Abstract
OBJECTIVE
Persistent organic pollutants (POPs), lipophilic chemicals that accumulate mainly in adipose tissue, have recently been linked to type 2 diabetes. However, evidence from prospective studies is sparse. This study was performed to evaluate prospective associations of type 2 diabetes with selected POPs among the elderly.
RESEARCH DESIGN AND METHODS
Nineteen POPs (14 polychlorinated biphenyl [PCB] congeners, 3 organochlorine pesticides, 1 brominated diphenyl ether, and 1 dioxin) were measured in plasma collected at baseline in 725 participants, aged 70 years, of the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS).
RESULTS
After adjusting for known type 2 diabetes risk factors, including obesity, odds ratios (ORs) (95% CIs) for type 2 diabetes at age 75 years (n = 36) according to the quintiles of a summary measure of concentrations of PCBs (vs. the lowest quintile) were 4.5, 5.1, 8.8 (1.8–42.7), and 7.5 (1.4–38.8) (Ptrend <0.01). Among organochlorine pesticides, adjusted ORs across concentrations of trans-nonachlor showed that Ptrend = 0.03. Adjusted ORs (95% CIs) across quintiles of the sum of three organochlorine pesticides were 1.1, 1.6, 1.5, and 3.4 (1.0–11.7) (Ptrend = 0.03). Neither brominated diphenyl ether 47 nor dioxin was significantly associated with incident diabetes. The sum of PCBs improved reclassification significantly when added to traditional risk factors for diabetes.
CONCLUSIONS
Despite the small number of incident cases, this study found that environmental exposure to some POPs substantially increased risk of future type 2 diabetes in an elderly population.
Persistent organic pollutants (POPs) are various chemicals that share characteristics of high lipophilicity, the ability to accumulate in fat, and resistance to biodegradation (1). Although most chlorinated POPs, such as polychlorinated biphenyls (PCBs) and organochlorine pesticides, were banned several decades ago, and the emission of dioxins are strictly regulated in most developed countries, the exposure to these chemicals in the general population still occurs because they have widely contaminated our food chain (1). Also, POPs that have accumulated in human adipose tissue as a result of previous high exposure have become a continuous source of internal exposure because POPs are slowly but continuously released from adipose tissue to the circulation.
There is growing evidence that low-dose exposure to POPs is linked to type 2 diabetes. Several epidemiological studies showed associations between type 2 diabetes and a variety of POPs, including PCBs, dioxins, and organochlorine pesticides, such as dichlorodiphenyltrichloroethane or chlordane (2–4). A cross-sectional study in the U.S. general population observed that obesity was not associated with type 2 diabetes among people with very low levels of POPs (5), which, if confirmed, would suggest that the POPs accumulated in adipose tissue play a critical role in the pathogenesis of type 2 diabetes (5). Following these cross-sectional findings, some prospective studies have observed that some organochlorine pesticides and PCBs predicted the future risk of type 2 diabetes in the general population, although the specific kinds of POPs that predict type 2 diabetes and the shapes of the dose-response curves varied across studies (6–8). One recent experimental study (9) demonstrated that rats exposed to mixed POPs developed abdominal obesity, hepatosteatosis, and insulin resistance, which are prediabetic conditions.
For several reasons, it is of interest to explore the association between POPs and type 2 diabetes in elderly individuals. First, aging is associated with relative insulin secretory defects, with increased insulin resistance, leading to a higher risk of type 2 diabetes (10). However, the well-known association between obesity and type 2 diabetes was found to be weaker in elderly people (11). Second, changes in body composition are known to occur with aging, including an increase of fat mass, central redistribution of adipose tissue, and loss of muscle mass (12). All of these changes can affect the pharmacodynamics of POPs as well as serum concentrations. Third, the duration of exposure to POPs increases with age. Finally, serum concentrations of POPs show strong positive correlations with age (5). Therefore, we evaluated both cross-sectional and prospective associations between type 2 diabetes and selected POPs among subjects, aged 70 years, living in the community of Uppsala, Sweden.
RESEARCH DESIGN AND METHODS
All subjects, aged 70 years, living in the community of Uppsala, Sweden, were eligible for the study. The subjects were chosen from the register of community living and were invited in a randomized order from April 2001 to June 2004. The subjects received an invitation letter within 2 months of their 70th birthday. Of 2,025 subjects invited, 1,016 subjects were investigated at baseline (participation rate 50.1%). From March 2006 to September 2009, when the subjects turned 75 years of age, reinvestigation of the cohort was performed with a follow-up rate of 81.4%.
