Abstract
OBJECTIVE
To compare in the Swiss population the results of several scores estimating the risk of developing type 2 diabetes.
RESEARCH DESIGN AND METHODS
This was a single-center, cross-sectional study conducted between 2003 and 2006 in Lausanne, Switzerland. Overall, 3,251 women and 2,937 men, aged 35–75 years, were assessed, of which 5,760 (93%) were free from diabetes and included in the current study. The risk of developing type 2 diabetes was assessed using seven different risk scores, including clinical data with or without biological data. Participants were considered to be eligible for primary prevention according to the thresholds provided for each score. The results were then extrapolated to the Swiss population of the same sex and age.
RESULTS
The risk of developing type 2 diabetes increased with age in all scores. The prevalence of participants at high risk ranged between 1.6 and 24.9% in men and between 1.1 and 15.7% in women. Extrapolated to the Swiss population of similar age, the overall number of participants at risk, and thus susceptible to intervention, ranged between 46,708 and 636,841. In addition, scores that included the same clinical variables led to a significantly different prevalence of participants at risk (4.2% [95% CI 3.4–5.0] vs. 12.8% [11.5–14.1] in men and 2.9% [2.4–3.6] vs. 6.0% [5.2–6.9] in women).
CONCLUSIONS
The prevalence of participants at risk for developing type 2 diabetes varies considerably according to the scoring system used. To adequately prevent type 2 diabetes, risk-scoring systems must be validated for each population considered.
Type 2 diabetes is a serious disease with increasing prevalence. This disease remains asymptomatic for years, being discovered only at a stage with preexisting complications (1). Recent studies (2) have shown that lifestyle or medication intervention could prevent the incidence of type 2 diabetes. Hence, screening tools are needed to identify participants with undiagnosed diabetes or those who are at risk for developing diabetes in the future. For this purpose, numerous risk scores recently have been proposed (3–6). Participants at high risk of developing type 2 diabetes, according to the risk score threshold, are thus amenable to preventive measures. A good diabetes risk score ideally should be easily completed by the physician and rely on easily and routinely accessible clinical and biological parameters, such as age, family history, hypertension, anthropometry, or lifestyle habits. Moreover, the risk score has to be accurate enough to provide targeted warnings for the patients. Some scores have been validated in selected populations (3–7), prompting their use in other countries (8,9). Nevertheless, recent studies (10) have shown that risk scores that are developed in the same country can lead to different results. Likewise, one equation validated in one country might not provide adequate estimates in another; for instance, the Framingham cardiovascular risk equations can over- or underestimate risk when directly applied to other populations (11). Finally, and to the best of our knowledge, no study has ever compared the results of differing scoring systems in Switzerland.
The current study aimed to compare the results of several scores that estimate the risk of developing type 2 diabetes using data from the Cohorte Lausannoise (CoLaus) study, a cross-sectional study conducted in Lausanne, Switzerland. The resulting number of subjects at risk for developing type 2 diabetes in Switzerland according to these different risk equations also was estimated.
RESEARCH DESIGN AND METHODS
Risk scores
We performed a PubMed search and selected risk scores for their relative novelty and their applicability to the Swiss population. The score from the Swiss Diabetes Association, available on the Internet (8), also was assessed. This score actually is an adaptation of the Finnish Diabetes Risk Score (FINDRISC) score (7). Overall, seven risk scores, including clinical (C) or clinical and biological variables (CB) were studied: 10-year risk scores from Kahn et al. (3) (C and CB); 8-year risk score from Wilson et al. (4) (CB); 9-year risk score from Balkau et al. (6) (C); the prevalent undiagnosed diabetes risk score from Griffin et al. (5) (C); the risk score from the Swiss Diabetes Association (8); and the FINDRISC (C), which is a 5- to 10-year risk score (7). The characteristics of the studies, where the scores were developed, and the variables included in each score are summarized in Supplementary Tables 1 and 2. From this point on, the scores will be referenced by the name of the first author, with a further differentiation by C or CB in the case of the Kahn and Balkau scores.
We used the thresholds recommended by the authors to define participants at high risk of developing type 2 diabetes (Supplementary Table 1). These thresholds were defined differently according to the study: Kahn (3), Wilson (4), and Balkau (6) used a probability, whereas the Swiss Diabetes Association and FINDRISC used a score above a given number of points. The initial publication from Griffin et al. (5) provided no threshold; hence, we used the 37% probability, which was used in another study (12). The scores from the Swiss Diabetes Association and FINDRISC included regular consumption of selected foods (fruits, vegetables, berries, and brown bread) and familial history of diabetes for second-degree parents (grandparents, cousins, and uncles). Because these data were not available in our study, the scoring system was adapted by reducing by one point the cutoff value for high-risk participants.
