Abstract
OBJECTIVE
It has been suggested that the high prevalence of subnormal free testosterone concentrations, along with low or inappropriately normal gonadotropins in men with type 2 diabetes, may be the result of an increase in plasma estradiol concentrations secondary to an increase in aromatase activity in the adipose tissue that leads to the suppression of the hypothalamo-hypophyseal-gonadal axis.
RESEARCH DESIGN AND METHODS
To investigate this hypothesis, plasma estradiol, testosterone, leutinizing hormone, follicle-stimulating hormone, and sex hormone–binding globulin (SHBG) concentrations were measured in fasting blood samples of 240 men with type 2 diabetes. Free estradiol concentrations were either calculated (n = 198) using total estradiol and SHBG measured by immunoassay or directly measured by liquid chromatography tandem mass spectrometry (LC-MS/MS) and equilibrium dialysis (n = 102).
RESULTS
The calculated free estradiol concentration in men with subnormal free testosterone concentrations was lower than that in men with normal free testosterone concentrations (median 0.047 vs. 0.063 ng/dL, P < 0.001). Directly measured (LC-MS/MS) free estradiol concentrations were also lower in men with subnormal free testosterone concentrations (median 0.025 vs. 0.045 ng/dL, P = 0.008). Free estradiol concentrations were directly related to free testosterone but not to BMI or age.
CONCLUSIONS
These data show that the suppression of the hypothalamo-hypophyseal-gonadal axis in patients with subnormal free testosterone concentrations and type 2 diabetes is not associated with increased estradiol concentrations. The pathogenesis of subnormal free testosterone concentrations in type 2 diabetes needs to be investigated further.
We have previously demonstrated that at least one-third of male patients with type 2 diabetes >18 years of age have subnormal free testosterone concentrations in association with inappropriately low gonadotropin concentrations (1,2). The high frequency of subnormal free testosterone concentrations in type 2 diabetes has been confirmed by several other studies from the U.S., the U.K., Brazil, Italy, and Australia (3,4). Because type 2 diabetes is a common condition affecting more than 20 million Americans, clinicians are likely to encounter a man with type 2 diabetes and subnormal free testosterone on a very frequent basis. Thus, the underlying mechanism is important; it may also affect the therapeutic strategies used in this condition. The patients with subnormal testosterone concentrations tend to be obese, and indeed, there is an inverse relationship of BMI with total and free testosterone concentrations (1–3). Because adipose tissue expresses the enzyme aromatase, which converts testosterone to estradiol, it has been suggested that the decrease in free testosterone concentrations in these patients may be the result of an excessive aromatase-dependent conversion of testosterone into estradiol (5–8). Elevated concentrations of estradiol may in turn suppress hypothalamic gonadotropin-releasing hormone and gonadotropin secretion from the pituitary gland (9). This would then explain the pathogenesis of subnormal free testosterone concentrations. We therefore investigated the hypothesis that the plasma concentrations of estradiol in patients with type 2 diabetes and subnormal free testosterone concentrations are elevated when compared with those who have normal testosterone concentrations.
RESEARCH DESIGN AND METHODS
The study was carried out at a tertiary diabetes referral center, Diabetes-Endocrinology Center of Western New York; Department of Endocrinology, Diabetes, and Metabolism, State University of New York at Buffalo; and at Kaleida Health. This is a cross-sectional study of 240 consecutive type 2 diabetic men who presented to the diabetes center between September 2008 and September 2010. It is our practice to screen all male type 2 diabetic patients for hypogonadism with total testosterone and free testosterone concentrations because of the high prevalence of subnormal free testosterone concentrations in our population and as recommended by the Endocrine Society (10). For a complete evaluation, we also measure sex hormone–binding globulin (SHBG), leutinizing hormone (LH), follicle-stimulating hormone (FSH), and prolactin in all male diabetic patients. For the past 2 years, we have also included estradiol in our routine measurements to determine if men with subnormal free testosterone concentrations have elevated estradiol concentrations. We excluded patients with history of panhypopituitarism or congenital hypogonadotropic hypogonadism; severe depression or psychiatric illness; head trauma, renal failure, hemochromatosis, cirrhosis, hepatitis C, or HIV; treatment with testosterone, steroids, or opiates; and foot ulcers; as well as patients with active infection or who had a recent surgery or hospitalization for any reason in the past 6 weeks. Health Insurance Portability and Accountability Act forms were signed by all patients. The collected data were kept confidential by the investigators, and patient identifiers were eliminated from the database once the master database had been created. Permission to review the charts of the patients to collect data was obtained from the human research committee of the State University of New York at Buffalo.