Among participants at baseline, 992 subjects had valid measurement of POPs; after excluding 3 subjects without information on fasting blood glucose, 989 subjects constituted the study sample for the cross-sectional analyses. The sample size for the prospective analyses was 725 subjects after excluding 112 type 2 diabetes cases at baseline and 152 subjects who were not followed up (of whom 52 had died). The study was approved by the ethics committee of the University of Uppsala, and the participants gave written informed consent.
Measurement
The participants were asked about their health behaviors, medical history, and regular medication. All subjects were investigated in the morning after an overnight fast, with no mediation or smoking allowed after midnight. BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Waist circumference was measured in the supine position midway between the lowest rib and the iliac crest. Serum cholesterol and triglyceride concentrations were determined with an enzymatic assay, and fasting blood glucose was analyzed with the hexokinase method using an Architect (Abbott Laboratories, Abbott Park, IL). Type 2 diabetes was defined as a fasting blood glucose ≥6.2 mmol/L or the use of insulin or oral hypoglycemic agents.
POPs analyses
POPs were measured in stored plasma samples collected at baseline. Analyses of POPs were performed using a Micromass Autospec Ultima (Waters, Mildford, MA) high-resolution chromatography coupled to a high-resolution mass spectrometry system based on the method by Sandau et al. (13), with some modifications. All details on POPs analyses were provided in the Supplementary Material. A total of 21 POPs were measured: 14 PCB congeners; 5 organochlorine pesticides; 1 octachlorodibenzo-p-dioxin; and 1 brominated diphenyl ether congener (Supplementary Table 1). Among 21 POPs measured, 2 organochlorine pesticides (trans-chlordane and cis-chlordane) with a detection rate <10% were not included in the final analyses.
Statistical analyses
Because POPs predominantly are carried in the lipid component of the blood, lipid-standardized concentrations, the concentrations of POPs divided by total serum lipid content, tended to be reported in epidemiological studies. However, POPs can disturb lipid metabolism (14,15), and dyslipidemia also is involved in the pathogenesis of type 2 diabetes (16). Therefore, wet-weight concentrations (POPs concentrations in pg/mL not divided by lipids) would have greater validity because lipid concentrations may be intermediate in a causal chain linking POPs and type 2 diabetes. In addition, one simulation study (17) reported that wet-weight concentrations adjusting for triglycerides and total cholesterol in a model as covariates induced less bias than lipid-standardized concentrations. Therefore, we present wet-weight concentrations (pg/mL), adjusting for triglycerides and total cholesterol by including these two lipid profiles in the models as covariates. In fact, analyses on the basis of lipid-standardized concentrations showed similar results in the current study (data not shown).
Analyses were done on individual POPs and summary POP measures. In the individual analyses, study subjects were categorized into quintiles of concentrations of each POP. Cutoff points of individual POPs were provided in the Supplementary Table 2. In the summary analyses, the individual ranks were summed (1–922) for each POP, as applied in previous studies (5,6), and summed values also were divided into quintiles. Odds ratios (ORs) and 95% CIs for the risk of type 2 diabetes were estimated using logistic regression. The covariates were sex, BMI (continuous), cigarette smoking (current, former, and never), alcohol consumption (grams per week), exercise (no, mild, moderate, and vigorous), triglycerides (continuous), and total cholesterol (continuous). We also noted ORs per 1-SD increase in POPs.
The additional value of adding POPs to the traditional risk factors was evaluated by C-statistics, and tests of reclassification (net reclassification index [NRI]) and discrimination (improved discrimination index [IDI]) according to the method proposed by Pencina et al. (18). For the NRI analyses, three risk groups were considered: <3% (low risk); 3–7% (intermediate risk); and >7% (high risk) risk of diabetes development. We considered P < 0.05 to be statistically significant.
RESULTS
Among 989 subjects for cross-sectional analyses, approximately one-half of the subjects were women and 22.1% were obese (BMI ≥30 kg/m2) (Table 1). Among prevalent diabetes cases, insulin and oral antiglycemic drugs were reported in 16.1 and 53.6%, respectively. The proportions who used antihypertensive or statin medication were 58.0 and 27.8%. Subjects eligible for the prospective analyses (n = 725) had baseline characteristics similar to those for the cross-sectional analysis (Table 1). Among incident diabetes cases, 11.1 and 30.6% of patients reported insulin and oral antiglycemic drugs.
Table 1.
