Abstract
OBJECTIVE
Discordance between HbA1c and fructosamine estimations in the assessment of glycemia is often encountered. A number of mechanisms might explain such discordance, but whether it is consistent is uncertain. This study aims to coanalyze paired glycosylated hemoglobin (HbA1c)-fructosamine estimations by using fructosamine to determine a predicted HbA1c, to calculate a glycation gap (G-gap) and to determine whether the G-gap is consistent over time.
RESEARCH DESIGN AND METHODS
We included 2,263 individuals with diabetes who had at least two paired HbA1c-fructosamine estimations that were separated by 10 ± 8 months. Of these, 1,217 individuals had a third pair. The G-gap was calculated as G-gap = HbA1c minus the standardized fructosamine-derived HbA1c equivalent (FHbA1c). The hypothesis that the G-gap would remain consistent in individuals over time was tested.
RESULTS
The G-gaps were similar in the first, second, and third paired samples (0.0 ± 1.2, 0.0 ± 1.3, and 0.0 ± 1.3, respectively). Despite significant changes in the HbA1c and fructosamine, the G-gap did not differ in absolute or relative terms and showed no significant within-subject variability. The direction of the G-gap remained consistent.
CONCLUSIONS
The G-gap appears consistent over time; thus, by inference any key underlying mechanisms are likely to be consistent. G-gap calculation may be a method of exploring and evaluating any such underlying mechanisms.
Glycated HbA1c results from the nonenzymatic concentration-dependent covalent bonding of glucose to hemoglobin within the erythrocytes. Despite the reliability and standardization of glycated HbA1c assays, clinicians still encounter discrepancy between glycated HbA1c results and other assessments of glycemia among their patients (1,2). Certain causes for such discrepancy have long been known, and many erythrocytic factors may affect HbA1c independently of glycemia (3–5). These include impacts on red cell life span and glucose gradient across the red cell membrane (6,7). Possible intracellular enzyme-dependent processes that promote deglycation have been proposed but are of uncertain importance (8–10). Thus, glycated HbA1c may not reflect prevailing glycemia because of a variation in many potential underlying mechanisms, with its concentrations being the net product of these reactions and processes. Thus, individuals may have a lower HbA1c or a higher HbA1c than predicted from prevailing glycemia (1–3,11).
The methodology to determine the deviation of glycated HbA1c from prevailing glycemia has largely centered on the co-use of serum fructosamine as another glycated protein product that is ostensibly not subject to intracellular enzymatic deglycation or other erythrocytic factors because it is extracellular (1,12). This deviation has been described as the glycation gap (G-gap). It should also be noted that serum fructosamine itself may be subject to variability in protein turnover, serum albumin concentration, and obesity (13,14).
We have previously demonstrated the possibility of clinical error due to the G-gap (1). It is also possible that the G-gap might alter an individual’s risk of vascular complications for any given level of long-term glycemia by modifying one of the key pathologic processes, namely, protein glycation and the formation of advanced glycation end products (12,15–17). Thus, determining the G-gap in any individual may be of importance. Identification of the G-gap might aid risk stratification for the development of diabetes complications or guide safer therapeutics.
These multiple varying impacting factors could impart randomness on the G-gap, and, if so, it would not be expected to be consistent over time. Demonstrating consistency in G-gap may be indicative of consistent underlying mechanisms rather than any such randomness, possibly reflecting a genetic or biochemical basis. Such consistency has been suggested by others with various methodologies in the normal population and in people with diabetes (2,3,12). Indeed, a possible genetic basis for the G-gap may exist. Twin studies have suggested it to have a significant inheritability (18), and genome-wide association studies have identified a number of loci that may be relevant to HbA1c variation (19).
The aim of this study is to determine the G-gap within individuals with diabetes and verify its consistency over time.
RESEARCH DESIGN AND METHODS
Patient selection
Among people with diabetes, we reviewed all of the HbA1c and serum fructosamine estimations performed at New Cross Hospital, Wolverhampton, over a 4-year period from the beginning of January 2006 to the end of December 2009 (n = 120,768) and selected those who had estimations of HbA1c and fructosamine performed on the same day from the same sample set. Of the 4,724 identified, we further selected 2,263 people who had at least two paired HbA1c-fructosamine estimations over this period of time, and of these, 1,217 had a third pair. People with an abnormal hemoglobin electrophoretic pattern and women known to be pregnant were excluded, but no other selection or exclusion criteria were applied. We do not have systematic data on therapeutics, but it is likely that a variety of treatment changes were made in a number of these individuals over this time frame.
