Abstract
OBJECTIVE
Diabetic foot infection is the predominant predisposing factor to nontraumatic lower-extremity amputation (LEA), but few studies have investigated which specific risk factors are most associated with LEA. We sought to develop and validate a risk score to aid in the early identification of patients hospitalized for diabetic foot infection who are at highest risk of LEA.
RESEARCH DESIGN AND METHODS
Using a large, clinical research database (CareFusion), we identified patients hospitalized at 97 hospitals in the U.S. between 2003 and 2007 for culture-documented diabetic foot infection. Candidate risk factors for LEA included demographic data, clinical presentation, chronic diseases, and recent previous hospitalization. We fit a logistic regression model using 75% of the population and converted the model coefficients to a numeric risk score. We then validated the score using the remaining 25% of patients.
RESULTS
Among 3,018 eligible patients, 21.4% underwent an LEA. The risk factors most highly associated with LEA (P < 0.0001) were surgical site infection, vasculopathy, previous LEA, and a white blood cell count >11,000 per mm3. The model showed good discrimination (c-statistic 0.76) and excellent calibration (Hosmer-Lemeshow, P = 0.63). The risk score stratified patients into five groups, demonstrating a graded relation to LEA risk (P < 0.0001). The LEA rates (derivation and validation cohorts) were 0% for patients with a score of 0 and ~50% for those with a score of ≥21.
CONCLUSIONS
Using a large, hospitalized population, we developed and validated a risk score that seems to accurately stratify the risk of LEA among patients hospitalized for a diabetic foot infection. This score may help to identify high-risk patients upon admission.
Lower-extremity amputation (LEA) is one of the complications of diabetes that is perhaps most feared by patients with this disease (1) and rightfully so. These LEAs are generally the end point of a characteristic sequence of events: a foot wound, usually a consequence of peripheral neuropathy, becomes infected and does not respond to treatment (2). More than 60% of nontraumatic LEAs in the U.S. occur among people with diabetes, in whom the rate is 6 to 10 times higher than for people without diabetes (3). After a first LEA, up to 50% of patients require another amputation within 3–5 years. Furthermore, the 5-year mortality after LEA is ~50% (4), with the risk considerably higher for diabetic compared with nondiabetic patients (5).
Considering the substantial morbidity and mortality associated with LEA in people with diabetes, the ability to identify which patients hospitalized for a diabetic foot infection are at highest risk for this complication could help clinicians direct special prevention efforts to these individuals. This information also could help identify the baseline risk for LEA among patients admitted to a medical center, allowing fairer comparisons of amputation rates at different centers. Although the factors associated with diabetic people developing a foot ulcer are well defined (1), risk factors for amputation are less clear. Previous studies have identified independent risk factors that include (in approximate order of odds ratio) a history of a foot ulcer (6), limb ischemia, underlying bone involvement, the presence of gangrene (e.g., a higher Wagner grade), deep wounds, older age, elevated inflammatory markers (7), poor glycemic control (8), a specific ethnicity or geographical region (9,10), nephropathy (8), and retinopathy (6). To determine whether we could develop and validate a scoring system to predict the risk of LEA, we examined data from a large group of patients hospitalized with a diabetic foot infection.
RESEARCH DESIGN AND METHODS
We used data from a clinical research database of patients hospitalized at 97 acute-care hospitals in the U.S. that was compiled by CareFusion (Department of Clinical Research, CareFusion, Marlborough, MA). The database includes extensive data in the following categories: clinical (including diagnoses and vital signs); laboratory (e.g., chemistry, hematology, and microbiology); and administrative (e.g., demographics, admission source, length of hospitalization, and discharge status). Eligible patients were those discharged from one of the designated hospitals between 1 January 2003 and 30 June 2007 with a principal diagnosis (ICD-9-CM) of diabetes and a secondary diagnosis indicating skin or soft-tissue infection (including cellulitis, infected ulcer, or surgical-site infection [SSI]) that was culture documented within 48 h of admission. This study was approved by the New England Institutional Review Board Human Subjects Research Committee (Wellesley, MA) and was conducted in compliance with the Health Insurance Portability and Accountability Act.