Recruitment
The CoLaus study is a cross-sectional study in the Caucasian population of Lausanne, Switzerland, a town of 117,161 inhabitants, of which 79,420 are of a Swiss nationality. This study was approved by the institutional ethics committee of the University of Lausanne. The study was designed to assess the prevalence and to identify the molecular determinants of cardiovascular risk factors. The population of Lausanne can be considered as representative of the whole country because a considerable proportion is non-Swiss or comes from other cantons (political regions of Switzerland). In 2006, of 128,231 Lausanne inhabitants, 38% were non-Swiss, 30% came from other cantons (including Italian and German-speaking cantons), and only 32% were actually from the Vaud canton (13).
The sampling procedure of the CoLaus study has been described previously (14). A complete list of Lausanne inhabitants, aged 35–75 years (n = 56,694), was provided by the population registry of the city. A simple, nonstratified random sample of 35% of the overall population was drawn. The following inclusion criteria were applied: 1) provided written informed consent; 2) was aged 35–75 years; 3) was willing to take part in the examination and donate blood samples; and 4) was of Caucasian origin, defined as having both parents and grandparents born in a restricted list of countries (available from the authors). Recruitment began in June 2003 and ended in May 2006. Participation rate was 41%, and 6,188 Caucasian participants (3,251 women and 2,937 men) took part in the study.
All participants attended the outpatient clinic of the University Hospital of Lausanne in the morning after an overnight fast (minimum fasting time 8 h). Data were collected by trained field interviewers in a single visit lasting ~60 min.
Clinical data
The participants first received a questionnaire to record information about their lifestyle factors, namely tobacco use, alcohol use, and physical activity. According to their smoking histories, participants were classified as never, current, or former smokers. Current smokers were defined as giving a positive answer to the statement “I currently smoke,” former smokers were defined as giving a positive answer to the statement “I don’t smoke anymore,” and never smokers were defined as giving a positive answer to the statement “I have never smoked.” Alcohol consumption included past and current drinking status as well as the number of alcoholic beverage units (wine, beer, and spirits) consumed over the week preceding the interview. A participant was considered to be physically active if he/she reported practicing at least 2 h of leisure-time physical activity per week.
During a second face-to-face meeting, the participants were asked if they or their first-degree family (i.e., parents, children) had presented with diabetes. The participants also were asked if they had been diagnosed with hypertension or if they currently were being treated for hypertension. Personal medicines, including prescription and self-prescribed drugs, were collected, together with their main indications. Only corticosteroids, being of systemic or topical use, were considered for testing the scores. Despite the fact that other medications, such as hydrochlorothiazide or ACE inhibitors, have been shown to influence diabetes status (15), they were not included in the risk scores.
Body weight and height were measured with participants standing without shoes in light indoor clothes. Body weight was measured in kilograms to the nearest 100 g, using a Seca scale, which was calibrated regularly. Height was measured to the nearest 5 mm using a Seca height gauge. Waist was measured with a nonstretchable tape over the unclothed abdomen at the narrowest point between the lowest rib and the iliac crest. Two measures were made, and the mean (expressed in centimeters) was used for analyses. Blood pressure and resting pulse were measured three times using an Omron HEM-907 automated oscillometric sphygmomanometer on the left arm, with an appropriately sized cuff, after at least 10 min rest in the seated position. The average of the last two measurements was used for analyses.
Biological analyses
Fasting plasma glucose, HDL cholesterol, LDL cholesterol, triglycerides, and uric acid levels were measured by the Centre Hospitalier Universitaire Vaudois Clinical Laboratory using fresh blood samples within 2 h of blood collection. All measurements were conducted in a Modular P apparatus (Roche Diagnostics, Basel, Switzerland). The following analytical procedures (with maximum interbatch and intrabatch coefficients of variation) were used: cholesterol by cholesterol oxidase-peroxide + 4-aminophenazone + phenol (PAP) (1.6–1.7%); HDL cholesterol by cholesterol oxidase-PAP plus polyethylene-glycol plus cyclodextrin (3.6–0.9%); glucose by glucose dehydrogenase (2.1–1.0%); triglycerides by glucose oxidase-PAP (2.9–1.5%); and uric acid by uricase-PAP (1.0–0.5%).