Analytical methods
HbA1c was reported in both the Diabetes Control and Complications Trial (DCCT) aligned and International Federation of Clinical Chemistry and Laboratory Medicine reference methods, but the International Federation of Clinical Chemistry and Laboratory Medicine values were available only from June 1, 2009. Thus, we have used the DCCT-aligned HbA1c in our analysis. HbA1c (DCCT-aligned) was measured using a high-performance liquid chromatography method on a Tosoh G7 analyzer (Tosoh Bioscience Ltd., Worcestershire, U.K.). The performance scores in the U.K. National External Quality Assurance Scheme were A scores <100 and B scores <2%. The between batch percentage coefficient of variation was 1.8 and 1.4 for an HbA1c of 5.7 and 9.5%, respectively. Fructosamine was measured using a nitrotetrazolium-blue reduction method on a Roche Modular P analyzer (Roche Diagnostics Ltd., West Sussex, U.K.) using a Cobas kit with between batch percentage coefficient of variation of 3.1 (at a level of 263 µmol/L) and 2.2 (at a level of 518 µmol/L).
Statistical analysis
All data were analyzed using SPSS version 18.0 (SPSS Inc., Chicago, IL). Results have been presented as the mean ± 1 SD unless otherwise stated, and statistical significance was taken at less than 0.05 probability.
Analysis of the simultaneously measured HbA1c and fructosamine values was used to calculate a predicted HbA1c from fructosamine estimation that was standardized to the mean and standard deviation of HbA1c. The standardized predicted HbA1c from these fructosamine values is denoted as the FHbA1c. This method has been described previously with FHbA1c calculated as ({[(fructosamine – mean fructosamine)/SD fructosamine] × SD HbA1c} + mean HbA1c) (1). The difference between glycation as assessed by the true HbA1c and the fructosamine-derived HbA1c (FHbA1c) is referred to as the glycation gap (G-gap). A negative G-gap would denote the true HbA1c appearing to read lower than the standardized predicted FHbA1c. A positive G-gap would denote the true HbA1c appearing to read higher than predicted by fructosamine. In all individuals, the G-gaps for the first, second, and third HbA1c-fructosamine pairs were calculated to test the hypothesis that the G-gap would be consistent in direction within individuals over time. If so, the product of any two concordant G-gaps, whether individual G-gap is negative or positive, would always be positive (i.e., above the x-axis: positive × positive = positive, negative × negative = positive) but negative (below the x-axis) with any discordance (negative × positive = negative).
RESULTS
The demographic details of the tested cohort are described in Table 1.
Table 1.
Demographic details of people with diabetes with the two (n = 2,263) or three (n = 1,217) paired HbA1c-fructosamine estimations
| Age (years) | 60.7 ± 14.2 (18–94) |
| Sex | |
| Male | 1,238 (54.7%) |
| Ethnic origin | |
| Caucasian | 1,058 (46.8%) |
| Asian | 408 (18%) |
| Afro-Caribbean | 191 (8.4%) |
| Other | 606 (26.8%) |
| Weight (kg) | 90.9 ± 21 |
| BMI (kg/m2) | 32.6 ± 6.9 |
| Diabetes type | |
| 1 | 418 (18.5%) |
| 2 | 1,826 (80.7%) |
| Other | 19 (0.8%) |
| Diabetes duration (years) | 15.8 ± 9.9 |
| Insulin treatment | 1,621 (71.6%) |
The data on the HbA1c, fructosamine, FHbA1c, and G-gap for all three paired samples are shown in Table 2. Pair 1 represents the latest value, and pair 3 represents the oldest value. The first and second pairs of HbA1c-fructosamine estimations were separated by a mean of 10 ± 8 months, and the first and third pairs of HbA1c-fructosamine estimations were separated by a mean of 14 ± 10.4 months. HbA1c and fructosamine estimations were significantly correlated at all three time points (r = 0.73, 0.72, and 0.73, respectively, all P < 0.001). Of note, the G-gaps showed a wide variation in the assessment of the glycemic control by HbA1c or fructosamine-derived estimations.
