Abstract
OBJECTIVE
To examine the effect of intensive glycemic control therapy (IT) on insulin sensitivity and β-cell function in newly diagnosed type 2 diabetic patients compared with subjects with normal glucose tolerance (NGT) and those with impaired glucose tolerance (IGT).
RESEARCH DESIGN AND METHODS
Forty-eight newly diagnosed type 2 diabetic patients were randomly assigned to IT for 2 weeks and followed up for 1 year. Intravenous glucose tolerance tests were conducted in NGT, IGT, and diabetic subjects. Blood glucose and insulin were measured before and after IT and at the 1-year follow-up.
RESULTS
IT lowered the homeostasis model assessment (HOMA) for insulin resistance (IR) significantly, from 3.12 ± 1.4 (mean ± SD) to 1.72 ± 0.8, a level comparable to the IGT (1.96 ± 1.1) and NGT (1.37 ± 0.6) subjects in the remission group; however, no HOMA-IR improvement was observed in nonremission subjects. HOMA-β in the remission group was improved (mean, interquartile range) from 18.4 (8.3–28.5) to 44.6 (32.1–69.1) and acute insulin response of insulin (AIRins) from 1.50 ± 0.22 to 1.83 ± 0.19 μIU/mL after IT, but was still significantly lower than those in NGT individuals (HOMA-β: 86.4 [56.7–185.2], P < 0.01; AIRins: 2.54 ± 0.39 μIU/mL, P < 0.01). After IT and at 1 year, the hyperbolic relationship between HOMA-β and HOMA sensitivity of remission subjects shifted close to that of IGT subjects.
CONCLUSIONS
IT in newly diagnosed type 2 diabetes not only partially restored β-cell function but also greatly restored insulin sensitivity. Compared with IGT and NGT subjects, β-cell function was less restored than insulin sensitivity after IT in the remission subjects.
Type 2 diabetes is a complex metabolic disease attributed to genetic and environmental susceptibility (1). Insulin resistance (IR) and β-cell dysfunction are both responsible for pathogenesis of type 2 diabetes (2–4). Qian et al. (5) reported that β-cell failure, instead of aggravated IR, might be the main reason for Chinese subjects with impaired glucose tolerance (IGT) to develop diabetes. Conventional first-line medications for type 2 diabetes include gliclazide, nateglinide, and metformin aimed to improve β-cell function and IR, but these treatments are usually long-term.
Recently, short-term intensive glycemic control therapy (IT) was reported to achieve long-term glycemic control (6–9) in a large percentage of patients with newly diagnosed type 2 diabetes, and thus, the therapy may be developed as an alternative effective treatment or cure for type 2 diabetes. However, the mechanism underlying the therapy is not well understood. Several studies reported an improvement of β-cell function by IT (8,10,11), but only a few studies have examined insulin sensitivity changes during and after IT (10,12). Li et al. (8) reported an improvement of homeostasis model assessment (HOMA)-IR in remission and nonremission groups after IT with continuous insulin infusion. However, Chen et al. (9) did not observe a significant change of HOMA-IR in diabetic patients, even though their β-cell function was improved after IT. Thus, the effect of short-term IT on IR in subjects with newly diagnosed type 2 diabetes is controversial and not well documented.
We hypothesized that improvement of IR is an important mechanism for IT-mediated long-term remission in subjects with newly diagnosed type 2 diabetes. Therefore, we determined the changes of both insulin sensitivity and β-cell function between remission and nonremission groups during and after short-term IT in patients with newly diagnosed type 2 diabetes and compared the restoration levels of β-cell function and insulin sensitivity in these patients with results in IGT and normal glucose tolerant (NGT) subjects. We found that in the remission group, IT significantly improved IR but only modestly restored β-cell function, suggesting that the nearly normal restoration of insulin sensitivity could be an important mechanism for the long-term remission achieved with IT.