Study design and statistical analysis
We randomly split the study population into two groups: one for model derivation (75% of the population) and the other for model validation (the remaining 25%). Candidate predictor variables used in the model were demographic data, a history of previous health care–associated infection, clinical presentation, concomitant chronic disease(s), previous LEA, and type of skin or soft-tissue infection (cellulitis, ulcer or other infection, or SSI). Our outcome measure of interest was LEA during the index hospitalization, which was identified by ICD-9 procedure codes (841.1 amputation, toe; 841.2 amputation, foot; 841.3 disarticulation, ankle; 841.4 amputation, Malleoli; 841.5 amputation, knee, below NEC [not elsewhere classified]; 841.6 disarticulation, knee; and 841.7 amputation, knee, above).
Using the derivation cohort, we conducted univariate analyses to determine the proportion of patients with each candidate predictor who underwent an LEA. We then fit a stepwise multivariate logistic regression model to identify independent predictors of LEA and to estimate their relative predictive weights (coefficients). Using previously published methods, we converted the coefficients for the independent predictors into a simplified risk score system (11). Specifically, we calculated the number of points assigned to each variable by dividing its regression coefficient by the smallest coefficient in the model then rounded this quotient to the nearest whole number. We then calculated each subject’s LEA risk score by summing up the points of all variables present on admission. We then validated the risk score system using the remaining 25% of the population.
We assessed model discrimination using the c-statistic, which defines how well a model or prediction rule can discriminate between patients who do and do not have an event and measures how well a clinical prediction rule correctly ranks patients in order by risk. We assessed model calibration using the Hosmer-Lemeshow goodness-of-fit test, which assesses whether the observed and expected event rates match in subgroups of the model population. The test specifically identifies subgroups as the deciles of fitted risk values, and models with similar expected and observed event rates (i.e., a large P value) are considered to be well calibrated. We then used the Cochran-Armitage trending statistic, which modifies the χ2 test to incorporate a suspected ordering, to assess the ability of the risk score system to differentiate low-risk from high-risk patients in a graded response. All analyses were conducted using Statistical Analysis System (version 9.01; SAS Institute, Cary, NC).
RESULTS
Patient characteristics
Among hospitalized patients, 3,018 met our inclusion criteria for a diabetic foot infection, with cellulitis (in 80%) and an infected ulcer (16%) being the most common diagnoses. We used 2,230 patients for the derivation cohort and 788 for the validation cohort; all baseline characteristics were similar between the two cohorts (Table 1). A total of 646 (21.4%) patients underwent an LEA during their index hospitalization; the number (and rate) in the derivation cohort was 463 (20.8%) compared with 183 (23.2%) for the validation cohort. For those patients undergoing an LEA, the median time from admission to amputation was 4 days, with an interquartile range of 2–7 days. A previous LEA of some type was noted in ~27% of patients in the derivation cohort and 26% in the validation cohort.
Table 1.
Characteristics for patients in the derivation and the validation cohorts
| Variable | Derivation cohort | Validation cohort |
|---|---|---|
| n | 2,230 | 788 |