Table 2.
HbA1c, fructosamine, FHbA1c, and G-gap results derived from the separate pairs of HbA1c-fructosamine estimations
| HbA1c-fructosamine pairs | First (latest) | Second | Third (oldest) |
|---|---|---|---|
| n | 2,263 | 2,263 | 1,217 |
| Glycated HbA1c (%) | 8.3 ± 1.7 (4.0–17.7) | 8.5 ± 1.8* (4.6–18.1) | 8.6 ± 1.8* (4.7–17.1) |
| Fructosamine (µmol/L) | 308 ± 77 (143–978) | 315 ± 81* (72–853) | 318 ± 82* (167–821) |
| FHbA1c (%HbA1c) | 8.3 ± 1.7 (4.6–23.4) | 8.5 ± 1.8* (3.2–20.4) | 8.6 ± 1.8* (5.3–19.7) |
| G-gap (%HbA1c) | 0.0 ± 1.2 (−8.2 to 5.9) | 0.0 ± 1.3 ns (−7.3 to 9.1) | 0.0 ± 1.3 ns (−7.9 to 4.3) |
| G-gap/HbA1c (%) | −1.6 ± 15.1 (−85 to 48) | −1.2 ± 15.4 ns (−119 to 64) | −0.8 ± 17.0 ns (−122 to 57) |
Statistical significance was determined separately for the first two and all three samples.
*P < 0.001.
A variety of factors may influence serum fructosamine (13,14). A univariate ANCOVA was used to assess the latest fructosamine value against multiple demographic and biochemical factors. The overall model showed a significant association (n = 1,500 with complete data, r = 0.38, r2 = 0.15, F = 36.9, df = 7, P < 0.001) with BMI (F = 38.1, P < 0.001), type of diabetes (F = 14.1, P < 0.001), age (F = 14.0, P < 0.001), ethnic origin (F = 13.2, P < 0.001), and duration of diabetes (F = 8.1, P < 0.01), but not sex, serum albumin, serum creatinine, or urine albumin creatinine ratio. This had no significant effect on the fructosamine value (actual vs. model predicted, 314 ± 76 vs. 314 ± 29 µmol/L, t = −0.00, P = 1.00, ns) and no impact on the recalculated FHbA1c (8.4 ± 1.7 vs. 8.4 ± 1.6, t = 1.06, P = 0.29, ns) or on the recalculated G-gap (−0.05 ± 1.2 vs. −0.00 ± 2.2, t = −1.04, P = 0.29, ns). By considering the G-gap completely separately, the overall model showed significant associations (n = 1,452 with complete data, r = 0.45, r2 = 0.21, F = 55.27, df = 7, P < 0.001) with BMI (F = 138.1, P < 0.001), serum creatinine (F = 69.2, P < 0.001), ethnic origin (F = 21.2, P < 0.001), type of diabetes (F = 17.9, P < 0.001), and urine albumin to creatinine ratio (F = 13.3, P < 0.001), but not sex, duration of diabetes, or serum albumin. Again, this had no significant impact on the G-gap (actual vs. model predicted, −0.04 ± 1.2 vs. −0.04 ± 0.57, t = 0.00, P = 1.00, ns).
Significant differences were found for HbA1c, fructosamine, and FHbA1c, whether testing the latest two or all three related samples, with a mean tendency to improved glycemic control over time (Table 2). Consequently, HbA1c (F = 41.2, P < 0.001) and fructosamine (F = 14.6, P < 0.001) both showed significant within-subject variation over time. Despite these shifts, the G-gap did not differ significantly either in absolute or relative terms. However, as examined in the latest (first) and preceding (second) pairs, there was a significant but weak correlation between absolute change in HbA1c and change in G-gap (r = 0.32, r2 = 0.11, P < 0.001). This was explored further by assessing those who had a shift of ≥1.5 HbA1c% (Table 3). As the HbA1c decrease or increased by ≥1.5% HbA1c%, significant changes did occur in the G-gap that followed the same direction of change. Furthermore, considering the latest data in those with an HbA1c less than (n = 1,148) or more than (n = 1,115) 8%, the HbA1c was 7.0 ± 0.7 vs 9.6 ± 1.4% (P < 0.001); the G-gap was −0.37 ± 1.01 vs. 0.30 ± 1.37 (P < 0.001), and the G-gap/HbA1c was −5 ± 15 vs. 3 ± 14 (P < 0.001).