RESEARCH DESIGN AND METHODS
Subjects
Human subjects were divided into type 2 diabetes, IGT, and NGT groups, based on World Health Organization 1999 diagnostic criteria (13). Forty-eight newly diagnosed type 2 diabetic patients (34 men, 14 women) were a subset of the multicenter study of 382 patients as reported in The Lancetby Weng et al. (10). They were 50.6 ± 7.9 years of age, with a BMI of 25.7 ± 3.3 kg/m2, nonketourine, and negative for islet cell antibodies and had not received antihyperglycemic therapy. Exclusion criteria included acute and severe chronic diabetes complications.
All NGT and IGT subjects were newly recruited and underwent a standard 75-g oral glucose tolerance test (OGTT). The 28 IGT subjects (17 men, 11 women) were 51.1 ± 7.6 years of age, with a BMI of 25.0 ± 2.6 kg/m2, fasting plasma glucose (FPG) of 5.6 ± 0.8 mmol/L, and 2-h postprandial plasma glucose (PPG) of 8.8 ± 1.3 mmol/L. The 12 NGT subjects (9 men, 3 women) were 39.5 ± 9.2 years of age, with a BMI of 24.7 ± 2.1 kg/m2, FPG of 4.6 ± 0.5 mmol/L, and PPG of 5.0 ± 0.5 mmol/L. Age and PPG, but not FPG and BMI, were statistically significant between the IGT and NGT groups. All studies were done at Drum Tower Hospital. All subjects had given written informed consent.
Study design
The patients were randomly assigned to three antihyperglycemic therapies: continuous subcutaneous insulin infusion (CSII), multiple daily insulin injections (MDI), or oral hypoglycemic agents (OHA). The CSII group received Novolin-R (Novo Nordisk, Bagsværd, Denmark) with an insulin pump. The MDI group was treated with Novolin-R before each meal and Novolin-N (Novo Nordisk) at bedtime. Initial insulin doses were 0.4–0.5 IU/kg per day. Total daily doses were divided into 50% basal and 50% bolus injection in the CSII group and into 30%-20%-20%-30% in the MDI group. In the OHA group, gliclazide (Servier, Tianjin, China) was initially administered at 80 mg twice daily to patients with a BMI of 20–25 kg/m2, and the dose was doubled depending on the blood glucose level. Metformin (Glucophage; Bristol-Myers Squibb, New York, NY) was initially given at 0.5 g twice daily day in the patients with a BMI of 25–35 kg/m2 and was increased up to a maximum of 2.0 g daily. Gliclazide and metformin were combined until hyperglycemia was controlled in patients who did not reach the glycemic control goal using one kind of oral hypoglycemic agent. The glycemic control target was defined as FPG <6.1 mmol/L and PPG <8.0 mmol/L. Treatments were maintained for 2 weeks after the target was reached.
Outpatient clinic follow-up
After 2 weeks of IT, medication was stopped, patients were advised to perform moderate exercise of 30 min of walking after meals and to eat a low-caloric, low-fat, high-fiber diet and more vegetables. Glycemic control was monitored monthly during the initial 3 months and at 3-month intervals thereafter. Hyperglycemia relapse was defined as FPG >7.0 mmol/L or PPG >11.0 mmol/L, and measurements were repeated 1 week later. Patients who maintained optimal glycemic control for at least 12 months without medication were defined as remission subjects, and those who relapsed during the 1-year follow-up were defined as nonremission subjects. The remission subjects received no medication, whereas the relapsed patients resumed OHA or IT during the 1-year follow-up period.
Measurements
All patients were studied in the morning after a 10-h overnight fast. Blood was drawn for the measurement of total cholesterol (TC), triglycerides (TG), HDL and LDL cholesterol, free fatty acid (FFA), FPG, PPG, glycosylated hemoglobin A1c (A1C), and insulin. Then NGT, IGT, and type 2 diabetic patients underwent intravenous glucose tolerance test (IVGTT) with a 25-g dose of glucose. Serum samples were obtained at 1, 2, 4, 6, and 10 min after the glucose injection for insulin determination. The blood measurements and IVGTT were repeated after 2-week normoglycemia and at the 1-year follow-up in type 2 diabetic patients.