Diabetes
Diabetes was defined as fasting plasma glucose ≥7.0 mmol/L and/or the presence of oral hypoglycemic or insulin treatment. Type 2 diabetes was defined in cases of diabetes without self-reported type 1 diabetes. Impaired fasting glucose was defined as fasting plasma glucose between 6.1 and 6.9 mmol/L without antidiabetes treatment.
Statistical analysis
Of the initial 6,188 participants, 21 (0.3%) had missing data for the variables of interest, 407 (6.6%) had diabetes, and 655 had impaired fasting glucose (10.6%). Diabetic participants were excluded, and the remaining 5,760 (93.1%) participants were used in the analyses. Characteristics of the patients included in our study are available in Supplementary Table 1.
The prevalence of participants at risk for developing type 2 diabetes according to each score was determined and expressed in percentages and 95% CIs. The number of participants at risk in Switzerland was then estimated for each score by applying the sex-specific and 10-year age-group–specific prevalence obtained to the corresponding diabetes-free population numbers, obtained by averaging the population estimates between 2003 and 2006, provided by the Swiss Federal Statistical Office (www.statistique.admin.ch). To assess the number of subjects without diabetes in the Swiss population, we assumed that the proportion of nondiabetic patients in our study was representative of the whole country. All statistical analyses were made using Stata version 11.1 (Stata, College Station, TX).
RESULTS
Prevalence of subjects at risk for type 2 diabetes
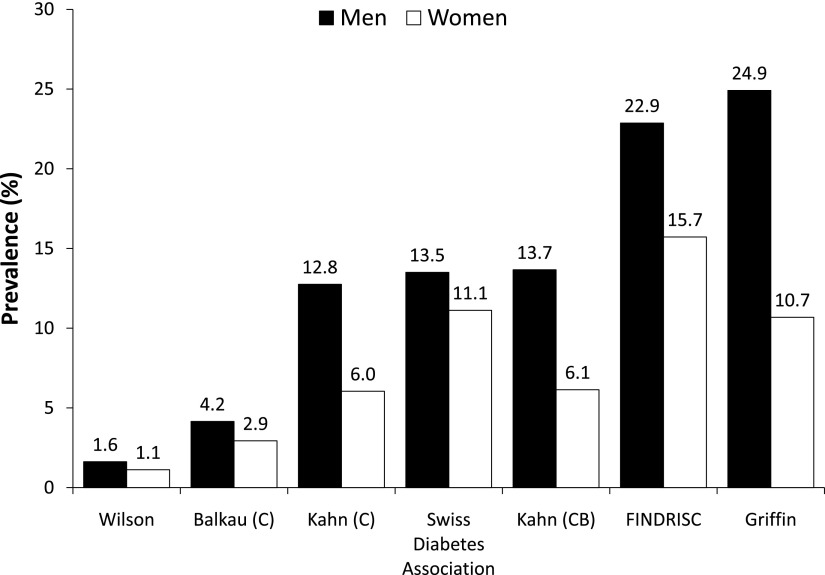
The prevalence of participants at risk for developing type 2 diabetes is shown in Fig. 1. In men, the prevalence of participants at high risk of developing type 2 diabetes was the following: 1.6% (1.2–2.2) (Wilson); 4.2% (3.4–5.0) (Balkau); 12.8% (11.5–14.1) (Kahn [C]); 13.5% (12.2–14.9) (Swiss Diabetes Association); 13.7% (12.4–15.0) (Kahn [CB]); 22.9% (21.3–24.5) (FINDRISC); and 24.9% (23.4–26.6) (Griffin). In women, the corresponding values were 1.1% (0.8–1.6) (Wilson); 2.9% (2.4–3.6) (Balkau); 6.0% (5.2–6.9) (Kahn [C]); 11.1% (10.0–12.3) (Swiss Diabetes Association); 6.1% (5.3–7.0) (Kahn [CB]); 15.7% (14.5–17.1) (FINDRISC); and 10.7% (9.6–11.8) (Griffin). Overall, men tended to present a higher risk of type 2 diabetes than women. Extrapolated to the Swiss population of the same age, the number of subjects at risk ranged from 46,708 to 636,841, more than a 13-fold variation (Table 1). Restricting the analysis to participants aged <65 years showed either slight increases (Wilson) or decreases (FINDRISC; Griffin) in the prevalence of subjects at risk for developing type 2 diabetes (Supplementary Table 4). Likewise, excluding from the analysis the 11 women with a possible pregnancy at examination did not change the results (data not shown).