Table 3.
G-gap in those subjects with movements of ≥1.5% HbA1c between the latest and previous HbA1c
| HbA1c decreased | HbA1c increased | ||
|---|---|---|---|
| n | 320 | 143 | |
| Glycated HbA1c (%) | Latest | 8.0 ± 1.6* | 10.9 ± 1.8* |
| Previous | 10.7 ± 2.1 | 8.3 ± 1.3 | |
| Fructosamine | Latest | 293 ± 70* | 396 ± 105* |
| Previous | 384 ± 103 | 296 ± 62 | |
| G-gap (%HbA1c) | Latest | 0.03 ± 1.2* | 0.7 ± 1.8* |
| Previous | 0.6 ± 1.8 | 0.2 ± 1.3 | |
| G-gap/HbA1c (%) | Latest | −1 ± 16* | 6.0 ± 15* |
| Previous | 5 ± 16 | 1 ± 14 |
*P < 0.001.
Nevertheless, despite the within-subject variations in HbA1c and fructosamine and the potential impact of changing and variable control, the G-gaps over time were all significantly intercorrelated (r = 0.71–0.77, P all <0.001); in ANOVA applied to regression for the HbA1c-fructosamine relationships over the three time points, there was no significant difference in the slopes (F = 0.25, P = 0.78, ns) or the intercepts (F = 0.0, P = 0.99, ns); and the G-gap showed no significant within-subject variation (F = 1.58, P = 0.21, ns) over time in three estimations.
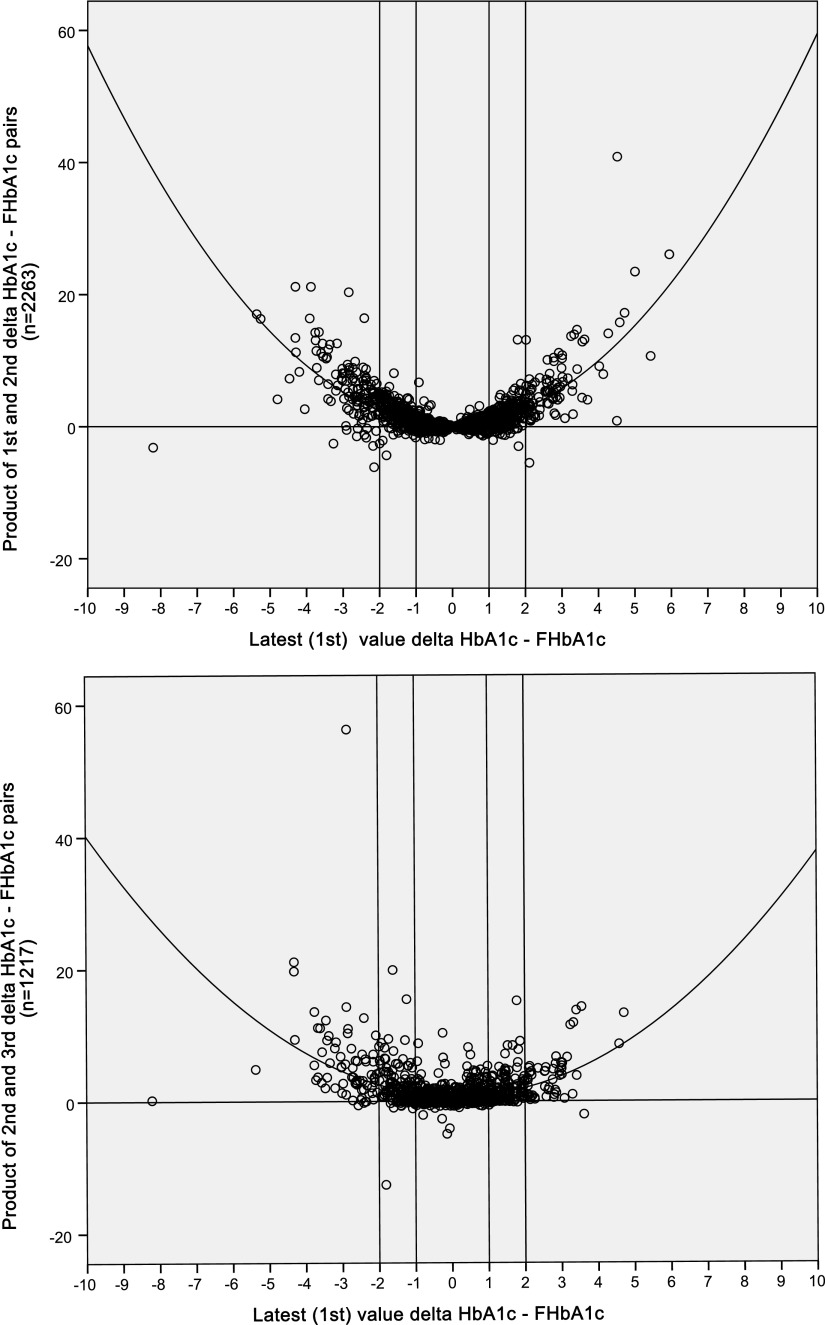
In relationship to the G-gap, it would be the direction of the difference and not its magnitude that would be more pertinent. To further explore evidence of consistency of the direction of the G-gap, Figure 1 demonstrates the product of the two G-gaps for the latest and previous (second) paired HbA1c-fructosamine estimations plotted against the latest G-gap. Discordance in the direction of the G-gap between the two samples is annotated by a negative product (see research design and methods). The value of this product was 1.2 ± 2.6 (−6.1 to 40.9) and a value of negative ≥1 occurred in only 29 (1.3%) of the cohort. The