| Mortality (death during hospitalization) | 30 (1.3) | 10 (1.3) |
| Amputation during index hospitalization | 463 (20.8) | 184 (23.3) |
| Median age (years [first through third quartiles]) | 60 (50–71) | 60 (50–71) |
| Male sex | 1,359 (60.9) | 493 (62.6) |
| Previous admission within ≤30 days | 214 (9.6) | 68 (8.6) |
| Transferred from an acute-care hospital | 19 (0.9) | 8 (1.0) |
| Transferred from a skilled nursing facility | 72 (3.2) | 32 (4.1) |
| Race/ethnicity | ||
| White | 1,574 (70.6) | 593 (75.3) |
| Black | 420 (18.8) | 115 (14.6) |
| Other | 236 (10.6) | 80 (10.2) |
| Comorbidities | ||
| Congestive heart failure | 522 (23.4) | 171 (21.7) |
| History of coronary disease | 532 (23.9) | 193 (24.5) |
| Immunosuppressive medication | 73 (3.3) | 33 (4.2) |
| Cancer | 46 (2.1) | 12 (1.5) |
| Peripheral vascular disease | 807 (36.2) | 287 (36.4) |
| Chronic liver disease | 31 (1.4) | 9 (1.1) |
| Chronic lung disease | 232 (10.4) | 97 (12.3) |
| Previous stroke | 234 (10.5) | 74 (9.4) |
| Chronic renal disease | 445 (20.0) | 153 (19.4) |
| History of amputation | 611 (27.4) | 203 (25.8) |
| Renal dialysis treatment | 53 (2.4) | 23 (2.9) |
| Type of skin and soft-tissue infection | ||
| Cellulitis | 1,788 (80.2) | 629 (79.8) |
| Infected ulcer | 360 (16.1) | 129 (16.4) |
| Surgical site | 82 (3.7) | 30 (3.8) |
| Severe infection clinical presentation | ||
| Systolic blood pressure <100 mmHg | 293 (13.1) | 110 (14.0) |
| Temperature <96°F or >100.5°F | 681 (30.5) | 238 (30.2) |
| Pulse <49 or >125 bpm | 128 (5.7) | 40 (5.1) |
| Respiration <10 or >29 breaths per minute | 86 (3.9) | 35 (4.4) |
| Altered mental status | 173 (7.8) | 66 (8.4) |
| Laboratory results | ||
| Albumin <2.8 g/dL | 237 (10.6) | 95 (12.1) |
| Blood urea nitrogen >40 mg/dL | 399 (17.9) | 121 (15.4) |
| Creatinine >3 mg/dL | 176 (7.9) | 65 (8.2) |
| Sodium >145 mEq/dL | 24 (1.1) | 11 (1.4) |
| Total bilirubin >0.8 mg/dL | 206 (9.2) | 89 (11.3) |
| pO2 <55 or >140 or O2 sat <90% | 37 (1.7) | 10 (1.3) |
| Prothrombin time international normalized ratio >1.2 or prothrombin time >14 s | 209 (9.4) | 68 (8.6) |
| Bands on leukocyte differential >13% | 80 (3.6) | 29 (3.7) |
| White blood cell count >11,000 per mm3 | 1,037 (46.5) | 397 (50.4) |
| Glucose on admission (mg/dL) | ||
| ≤70 | 100 (4.5) | 35 (4.4) |
| 71–135 | 331 (14.8) | 124 (15.7) |
| 136–240 | 717 (32.2) | 236 (29.9) |
| >240 | 1,082 (48.5) | 393 (49.9) |
Data are n (%), unless otherwise indicated. The P values for each variable is >0.05, indicating that the derivation and validation cohorts are similar.
For the entire study cohort, the patients who underwent an LEA were significantly older (median age in years [interquartile range] 62 [53–72] vs. 60 [50–71]; P < 0.0001), and their in-hospital mortality rate was significantly higher (2.3 vs. 1.1%; P < 0.05) compared with patients who did not require amputation. The most common finding on culture was a polymicrobial (two or more different microorganisms) infection, which accounted for ~57% of all patients. A detailed accounting of pathogen distribution is shown in Supplementary Appendix A.
Univariate analysis of risk factors associated with LEA
As shown in Table 2, in the derivation cohort the univariate analysis revealed that the following factors were significantly associated with LEA (P < 0.05): older age; male sex; transfer from another hospital or nursing home; previous LEA; coronary, renal, or peripheral vascular disease; low serum albumin; elevated values for white blood cell count, prothrombin time or international normalized ratio, or creatinine; elevated body temperature; the presence of a foot ulcer; and the presence of an SSI.
Table 2.