Insulin was measured by a radioimmunoassay (Diagnostic Products Corporation, Los Angeles, CA), and A1C was measured by the Variant A1C Assay kit (Bio-Rad Laboratories, Hercules, CA). Other measurements were conventionally conducted.
Calculations
Acute insulin response of insulin (AIRins) during IVGTT was used to assess the first phase of β-cell insulin secretion, which was calculated as the incremental trapezoidal area during the first 10 min. HOMA (14) was used to estimate HOMA-IR: (FPG × fasting insulin/22.5); the β-cell function index: (HOMA-β = 20 × fasting insulin/[fasting plasma glucose − 3.5]; sensitivity: (HOMA-S = 1/HOMA-IR); and the disposition index (DI): HOMA-S × AIRins (15).
Statistical analysis
Statistical analyses were performed with SPSS 17.0 software (SPSS Inc., Chicago, IL). All numeric variables with normal distribution (age, BMI, FPG, PPG, TC, TG, HDL, LDL, FFA, A1C, and HOMA-IR) were expressed as the mean ± SD, whereas variables with skewed distribution (HOMA-β and DI) were expressed as the median (interquartile range [IQR]) and were logarithm-transformed before analyses. Data for AIRins were logarithm-transformed to achieve a normal distribution and expressed as the mean ± SD. Statistical significance was analyzed by the Student unpaired or paired t test, ANOVA or ANCOVA, with age and BMI as the covariates. The percentages of data were expressed as mean (95% CI). The hyperbolic regression curve for HOMA-S and HOMA-β relationship and the group differences were analyzed with HOMA-S as the dependent variable, and HOMA-β and group were analyzed as the independent variables by Stata 10 software (StataCorp, College Station, TX). A value of P < 0.05 was considered statistically significant.
RESULTS
The patients achieved target glycemic goal in 6.4 ± 3.4 days in CSII group, in 7.1 ± 3.1 days in MDI group, and in 11.6 ± 5.5 days in the OHA group (P < 0.05 for CSII/MDI vs. OHA). After short-term IT, 21 of 48 newly diagnosed type 2 diabetic patients (44%), comprising 8 CSII, 6 MDI, and 7 OHA subjects, achieved remission for 1 year, and 27 patients (8 in CSII, 12 in MDI, and 7 in OHA group) had relapsed (nonremission). There were no apparent differences in therapeutic effectiveness among the three different therapies (data not shown), and thus, all subjects with or without remission were pooled for subsequent analysis.
Clinical characteristics before IT and 2 weeks after IT, and at 1-year follow-up, are summarized in Table 1. Blood glucose levels and lipid profiles were similar at baseline between the remission and nonremission groups (P > 0.05). The remission group had markedly lower FPG, PPG, and TG after IT than the nonremission group (P < 0.01), whereas no significant differences were noted in BMI, TC, HDL, LDL, FFA, and A1C between the two groups at 2 weeks after IT. At the 1-year follow-up, the remission group had significantly lower FPG, PPG, and A1C levels than the nonremission group (Table 1).
Table 1.