Figure 1.
Proportion of participants at high risk of developing type 2 diabetes according to each score, by sex. C and CB are only specified in the case of various equations provided by the authors.
Table 1.
Number of participants in the Swiss population at high risk of developing type 2 diabetes according to each score, by sex and age-group
| Sex and age-group | Total Swiss population | Wilson | Balkau (C) | Kahn (C) | Swiss Diabetes Association | Kahn (CB) | Griffin | FINDRISC |
|---|---|---|---|---|---|---|---|---|
| Men | ||||||||
| Age (years) | ||||||||
| 35–44 | 620,900 | 4,964 | 12,046 | 36,924 | 18,462 | 34,745 | 35,471 | 29,116 |
| 45–54 | 529,600 | 11,275 | 15,066 | 44,500 | 55,775 | 60,165 | 80,869 | 86,506 |
| 55–64 | 441,700 | 9,281 | 24,394 | 84,151 | 77,181 | 85,325 | 145,119 | 146,256 |
| 65–75 | 304,300 | 1,360 | 18,430 | 47,762 | 75,055 | 47,762 | 157,613 | 94,240 |
| Total | 1,896,500 | 26,879 | 69,937 | 213,338 | 226,474 | 227,998 | 419,072 | 356,118 |
| Women | ||||||||
| Age (years) | ||||||||
| 35–44 | 615,900 | 4,262 | 8,523 | 15,647 | 11,385 | 10,654 | 7,854 | 21,309 |
| 45–54 | 522,500 | 5,133 | 11,434 | 20,022 | 37,148 | 23,427 | 25,714 | 55,443 |
| 55–64 | 449,100 | 7,670 | 19,132 | 48,363 | 77,083 | 46,914 | 71,330 | 104,865 |
| 65–75 | 361,700 | 2,765 | 13,166 | 20,802 | 72,050 | 25,641 | 88,014 | 99,106 |
| Total | 1,949,200 | 19,829 | 52,256 | 104,834 | 197,666 | 106,636 | 192,912 | 280,722 |
| Total | 3,845,700 | 46,708 | 122,192 | 318,172 | 424,140 | 334,634 | 611,984 | 636,841 |
C and CB are only specified in the case of various equations provided by the authors.
Comparison between scores
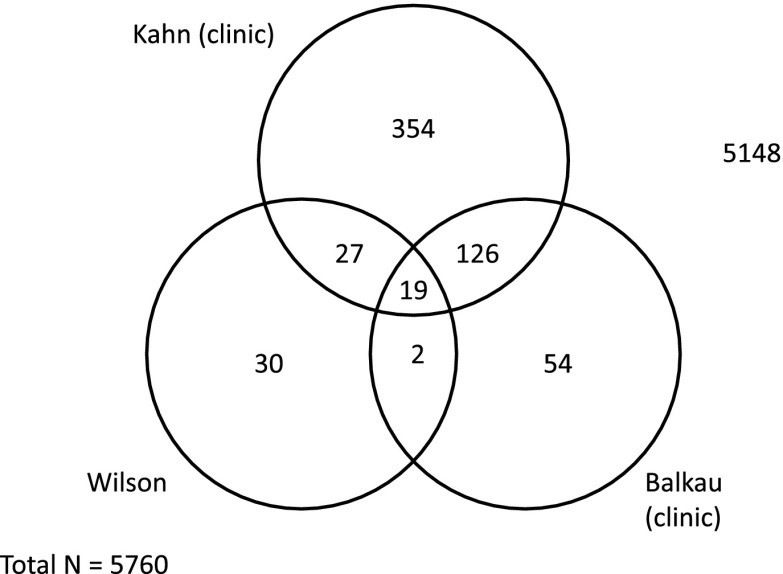
We also checked whether the same participants were considered to be at risk according to the different scores. For this, we compared the participants at risk for type 2 diabetes according to the scores that led to the lowest prevalence (Wilson and Balkau) and also according to the scores that included the same clinical variables (Balkau and Kahn [C]). The results are presented in Fig. 2. The scores classified a total of 612 participants as being at risk: n = 78, Wilson; n = 201, Balkau; and n = 558, Kahn (C). Of 78 participants at risk according to Wilson et al. (4), only 21 (26.9%) also were considered at risk according to Balkau. Likewise, of 201 participants at risk according to Balkau, only 145 (72%) also were considered at risk according to Kahn (C). Only 19 patients were simultaneously classified as high-risk by all three scores.