Baseline characteristics of study subjects used in cross-sectional and prospective analyses
| Cross-sectional analyses | Prospective analyses | |
|---|---|---|
| Characteristic of all subjects | ||
| n | 989 | 725 |
| Women | 50.0 | 51.7 |
| BMI ≥30 kg/m2 | 22.1 | 20.1 |
| Current smokers | 10.5 | 9.0 |
| Moderate or vigorous exercise | 25.1 | 28.5 |
| BMI (kg/m2) | 27.0 ± 4.3 | 26.9 ± 4.0 |
| Alcohol consumption (grams per week) | 6.3 ± 7.2 | 6.6 ± 7.5 |
| Total cholesterol (mmol/L) | 5.3 ± 1.6 | 5.5 ± 1.0 |
| Fasting triglycerides (mmol/L) | 1.3 ± 0.6 | 1.2 ± 0.6 |
| Fasting blood glucose (mmol/L) | 5.3 ± 1.6 | 4.9 ± 0.5 |
| Clinical features of diabetes cases* | ||
| n | 112 | 36 |
| Duration of diabetes (years) | 6.0 ± 6.1 | 0.7 ± 1.4 |
| BMI (kg/m2) | 29.1 ± 5.3 | 29.0 ± 5.6 |
| Oral antidiabetes medication | 53.6 | 30.6 |
| Insulin medication | 16.1 | 11.1 |
| Newly diagnosed diabetes | 43.8 | 36.1 |
| Family history of diabetes | 42.5 | 38.6 |
| Antihypertensive drug medication | 58.0 | 77.8 |
| Statin medication | 27.7 | 36.1 |
Data are means ± SD or percent. Prospective analyses omitted prevalent diabetes at baseline as well as participants who were not followed up.
*Even though characteristics of all subjects for prospective analyses were baseline characteristics at age 70 years, clinical features of incident diabetes cases were follow-up information at age 75 years.
Cross-sectional analyses
Supplementary Table 3 shows the cross-sectional associations between POPs and prevalent type 2 diabetes (n = 112). Among 14 PCBs, 9 PCBs showed significant ORs in the highest quintile, although Ptrend was significant only for PCB congeners 105, 118, and 153. The adjusted ORs (95% CIs) for prevalent type 2 diabetes, according to quintiles of the sum of individual ranks of the 14 PCBs, were 1.0, 1.6, 1.3, and 2.1 (1.1–4.4) (Ptrend = 0.20). Among organochlorine pesticides, both trans-nonachlor and p,p´-2,2-Bis(4-chlorophenyl)-1,1-dichloroethene (DDE) showed statistically significant associations with prevalent type 2 diabetes. The adjusted ORs (95% CIs) of the summary measure of three organochlorine pesticides were 1.0, 0.9, 1.8, 1.7, and 2.5 (1.2–5.2) (Ptrend <0.01). Neither octachlorodibenzo-p-dioxin nor brominated diphenyl ether 47 was associated with prevalent type 2 diabetes (Supplementary Table 4).
Prospective analyses
During the 5-year follow-up, 36 subjects developed diabetes (cumulative incidence 5.0%). Plasma concentrations of POPs, in particular PCBs, strongly predicted future risk of type 2 diabetes (Table 2). Although the adjusted ORs from the second to fifth quintiles were substantially different for individual PCBs, five PCBs showed a significant P value for trend (congeners 74, 99, 138, 194, and 206). For some PCBs with a nonsignificant Ptrend (for example PCB105, PCB118, and PCB156), adjusted ORs in the second quintile already were significantly increased but did not further increase in the higher quintiles. When we combined by summing the individual rank of each PCB, adjusted ORs (95% CIs) were 1.0, 4.5, 5.1, 8.8 (1.8–42.7), and 7.5 (1.4–38.8) (Ptrend <0.01). Among organochlorine pesticides, only trans-nonachlor showed a significant Ptrend. Both p,p´-DDE and hexachlorobenzene showed nonsignificant positive trends. Adjusted ORs (95% CIs) for the summary measure were 1.0, 1.1, 1.6, 1.5, and 3.4 (1.0–11.7) (Ptrend = 0.03). Neither octachlorodibenzo-p-dioxin nor brominated diphenyl ether 47 was associated with incident type 2 diabetes (Supplementary Table 5).
Table 2.