Comparison of clinical characteristics between the remission and nonremission groups during IT and at 1-year follow-up
| Variable | Total | Remission group | Nonremission group | P |
|---|---|---|---|---|
| n | 48 | 21 | 27 | |
| Sex | ||||
| Male | 34 | 15 | 19 | |
| Female | 14 | 6 | 8 | |
| Age (years) | 50.6 ± 7.9 | 49.4 ± 8.8 | 51.5 ± 7.1 | 0.376 |
| BMI (kg/m2) | ||||
| Before therapy | 25.7 ± 3.3 | 26.3 ± 3.8 | 25.3 ± 2.9 | 0.326 |
| After therapy | 25.6 ± 3.1 | 26.0 ± 3.3 | 25.2 ± 2.9 | 0.393 |
| At 1 year | 25.2 ± 3.3 | 25.2 ± 3.5 | 25.2 ± 3.2 | 0.974 |
| FPG (mmol/L) | ||||
| Before therapy | 11.5 ± 2.8 | 11.7 ± 2.9 | 11.7 ± 2.4 | 0.993 |
| After therapy | 7.5 ± 2.1* | 6.2 ± 0.8* | 8.5 ± 2.2* | 0.000 |
| At 1 year | 6.8 ± 1.4* | 6.3 ± 1.3* | 7.5 ± 1.4* | 0.011 |
| PPG (mmol/L) | ||||
| Before therapy | 14.8 ± 5.8 | 15.1 ± 6.9 | 14.5 ± 4.9 | 0.721 |
| After therapy | 7.6 ± 3.1* | 6.1 ± 1.5* | 8.8 ± 3.4* | 0.002 |
| At 1 year | 7.7 ± 2.7* | 6.5 ± 1.78* | 9.2 ± 2.9* | 0.001 |
| TG (mmol/L) | ||||
| Before therapy | 2.0 ± 1.0 | 1.7 ± 0.5 | 2.2 ± 1.3 | 0.073 |
| After therapy | 1.5 ± 0.8* | 1.2 ± 0.4* | 1.7 ± 1.0* | 0.040 |
| At 1 year | 1.6 ± 0.7† | 1.5 ± 0.7‡ | 1.6 ± 0.8† | 0.751 |
| TC (mmol/L) | ||||
| Before therapy | 5.0 ± 1.0 | 4.9 ± 1.0 | 5.1 ± 0.9 | 0.442 |
| After therapy | 4.6 ± 0.9* | 4.5 ± 0.9 | 4.7 ± 0.8† | 0.601 |
| At 1 year | 4.9 ± 0.9 | 4.7 ± 0.8‡ | 5.2 ± 0.9§ | 0.047 |
| HDL (mmol/L) | ||||
| Before therapy | 1.2 ± 0.3 | 1.2 ± 0.2 | 1.2 ± 0.3 | 0.578 |
| After therapy | 1.3 ± 0.3* | 1.2 ± 0.3† | 1.3 ± 0.4† | 0.515 |
| At 1 year | 1.3 ± 0.4 | 1.2 ± 0.2 | 1.4 ± 0.5 | 0.189 |
| LDL (mmol/L) | ||||
| Before therapy | 3.0 ± 0.8 | 3.2 ± 0.8 | 3.0 ± 0.8 | 0.408 |
| After therapy | 2.7 ± 0.8* | 2.8 ± 0.7† | 2.6 ± 0.8* | 0.345 |
| At 1 year | 2.8 ± 0.8* | 2.8 ± 0.7* | 2.9 ± 0.9 | 0.850 |
| FFA (mmol/L) | ||||
| Before therapy | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.7 ± 0.2 | 0.462 |
| After therapy | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.745 |
| A1C (%) | ||||
| Before therapy | 10.0 ± 2.2 | 10.5 ± 2.3 | 9.7 ± 2.0 | 0.184 |
| After therapy | 7.9 ± 1.5* | 8.1 ± 1.6* | 7.7 ± 1.4* | 0.357 |
| At 1 year | 6.5 ± 0.7*§ | 6.2 ± 0.7*§ | 6.8 ± 0.6* | 0.004 |
Categorical data are expressed as number and continuous data as means ± SD. P value for remission vs. nonremission group.
*P < 0.01 vs. before therapy.
†P < 0.05 vs. before therapy.
‡P < 0.05 vs. after therapy.
§P < 0.01 vs. after therapy.