Univariate analysis of risk factors associated with LEA in the derivation cohort
| Variable | Derivation cohort (% [n LEA/n evaluable]) (n = 2,230) | P* |
|---|---|---|
| n cases | 20.8 (463/2,230) | |
| Mortality (death during hospitalization) | 33.3 (10/30) | 0.1094 |
| Age ≥50 years | 23.1 (394/1,708) | <0.0001 |
| Male sex | 22.3 (303/1,359) | 0.0282 |
| Previous admission ≤30 days | 24.8 (53/214) | 0.1322 |
| Transferred from an acute-care hospital | 63.2 (12/19) | 0.0001 |
| Transferred from a skilled nursing facility | 40.3 (29/72) | 0.0002 |
| Comorbidities | ||
| Congestive heart failure | 24.3 (127/522) | 0.0227 |
| History of coronary disease | 26.1 (139/532) | 0.0006 |
| Immunosuppressive medication | 26.0 (19/73) | 0.3032 |
| Cancer | 23.9 (11/46) | 0.5829 |
| Peripheral vascular disease | 32.2 (260/807) | <0.0001 |
| Chronic liver disease | 25.8 (8/31) | 0.5033 |
| Chronic lung disease | 24.1 (56/232) | 0.1993 |
| Previous stroke | 23.9 (56/234) | 0.2025 |
| Chronic renal disease | 28.8 (128/445) | <0.0001 |
| History of amputation | 31.3 (191/611) | <0.0001 |
| Renal dialysis treatment | 41.5 (22/53) | 0.0005 |
| Type of skin and soft tissue infection | <0.0001 | |
| Cellulitis | 16.9 (302/1,788) | |
| Infected ulcer | 32.8 (118/360) | |
| Surgical site | 52.4 (43/82) | |
| Acute clinical presentation | ||
| Systolic blood pressure <100 mmHg | 24.6 (72/293) | 0.0892 |
| Temperature <96°F or >100.5°F | 27.0 (184/681) | <0.0001 |
| Pulse <49 or >125 bpm | 24.2 (31/128) | 0.3138 |
| Respiration <10 or >29 breaths per minute | 25.6 (22/86) | 0.2776 |
| Altered mental status | 23.7 (41/173) | 0.3293 |
| Laboratory results | ||
| Albumin <2.8 g/dL | 36.7 (87/237) | <0.0001 |
| Blood urea nitrogen >40 mg/dL | 23.6 (94/399) | 0.1341 |
| Creatinine >3 mg/dL | 35.8 (63/176) | <0.0001 |
| Sodium >145 mEq/dL | 25.0 (6/24) | 0.6133 |
| Total bilirubin >0.8 mg/dL | 23.8 (49/206) | 0.2791 |
| pO2 <55 or >140 or O2 sat <90% | 21.6 (8/37) | 0.8398 |
| Prothrombin time international normalized ratio >1.2 or prothrombin time >14 s | 37.8 (79/209) | <0.0001 |
| Bands on leukocyte differential >13% | 26.3 (21/80) | 0.2092 |
| White blood cell count >11,000 per mm3 | 30.3 (314/1,037) | <0.0001 |
| Glucose on admission (mg/dL) | 0.0603 | |
| ≤70 | 12.0 (12/100) | |
| 71–135 | 18.1 (60/331) | |
| 136–240 | 22.0 (158/717) | |
| >240 | 21.5 (233/1,082) |
*Fisher exact test.
Multivariable LEA predictive model
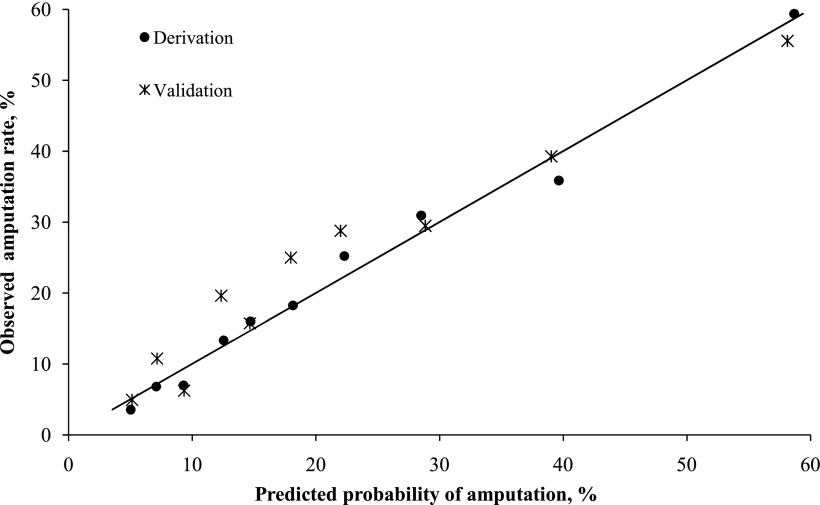
Using stepwise regression analysis, we found 11 independent predictors of LEA (Table 3). The most highly significant (P < 0.0001) were SSI, vasculopathy, previous LEA, and white blood cell count >11,000 per mm3. The predictive model developed using these predictors had very good discrimination (c-statistic 0.76) and excellent calibration between predicted and observed LEA rates (Hosmer-Lemeshow test showing that they did not significantly differ across risk deciles; P = 0.63) (Fig. 1). Patients in the highest risk decile had a predicted probability of LEA of 59.4% and an observed LEA rate of 58.7%, whereas those in the lowest decile had a predicted probability of LEA of 4% and an observed LEA rate of 5%. The predictive model yielded a good calibration when applied to the validation cohort (Hosmer-Lemeshow test; P = 0.33).
