Abstract
OBJECTIVE
The definition of obesity (BMI ≥30 kg/m2), a key risk factor of diabetes, is widely used in white populations; however, its appropriateness in nonwhite populations has been questioned. We compared the incidence rates of diabetes across white, South Asian, Chinese, and black populations and identified equivalent ethnic-specific BMI cutoff values for assessing diabetes risk.
RESEARCH DESIGN AND METHODS
We conducted a multiethnic cohort study of 59,824 nondiabetic adults aged ≥30 years living in Ontario, Canada. Subjects were identified from Statistics Canada’s population health surveys and followed for up to 12.8 years for diabetes incidence using record linkages to multiple health administrative databases.
RESULTS
The median duration of follow-up was 6 years. After adjusting for age, sex, sociodemographic characteristics, and BMI, the risk of diabetes was significantly higher among South Asian (hazard ratio 3.40, P < 0.001), black (1.99, P < 0.001), and Chinese (1.87, P = 0.002) subjects than among white subjects. The median age at diagnosis was lowest among South Asian (aged 49 years) subjects, followed by Chinese (aged 55 years), black (aged 57 years), and white (aged 58 years) subjects. For the equivalent incidence rate of diabetes at a BMI of 30 kg/m2 in white subjects, the BMI cutoff value was 24 kg/m2 in South Asian, 25 kg/m2 in Chinese, and 26 kg/m2 in black subjects.
CONCLUSIONS
South Asian, Chinese, and black subjects developed diabetes at a higher rate, at an earlier age, and at lower ranges of BMI than their white counterparts. Our findings highlight the need for designing ethnically tailored prevention strategies and for lowering current targets for ideal body weight for nonwhite populations.
Type 2 diabetes is a growing epidemic. There currently are an estimated 285 million people with known type 2 diabetes (henceforth “diabetes”) worldwide; this number is projected to rise to ~438 million by 2030, with a disproportionate burden expected in developing countries, particularly India and China, and among ethnic minorities living in wealthier nations (1). Earlier cross-sectional studies (2–4) have reported ethnic differences in the prevalence of diabetes; however, to date there have been no longitudinal studies comparing the incidence rate and age at diagnosis of diabetes across the world’s four major ethnic groups (white, South Asian, Chinese, and black populations) and among individuals from these four groups living in the same social macroenvironment.
Although the definition of obesity (BMI ≥30 kg/m2), a key risk factor of diabetes, has been validated in white populations (5), its appropriateness in Asian populations has been questioned (6). Recognizing this, a World Health Organization (WHO) expert panel was convened in 2002 to discuss the potential for developing Asian-specific BMI cutoff points for obesity (7). The consultation concluded that with the data available at the time, there was no clear BMI cutoff point that would be universally applicable to Asians and that the prespecified BMI ranges would be retained (i.e., underweight <18.5 kg/m2, normal 18.5 to <25 kg/m2, overweight 25 to <30 kg/m2, and obese ≥30 kg/m2) for assessing the risk of obesity-related chronic diseases. Nevertheless, the WHO expert panel recommended potential BMI categories for public health action in people of Asian descent (i.e., underweight <18.5 kg/m2, increasing but acceptable risk 18.5 to <23 kg/m2, increased risk 23 to <27.5 kg/m2, and high risk ≥27.5 kg/m2) (7). The WHO panel also emphasized the need for longitudinal studies using disease outcomes data to better understand the relationship between BMI and obesity-related diseases and to derive and validate ethnic-specific BMI cutoff points. Because of these recommendations, several studies have attempted to redefine obesity for Asians. A systematic review (8) of these studies found that, although most studies were in favor of lowering the BMI cutoff point for obesity in Asians, the majority of these studies used cross-sectional data and some did not report health outcomes.
The two main objectives of this multiethnic cohort study were 1) to compare the incidence and age at diagnosis of diabetes across white, South Asian, Chinese, and black subjects living in Ontario, Canada, one of the world’s most ethnically diverse regions; and 2) to derive ethnically appropriate population-based BMI cutoff values for obesity in assessing diabetes risk using clinically ascertained diabetes. A cutoff point of a BMI of 30 kg/m2 was chosen because it represents the current standard for obesity, a key risk factor of diabetes.
RESEARCH DESIGN AND METHODS
The study cohort included individuals drawn from Statistics Canada’s 1996 National Population Health Survey (NPHS) and the Canadian Community Health Survey (CCHS) cycles 1.1 (2001), 2.1 (2003), and 3.1 (2005) (9). The NPHS/CCHS are periodic government–funded surveys that provide population-based estimates of the health status of Canadians. These surveys were conducted in over 25 languages and had response rates between 75.1 and 94.4%.