Insulin sensitivity
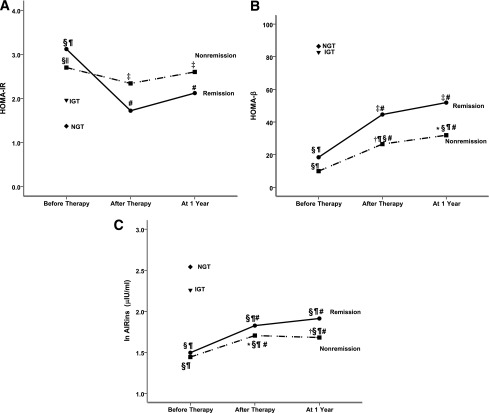
HOMA-IR was measured before, during, and after IT (Fig. 1A). Before IT, HOMA-IR in both remission (3.12 ± 1.4) and nonremission (2.70 ± 1.7) groups was significantly higher than that in IGT (1.96 ± 1.1) and NGT (1.37 ± 0.6) subjects. After IT, HOMA-IR in the remission group after treatment (1.72 ± 0.8) and at 1 year (2.12 ± 1.3) was comparable with that in IGT and NGT groups (P > 0.05). However, in the nonremission group, HOMA-IR was reduced from 2.70 ± 1.7 before IT to 2.34 ± 1.4 immediately after IT and to 2.60 ± 1.9 at the 1-year follow-up. By percentage, a great decrease of HOMA-IR of 38.7% (95% CI 24–54) after therapy and 31.1% (16–46) at the 1-year follow-up, compared with before treatment (P < 0.01), was observed in the remission group. A small and statistically insignificant decrease of 11.9% (–21 to 33) after therapy and 5.8% (–58 to 34) at the 1-year follow-up was noted in the nonremission group.
Figure 1.
Changes of HOMA-IR (A), HOMA-β (B), and AIRins (C) during IT and at 1-year follow-up in the remission (●) and nonremission (■) groups compared with NGT (♦) and IGT (▼) subjects. *P < 0.05 vs. remission group. †P < 0.01 vs. remission group. ‡P < 0.05 vs. NGT. §P < 0.01 vs. NGT. ||P < 0.05 vs. IGT. ¶P < 0.01 vs. IGT. #P < 0.01 vs. before therapy. HOMA-IR values are expressed as means, HOMA-β data are logarithm-transformed for analysis and expressed as median, and AIRins values are logarithm-transformed for analysis and expressed as means.
β-Cell function
Before IT, HOMA-β was higher in the remission group than in the nonremission group (Fig. 1B), although the difference was not statistically significant. The index in the remission (18.4 [IQR 8.3–28.5]) and nonremission (9.91 [6.2–17.4]) groups was significantly lower than that in the IGT (82.7 [32.1–127.1]) and NGT (86.4 [56.7–185.2]) subjects (P < 0.01). Compared with pretherapy, a significant increase of HOMA-β after therapy and at 1 year was observed in the remission and nonremission groups (P < 0.01); IT restored more HOMA-β in the remission group (44.6 [32.1–69.1] after therapy; 51.9 [28.8–79.8] at 1 year) than the nonremission group (26.5 [14.9–43.8] after therapy; 31.9 [18.8–52.7] at 1 year). Apparently, more restoration of HOMA-β was observed in the remission than that in the nonremission group, even though there was no statistical difference in HOMA-β between the two groups.
The restoration of HOMA-β was modest after IT, at ~24.0% (95% CI 16–32) of NGT in the remission group and 12.7% (3–22) in the nonremission group.
Next, we measured AIRins during IVGTTs. At the baseline measurement, AIRins (μIU/mL) was significantly lower in the remission (1.50 ± 0.22) and nonremission (1.44 ± 0.32) groups compared with the IGT (2.26 ± 1.44) and NGT (2.54 ± 0.39) subjects (P < 0.01). IT improved AIRins significantly in both groups (Fig. 1C). However, the restoration of AIRins was very limited: the average value at the 1-year follow-up was still ~18.4% (95% CI 13–24) of NGT in the remission group and 9.5% (4–15) in the nonremission group.