relationship was significantly described by a quadratic curve (n = 2,263, r = 0.72, r2 = 0.52, F = 1,233.6, P < 0.001). The relationship between the latest G-gap and the product of G-gaps for the latest (first) and last (third) was similar (n = 1,217, r = 0.62, r2 = 0.39, F = 390.0, P < 0.001). In both of these latter two analyses, the anchor (latest) HbA1c is a component of the assessed product. Finally, we determined the relationship of the latest HbA1c to the unassociated product of the second and final (third) HbA1c, and this again was similarly associated (n = 1,217, r = 0.45, r2 = 0.21, F = 156.6, P < 0.001).
Figure 1.
Scatter diagrams plotting the G-gap for the first HbA1c-fructosamine pair against product of two G-gaps (first vs. second, top, and second vs. third, bottom).
The distribution of the G-gap was categorized as ≥−1, <−1 to <+1, and ≥+1. The G-gap concordance and discordance between the first two paired samples is shown in Table 4. Discordance was defined as a shift of two categories and occurred in 11 individuals (0.5%), whereas tight (no shift in category) and good (shift in one category) concordance occurred in 71.2 and 28.3%, respectively.
Table 4.
The magnitude of the G-gap and its concordance within individuals (n = 2,263) in two paired HbA1c-fructosamine samples separated by 10 ± 8 months
| G-gap in the second HbA1c-fructosamine pair |
||||
|---|---|---|---|---|
| ≥−1 | <−1 to <+1 | ≥+1 | Total | |
| G-gap in the first HbA1c-fructosamine pair | ||||
| ≥−1 | 279 (12.3%) | 135 (6.0%) | 7 (0.3%) | 421 (18.6%) |
| <−1 to <+1 | 153 (6.8%) | 1,098 (48.5%) | 197 (8.7%) | 1,448 (64.0%) |
| ≥+1 | 4 (0.2%) | 154 (6.8%) | 236 (10.4%) | 394 (17.4%) |
| Total | 436 (19.3%) | 1,387 (61.3%) | 440 (19.4%) | 2,263 (100%) |
Results are the number (%).