Patients were asked to provide fasting blood samples between 8:00 and 10:00 a.m. to measure serum total testosterone and estradiol, SHBG, LH, and FSH. All measurements were carried out by Quest Diagnostics. Total testosterone concentrations were measured by liquid chromatography tandem mass spectrometry (LC-MS/MS). A detailed description of the methodology has previously been published (11). The sensitivity of the assay (limit of quantification [LOQ]), set at a coefficient of variation (CV) of ≤20%, was 0.3 ng/dL. The intra-assay CV ranged from 7.6 to 10.8% and interassay CV ranged from 9.8 to 13.4% at total testosterone concentrations between 10 and 1,200 ng/dL. Reference range for total testosterone (250–1,100 ng/dL) was determined from 264 apparently healthy men.
Total estradiol was measured by solid phase competitive chemiluminescence enzyme immunoassay in 198 men. The normal range of total estradiol (2.0–5.5 ng/dL) is based on serum samples of 115 apparently healthy men. The sensitivity and assay range for total estradiol is 0.7–100 ng/dL. The total CV of the assay is 12%. The assay is highly specific for estradiol and has negligible cross-reactivity with estrone or metabolites of estrogens.
Although most prior studies have measured total estradiol by chemiluminescence enzyme immunoassays or radioimmunoassay (5,12), LC-MS/MS is more sensitive and specific and is now considered the method of choice for measuring estradiol, especially in subjects with low levels, such as children and adult men. For this reason we switched to the LC-MS/MS estradiol assay during the 2nd year of our study. The LC-MS/MS methodology used to measure total estradiol is similar to that previously described for measuring total testosterone by LC-MS/MS. The CV of the assay is 15% at an estradiol concentration of 1.5 ng/dL and 13% at 20 ng/dL. The LOQ for estradiol in this assay is 0.2 ng/dL. With this assay, the reference range for total estradiol in men is <2.9 ng/dL. This was based on a study performed by Quest Diagnostics on 39 healthy men (R.F., personal communication). Of normal men, 52% have undetectable estradiol concentrations. To provide comparative data on estradiol concentrations for our study subjects, we measured estradiol in blood samples from 18 lean, healthy young men. For this purpose, we used frozen blood samples from previous studies done at our research center.
Because LC-MS/MS assays are more specific than the antibody-based assays, the reference ranges for LC-MS/MS assays are generally lower than those for immunoassays. In our study, 102 men had estradiol concentrations measured by LC-MS/MS. Of these men, seven had estradiol concentrations below the LOQ (0.2 ng/dL) of the assay, and five of these seven men also had subnormal free testosterone concentrations. Concentrations below LOQ are often encountered in studies, and discarding these observations would create a bias, making the remaining lower concentrations selectively too high. Conversely, assigning them a value of 0 would also introduce a bias, making the concentrations on the lower side falsely low. A commonly recommended method is to assign a value of LOQ/2 for the purpose of statistical analysis (13). Therefore, men with estradiol concentrations below LOQ were assigned a value of 0.1 ng/dL for analysis.
Free and bioavailable testosterone and estradiol concentrations were calculated from the concentrations of total testosterone, total estradiol, SHBG, and albumin by the formula of Södergård, Vermeulen, and colleagues (5,14,15). The formula to calculate free testosterone was [FT] = {[T] − (N[FT])} / Kt {B[SHBG] − [T] + N[FT]} where Kt is the association constant of T for SHBG (1 × 109 L/mol), T is total testosterone, B is number of binding sites per SHBG dimer, FT is free testosterone, and N is 1 + Ka[A] (where Ka is the association constant of T for albumin [3.6 × 104 L/mol] and A is albumin), and testosterone and protein concentrations are in mol/L. Because 1 mol of SHBG homodimer binds to 2 mol of testosterone, a value of 2 was used for B in the equation (11). With this adjustment for the number of SHBG binding sites, free testosterone measured by equilibrium dialysis or calculated with the above formula has been found to be very similar, correlating with an r2 of >0.98, slope of 1.02, and intercept of 0.16 (11). To calculate free estradiol, first the testosterone binding was calculated, then estradiol binding was calculated on the remainder of nontestosterone-bound SHBG using the same formula as above, but this time with the weaker association constants for estradiol for SHBG (0.6 × 109 L/mol) and for albumin (0.61 × 104 L/mol) (5,12,16). The normal range used for free estradiol was 0.03–0.13 ng/dL. This is based on measurements obtained from 207 healthy men by Hofstra et al. (5). Bioavailable testosterone and estradiol were similarly calculated.