The hyperbolic relationship between HOMA-β and HOMA-S
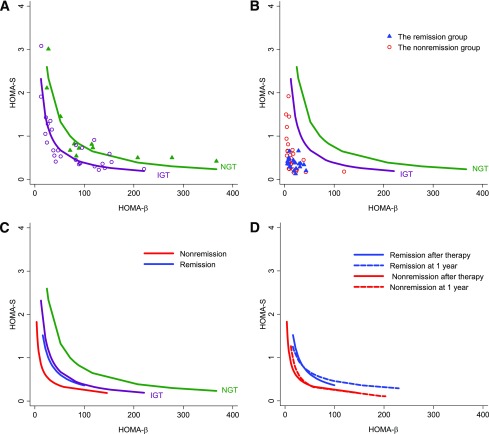
Finally, we determined hyperbola, which describes a nonlinear inverse relationship between insulin sensitivity and β-cell function. As expected, the hyperbolas of IGT subjects were to the left of NGT (P < 0.01; Fig. 2A). Before IT, the tracks in both the remission and nonremission groups obviously shifted left to NGT and IGT curves (Fig. 2B). After IT, the hyperbola in the remission group was restored to the IGT curve (P > 0.05) but was still to the left of the NGT curve (P < 0.01). However, the hyperbola in the nonremission group after therapy remained left of the IGT, NGT, and remission hyperbolas (P < 0.01, respectively; Fig. 2C). The curve at 1 year almost overlapped with the curve right after IT within the remission or nonremission group (P > 0.05, respectively; Fig. 2D). The DI in the remission group was significantly higher than that in the nonremission group after IT and at 1 year (Table 2).
Figure 2.
The hyperbolic relationship between HOMA-β and HOMA-S. A: The IGT hyperbola (purple line and circles; regression R2 = 0.910, P < 0.01) was to the left of NGT (green line and triangles, R2 = 0.945, P < 0.01). B: Before therapy, the remission (blue triangle) and nonremission (red circles) tracks both apparently shifted left to the IGT and NGT hyperbolas. C: Two weeks after IT , the remission hyperbola (blue line, R2 = 0.868, P < 0.01) shifted to IGT (P > 0.05) but was still left of the NGT hyperbola (P < 0.01), whereas nonremission hyperbola (red line, R2 = 0.711, P < 0.01) remained to the left of the remission, IGT, and NGT hyperbolas (P < 0.01, respectively). D: Curves at 1 year roughly overlap with those at 2 weeks after intensive therapy for the remission group (P > 0.05, blue dashed line vs. solid line) and the nonremission group (P > 0.05, red dashed line vs. solid line).
Table 2.
Changes of DI during IT and at 1-year follow-up in the remission and nonremission groups compared with NGT and IGT subjects
| Diabetes |
NGT (n = 12) | IGT (n = 28) | ||
|---|---|---|---|---|
| Remission (n = 21) | Nonremission (n = 27) | |||
| DI | 331.5 (131.6–444.8) | 112.1 (69.2–216.2) | ||
| Before therapy | 11.7 (7.3–15.9)*† | 12.4 (9.2–16.2)*† | ||
| After therapy | 34.6 (31.2–61.2)*†‡ | 25.6 (21.5–33.6)*†‡§ | ||
| At 1 year | 41.2 (30.8–80.8)*†‡ | 21.3 (18.2–37.3)*†‡§ | ||
Data for DI are logarithm-transformed for analysis and expressed as median (IQR).
*P < 0.01 vs. NGT.
†P < 0.01 vs. IGT.
‡P < 0.01 vs. before therapy, adjusted for BMI and age.
§P < 0.01 vs. remission group.
CONCLUSIONS
Type 2 diabetes is a heterogeneous disease (16) attributed to IR and β-cell dysfunction (2–4), although their relative contributions to glucose worsening depend on the duration of the disease (17). Indeed, in our newly diagnosed type 2 diabetic patients, higher IR and lower β-cell function than in NGT and IGT subjects were both observed before IT. In this study, we aimed to understand the possible beneficial mechanism of the IT-induced remission by examining the change of insulin sensitivity and β-cell function indexes during IT and comparing these indexes with those of IGT and NGT subjects, and we determined the degree of restoration, respectively, after the therapy.