Figure 2.
Number of patients at high risk of developing type 2 diabetes according to three scores.
CONCLUSIONS
To our knowledge, this is one of the few studies that assessed the effect of differing type 2 diabetes risk–scoring systems in a given population. In agreement with previous studies (10,16), our results indicate that the prevalence of subjects at risk for developing type 2 diabetes varies considerably according to the scoring system used. This has a considerable impact in the number of subjects susceptible of benefiting from measures regarding the primary prevention of type 2 diabetes.
The risk-scoring systems compared in this study shared several types of variables (Supplementary Table 1). For instance, all of them included a genetic background (personal or family history), which can be explained by the association between certain genes and diabetes (17), and most of them also included age, which has been shown to be related to the risk of diabetes. Most scores also included obesity markers, such as BMI or waist circumference, as well as cardiovascular risk factors, such as hypertension and dyslipidemia, all of which are involved in the metabolic syndrome definition (18). Finally, some scores included lifestyle habits, whether protective, such as alcohol consumption and physical activity, or deleterious, such as smoking, also in agreement with previous findings (19). It should be noticed that in some studies nondrinkers and former drinkers were included in the same group (3) and that the nonlinear, U-shaped association between alcohol consumption and the risk of developing type 2 diabetes (20) was not considered. Overall, these findings suggest that any generic scoring system that included clinical variables known to be related to type 2 diabetes (age, obesity, cardiovascular risk factors, and lifestyle) could be used to derive diabetes risk scores but that the relative weight of each variable might be different according to the population considered. For instance, age, obesity, and the other factors mentioned vary by country, and this may result in a differential importance to predict diabetes. Finally, the inclusion of other variables, such as biological and genetic markers, also should be considered but is beyond the scope of our study (21).
The two scoring systems that did not include age (i.e., Wilson and Balkau) gave the lowest prevalence rates of subjects at risk for type 2 diabetes, whereas the Griffin score, which includes a linear relationship with age (5), provided the greatest prevalence. It is interesting to note that the scoring systems using age-groups instead of age (3,8,9) provided intermediate prevalence rates. Considering how easy it is to collect age and the increasing prevalence of type 2 diabetes with age (18), we can postulate that this variable should be included in any risk-scoring system. Another possible explanation for the low prevalence rates of participants at risk using the Wilson score is the fact that it includes a low HDL cholesterol level, whose prevalence was 36.9% in the original study. However, in the CoLaus study (14), the prevalence of low HDL cholesterol was only 2.8%, which might lead to spurious results. Overall, these findings further stress the importance of not only including certain variables but also their relative weighting and even the way they are coded to compute the risk of developing type 2 diabetes.
Only two scoring systems (Wilson and Kahn [CB]) included fasting glucose. This was somewhat unexpected because fasting glucose has a strong predictive value for diabetes (22). Indeed, in the study from Balkau et al. (6), fasting glucose was considered to be the best predictor, but no scoring system that included fasting glucose was provided, possibly because of the fact that the objective was to derive a clinically based scoring system.
Most values for each variable included in the scoring systems were derived from logistic or Cox regression coefficients. Still, it should be noticed that some scoring systems (i.e., Swiss Diabetes Association) include variables (i.e., familial history) for which the scores were not based upon statistical analysis but on an “educated” proposal by the authors (7). As a subject might shift from a low-risk to a high-risk category by one single scoring unit, care should be taken when such non–evidence-based scores are applied. Furthermore, the FINDRISC score has been used (and in some cases modified) by others (8,9) without any complementary statistical analysis or validation; therefore, the results obtained by these modified, nonvalidated scores might be questionable. Finally, many scoring systems did not take into account the nonlinear association between some variables (i.e., alcohol consumption) and diabetes risk; the reason might be that introducing nonlinearity complicates the scoring system, but no precise rationale could be obtained from the literature.