Tracer equilibrium dialysis is considered the gold standard for measuring free steroid hormone concentrations, and during the second year of the study, this methodology was used to determine the free testosterone and free estradiol levels in our subjects. Because patients were getting the laboratory tests as part of their clinical work-up, samples were collected at the local patient service center and allowed to clot (15 min) and centrifuged, and the supernatant was sent to the Pittsburgh business unit refrigerated (cold packs). The Pittsburgh business unit then sent the refrigerated samples to the appropriate Nichols site. The free testosterone was done at Nichols Institute in Chantilly, and the free estradiol was done at Nichols Institute in San Juan Capistrano, California. For free estradiol, samples are stable for 4 h at room temperature, 3 days refrigerated, and 2 years frozen. For free testosterone, the samples are stable for 7 days room temperature, 7 days refrigerated, and 2 years frozen. The methodology for free testosterone measurement at Nichols Institute has been published (11). The normal range for free testosterone is 3.5–15.5 ng/dL. The same methodology was used to measure free estradiol (R.F., personal communication). The normal range provided by Quest Diagnostics for free estradiol is 0–0.045 ng/dL, respectively.
SHBG (normal range 13–71 nmol/L), LH, and FSH concentrations were measured by a solid-phase, chemiluminescent immunometric assay (IMMULITE 2500; Siemens, Deerfield, IL). Hypogonadotropic hypogonadism was defined as free testosterone concentration <3.5 ng/dL along with an inappropriately low LH of <10 units/L (10).
The mean age and BMI of the 240 type 2 diabetic men were 56 ± 12 years (range: 23–83 years) and 35 ± 7 kg/m2 (range: 17–59 kg/m2). The mean age and BMI of the 18 lean, healthy men were 31 ± 8 years (range: 24–56 years) and 23 ± 2 kg/m2 (range: 20–25 kg/m2).
Statistical analysis
Group comparisons were performed by one-way ANOVA, t tests, Mann-Whitney U tests, and χ2 tests as appropriate. Adjustment for age and BMI in group comparisons was done with ANCOVA and generalized linear model analysis. Data that were not normally distributed (Kolmogorov-Smirnov test) were log transformed to perform the parametric statistical tests. Estradiol, testosterone, and SHBG were not normally distributed and were log transformed. Pearson correlation and multiple linear regression analyses between variables were done using SPSS software (SPSS Inc., Chicago, IL). Data are presented as means ± SD for normally distributed data and median [25th–75th percentile] for nonnormal data. P < 0.05 was considered significant.
RESULTS
Comparison of total estradiol measured by immunoassay (group 1)
Out of the total sample size of 240 men, 198 men had total estradiol measured by solid phase competitive chemiluminescence enzyme immunoassay. Free and bioavailable estradiol and testosterone were calculated as described above. The median total, free, and bioavailable estradiol concentrations were 3.7 [3.0–4.6], 0.061 [0.043–0.075], and 2.26 [1.66–2.87] ng/dL, respectively. The median total, free, and bioavailable testosterone concentrations were 309 [234–417], 4.53 [3.36–6.11], and 100 [74–142] ng/dL, respectively. Fifty men had subnormal (<3.5 ng/dL) free testosterone concentrations and low or inappropriately normal LH concentrations. These men tended to be older and had a higher BMI than men with normal free testosterone concentrations. The age- and BMI-adjusted estradiol and testosterone concentrations of type 2 diabetic men with and without subnormal free testosterone concentrations are shown in Table 1. Contrary to the hypothesis tested, the mean estradiol concentration of men with subnormal free testosterone concentrations was significantly lower than that of men with normal free testosterone concentrations. Five men had free estradiol concentrations above the normal range. All of them had normal free testosterone concentrations.
Table 1.