In our study, IT achieved a remission rate of ~44% and improved β-cell function, as measured by HOMA-β and AIRins, which is in line with previous findings (7–9). A notable finding of this study is a marked decrease of HOMA-IR to a level of NGT after IT and to that of IGT at 1-year follow-up in the remission subjects, but not in the nonremission subjects. Improvement of IR after normoglycemia in long-term treatment of type 2 diabetes has been reported (18,19). Yki-Järvinen et al. (18) and Glaser et al. (19) reported that IT in type 2 diabetic patients in whom sulfonylurea therapy was not successful appears to achieve remission by lowering IR. However, only a few of studies examined the effect of short-term IT on insulin sensitivity in newly diagnosed type 2 diabetic patients and the results are not consistent. Li et al. (8) reported an improvement of HOMA-IR in remission and nonremission groups after IT with a continuous insulin infusion. However, Chen et al. (9) observed no significant change of HOMA-IR in subjects whose β-cell function was improved after intensive IT. The reason for the discrepancy is unclear and probably because of the difference of patient populations.
In this study, HOMA-IR in the remission group appeared higher than that in the nonremission group before IT, but the index greatly improved to the level of IGT and NGT subjects right after the therapy and remained lower at the 1-year follow-up. The β-cell functional indexes HOMA-β and AIRins showed that the remission group preserved more β-cell function before IT. IT restored more β-cell function in the remission than that in the nonremission subjects. By comparing the indexes in IGT and NGT subjects, we found that insulin sensitivity was nearly fully restored, but β-cell function was modestly improved after IT in the remission subjects. Although the recovery of β-cell function is understandably required for any remission of diabetes (8,10), the nearly normal restoration of insulin sensitivity could be an important beneficial mechanism for IT-induced remission because the decrease of IR would alleviate β-cell load. The mechanism by which IT decreases IR is not well-known, but our findings agree with the report of Pratipanawatr et al. (20) that normalization of blood glucose profiles in type 2 diabetic patients can reduce hyperglycemia-induced IR.
Kahn et al. (21) first described a hyperbolic relationship between insulin sensitivity and HOMA-β in a cohort of 96 nondiabetic subjects, and a left shift indicates inadequate β-cell compensation to IR. The observations have been supported by many human studies (22–25). In this study, we found that the hyperbola and DI in the remission group were restored to that in the IGT group and was almost close to the normal hyperbola in some patients, suggesting that restoration of a nearly normal glucose–insulin relationship in the remission group.
In conclusion, we demonstrated that short-term IT resulted in a greater improvement of IR resistance than β-cell function and that improvement for IR could be an important determining factor for the IT-induced remission in newly diagnosed diabetic patients.
Acknowledgments
The study was partially supported by Jiangsu Natural Science Fund BK2006006, Nanjing Targeted Science and Technology Development Fund ZKX10016, and the Ministry of Health of the People's Republic of China for Key Projects of Clinical Disciplines of Hospitals Affiliated to Ministry of Health (2010-2012) (J.W.).
No potential conflicts of interest relevant to this article were reported.
Y.H. analyzed the data and wrote the manuscript. L.L. contributed to the data analysis and discussion. Y.X. contributed to the statistical methods and data analysis. T.Y., G.T., H.H., and Y.B. contributed to the data collection. J.W. designed the study. D.Z. designed the study and reviewed the manuscript.
