2007;7:753–766. doi: 10.1038/nri2174. [DOI] [PubMed] [Google Scholar]
- 2.Fujita T. A nonself RNA pattern: Tri-p to panhandle. Immunity. 2009;31:4–5. doi: 10.1016/j.immuni.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Ding SW, Voinnet O. Antiviral immunity directed by small RNAs. Cell. 2007;130:413–426. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kemp C, Imler JL. Antiviral immunity in Drosophila. Curr Opin Immunol. 2009;21:3–9. doi: 10.1016/j.coi.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann JA, Imler JL. Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat Immunol. 2006;7:590–597. doi: 10.1038/ni1335. [DOI] [PubMed] [Google Scholar]
- 6.van Rij RP, et al. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 2006;20:2985–2995. doi: 10.1101/gad.1482006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang XH, et al. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider DS, Ayres JS. Two ways to survive infection: What resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croker B, et al. ATP-sensitive potassium channels mediate survival during infection in mammals and insects. Nat Genet. 2007;39:1453–1460. doi: 10.1038/ng.2007.25. [DOI] [PubMed] [Google Scholar]
- 10.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 11.Kane GC, et al. Gene knockout of the KCNJ8-encoded Kir6.1 K(ATP) channel imparts fatal susceptibility to endotoxemia. FASEB J. 2006;20:2271–2280. doi: 10.1096/fj.06-6349com. [DOI] [PubMed] [Google Scholar]
- 12.Döring F, Wischmeyer E, Kühnlein RP, Jäckle H, Karschin A. Inwardly rectifying K+ (Kir) channels in Drosophila. A crucial role of cellular milieu factors Kir channel function. J Biol Chem. 2002;277:25554–25561. doi: 10.1074/jbc.M202385200. [DOI] [PubMed] [Google Scholar]
- 13.Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- 14.Kopek BG, Perkins G, Miller DJ, Ellisman MH, Ahlquist P. Three-dimensional analysis of a viral RNA replication complex reveals a virus-induced mini-organelle. PLoS Biol. 2007;5:e220. doi: 10.1371/journal.pbio.0050220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akasaka T, et al. The ATP-sensitive potassium (KATP) channel-encoded dSUR gene is required for Drosophila heart function and is regulated by tinman. Proc Natl Acad Sci USA. 2006;103:11999–12004. doi: 10.1073/pnas.0603098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heo I, Kim VN. Regulating the regulators: Posttranslational modifications of RNA silencing factors. Cell. 2009;139:28–31. doi: 10.1016/j.cell.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Xin M, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinon F, Mayor A, Tschopp J. The inflammasomes: Guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 19.Lamkanfi M, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poeck H, et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010;11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 21.Redman PT, et al. Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2.1. Proc Natl Acad Sci USA. 2007;104:3568–3573. doi: 10.1073/pnas.0610159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye J, Chen S, Maniatis T. Cardiac glycosides are potent inhibitors of interferon-β gene expression. Nat Chem Biol. 2011;7:25–33. doi: 10.1038/nchembio.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deddouche S, et al. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in drosophila. Nat Immunol. 2008;9:1425–1432. doi: 10.1038/ni.1664. [DOI] [PubMed] [Google Scholar]
- 24.Ocorr K, et al. Genetic control of heart function and aging in Drosophila. Trends Cardiovasc Med. 2007;17:177–182. doi: 10.1016/j.tcm.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 26.Andréoletti L, Lévêque N, Boulagnon C, Brasselet C, Fornes P. Viral causes of human myocarditis. Arch Cardiovasc Dis. 2009;102:559–568. doi: 10.1016/j.acvd.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360:1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–155. doi: 10.1146/annurev.pathmechdis.3.121806.151534. [DOI] [PubMed] [Google Scholar]
- 29.Deonarain R, Cerullo D, Fuse K, Liu PP, Fish EN. Protective role for interferon-beta in coxsackievirus B3 infection. Circulation. 2004;110:3540–3543. doi: 10.1161/01.CIR.0000136824.73458.20. [DOI] [PubMed] [Google Scholar]
- 30.Fuse K, et al. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of Coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation. 2005;112:2276–2285. doi: 10.1161/CIRCULATIONAHA.105.536433. [DOI] [PubMed] [Google Scholar]
- 31.Kumar H, et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med. 2006;203:1795–1803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato H, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Molina MR, Cripps RM. Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mech Dev. 2001;109:51–59. doi: 10.1016/s0925-4773(01)00509-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.