Comparison of type 2 diabetic men with and without subnormal free testosterone concentrations
| Group 1 testosterone concentrations |
Group 2 testosterone concentrations |
|||||
|---|---|---|---|---|---|---|
| Subnormal free | Normal free | P value | Subnormal free | Normal free | P value | |
| n | 50 | 148 | 30 | 72 | ||
| Age (years) | 59 ± 11 | 55 ± 12 | 0.08 | 61 ± 10 | 53 ± 11 | 0.003 |
| BMI (kg/m2) | 37.2 ± 8.8 | 34.2 ± 6.6 | 0.02 | 36.3 ± 7.5 | 35.6 ± 7.6 | 0.68 |
| Total testosterone (ng/dL) | 198 [136–259], 217 ± 171 | 347 [276–449], 385 ± 166 | <0.001 | 175 [128–234], 175 ± 78 | 292 [235–367], 319 ± 129 | <0.001 |
| Free testosterone (ng/dL) | 2.8 [2.0–3.04], 2.8 ± 0.76 | 5.18 [4.19–6.58], 5.5 ± 1.81 | <0.001 | 2.77 [2.34–3.33], 2.95 ± 0.60 | 5.76 [4.75–6.77], 5.87 ± 1.57 | <0.001 |
| Bioavailable testosterone (ng/dL) | 62 [47–69], 64 ± 17 | 118 [94–154], 125 ± 42 | <0.001 | |||
| Total estradiol (ng/dL) | 3.4 [2.2–4.8], 3.4 ± 1.5 | 3.9 [3.1–4.6], 4.0 ± 1.5 | 0.006 | 1.25 [0.04–2.60], 1.86 ± 2.03 | 1.95 [1.23–3.08], 2.10 ± 1.21 | 0.03 |
| Free estradiol (ng/dL) | 0.047 [0.035–0.068], 0.053 ± 0.026 | 0.063 [0.046–0.077], 0.066 ± 0.027 | 0.003 | 0.025 [0.012–0.047], 0.038 ± 0.039 | 0.045 [0.028–0.071], 0.048 ± 0.027 | 0.008 |
| Bioavailable estradiol (ng/dL) | 1.86 [1.32–2.55], 2.0 ± 0.94 | 2.39 [1.84–2.95], 2.5 ± 1.02 | 0.001 | |||
| SHBG (nmol/L) | 27 [20–38], 33 ± 27 | 26 [17–36], 28 ± 14 | 0.42 | 23 [15–36], 26 ± 14 | 23 [15–29], 25 ± 11 | 0.45 |
| Free estradiol/free testosterone (%) | 1.9 [1.3–3.0], 2.2 ± 1.4 | 1.2 [0.9–1.6], 1.3 ± 0.6 | 0.002 | 1.2 [0.6–1.9], 1.4 ± 1.5 | 0.8 [0.4–1.3], 0.9 ± 0.5 | 0.006 |
| LH (IU/L) | 4.0 [1.7–5.9], 4.1 ± 2.8 | 4.2 [2.9–6.5], 5.2 ± 3.8 | 0.10 | 4.8 [3.0–7.0], 4.6 ± 2.4 | 4.5 [3.2–6.5], 5.6 ± 3.9 | 0.13 |
| FSH (IU/L) | 5.6 [3.2–8.4], 5.9 ± 3.6 | 5.9 [3.2–9.1], 7.1 ± 6.2 | 0.69 | 6.8 [4.9–11.9], 7.3 ± 5.0 | 4.5 [3.5–9.0], 7.3 ± 7.5 | 0.36 |
| Prolactin (mg/L) | 7.0 [5.0–8.4], 8.2 ± 7.2 | 6.6 [5.1–8.6], 7.9 ± 5.6 | 0.88 | 7.0 [4.7–8.3], 6.6 ± 2.4 | 6.3 [4.7–9.0], 7.3 ± 3.8 | 0.41 |
| HbA1c (%) | 6.8 [6.1–8.0], 7.5 ± 1.9 | 7.3 [6.4–9.0], 7.9 ± 2.0 | 0.34 | 7.6 [6.8–9.5], 8.2 ± 2.1 | 7.9 [6.9–9.8], 8.2 ± 1.9 | 0.98 |
| Duration of diabetes (years) | 7 [2–20], 11 ± 9 | 8 [4–13], 9 ± 7 | 0.52 | 11 [2–20], 11 ± 11 | 8 [4–13], 9 ± 8 | 0.56 |
| Use of insulin (%) | 56 | 66 | 0.40 | 70 | 56 | 0.73 |
| Mean insulin dose (IU) | 92 ± 114 | 69 ± 58 | 0.35 | 58 ± 50 | 66 ± 59 | 0.58 |
| Use of oral hypoglycemics (%) | 85 | 88 | 0.99 | 80 | 86 | 0.66 |
| Use of exenatide (%) | 17 | 19 | 0.79 | 20 | 18 | 0.88 |
Group 1: Total estradiol concentrations were measured by immunoassay. Free testosterone and free estradiol concentrations were calculated. Group 2: Total estradiol concentrations were measured by LC-MS/MS. Free testosterone and free estradiol concentrations were measured by equilibrium dialysis. Data are expressed as means ± SD. SHBG, testosterone, and estradiol concentrations were not normally distributed; therefore, medians [25th–75th percentile] are also mentioned. Statistical comparisons of hormonal concentrations were performed after log transformation and adjustment for age and BMI differences in the two groups. Total estradiol and total testosterone concentrations were also adjusted for SHBG. P values are given for comparison between men with and without subnormal free testosterone concentrations.