Table 3.
Multivariable model and risk score for LEA
| Risk factor | Coefficient | Odds ratio (95% CI) | P | Risk score weight* |
|---|---|---|---|---|
| Chronic renal disease or creatinine >3 mg/dL† | 0.1372 | 1.15 (0.89–1.49) | 0.2998 | 1 |
| Male sex† | 0.1988 | 1.22 (0.97–1.54) | 0.0963 | 1 |
| Temperature <96°F or >100.5°F | 0.2830 | 1.33 (1.05–1.68) | 0.0187 | 2 |
| Age ≥50 years | 0.5477 | 1.73 (1.28–2.34) | 0.0004 | 4 |
| Infected ulcer versus cellulitis | 0.5168 | 1.68 (1.27–2.21) | 0.0002 | 4 |
| History of amputation | 0.5020 | 1.65 (1.29–2.11) | <0.0001 | 4 |
| Albumin <2.8 g/dL | 0.6203 | 1.86 (1.35–2.56) | 0.0001 | 5 |
| History of peripheral vascular disease | 0.7485 | 2.11 (1.66–2.69) | <0.0001 | 5 |
| White blood cell count ≥11 (1,000 per mm3) | 0.9596 | 2.61 (2.07–3.30) | <0.0001 | 7 |
| Surgical site vs. cellulitis | 1.3845 | 3.99 (2.44–6.55) | <0.0001 | 10 |
| Transferred from other acute-care facility | 1.6418 | 5.16 (1.78–15.02) | 0.0026 | 12 |
*We used the method described by Sullivan et al. (11) to calculate the risk score weight: Step 1: divide each regression coefficient by the smallest coefficient in the model (in our model, this is chronic renal disease or creatinine >3 mg/dL). Step 2: round this quotient to the nearest whole number. For example, to calculate the score weight of male sex, we divided its coefficient of 0.1988 by 0.1371, resulting in a quotient of 1.44. Rounding this quotient to its nearest integer resulted in 1 for the score weight of this variable. We then calculated each subject’s overall LEA risk score by summing the points of all variables present on admission.
†We retained these two variables for clinical plausibility despite the fact that they are not statistically significant at the 0.05 level in the model.
Figure 1.
Comparison of the predicted probability of LEA against the observed amputation rate for both derivation and validation cohorts, by decile. The diagonal line represents perfect correlation of predicted and observed LEA rates. Model Hosmer-Lemeshow goodness-of-fit test χ2 = 6.2, P = 0.63 vs. χ2 = 9.2, P = 0.33 for the derivation vs. the validation cohorts, indicating excellent fit of the model.