The prevalence rates of participants at risk for developing type 2 diabetes varied almost 13-fold according to the scoring system used, leading to considerable differences in the number of subjects amenable to prevention measures in the corresponding Swiss population. This great variability can be partly explained by the differences between the scoring systems. First, the variables used and their corresponding coefficients varied considerably. Second, the thresholds used to define subjects at high risk also varied (30–46%), as shown in Table 1. Third, and as stated previously, the scoring systems were developed and validated in a given population, and applying them to a different population can lead to inconsistent results, as it has been underlined in a previous German study (16). Overall, our results suggest that the indiscriminate use of a nonvalidated scoring system might lead to considerable differences in the number of subjects to prevent, with a likely under- or overuse of the limited available preventive resources.
The agreement between the different scoring systems was disappointing. Indeed, we initially expected that two scoring systems detecting a low number of subjects at risk for diabetes would detect the same patients, but Fig. 2 shows that it is not the case. Likewise, even two scoring systems that included broadly the same variables (i.e., Balkau and Kahn [C]) failed to detect the same participants. Hence, our results indicate that different scoring systems detect different subjects at risk for developing type 2 diabetes and thus are not interchangeable. Adequate validation of these scoring systems using prospective data is therefore necessary to select the best system applicable to the population under study. The ongoing follow-up of the CoLaus cohort will allow this validation.
Our study has several limitations. First, the predicting ability of the tested scores could not be achieved in the current study. Thus, it is unclear which of them will be the most accurate. The ongoing follow-up of the CoLaus cohort will enable such a comparison. Second, the prevalence rates according to Swiss Diabetes Association and FINDRISC may be over- or underestimated as a result of our lack of dietary data and second-degree familial history. Of interest, sensitivity analyses showed that decreasing the threshold of these scores by two and three points led to a 50% increase in the number of subjects at risk for developing type 2 diabetes (Supplementary Table 6). These findings suggest that minor changes in the scoring system can lead to considerable changes in the number of subjects at risk for developing type 2 diabetes and that any risk-scoring system should be adequately validated before being applied in a given population. Third, although the participation rate was similar to other epidemiological studies (23), it was rather low (41%), which might limit the generalization of findings. Indeed, the CoLaus study may not be representative of the Swiss population, but seeing the great variability in the number of high-risk patients, this mistake may not be of great relevance. In addition, there was no sex or ZIP code distribution difference between the source population, the random sample, and the CoLaus participants. On the other hand, the CoLaus sample had more women and was slightly younger than the corresponding Swiss population aged 35–75 years (Supplementary Table 7). Hence, it can be argued that although the CoLaus sample is not fully representative of the Swiss population, the differences in population structure are relatively small. Likewise, our population was at relatively low risk for diabetes, and it is unknown how this might influence the performance of some of the risk scores if applied in other populations or ethnic groups. Still, most equations we used have been developed in European countries and should thus be generalizable to the European population. On the other hand, it has been shown that risk scores developed in the same country lead to different results (10). Thus and again, a precise validation within a given of any risk score should be conducted before its application in clinical or public health practice. Fourth, diabetic subjects were excluded on the basis of fasting but not on 2-h plasma glucose; hence, diabetic subjects by 2-h glucose (but not by fasting glucose) were retained in the analysis. Nevertheless, because the number of subjects with type 2 diabetes (by 2-h glucose) is fixed, our results still indicate that the number of subjects at risk for developing type 2 diabetes varies considerably according to the risk score used. Finally, this article only included leisure-time physical activity, and occupational physical activity was not considered. Hence, it is possible that the risk of developing type 2 diabetes might be overestimated when using the equations that include physical activity.
In summary, our results indicate that the prevalence of participants at risk for developing type 2 diabetes varies considerably according to the scoring system used. To adequately prevent type 2 diabetes, risk-scoring systems should be validated for each population considered.
Supplementary Material
Acknowledgments
The CoLaus study was supported by research grants from the Swiss National Science Foundation (grant no. 33CSCO-122661), from GlaxoSmithKline, and from the Faculty of Biology and Medicine of Lausanne, Lausanne, Switzerland. P.V. and G.W. received an unrestricted grant from GlaxoSmithKline to build the CoLaus study. No other potential conflicts of interest relevant to this article were reported.
R.S. researched data and wrote the manuscript. P.V., G.W., and P.M.-V. reviewed and edited the manuscript and contributed to discussion.
The authors express their gratitude to the participants in the Lausanne CoLaus study and to the investigators who have contributed to the recruitment, in particular Yolande Barreau, Anne-Lise Bastian, Binasa Ramic, Martine Moranville, Martine Baumer, Marcy Sagette, Jeanne Ecoffey, and Sylvie Mermoud, all from the CoLaus team, for data collection.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0206/-/DC1.