CONCLUSIONS
By using multiple simultaneously measured estimations of HbA1c and fructosamine in the same individuals over an extended time period, we have calculated differences in the estimation of prevailing glycemia yielded from the two methods (1,12). This difference is referred to as the G-gap (12,18). We have confirmed that the G-gap is often substantial. The G-gap appears to be weakly associated with fluctuations in HbA1c levels over time, may vary with magnitude of glycemic attainment, and may be influenced by associated demographic and biochemical parameters. However, despite significant changes in HbA1c and fructosamine over time, we now demonstrate that there is no significant within-subject variability in the G-gap and that the direction of the G-gap is consistent. This infers that the underlying mechanisms contributing to the G-gap may also be consistent.
Any comparison of glycated HbA1c and fructosamine to determine the G-gap must recognize that the degree of agreement between fructosamine and HbA1c in various studies may have considerable spread (1,12,20), reflecting glycemic status over greatly different time frames as a consequence of widely differing half lives. Correlations between fructosamine and HbA1c are of the order of r = 0.8, meaning that 64% of the mutual variance is explained; however, the weakness in this methodology would tend to negate or underestimate the consistency of our findings.
Others have reported the potential consistency in the G-gap in populations including people with or without diabetes (2,3,11,12). In a population not known to have diabetes, Yudkin et al. (3) and Gould et al. (11) showed that the discrepancy between the HbA1c relative to fasting and 2-h blood glucose levels in the oral glucose tolerance test remained consistent over a 4.4-year period. Cohen et al. (12) reported the reproducibility of the G-gap in 65 paired HbA1c-fructosamine estimations separated by 23 weeks in a population with diabetes. Hempe et al. (2) used HbA1c and a predicted HbA1c from date-matched mean blood glucose estimations and referred to the discrepancy in the two measures of glycemia as the “hemoglobin glycation index” (HGI). When the HGI was studied in 128 children with type 1 diabetes, it was noted to be consistent over a 2-year study period. Furthermore, a study looking at the G-gap and the HGI in 62 patients with type 1 diabetes has confirmed that the two indices are highly correlated and consistent (21). The possibility of a genetic basis for the G-gap is further supported in a study in twins, in whom the G-gap was suggested to be 69% inheritable (18). Our data support these previous findings and have the largest epidemiologic base, with repeat observations over a longer period of time and is the first exposition of the product method of assessing consistency of direction of the G-gap.
On the basis of our study and available published evidence, we conclude that the G-gap does remain consistent over a time period and that its magnitude can be large. The G-gap is easily assessed. This may provide a platform to further explore underlying genetic and metabolic mechanisms, allow determination of any impact on long-term macro- and microvascular risk consequent on a variation in this key metabolic mechanism, and permit consideration of targeted interventions and new therapeutic approaches.
Acknowledgments
This study was supported by a grant from the South Staffordshire Medical Foundation, U.K.
No potential conflicts of interest relevant to this article were reported.
A.U.N., M.R.H., and B.M.S. researched the data. A.U.N., D.R.M., and B.M.S. contributed to the discussion. A.U.N., B.M.S., and A.N. analyzed the data. A.U.N. and B.M.S. wrote the manuscript. A.U.N., M.R.H., D.R.H., A.N., and B.M.S. edited and reviewed the manuscript.
Parts of this study were presented in abstract form at the 46th annual European Association for the Study of Diabetes meeting, Stockholm, Sweden, 20–24 September 2010 (Diabetologia 2010;53[Suppl. 1]:400).