We compared the ratio of free estradiol and free testosterone concentrations among the men with normal or subnormal free testosterone concentrations. This free estradiol to free testosterone (FE/FT) ratio can serve as a surrogate marker of aromatase activity. As shown in Table 1, free estradiol concentrations were 1.9 and 1.2% (P < 0.001) of the free testosterone concentrations in men with and without subnormal free testosterone concentrations, respectively.
Nine men had subnormal free testosterone concentrations and supranormal LH concentrations (>10 units/L). The median free estradiol concentration in these men (0.046 [0.028–0.059] ng/dL) was lower than that in eugonadal men (P = 0.05) but similar to those of men with subnormal free testosterone concentrations (P = 0.88).
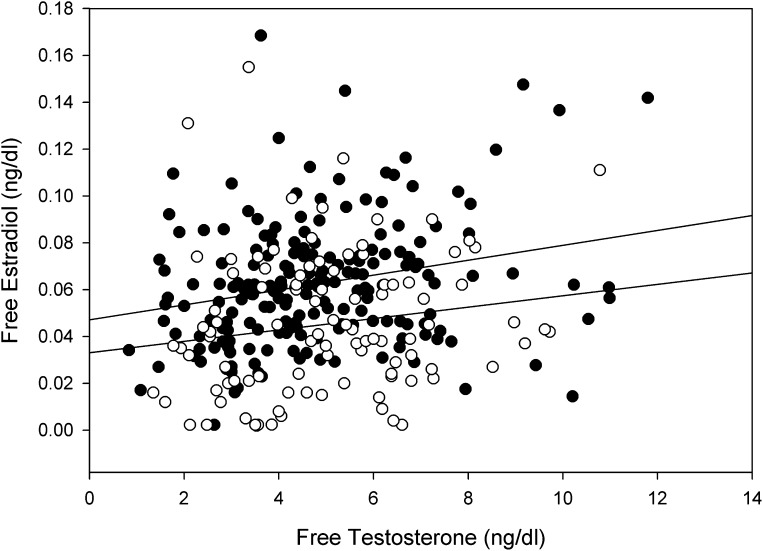
On univariate analyses, BMI was negatively related to free testosterone (r = −0.18, P = 0.02) but not to free estradiol concentrations (r = 0.06, P = 0.42). Free estradiol concentrations were directly related to free testosterone (r = 0.24, P = 0.001) (Fig. 1). Age was related negatively to free testosterone (r = −0.24, P = 0.001) but not to free estradiol (r = −0.14, P = 0.06). BMI was positively related to FE/FT ratio (r = 0.16, P = 0.03). In multiple regression analysis, free estradiol was independently and directly related to free testosterone (β = 0.24, P = 0.001) but not to BMI (β = 0.10, P = 0.17) or age (β = −0.07, P = 0.37).
Figure 1.
Direct relationship between free testosterone and free estradiol. ●, Calculated free testosterone and free estradiol (r = 0.24, P = 0.001). ○, Free testosterone and free estradiol directly measured by equilibrium dialysis (r = 0.24, P = 0.02). Upper limit of normal for calculated free estradiol is 0.13 ng/dL and for measured free estradiol is 0.045 ng/dL.
Total estradiol measured by LC-MS/MS (group 2)
In 102 men, total estradiol concentrations were measured by LC-MS/MS. All these men also had free estradiol and free testosterone measured by equilibrium dialysis. The median total and free estradiol concentrations were 1.90 [0.9–3.0] and 0.042 [0.021–0.067] ng/dL, respectively. The median total and free testosterone concentrations were 262 [205–349] and 4.92 [3.56–6.38] ng/dL, respectively. Thirty men had subnormal free testosterone concentrations (Table 1). Consistent with the results in group 1, we found lower estradiol concentrations in men with subnormal free testosterone concentrations. Free estradiol concentrations were directly related to free testosterone (r = 0.24, P = 0.02) (Fig. 1) but not to age (r = −0.13, P = 0.18) or BMI (r = 0.06, P = 0.54). Free testosterone concentrations were negatively related to both age (r = −0.31, P = 0.001) and BMI (r = −0.17, P = 0.03). In multiple regression analysis, free estradiol was directly related to free testosterone (β = 0.24, P = 0.03) but not to BMI (β = 0.08, P = 0.42) or age (β = −0.04, P = 0.68). BMI was not related to FE/FT ratio (r = 0.12, P = 0.24). Total estradiol and total testosterone concentrations were positively related to each other (r = 0.33, P = 0.001), independently of age, BMI, or SHBG. We found 47% of eugonadal men and 27% of men with subnormal free testosterone concentrations had supranormal free estradiol concentrations (P = 0.09), and 3% of eugonadal men and 17% of men with subnormal free testosterone concentrations had undetectable estradiol concentrations (P = 0.04).