Adjusted* ORs (95% CIs) of incident diabetes according to quintiles of plasma concentrations of PCBs, organochlorine pesticides, and summary measures of PCBs or organochlorine pesticides
| Quintiles of plasma concentrations of POPs | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Ptrend | |
| PCBs congener (number of chlorine atoms) | ||||||
| PCB74 (4) | ||||||
| Cases/n at risk | 1/136 | 8/149 | 8/151 | 11/144 | 8/145 | |
| Adjusted OR (95% CI) | Reference | 7.7 (0.9–64.1) | 8.5 (1.0–71.8) | 11.5 (1.4–94.9) | 9.0 (1.0–78.6) | 0.05 |
| PCB99 (5) | ||||||
| Cases/n at risk | 2/142 | 5/153 | 8/145 | 14/148 | 7/137 | |
| Adjusted OR (95% CI) | Reference | 2.5 (0.5–13.5) | 4.3 (0.9–21.2) | 6.9 (1.5–31.6) | 3.3 (0.6–17.3) | 0.04 |
| PCB105 (5)† | ||||||
| Cases/n at risk | 0/134 | 12/158 | 7/148 | 8/148 | 9/137 | |
| Adjusted OR (95% CI) | Reference | 10.7 (1.3–84.4) | 7.0 (0.8–59.3) | 7.5 (0.9–63.6) | 8.0 (0.9–68.2) | 0.30 |
| PCB118 (5) | ||||||
| Cases/n at risk | 2/141 | 9/152 | 6/143 | 11/150 | 8/138 | |
| Adjusted OR (95% CI) | Reference | 4.0 (0.8–19.2) | 2.9 (0.6–15.1) | 5.2 (1.1–25.5) | 3.6 (0.7–18.8) | 0.19 |
| PCB138 (6) | ||||||
| Cases/n at risk | 3/150 | 4/153 | 7/148 | 14/135 | 8/139 | |
| Adjusted OR (95% CI) | Reference | 1.5 (0.3–7.0) | 2.5 (0.6–10.1) | 5.9 (1.6–21.7) | 3.2 (0.8–13.2) | 0.01 |
| PCB153 (6) | ||||||
| Cases/n at risk | 5/149 | 4/152 | 6/148 | 14/136 | 7/140 | |
| Adjusted OR (95% CI) | Reference | 0.8 (0.2–3.2) | 1.5 (0.4–5.1) | 3.4 (1.1–10.2) | 1.7 (0.5–6.2) | 0.06 |
| PCB156 (6) | ||||||
| Cases/n at risk | 4/147 | 7/153 | 9/142 | 9/140 | 7/143 | |
| Adjusted OR (95% CI) | Reference | 2.1 (0.6–7.6) | 3.2 (0.9–11.1) | 3.0 (0.8–10.8) | 2.6 (0.7–10.3) | 0.14 |
| PCB157 (6) | ||||||
| Cases/n at risk | 4/152 | 7/148 | 7/140 | 10/139 | 8/146 | |
| Adjusted OR (95% CI) | Reference | 2.1 (0.6–7.4) | 2.3 (0.6–8.4) | 3.5 (1.0–12.4) | 2.9 (0.8–10.9) | 0.07 |
| PCB170 (7) | ||||||
| Cases/n at risk | 3/154 | 6/143 | 11/145 | 10/147 | 6/136 | |
| Adjusted OR (95% CI) | Reference | 2.8 (0.7–11.9) | 5.6 (1.4–21.8) | 5.0 (1.2–20.5) | 3.6 (0.8–16.8) | 0.06 |
| PCB180 (7) | ||||||
| Cases/n at risk | 3/180 | 10/147 | 6/144 | 9/142 | 8/139 | |
| Adjusted OR (95% CI) | Reference | 4.6 (1.2–17.8) | 2.8 (0.7–12.1) | 4.6 (1.1–18.8) | 4.8 (1.1–20.9) | 0.07 |
| PCB189 (7) | ||||||
| Cases/n at risk | 4/139 | 6/144 | 11/151 | 8/153 | 7/138 | |
| Adjusted OR (95% CI) | Reference | 1.9 (0.5–7.1) | 3.5 (1.0–11.9) | 2.9 (0.8–10.9) | 2.8 (0.7–11.2) | 0.12 |
| PCB194 (8) | ||||||
| Cases/n at risk | 4/141 | 5/149 | 11/155 | 5/141 | 11/139 | |
| Adjusted OR (95% CI) | Reference | 1.4 (0.4–5.6) | 3.5 (1.0–11.7) | 2.2 (0.5–9.0) | 6.0 (1.6–22.9) | <0.01 |
| PCB206 (9) | ||||||
| Cases/n at risk | 3/146 | 8/143 | 6/153 | 10/139 | 9/144 | |