References
- 1.American Diabetes Association. Standards of medical care in diabetes: 2009. Diabetes Care 2009;32(Suppl. 1):S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn HS, Cheng YJ, Thompson TJ, Imperatore G, Gregg EW. Two risk-scoring systems for predicting incident diabetes mellitus in U.S. adults age 45 to 64 years. Ann Intern Med 2009;150:741–751 [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RB, Sr. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med 2007;167:1068–1074 [DOI] [PubMed] [Google Scholar]
- 5.Griffin SJ, Little PS, Hales CN, Kinmonth AL, Wareham NJ. Diabetes risk score: towards earlier detection of type 2 diabetes in general practice. Diabetes Metab Res Rev 2000;16:164–171 [DOI] [PubMed] [Google Scholar]
- 6.Balkau B, Lange C, Fezeu L, et al. Predicting diabetes: clinical, biological, and genetic approaches: data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2008;31:2056–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725–731 [DOI] [PubMed] [Google Scholar]
- 8.Diabète de type 2: quel est votre risque? [Internet], 2011. Available from http://www.diabetesgesellschaft.ch/fr/informations/test-diabete/. Accessed 26 May 2011
- 9.Findrisk (“finde das risiko”) fragebogen: optimierte deutsche version [Internet], 2011. Available from www.diabetes-heute.de. Accessed 27 May 2011
- 10.Mann DM, Bertoni AG, Shimbo D, et al. Comparative validity of 3 diabetes mellitus risk prediction scoring models in a multiethnic US cohort: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2010;171:980–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hense HW, Schulte H, Löwel H, Assmann G, Keil U. Framingham risk function overestimates risk of coronary heart disease in men and women from Germany: results from the MONICA Augsburg and the PROCAM cohorts. Eur Heart J 2003;24:937–945 [DOI] [PubMed] [Google Scholar]
- 12.Rahman M, Simmons RK, Harding AH, Wareham NJ, Griffin SJ. A simple risk score identifies individuals at high risk of developing type 2 diabetes: a prospective cohort study. Fam Pract 2008;25:191–196 [DOI] [PubMed] [Google Scholar]
- 13.Evolution mensuelle de la population [Internet], 2008. Available from http://www.lausanne.ch/view.asp?docId=22884&domId=63584&language=F. Accessed 27 May 2011
- 14.Firmann M, Mayor V, Vidal PM, et al. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord 2008;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padwal R, Laupacis A. Antihypertensive therapy and incidence of type 2 diabetes: a systematic review. Diabetes Care 2004;27:247–255 [DOI] [PubMed] [Google Scholar]
- 16.Rathmann W, Martin S, Haastert B, et al. ; KORA Study Group. Performance of screening questionnaires and risk scores for undiagnosed diabetes: the KORA Survey 2000. Arch Intern Med 2005;165:436–441 [DOI] [PubMed] [Google Scholar]
- 17.Frayling TM. Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet 2007;8:657–662 [DOI] [PubMed] [Google Scholar]
- 18.Joseph J, Svartberg J, Njolstad I, Schirmer H. Risk factors for type 2 diabetes in groups stratified according to metabolic syndrome: a 10-year follow-up of the Tromso study. Eur J Epidemiol 2011;26:117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007;298:2654–2664 [DOI] [PubMed] [Google Scholar]
- 20.Clerc O, Nanchen D, Cornuz J, et al. Alcohol drinking, the metabolic syndrome and diabetes in a population with high mean alcohol consumption. Diabet Med 2010;27:1241–1249 [DOI] [PubMed] [Google Scholar]
- 21.Schulze MB, Weikert C, Pischon T, et al. Use of multiple metabolic and genetic markers to improve the prediction of type 2 diabetes: the EPIC-Potsdam Study. Diabetes Care 2009;32:2116–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt MI, Duncan BB, Bang H, et al. ; Atherosclerosis Risk in Communities Investigators. Identifying individuals at high risk for diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care 2005;28:2013–2018 [DOI] [PubMed] [Google Scholar]
- 23.Tolonen H, Koponen P, Aromaa A, et al. ; Feasibility of a European Health Examination Survey (FEHES) Project. Review of Health Examination Surveys in Europe. Helsinki, Finland, National Public Health Institute (KTL), 2008, p. 1–379 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.