Four men had subnormal free testosterone concentrations with elevated LH concentrations. Their median total and free estradiol concentrations were 1.60 [0.77–3.10] and 0.036 [0.017–0.056], respectively. These values were similar to those found in men with subnormal free testosterone concentrations and hypogonadotropism.
Estradiol concentrations measured by LC-MS/MS in lean, healthy men
The median total and free estradiol concentrations in healthy men were 1.45 [0.70–2.35] and 0.037 [0.022–0.072] ng/dL, respectively. The mean total and free estradiol concentrations in healthy men were 1.68 ± 1.11 and 0.050 ± 0.031 ng/dL, respectively. These concentrations were not significantly different than those in type 2 diabetic men with normal or subnormal free testosterone concentrations, with or without adjustment for age. One subject had undetectable estradiol concentrations. Using the reference ranges mentioned in research design and methods, one subject had supranormal total estradiol, whereas eight men had supranormal free estradiol concentrations.
Comparison of total estradiol measured by immunoassay and LC-MS/MS
Of the subjects, 60 men had total estradiol measured by both the assays. The mean and median total estradiol concentrations measured by immunoassay were 3.95 ± 1.31 and 3.9 [3.1–4.8] ng/dL, respectively. In contrast, the mean and median total estradiol concentrations measured by LC-MS/MS were lower by almost half (2.16 ± 1.66 and 1.9 [0.8–3.2] ng/dL, respectively; P < 0.001 for comparison with immunoassay). The total estradiol concentrations measured by the two assays correlated only weakly (r = 0.37, P = 0.004).
CONCLUSIONS
Our data show clearly that estradiol concentrations in patients with type 2 diabetes with subnormal free testosterone concentrations are significantly lower than in those with normal free testosterone concentrations. Furthermore, we show that in patients with type 2 diabetes, estradiol and testosterone concentrations are significantly and positively correlated. It would thus appear that the suppression of LH, FSH, and testosterone in patients with type 2 diabetes is not the result of an excessive concentration of estradiol but instead, the consequence of some as yet unidentified hypothalamic mechanism. The direct relationship between plasma estradiol and testosterone concentrations is consistent with the concept that estradiol concentrations are dependent upon testosterone, the substrate for estradiol synthesis. Furthermore, BMI was related negatively to testosterone but not to estradiol concentrations. The absence of a relationship between BMI and estrogen in our study is probably accounted for by the relationships between BMI and testosterone on one hand and estradiol and testosterone on the other hand, which exert opposing influences on estradiol concentrations. This is important because it has been assumed hitherto that low concentrations of testosterone in obesity are due to an increase in aromatase activity and a parallel increase in estradiol concentrations, which suppress the hypothalamo-hypophyseal-gonadal axis. It is, however, of interest that FE/FT ratio was significantly related to BMI. If, indeed, this ratio can be taken to be a reflection of aromatase activity, our data suggest that in spite of an increase in this ratio, plasma concentrations of estradiol do not increase as a result of the lack of the substrate, testosterone.
Studies in the past have found that estrogen concentrations are elevated in obese men as compared with lean men and that estradiol concentrations correlate positively with BMI (5,17). These studies were not done with the more specific assay for estradiol, LC-MS/MS. However, few studies have compared estrogen concentrations in hypogonadal and eugonadal obese men. One study found that calculated free estradiol concentrations were similar in obese men with low or normal free testosterone concentrations (5). A recent study that measured estradiol by LC-MS/MS (European Male Ageing Study) found lower total estradiol concentrations in men with low testosterone (18), as compared with men with normal testosterone concentrations. Consistent with our data, the estradiol concentration was low regardless of whether the subnormal testosterone was associated with elevated or low gonadotropin concentrations. We have not found any study showing elevated estradiol concentrations in men with subnormal testosterone concentrations. Aromatase inhibitors, such as letrozole, have been shown to increase testosterone and decrease estradiol concentrations in obese men with low testosterone (19). This, however, cannot be taken as evidence of estradiol being the cause of low testosterone in those men because eugonadal men also respond to aromatase inhibition by an increase in testosterone concentrations (20).
We measured estradiol concentrations in samples from 18 lean, healthy, and presumably eugonadal men. The prevalence of undetectable estradiol concentrations was similar in healthy men (5.6%) and in men with type 2 diabetes (7%). The total and free estradiol concentrations were also not different from the estradiol concentrations in men with type 2 diabetes. However, the small sample size of the healthy men limits any conclusions that can be drawn from comparisons. Men with type 2 diabetes are more obese and would likely aromatize a larger percentage of their testosterone concentrations into estradiol. However, they are also more likely to have lower testosterone concentrations than lean nondiabetic men (21). This may limit the increase in estradiol concentrations. The aim of our study was to compare the estradiol concentrations in type 2 diabetic men with normal or subnormal free testosterone concentrations, rather than to compare the estradiol concentrations in lean versus obese men. Furthermore, the concept of increased estradiol concentrations in obesity is based on studies that have used the relatively nonspecific immunoassays. Future studies examining this concept should be done with LC-MS/MS and should also take into account the difference in testosterone concentrations in lean and obese men.