Simplified risk score strata
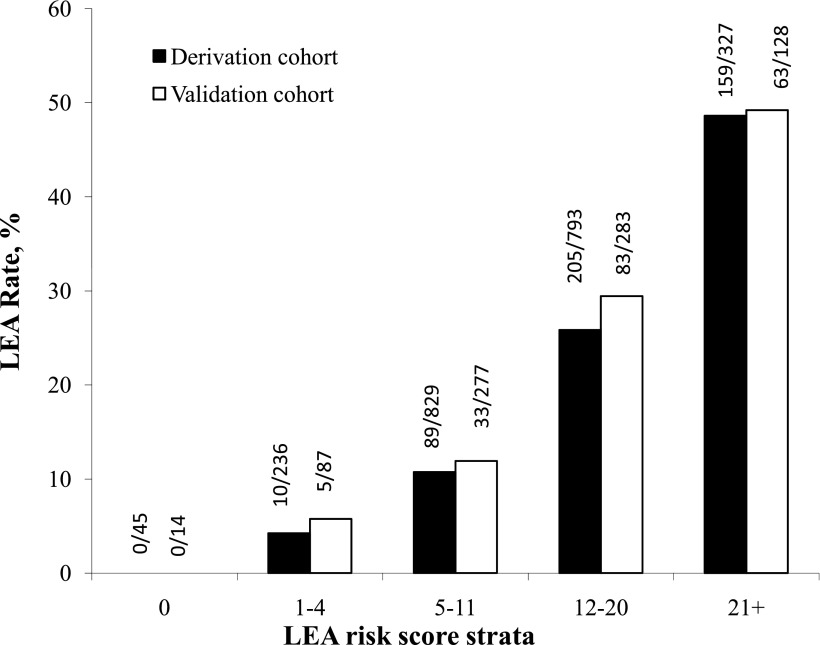
For each patient, we summed all the variables present on admission to create an LEA risk score. We then grouped the risk scores into five risk strata. The use of a five-level risk strata allows easy application of risk stratification for comparisons of outcomes. LEA rates in the derivation and validation cohorts increased significantly by risk score strata (P < 0.0001 by the Cochran-Armitage trending test for both derivation and validation cohorts) (Fig. 2). For the derivation cohort, the risk of LEA for patients aged <50 years and without any of the other 10 factors in the risk score system was essentially zero. In contrast, for those whose score was ≥21, LEA risk was ~50%. The findings in the validation cohort were similar to those in the derivation cohort.
Figure 2.
Observed LEA rates by risk score strata for both derivation and validation cohorts (Cochran-Armitage trending test, P < 0.0001).
CONCLUSIONS
In this large cohort of patients hospitalized for a diabetic foot infection, more than one-fifth required an LEA. In reviewing numerous clinical and laboratory variables present at hospital admission in our derivation cohort, we identified 11 significant independent risk factors for LEA. By rounding the logistic regression coefficients into integers, we developed a simple LEA risk score system with five strata that we demonstrated was highly predictive of the risk for LEA. Using the patients in the validation cohort, we were then able to demonstrate that this risk score was indeed valid and well calibrated.
Most of the factors included in our risk score have been reported as risks for LEA in smaller, previously published studies (7,12,13). The presence of infection and peripheral vascular disease are the most powerful predictors. Most patients with diabetic foot ulcers do not have a fever or leukocytosis (14), which define a severe infection according to the Infectious Diseases Society of America and the International Working Group on the Diabetic Foot criteria. These severe diabetic foot infections are associated with a greater risk of LEA than those of mild or moderate severity. Previous studies (15,17) also have identified renal insufficiency as being associated with an increased risk of amputations. Other studies have identified increasing age (18), male sex (16), and hypoalbuminemia (19) as risks for LEA. Likewise, a previous LEA is a strong risk factor predicting the need for another amputation (12). In none of these previous studies, however, did the authors attempt to construct a scoring system to predict amputation risk for both men and women.
In a previous study (20), we showed that patients with SSIs who were transferred from another acute-care facility had worse clinical and economic outcomes, perhaps because patients with infections of greater severity are more likely to be transferred to hospitals with more intensive resources or greater expertise. A recent meta-analysis (21) demonstrated a direct association between hyperglycemia (as measured by hemoglobin A1c) and LEA. Unfortunately, we did not have hemoglobin A1c values on most of our patients, but we did note a nonsignificant trend toward higher amputation rates in those with increased blood glucose levels on admission. All of our patients had an infection; therefore, that variable was not among those included in our scoring system. It is noteworthy that the type of infection was associated with amputation risk, with SSIs at the highest risk, followed by infected ulcers, when compared with cellulitis. Our assumption is that these SSIs may be associated with failed lower-extremity bypass procedures. We found no other studies that investigated the risk of adverse outcomes in patients with an infected ulcer compared with cellulitis or SSIs. We did find other studies (7,14,22,23) that reported that deep infections (especially those involving bone) and necrotizing infections more often resulted in amputations.