| Adjusted OR (95% CI) | Reference | 3.2 (0.8–12.7) | 2.1 (0.5–9.0) | 5.5 (1.4–21.9) | 5.0 (1.2–21.1) | 0.02 |
| PCB209 (10) | ||||||
| Cases/n at risk | 2/141 | 8/148 | 12/147 | 6/143 | 8/146 | |
| Adjusted OR (95% CI) | Reference | 3.9 (0.8–19.1) | 7.1 (1.5–33.0) | 3.8 (0.7–20.2) | 6.2 (1.2–33.0) | 0.06 |
| Organochlorine pesticides | ||||||
| p,p’-DDE | ||||||
| Cases/n at risk | 5/149 | 4/148 | 8/147 | 8/143 | 11/138 | |
| Adjusted OR (95% CI) | Reference | 0.8 (0.2–2.9) | 1.4 (0.4–4.5) | 1.4 (0.4–4.4) | 2.1 (0.7–6.3) | 0.11 |
| Trans-nonachlor | ||||||
| Cases/n at risk | 4/148 | 5/146 | 2/147 | 17/147 | 8/137 | |
| Adjusted OR (95% CI) | Reference | 1.2 (0.3–4.7) | 0.5 (0.1–2.6) | 4.2 (1.3–13.3) | 1.8 (0.5–6.8) | 0.03 |
| Hexachlorobenzene | ||||||
| Cases/n at risk | 5/143 | 5/149 | 10/149 | 5/134 | 11/150 | |
| Adjusted OR (95% CI) | Reference | 1.0 (0.3–3.5) | 1.9 (0.6–5.9) | 1.1 (0.3–4.2) | 2.1 (0.6–7.1) | 0.21 |
| Summary measure | ||||||
| All 14 PCBs | ||||||
| Cases/n at risk | 2/150 | 6/140 | 7/146 | 12/147 | 9/142 | |
| Adjusted OR (95% CI) | Reference | 4.5 (0.9–23.5) | 5.1 (1.0–26.0) | 8.8 (1.8–42.7) | 7.5 (1.4–38.8) | <0.01 |
| All 3 organochlorine pesticides | ||||||
| Cases/n at risk | 4/152 | 5/151 | 7/142 | 6/139 | 14/141 | |
| Adjusted OR (95% CI) | Reference | 1.1 (0.3–4.5) | 1.6 (0.4–5.8) | 1.5 (0.4–5.8) | 3.4 (1.0–11.7) | 0.03 |
*Adjusted for sex, BMI, cigarette smoking, exercise, alcohol consumption, triglycerides, and total cholesterol.
†PCB105 had zero cases in the lowest quintile; for statistical assessment, we artificially added one case to that quintile so that OR estimates were not infinite; covariates were evaluated at the median values of noncases in the lowest quintile.
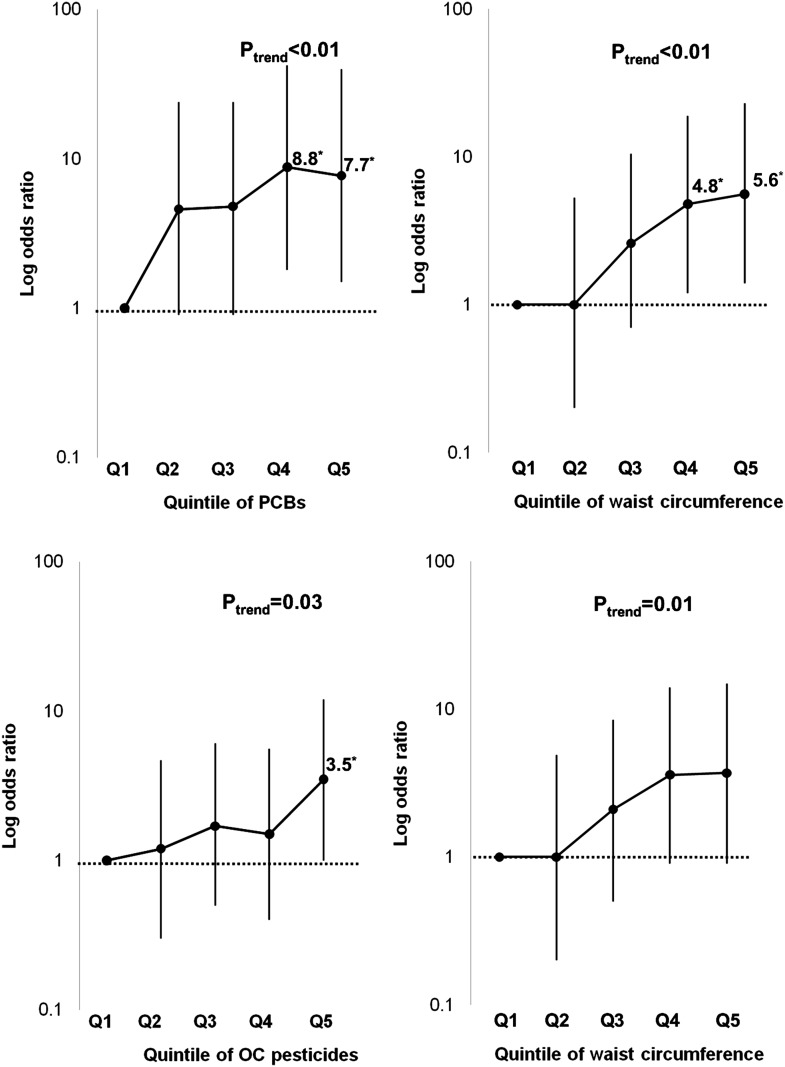
In a single regression including both the summary measure of PCB and waist circumference, the strength of association of the summary measure of PCBs was as strong as that of waist circumference (Fig. 1) (Supplementary Table 6). In particular, the adjusted OR in the second quintile of summary PCBs was similar to that in the fourth quintile of waist circumference. The summary measure of organochlorine pesticides showed a weaker association with diabetes than waist circumference (Fig. 1). ORs per 1-SD increase in summary PCBs and waist circumference were 1.7 (95% CI 1.1–2.5) and 2.0 (1.4–2.9), respectively. Those figures for summary organochlorine pesticides and waist circumference were 1.5 (1.0–2.2) and 1.7 (1.2–2.5). We assessed a possible interaction between POPs and adiposity in predicting diabetes; there was no reportable interaction (data not shown), although power was low for this test given the small number of incident diabetes cases.