The specific mechanism involved in the pathogenesis of frequent occurrence of subnormal free testosterone in type 2 diabetes is not known. We have previously suggested that this mechanism may be related to the observations made by Brüning et al. (22), which demonstrated that the selective deletion of the insulin receptor in the neurons of mice leads to features of hypogonadotropic hypogonadism in addition to an increased weight and mild metabolic syndrome. These mice have undeveloped seminiferous tubules and markedly impaired spermatogenesis. Because type 2 diabetes is an insulin resistant state with an interference with insulin signal transduction and because insulin is known to facilitate gonadotropin-releasing hormone secretion by hypothalamic neurons, in vitro, it is possible that this syndrome is a manifestation of insulin resistance at the neuronal level, which results in subnormal secretion of gonadotropin-releasing hormone from the hypothalamus (23). This would in turn result in diminished secretion of LH and FSH. Thus, interference with insulin signal transduction, as is known to occur in type 2 diabetes, may result in low testosterone. It is also recognized that the mechanism underlying insulin resistance in obesity and type 2 diabetes is dependent on inflammatory mediators, which are significantly increased in this condition at the molecular, cellular, and plasma level (24). Because C-reactive protein concentrations are markedly elevated in patients with subnormal free testosterone concentrations and are related inversely to testosterone concentrations (4,25), it is possible that there exists an inflammation-based interference of insulin signal transduction at the hypothalamic level. Further studies are necessary to explore these hypotheses.
The majority of men in the study had total estradiol concentrations measured by immunoassay, and the free concentrations of estradiol were calculated. This may be considered a weakness, but it is also a strength of the study because we have been able to present comparative data applying the usually used immunoassay and LC-MS/MS, the preferred assay to measure total estradiol and equilibrium dialysis, to measure free estradiol. The data emerging from both assay methods are consistent and make it clear that total and free estradiol concentrations are significantly lower in patients with subnormal free testosterone concentrations; therefore, an elevated estradiol concentration is not the mechanism suppressing the hypothalamo-hypophyseal-gonadal axis in these patients. Our data also show that the concentrations of estradiol, both total and free, are significantly lower when measured by LC-MS/MS. They also show that the immunoassay’s measurements are probably inaccurate.
It is well known that there is significant day-to-day variability in hormone concentrations, especially testosterone. As in most epidemiologic or cross-sectional studies, the hormone concentrations were measured only once in this study. In view of the variability in testosterone concentrations, this is a limitation. However, it is not likely that the prevalence of subnormal free testosterone concentrations would have altered after repeated measurements because the probability of testosterone concentrations rising or falling with repeated measurements is statistically equal. The issue of repeated measurements is important in the context of diagnosing hypogonadism clinically in individual patients. The fact that our study included a moderately large number of participants also helps to diminish the effect of hormonal variability on study results. Our study population was composed of patients from a diabetes referral center and, therefore, may not be applicable to the general population of diabetic men. However, we have previously shown and confirmed again that HbA1c, medications, or the duration of type 2 diabetes do not appear to affect gonadal status in patients with type 2 diabetes (1). We also cannot exclude any effect of diet on the variables measured in the study because we do not have detailed dietary habits of participants available to us. However, any patient on over-the-counter androgen supplements was excluded from our study.
In conclusion, subnormal free testosterone concentrations in men with type 2 diabetes are associated with significantly lower estradiol concentrations than those with normal free testosterone concentrations. Therefore, low testosterone concentrations are not the consequence of estradiol dependent suppression of the hypothalamo-hypophyseal-gonadal axis. Furthermore, there is a significant direct relationship between estradiol concentrations and testosterone, consistent with the dependence of estradiol concentrations on the availability of testosterone. Clearly, the mechanism underlying frequent prevalence of subnormal free testosterone concentrations in type 2 diabetes requires further investigation.
Acknowledgments
S.D. has received a grant from the American Diabetes Association (110JF13). P.D. has received grants from the National Institutes of Health (R01-DK-069805-01A1 and R01-DK-075877-01A2) and the American Diabetes Association (708CR13) and is also supported by grants from Sanofi-Aventis, Merck, Amylin, and Solvay Pharmaceuticals. S.D. has received speaker fees from Abbott Laboratories. No other potential conflicts of interest relevant to this article were reported.