We thank Dr. Dawei Gong, University of Maryland School of Medicine, for critical reading of the manuscript, and Dr. Honggang Yi, the Department of Epidemiology and Biostatistics, School of Public Health, Nanjing Medical University, for statistics support.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2008;31(Suppl. 1):S55–S60 [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 2003;46:3–19 [DOI] [PubMed] [Google Scholar]
- 3.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Charles MA, Bennett PH. A two-step model for development of non-insulin-dependent diabetes. Am J Med 1991;90:229–235 [PubMed] [Google Scholar]
- 4.Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med 2002;347:1342–1349 [DOI] [PubMed] [Google Scholar]
- 5.Qian L, Xu L, Wang X, et al. Early insulin secretion failure leads to diabetes in Chinese subjects with impaired glucose regulation. Diabetes Metab Res Rev 2009;25:144–149 [DOI] [PubMed] [Google Scholar]
- 6.Alvarsson M, Sundkvist G, Lager I, et al. Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care 2003;26:2231–2237 [DOI] [PubMed] [Google Scholar]
- 7.Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care 2004;27:1028–1032 [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Xu W, Liao Z, et al. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of β-cell function. Diabetes Care 2004;27:2597–2602 [DOI] [PubMed] [Google Scholar]
- 9.Chen HS, Wu TE, Jap TS, Hsiao LC, Lee SH, Lin HD. Beneficial effects of insulin on glycemic control and β-cell function in newly diagnosed type 2 diabetes with severe hyperglycemia after short-term intensive insulin therapy. Diabetes Care 2008;31:1927–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008;371:1753–1760 [DOI] [PubMed] [Google Scholar]
- 11.McFarlane SI, Chaiken RL, Hirsch S, Harrington P, Lebovitz HE, Banerji MA. Near-normoglycaemic remission in African-Americans with type 2 diabetes mellitus is associated with recovery of beta cell function. Diabet Med 2001;18:10–16 [DOI] [PubMed] [Google Scholar]
- 12.Samanta A, Burden AC, Jones GR, Clarkson L. The effect of short term intensive insulin therapy in non-insulin-dependent diabetics who had failed on sulphonylurea therapy. Diabetes Res 1986;3:269–271 [PubMed] [Google Scholar]
- 13.World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of WHO consultation [Internet]. Available from http://www.staff.ncl.ac.uk/philip.home/who_dmg.pdf.
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 15.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 1981;68:1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care 1992;15:318–368 [DOI] [PubMed] [Google Scholar]
- 17.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care 2001;24:89–94 [DOI] [PubMed] [Google Scholar]
- 18.Yki-Järvinen H, Esko N, Eero H, Marja-Riitta T. Clinical benefits and mechanisms of a sustained response to intermittent insulin therapy in type 2 diabetic patients with secondary drug failure. Am J Med 1988;84:185–192 [DOI] [PubMed] [Google Scholar]
- 19.Glaser B, Leibovich G, Nesher R, Hartling S, Binder C, Cerasi E. Improved beta-cell function after intensive insulin treatment in severe non-insulin-dependent diabetes. Acta Endocrinol (Copenh) 1988;118:365–373 [DOI] [PubMed] [Google Scholar]
- 20.Pratipanawatr T, Cusi K, Ngo P, Pratipanawatr W, Mandarino LJ, DeFronzo RA. Normalization of plasma glucose concentration by insulin therapy improves insulin-stimulated glycogen synthesis in type 2 diabetes. Diabetes 2002;51:462–468 [DOI] [PubMed] [Google Scholar]
- 21.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 22.Wagenknecht LE, Mayer EJ, Rewers M, et al. The Insulin Resistance Atherosclerosis Study (IRAS) objectives, design, and recruitment results. Ann Epidemiol 1995;5:464–472 [DOI] [PubMed] [Google Scholar]
- 23.Buchanan TA, Xiang AH, Peters RK, et al. Response of pancreatic β-cells to improved insulin sensitivity in women at high risk for type 2 diabetes. Diabetes 2000;49:782–788 [DOI] [PubMed] [Google Scholar]
- 24.Mittelman SD, Van Citters GW, Kim SP, et al. Longitudinal compensation for fat-induced insulin resistance includes reduced insulin clearance and enhanced β-cell response. Diabetes 2000;49:2116–2125 [DOI] [PubMed] [Google Scholar]
- 25.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes 2002;51(Suppl. 1):S212–S220 [DOI] [PubMed] [Google Scholar]