Figure 1.
Comparison of adjusted ORs and 95% CIs between quintiles of the summary measure of PCBs or organochlorine (OC) pesticides and quintiles of waist circumference on the risk of future type 2 diabetes. Findings in each panel are from a single regression model that includes the sum of either PCBs or organochlorine pesticides and waist circumference, adjusted for sex, cigarette smoking, exercise, alcohol consumption, total cholesterol, and triglycerides. *ORs are statistically significant.
The addition of the sum of PCBs to a model containing BMI, smoking, serum cholesterol, serum triglycerides, sex, exercise habits, and alcohol intake as risk factors for diabetes development increased the receiver-operating curve area under the curve from 0.68 (95% CI 0.58–0.76) to 0.72 (0.63–0.80). Adding the sum of organochlorine pesticides to the risk factors increased the receiver-operating curve area under the curve less (0.69 [0.60–0.77]). To evaluate whether the addition of information of POPs to risk factors improved the risk classification with regard to diabetes development, IDI and NRI were used. Adding the sum of PCBs to the risk factors described above resulted in significantly improved reclassification with both methods (estimate 0.013, SE 0.0065, and P = 0.034 for IDI and estimate 0.22, SE 0.11, and P = 0.045 for NRI). When we used 7% as the cutoff for high risk and 3% as the cutoff for low risk, NRI analysis showed that of 36 cases 10 were reclassified to higher risk versus 5 reclassified to lower risk, by the addition of the sum of PCBs. Of 689 noncases, reclassification generally was reversed; 110 reclassified to higher risk versus 166 reclassified to lower risk (Supplementary Table 7). NRI was substantially greater within the low-risk and moderate-risk strata (0.35 and 0.52 vs. 0.07 in the highest-risk group) and as an average across risk strata (0.32). In a similar analysis, adding the sum of organochlorine pesticides to the traditional risk factors, a similar improvement in reclassification was seen using IDI but was less powerful using NRI (estimate 0.0097, SE 0.0045, and P = 0.034 for IDI and estimate 0.16, SE 0.086, and P = 0.063 for NRI).
CONCLUSIONS
In this study, plasma concentrations of POPs, especially PCBs and organochlorine pesticides, strongly predicted incident type 2 diabetes during a 5-year follow-up. Although there were some differences in terms of the strengths of the associations, most POPs measured in the current study showed positive associations. Although PCBs with differing biological characteristics were included in this study, dioxin-like PCBs (PCB105, PCB118, PCB156, PCB157, and PCB189) and nondioxin-like PCBs showed similar associations. It is important to note that prediction using the summary measure of PCBs improved risk classification compared with traditional risk factors for diabetes. Although the cross-sectional associations also showed significant results, the strengths of associations in this study of elderly subjects, in whom selective survivorship could play an important role, were weaker than those of prospective analyses.
Although obesity is critical in the pathogenesis of type 2 diabetes, one recent prospective study (11) reported that the association between adiposity and type 2 diabetes was weaker in elderly subjects. When the pathogenesis of type 2 diabetes is classified into two stages, insulin resistance and insulin secretory defects, the latter part seems to be more important among elderly people (10). Indeed, it is well known that many chemicals, including streptozotocin, alloxan, and vacor, can induce β-cell destruction (19). Although there has been no experimental study on the effects of POPs on pancreatic β-cells, one experimental study (9) observed alterations in mitochondrial function and oxidative capacity in the liver of rats exposed to a mixture of POPs. Similar mitochondrial defects may happen in the pancreatic β-cell after long-term exposure to low-dose POPs in humans, possibly more severely in elderly people because of the general decrease of antioxidant capacity with aging (20). Mitochondrial function has been implicated as a key for pancreatic β-cells to secrete insulin (21).