Our study included survey participants living in Ontario who were aged ≥30 years at the time of survey and who identified themselves as white, South Asian (i.e., of Indian, Pakistani, Bangladeshi, or Sri Lankan origin), Chinese, or black. The surveys collected information on various sociodemographic characteristics (e.g., income, education) and risk factors (e.g., BMI from self-reported height and weight, current smoking, and inadequate physical activity). Additional details on these variables are published elsewhere (4).
Data linkages
Each subject’s survey data were anonymously linked to health-administrative databases using their encrypted 10-digit health card number. Self-reported data on prevalent comorbidities were augmented using information found in administrative records (i.e., the Ontario Hypertension Database for hypertension [10] and the hospital discharge abstract database of the Canadian Institute for Health Information for heart disease [ICD-9 codes 410, 411, 413, and 428 or ICD-10 codes I20–I22 and I50], stroke [ICD-9 codes 430–438 or ICD-10 codes I60–I69], and cancer [ICD-9 code 14 or ICD-10 code C]). Records also were linked to the Ontario Registered Persons Database for vital status.
Subjects with prevalent diabetes, heart disease, stroke, or cancer, identified either by self-report or using administrative health records, and those with missing values for important risk factors (i.e., BMI, income adequacy, and urban/rural dwelling) were excluded from the study. The percentage of observations excluded as a result of missing BMI values were similar across ethnic groups (i.e., 2.5% white, 2.0% South Asian, 2.9% Chinese, and 3.5% black).
Incident cases of diabetes were ascertained by means of record linkages with the population-based Ontario Diabetes Database. The Ontario Diabetes Database is created using a validated administrative data algorithm that identifies diabetes from multiple administrative data sources with high sensitivity (86%) and specificity (97%) (11). Subjects were followed from the survey interview date to the diabetes diagnosis date, death date, or 31 March 2009, whichever occurred first.
Statistical analysis
Cumulative incidence curves were constructed using fitted Cox proportional hazards models to derive unadjusted and adjusted marginal estimates of cumulative incidence of diabetes for each ethnic group (12). From the fitted regression model, we estimated the predicted probabilities of diabetes within the 12.8 years of follow-up, assuming that all subjects were white. The mean of these probabilities across the entire sample represented the marginal probability of an event occurring within the study period, assuming everyone was white. This method was repeated for South Asian, Chinese, and black subjects.
Multivariate Cox proportional hazards regression methods were used to assess whether ethnicity was an independent predictor of incident diabetes. Sensitivity analyses were performed in which models were additionally adjusted for the highest level of education in the household, number of years lived in Canada, age-sex interaction, and age-BMI interaction. Moreover, we calculated hazard ratios (HRs) adjusted for covariates expressed as continuous rather than categorical measures.
To determine ethnic-specific BMI cutoff points, we performed a Poisson regression model in which the effect of BMI on diabetes incidence was modeled using restricted cubic splines with four knots (13). The incidence of diabetes was the dependent variable (the offset variable was person-years of follow-up), whereas BMI, ethnicity, BMI-ethnicity interaction, age, age-BMI interaction, sex, survey year, income adequacy, and urban/rural dwelling were the independent variables. We calculated the predicted incidence rate of diabetes for white subjects at a BMI of 30.0 kg/m2 and identified the corresponding BMI values for the other three ethnic groups. A sensitivity analysis using five knots to model the cubic splines also was performed.
Statistical analyses were performed using SAS version 9.2 statistical software (SAS Institute, Inc., Cary, NC). All analyses were weighted by Statistics Canada’s sample weights to account for the complex survey sampling design and to allow for estimates to be generalizable to the overall Ontario population. Bootstrap methods, using 500 sets of bootstrap-sampling weights and appropriate z tests, were used to test statistical significance (14). A two-sided P value <0.05 was considered statistically significant.
Ethics approval for this study was obtained from the research ethics board at Sunnybrook Health Sciences Centre. Statistics Canada obtained informed consent from all study participants for administrative data linkages.
RESULTS
Study participants
Table 1 displays the baseline characteristics of the study cohort of 57,210 white, 1,001 South Asian, 866 Chinese, and 747 black participants living in Ontario. At baseline, the median BMI was lowest among Chinese participants (Supplementary Fig. 1). The median follow-up time was 6 years for each ethnic group.
Table 1.