S.D. planned and executed the study, wrote the manuscript, and performed the statistical analysis. R.F. executed the study and wrote the manuscript. M.V. and H.G. executed the study. A.C. interpreted data and wrote the manuscript. P.D. put forth the hypothesis, planned and interpreted the study, and wrote the manuscript.
Parts of this study were presented in poster form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors thank Dr. T. Fiers (University Hospital Ghent, Ghent, Belgium) for providing the computer program for calculation of free estradiol.
References
- 1.Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 2004;89:5462–5468 [DOI] [PubMed] [Google Scholar]
- 2.Chandel A, Dhindsa S, Topiwala S, Chaudhuri A, Dandona P. Testosterone concentration in young patients with diabetes. Diabetes Care 2008;31:2013–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007;30:911–917 [DOI] [PubMed] [Google Scholar]
- 4.Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008;93:1834–1840 [DOI] [PubMed] [Google Scholar]
- 5.Hofstra J, Loves S, van Wageningen B, Ruinemans-Koerts J, Jansen I, de Boer H. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth J Med 2008;66:103–109 [PubMed] [Google Scholar]
- 6.Wake DJ, Strand M, Rask E, et al. Intra-adipose sex steroid metabolism and body fat distribution in idiopathic human obesity. Clin Endocrinol (Oxf) 2007;66:440–446 [DOI] [PubMed] [Google Scholar]
- 7.Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I. Estradiol in elderly men. Aging Male 2002;5:98–102 [PubMed] [Google Scholar]
- 8.Cohen PG. The hypogonadal-obesity cycle: role of aromatase in modulating the testosterone-estradiol shunt—a major factor in the genesis of morbid obesity. Med Hypotheses 1999;52:49–51 [DOI] [PubMed] [Google Scholar]
- 9.Pitteloud N, Dwyer AA, DeCruz S, et al. Inhibition of luteinizing hormone secretion by testosterone in men requires aromatization for its pituitary but not its hypothalamic effects: evidence from the tandem study of normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab 2008;93:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2006;91:1995–2010 [DOI] [PubMed] [Google Scholar]
- 11.Salameh WA, Redor-Goldman MM, Clarke NJ, Reitz RE, Caulfield MP. Validation of a total testosterone assay using high-turbulence liquid chromatography tandem mass spectrometry: total and free testosterone reference ranges. Steroids 2010;75:169–175 [DOI] [PubMed] [Google Scholar]
- 12.van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab 2000;85:3276–3282 [DOI] [PubMed] [Google Scholar]
- 13.Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 2001;28:481–504 [DOI] [PubMed] [Google Scholar]
- 14.Södergård R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 1982;16:801–810 [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672 [DOI] [PubMed] [Google Scholar]
- 16.Rinaldi S, Geay A, Déchaud H, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev 2002;11:1065–1071 [PubMed] [Google Scholar]
- 17.Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab 1979;48:633–638 [DOI] [PubMed] [Google Scholar]
- 18.Tajar A, Forti G, O’Neill TW, et al. ; EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010;95:1810–1818 [DOI] [PubMed] [Google Scholar]
- 19.Loves S, Ruinemans-Koerts J, de Boer H. Letrozole once a week normalizes serum testosterone in obesity-related male hypogonadism. Eur J Endocrinol 2008;158:741–747 [DOI] [PubMed] [Google Scholar]
- 20.T’Sjoen GG, Giagulli VA, Delva H, Crabbe P, De Bacquer D, Kaufman JM. Comparative assessment in young and elderly men of the gonadotropin response to aromatase inhibition. J Clin Endocrinol Metab 2005;90:5717–5722 [DOI] [PubMed] [Google Scholar]
- 21.Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010;33:1186–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brüning JC, Gautam D, Burks DJ, et al. Role of brain insulin receptor in control of body weight and reproduction. Science 2000;289:2122–2125 [DOI] [PubMed] [Google Scholar]
- 23.Salvi R, Castillo E, Voirol MJ, et al. Gonadotropin-releasing hormone-expressing neurons immortalized conditionally are activated by insulin: implication of the mitogen-activated protein kinase pathway. Endocrinology 2006;147:816–826 [DOI] [PubMed] [Google Scholar]
- 24.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 2004;25:4–7 [DOI] [PubMed] [Google Scholar]
- 25.Bhatia V, Chaudhuri A, Tomar R, Dhindsa S, Ghanim H, Dandona P. Low testosterone and high C-reactive protein concentrations predict low hematocrit in type 2 diabetes. Diabetes Care 2006;29:2289–2294 [DOI] [PubMed] [Google Scholar]