Aside from studies performed among people with high exposure to several selected POPs in the occupational or accidental setting, there have been three prospective studies that examined associations of type 2 diabetes with organochlorine pesticides and PCBs in general populations (6–8). Compared with occupational or accidental exposures, the background exposure in the general population is characterized by low-dose and long-term exposure to a mixture of various POPs. Consistent with the current findings, all three prospective studies have reported that some organochlorine pesticides and PCBs predicted the future risk of type 2 diabetes, but the details differed among the studies.
A prospective study in a cohort of Great Lakes sport fish consumers in Michigan in the U.S. demonstrated a strong association of incident diabetes with p,p’-DDE but not PCBs (7). Likewise, in a nested case-control study among a cohort of Swedish women, p,p’-DDE was also a risk factor for developing type 2 diabetes later in life but not PCB153 (8). In these two studies, no other organochlorine pesticides, except p,p’-DDE, were measured. In a nested case-control study performed in young adults within the Coronary Artery Risk Development in Young Adults (CARDIA) cohort, trans-nonachlor and highly chlorinated PCBs predicted type 2 diabetes, whereas p,p’-DDE did not (6). In the current study, the association between p,p’-DDE and type 2 diabetes was not as strong as that seen in the Michigan study. Unlike the CARDIA study, both moderately and highly chlorinated PCBs predicted future risk of type 2 diabetes. However, trans-nonachlor came out as the most significant among the organochlorine pesticides, similar to the CARDIA study.
The shapes of dose-response relations also differed among the studies. The current study and the Michigan study showed similar dose-response curves (7). The risk of future diabetes was substantially increased with only a slight increase of concentrations of POPs. However, after that, only a slight increase in risk was seen when the concentrations of POPs became higher. Although the CARDIA study also observed the increased risk at the low dose of some POPs, after that the risk even decreased as concentrations of POPs increased (6). Considering all these findings, the bottom line may be that the biological effects of POPs already are induced at very low concentrations of certain POPs.
At present, we do not have any clear explanation for why different POPs predicting type 2 diabetes and/or shapes of dose-response relations were different among epidemiological studies. The inconsistency may be related to the idea that POPs are involved in the pathogenesis of type 2 diabetes by interfering with endocrine signaling pathways (22). First, low-dose effects have been proposed as possible biological responses of endocrine disruptors (23). Also, endocrine function generally declines with age because hormone receptors become less sensitive and levels of most hormones change with age (24). Therefore, the different age distribution among the study populations may have led to different results even when comparing similar concentrations of POPs. In addition, endocrine-disturbing effects of one specific POP may differ depending on concentrations of other related endocrine disruptors. Humans are exposed to a mixture of various POPs with unique exposure patterns depending on the study population. For example, concentrations of p,p´-DDE in the CARDIA study was higher than in those of the current study and the Michigan study. However, concentrations of PCBs with six or seven chlorine atoms were much higher in the current study than in the CARDIA study, whereas those with four or five chlorine atoms were similar between these two studies. Although concentration of any particular POP might be similar between two populations, the strength of association between this POP and diabetes can differ depending on concentrations of other POPs.
There are several study limitations. First, there were only 36 incident type 2 diabetes cases. Therefore, the CIs were wide, and some associations may be chance findings despite strong statistical significance. In addition, although our general hypothesis of associations between POPs and incident diabetes were made a priori, we did perform many statistical tests, and caution should be exercised in interpreting the findings. Second, some diabetes cases would be misclassified as noncases because we did not perform an oral glucose tolerance test. Isolated postprandial hyperglycemia with normal fasting glucose is common among the elderly (25). Third, because concentrations of POPs tend to be highly correlated in the general population, any POP that is strongly correlated with other biologically important POPs may come out statistically significant in epidemiological studies. Although the measured POPs in the current study are a selection of the POPs referred to in the Stockholm Convention and are representative markers for the group of compounds, the real exposure of the subject is, of course, more complex.
In conclusion, the current study observed that PCBs and some organochlorine pesticides predicted the future risk of type 2 diabetes. Although careful interpretation may be needed because of the small number of incident type 2 diabetes cases, the association between PCBs and incident type 2 diabetes seemed to be at least as strong as the association of adiposity with incident diabetes in this elderly population. The environmental exposure to PCBs and some organochlorine pesticides may play a role in the development of type 2 diabetes.
Supplementary Material
Acknowledgments
This study was supported by the Swedish Research Council and the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning.
No potential conflicts of interest relevant to this article were reported.