The simplified five risk strata that we devised correlated strongly with LEA rates. This may have important clinical implications on how to allocate resources. In particular, a patient with a low score may need fewer medical resources than a patient with a high LEA risk score. At the other extreme, to try to avoid the tragedy of amputation, health care providers should concentrate efforts on a patient with a risk score of >21, who has a 50% chance of an LEA. Our finding that patients transferred from another acute-care hospital had the highest odds ratio for LEA highlights the need of risk adjustment to appropriately evaluate outcomes for hospitals treating the most severe patients. Because LEA rates are sometimes used to compare quality of care for patients with diabetic foot complications, our risk adjustment score could be used to ensure that centers treating higher-risk patients are not unfairly penalized. Furthermore, although this has not been tested, the score might be helpful to clinicians in deciding which patients with diabetic foot infections may need to be hospitalized. The LEA risk score system has the benefit of being simple to use; each of the risk factors is readily available, usually at the time of admission or soon afterward.
Our study is limited by the fact that our analysis was retrospective, and, although fairly inclusive, we could have missed potentially significant factors. For example, the individual reasons for amputation and whether amputation was elective or urgent were not captured in the database. One major risk factor that we did not capture that could have an effect on our risk score is a history of a previous lower-extremity revascularization procedure, which was a significant factor in other reports (24,25). Selection bias is another potential limitation when using administrative data to identify patients with skin or soft-tissue infections. To minimize potential bias related to the use of ICD-9 coding, we limited our study to culture-confirmed infections.
In conclusion, we used a large clinical database to develop and validate a risk score that seems to accurately stratify LEA risk among patients hospitalized for a diabetic foot infection. This score may help clinicians identify patients at highest risk of LEA upon admission. Once patient identification is achieved, methods to reduce the risk can be investigated. We would like to see our risk score validated prospectively, including in patients treated on an outpatient basis.
Supplementary Material
Acknowledgments
This project was funded in part by the Ortho-McNeil Janssen Scientific Affairs (Raritan, NJ). B.A.L. serves as a consultant to the Ortho-McNeil Janssen Scientific Affairs. X.S., R.S.J., K.G.D., and Y.P.T. are employees of CareFusion. No other potential conflicts of interest relevant to this article were reported.
B.A.L. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. J.A.W. contributed to discussion and wrote, reviewed, and edited the manuscript. X.S., R.S.J., and K.G.D. researched data, contributed to discussion, and reviewed and edited the manuscript. Y.P.T. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript.
Parts of this study were presented in abstract form at the 47th Annual Meeting of the Infectious Diseases Society of America, Philadelphia, Pennsylvania, 29 October to 1 November 2009.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0331/-/DC1.
References
- 1.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–228 [DOI] [PubMed] [Google Scholar]
- 2.Reiber GE, Pecoraro RE, Koepsell TD. Risk factors for amputation in patients with diabetes mellitus: a case-control study. Ann Intern Med 1992;117:97–105 [DOI] [PubMed] [Google Scholar]
- 3.Icks A, Haastert B, Trautner C, Giani G, Glaeske G, Hoffmann F. Incidence of lower-limb amputations in the diabetic compared to the non-diabetic population. Findings from nationwide insurance data, Germany, 2005-2007. Exp Clin Endocrinol Diabetes 2009;117:500–504 [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DG, Wrobel J, Robbins JM. Guest editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J 2007;4:286–287 [DOI] [PubMed] [Google Scholar]
- 5.Schofield CJ, Libby G, Brennan GM, MacAlpine RR, Morris AD, Leese GP; DARTS/MEMO Collaboration. Mortality and hospitalization in patients after amputation: a comparison between patients with and without diabetes. Diabetes Care 2006;29:2252–2256 [DOI] [PubMed] [Google Scholar]