References
- 1.Macdonald DR, Hanson AM, Holland MR, Singh BM. Clinical impact of variability in HbA1c as assessed by simultaneously measuring fructosamine and use of error grid analysis. Ann Clin Biochem 2008;45:421–425 [DOI] [PubMed] [Google Scholar]
- 2.Hempe JM, Gomez R, McCarter RJ, Jr, Chalew SA. High and low hemoglobin glycation phenotypes in type 1 diabetes: a challenge for interpretation of glycemic control. J Diabetes Complications 2002;16:313–320 [DOI] [PubMed] [Google Scholar]
- 3.Yudkin JS, Forrest RD, Jackson CA, Ryle AJ, Davie S, Gould BJ. Unexplained variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Diabetologia 1990;33:208–215 [DOI] [PubMed] [Google Scholar]
- 4.Jeffcoate SL. Diabetes control and complications: the role of glycated haemoglobin, 25 years on. Diabet Med 2004;21:657–665 [DOI] [PubMed] [Google Scholar]
- 5.Kilpatrick ES. Problems in the assessment of glycaemic control in diabetes mellitus. Diabet Med 1997;14:819–831 [DOI] [PubMed] [Google Scholar]
- 6.Cohen RM, Franco RS, Khera PK, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 2008;112:4284–4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khera PK, Joiner CH, Carruthers A, et al. Evidence for interindividual heterogeneity in the glucose gradient across the human red blood cell membrane and its relationship to hemoglobin glycation. Diabetes 2008;57:2445–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delpierrre G, Vertommen D, Communi D, Rider MH, Van Schaftingen E. Identification of fructosamine residues deglycated by fructosamine-3-kinase in human hemoglobin. J Biol Chem 2004;279:27613–27620 [DOI] [PubMed] [Google Scholar]
- 9.Delpierre G, Veiga-da-Cunha M, Vertommen D, Buysschaert M, Van Schaftingen E. Variability in erythrocyte fructosamine 3-kinase activity in humans correlates with polymorphisms in the FN3K gene and impacts on haemoglobin glycation at specific sites. Diabetes Metab 2006;32:31–39 [DOI] [PubMed] [Google Scholar]
- 10.Mohás M, Kisfali P, Baricza E, et al. A polymorphism within the fructosamine-3-kinase gene is associated with HbA1c Levels and the onset of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2010;118:209–212 [DOI] [PubMed] [Google Scholar]
- 11.Gould BJ, Davie SJ, Yudkin JS. Investigation of the mechanism underlying the variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Clin Chim Acta 1997;260:49–64 [DOI] [PubMed] [Google Scholar]
- 12.Cohen RM, Holmes YR, Chenier TC, Joiner CH. Discordance between HbA1c and fructosamine: evidence for a glycosylation gap and its relation to diabetic nephropathy. Diabetes Care 2003;26:163–167 [DOI] [PubMed] [Google Scholar]
- 13.Dominiczak MH, Orrell JM, Finlay WEI. The effect of hypoalbuminaemia, hyperbilirubinaemia and renal failure on serum fructosamine concentration in non-diabetic individuals. Clin Chim Acta 1989;182:123–129 [DOI] [PubMed] [Google Scholar]
- 14.Ardawi MSM, Nasrat HAN, Bahnassy AA. Fructosamine in obese normal subjects and type 2 diabetes. Diabet Med 1994;11:50–56 [DOI] [PubMed] [Google Scholar]
- 15.McCarter RJ, Hempe JM, Gomez R, Chalew SA. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care 2004;27:1259–1264 [DOI] [PubMed] [Google Scholar]
- 16.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 1988;318:1315–1321 [DOI] [PubMed] [Google Scholar]
- 17.Goh SY, Cooper ME. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab 2008;93:1143–1152 [DOI] [PubMed] [Google Scholar]
- 18.Cohen RM, Snieder H, Lindsell CJ, et al. Evidence for independent heritability of the glycation gap (glycosylation gap) fraction of HbA1c in nondiabetic twins. Diabetes Care 2006;29:1739–1743 [DOI] [PubMed] [Google Scholar]
- 19.Soranzo N, Sanna S, Wheeler E, et al. ; WTCCC. Common variants at 10 genomic loci influence hemoglobin A1(C) levels via glycemic and nonglycemic pathways. Diabetes 2010;59:3229–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narbonne H, Renacco E, Pradel V, Portugal H, Vialettes B. Can fructosamine be a surrogate for HbA1c in evaluating the achievement of therapeutic goals in diabetes? Diabetes Metab 2001;27:598–603 [PubMed] [Google Scholar]
- 21.Chalew SA, McCarter RJ, Thomas J, Thomson JL, Hempe JM. A comparison of the Glycosylation Gap and Haemoglobin Glycation Index in patients with diabetes. J Diabetes Complications 2005;19:218–222 [DOI] [PubMed] [Google Scholar]