D.-H.L. performed data analysis and wrote the initial draft of the manuscript. P.M.L. conceived the project and contributed to the critical revision of the manuscript for important intellectual content. D.R.J. performed data analysis and contributed to the critical revision of the manuscript for important intellectual content. S.S. and B.v.B. performed POPs laboratory analyses and contributed to the critical revision of the manuscript for important intellectual content. L.L. is the principal investigator of the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study and conceived the project, performed data analysis, and contributed to the critical revision of the manuscript for important intellectual content. Also, L.L. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-2116/-/DC1.
References
- 1.Abelsohn A, Gibson BL, Sanborn MD, Weir E. Identifying and managing adverse environmental health effects: 5. persistent organic pollutants. CMAJ 2002;166:1549–1554 [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DH, Lee IK, Song K, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999-2002. Diabetes Care 2006;29:1638–1644 [DOI] [PubMed] [Google Scholar]
- 3.Longnecker MP, Michalek JE. Serum dioxin level in relation to diabetes mellitus among Air Force veterans with background levels of exposure. Epidemiology 2000;11:44–48 [DOI] [PubMed] [Google Scholar]
- 4.Rylander L, Rignell-Hydbom A, Hagmar L. A cross-sectional study of the association between persistent organochlorine pollutants and diabetes. Environ Health 2005;4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DH, Jacobs DR, Jr, Porta M. Could low-level background exposure to persistent organic pollutants contribute to the social burden of type 2 diabetes? J Epidemiol Community Health 2006;60:1006–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DH, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR, Jr. Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case-control study. Environ Health Perspect 2010;118:1235–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turyk M, Anderson H, Knobeloch L, Imm P, Persky V. Organochlorine exposure and incidence of diabetes in a cohort of Great Lakes sport fish consumers. Environ Health Perspect 2009;117:1076–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rignell-Hydbom A, Lidfeldt J, Kiviranta H, et al. Exposure to p,p’-DDE: a risk factor for type 2 diabetes. PLoS ONE 2009;4:e7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruzzin J, Petersen R, Meugnier E, et al. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect 2010;118:465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steenland K, Piacitelli L, Deddens J, Fingerhut M, Chang LI. Cancer, heart disease, and diabetes in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Natl Cancer Inst 1999;91:779–786 [DOI] [PubMed] [Google Scholar]
- 11.Biggs ML, Mukamal KJ, Luchsinger JA, et al. Association between adiposity in midlife and older age and risk of diabetes in older adults. JAMA 2010;303:2504–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris TB. Invited commentary: body composition in studies of aging: new opportunities to better understand health risks associated with weight. Am J Epidemiol 2002;156:122–124; discussion 125–126 [DOI] [PubMed] [Google Scholar]
- 13.Sandau CD, Sjödin A, Davis MD, et al. Comprehensive solid-phase extraction method for persistent organic pollutants: validation and application to the analysis of persistent chlorinated pesticides. Anal Chem 2003;75:71–77 [DOI] [PubMed] [Google Scholar]
- 14.Obana H, Hori S, Tanaka R. The effects of “yusho” type PCB on triglyceride lipase and fatty acid composition. Environ Res 1987;42:500–508 [DOI] [PubMed] [Google Scholar]
- 15.Lee DH, Lee IK, Porta M, Steffes M, Jacobs DR, Jr. Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among non-diabetic adults: results from the National Health and Nutrition Examination Survey 1999-2002. Diabetologia 2007;50:1841–1851 [DOI] [PubMed] [Google Scholar]
- 16.Howard BV. Insulin resistance and lipid metabolism. Am J Cardiol 1999;84:28J–32J [DOI] [PubMed] [Google Scholar]
- 17.Schisterman EF, Whitcomb BW, Louis GM, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect 2005;113:853–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207–212 [DOI] [PubMed]
- 19.Kraine MR, Tisch RM. The role of environmental factors in insulin-dependent diabetes mellitus: an unresolved issue. Environ Health Perspect 1999;107(Suppl. 5):777–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dröge W. Oxidative stress and aging. Adv Exp Med Biol 2003;543:191–200 [DOI] [PubMed] [Google Scholar]
- 21.Lu H, Koshkin V, Allister EM, Gyulkhandanyan AV, Wheeler MB. Molecular and metabolic evidence for mitochondrial defects associated with β-cell dysfunction in a mouse model of type 2 diabetes. Diabetes 2010;59:448–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharp D. Environmental toxins, a potential risk factor for diabetes among Canadian Aboriginals. Int J Circumpolar Health 2009;68:316–326 [DOI] [PubMed] [Google Scholar]
- 23.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures: I. mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect 2003;111:994–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noth RH, Mazzaferri EL. Age and the endocrine system. Clin Geriatr Med 1985;1:223–250 [PubMed] [Google Scholar]
- 25.Adam JM, Josten D. Isolated post-challenge hyperglycemia: concept and clinical significance. Acta Med Indones 2008;40:171–175 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.