- 6.Krittiyawong S, Ngarmukos C, Benjasuratwong Y, et al. Thailand diabetes registry project: prevalence and risk factors associated with lower extremity amputation in Thai diabetics. J Med Assoc Thai 2006;89(Suppl. 1):S43–S48 [PubMed] [Google Scholar]
- 7.Yesil S, Akinci B, Yener S, et al. Predictors of amputation in diabetics with foot ulcer: single center experience in a large Turkish cohort. Hormones (Athens) 2009;8:286–295 [DOI] [PubMed] [Google Scholar]
- 8.Shojaiefard A, Khorgami Z, Larijani B. Independent risk factors for amputation in diabetic foot. Int J Diabetes Dev Ctries 2008;28:32–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaturvedi N, Stevens LK, Fuller JH, Lee ET, Lu M; WHO Multinational Study of Vascular Disease in Diabetes. Risk factors, ethnic differences and mortality associated with lower-extremity gangrene and amputation in diabetes. Diabetologia 2001;44(Suppl. 2):S65–S71 [DOI] [PubMed] [Google Scholar]
- 10.Gonsalves WC, Gessey ME, Mainous AG, 3rd, Tilley BC. A study of lower extremity amputation rates in older diabetic South Carolinians. J S C Med Assoc 2007;103:4–7 [PubMed] [Google Scholar]
- 11.Sullivan LM, Massaro JM, D’Agostino RB, Sr. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med 2004;23:1631–1660 [DOI] [PubMed] [Google Scholar]
- 12.Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower-extremity amputation in diabetes: the independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care 1999;22:1029–1035 [DOI] [PubMed] [Google Scholar]
- 13.Nather A, Bee CS, Huak CY, et al. Epidemiology of diabetic foot problems and predictive factors for limb loss. J Diabetes Complications 2008;22:77–82 [DOI] [PubMed] [Google Scholar]
- 14.Wang PH, Yu DM, Chu YJ, et al. Research on the clinical features and effective factors of 249 diabetic patients with deep foot infection. Zhonghua Yi Xue Za Zhi 2007;87:1828–1831 [in Chinese] [PubMed] [Google Scholar]
- 15.Akinci B, Yesil S, Bayraktar F, et al. The effect of creatinine clearance on the short-term outcome of neuropathic diabetic foot ulcers. Prim Care Diabetes 2010;4:181–185 [DOI] [PubMed] [Google Scholar]
- 16.Markowitz JS, Gutterman EM, Magee G, Margolis DJ. Risk of amputation in patients with diabetic foot ulcers: a claims-based study. Wound Repair Regen 2006;14:11–17 [DOI] [PubMed] [Google Scholar]
- 17.Ndip A, Rutter MK, Vileikyte L, et al. Dialysis treatment is an independent risk factor for foot ulceration in patients with diabetes and stage 4 or 5 chronic kidney disease. Diabetes Care 2010;33:1811–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skoutas D, Papanas N, Georgiadis GS, et al. Risk factors for ipsilateral reamputation in patients with diabetic foot lesions. Int J Low Extrem Wounds 2009;8:69–74 [DOI] [PubMed] [Google Scholar]
- 19.Flores Rivera AR. Risk factors for amputation in diabetic patients: a case-control study. Arch Med Res 1998;29:179–184 [PubMed] [Google Scholar]
- 20.Weigelt JA, Lipsky BA, Tabak YP, Derby KG, Kim M, Gupta V. Surgical site infections: causative pathogens and associated outcomes. Am J Infect Control 2010;38:112–120 [DOI] [PubMed] [Google Scholar]
- 21.Adler AI, Erqou S, Lima TA, Robinson AH. Association between glycated haemoglobin and the risk of lower extremity amputation in patients with diabetes mellitus—review and meta-analysis. Diabetologia 2010;53:840–849 [DOI] [PubMed] [Google Scholar]
- 22.Aragón-Sánchez J. Treatment of diabetic foot osteomyelitis: a surgical critique. Int J Low Extrem Wounds 2010;9:37–59 [DOI] [PubMed] [Google Scholar]
- 23.Eneroth M, Larsson J, Apelqvist J. Deep foot infections in patients with diabetes and foot ulcer: an entity with different characteristics, treatments, and prognosis. J Diabetes Complications 1999;13:254–263 [DOI] [PubMed] [Google Scholar]
- 24.Cruz CP, Eidt JF, Capps C, Kirtley L, Moursi MM. Major lower extremity amputations at a Veterans Affairs hospital. Am J Surg 2003;186:449–454 [DOI] [PubMed] [Google Scholar]
- 25.Taylor SM, York JW, Cull DL, Kalbaugh CA, Cass AL, Langan EM, 3rd. Clinical success using patient-oriented outcome measures after lower extremity bypass and endovascular intervention for ischemic tissue loss. J Vasc Surg 2009;50:534–541, discussion 541 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.