Baseline characteristics of the study cohort by ethnic group, Ontario, Canada, 1996–2005*
| White | South Asian | Chinese | Black | |
|---|---|---|---|---|
| n | 57,210 | 1,001 | 866 | 747 |
| Sociodemographic characteristics | ||||
| Age at baseline (years) | ||||
| Mean | 48.5 | 43.7 | 44.5 | 44.5 |
| Median (interquartile range) | 46 (38–57) | 42 (36–49) | 42 (36–50) | 42 (36–51) |
| Male sex | 49.1 | 56.8 | 51.0 | 50.1 |
| Year of interview | ||||
| 1996 | 22.2 | 14.4 | 11.2 | 19.6 |
| 2001 | 27.1 | 26.9 | 27.1 | 28.5 |
| 2003 | 26.9 | 28.0 | 33.5 | 26.6 |
| 2005 | 23.9 | 30.7 | 28.2 | 25.3 |
| Urban dwelling | 67.4 | 84.1 | 88.1 | 78.9 |
| Income adequacy† | ||||
| 1 (lowest) | 6.2 | 13.2 | 6.3 | 14.9 |
| 2 (lower-mid) | 16.4 | 26.4 | 26.9 | 27.6 |
| 3 (mid-higher) | 35.5 | 35.9 | 34.7 | 33.9 |
| 4 (highest) | 42.0 | 24.5 | 32.1 | 23.6 |
| Individual income (CAN$) (mean) | 43,950 | 33,402 | 33,060 | 33,572 |
| Highest level of education in household | ||||
| Less than secondary school diploma | 7.7 | 4.9 | 4.1 | 7.5 |
| Secondary school diploma | 13.2 | 10.3 | 10.8 | 11.2 |
| Some postsecondary | 5.8 | 3.4 | 3.0 | 9.9 |
| Postgraduate degree | 73.3 | 81.4 | 82.1 | 71.4 |
| Immigrant type, number of years in Canada | ||||
| Immigrant, <10 | 2.5 | 41.5 | 35.0 | 18.3 |
| Immigrant, 10 to <30 | 6.3 | 47.4 | 48.3 | 48.6 |
| Immigrant, ≥30 | 12.8 | 8.1 | 10.1 | 23.9 |
| Nonimmigrant | 78.5 | 3.0 | 6.6 | 9.3 |
| Risk factors | ||||
| BMI (in kg/m2) | ||||
| Mean | 26.1 | 24.6 | 22.6 | 26.1 |
| Median (interquartile range) | 26 (23–28) | 24 (22–27) | 22 (20–24) | 26 (23–28) |
| Obesity (BMI ≥30 kg/m2) | 16.5 | 6.9 | 2.2 | 14.7 |
| Currently smoking | 26.4 | 11.9 | 11.3 | 14.9 |
| History of hypertension | 20.4 | 17.1 | 15.2 | 20.8 |
| Inadequate physical activity† | 65.0 | 78.8 | 78.9 | 70.7 |
| Inadequate fruit and vegetable consumption† | 21.7 | 16.4 | 26.8 | 22.7 |
| Psychosocial stress† | 26.4 | 23.2 | 18.9 | 21.5 |
| Alcohol consumption (drinks per week) | ||||
| <3 | 61.0 | 84.8 | 92.2 | 85.4 |
| 3–14 | 33.3 | 14.1 | 7.3 | 13.7 |
| >14 | 5.7 | 1.1 | 0.6 | 0.8 |
| Number of alcoholic drinks per week | ||||
| Mean | 3.9 | 1.1 | 0.7 | 1.3 |
| Median (interquartile range) | 1 (0–5) | 0 (0–1) | 0 (0–0) | 0 (0–1) |
Data are percentages, unless otherwise indicated. Data were derived from the Ontario components of Statistics Canada’s NPHS and CCHS, 1996–2005.
*The study cohort included 57,210 white, 1,001 South Asian, 866 Chinese, and 747 black participants living in Ontario. All estimates were weighted by the survey sample weight to allow for estimates to be generalizable to the overall Ontario population.
†Definitions: income adequacy, based on annual household income and the number of people in the household [18]; inadequate physical activity, ≤15 min/day; inadequate fruit and vegetable intake, less than three times per day; psychosocial stress, feeling “extremely” or “quite a bit” versus “not at all,” “not very,” or “a bit” stressed most days.
Diabetes incidence
Over the 12.8-year study period, 4,076 subjects were diagnosed with diabetes. The crude incidence rate of diabetes (per 1,000 person-years) was highest among South Asian subjects (20.8 [95% CI 16.1–25.4]), followed by black (16.3 [11.8–21.6]), white (9.5 [9.1–9.9]), and Chinese (9.3 [5.8–13.1]) subjects (Table 2). Diabetes was more common among men than women in most ethnic groups, with the exception of the black group in which women had a 33% higher rate of diabetes than men. These differences, however, were statistically nonsignificant. There was a clear inverse association between income adequacy and diabetes incidence among white subjects; however, the gradient was less clear among South Asian and black subjects and was reversed among Chinese subjects. Across all groups, a higher level of household education seemed to be protective against the risk of diabetes. Among immigrants, the incidence of diabetes seemed to increase with longer duration of residence in Canada, which is consistent with the hypothesis that immigrant health declines with increasing duration of exposure to Western culture (15).
Table 2.
Ethnic-specific incidence rates (per 1,000 person-years) of diabetes for subjects aged ≥30 years, overall and by sociodemographic characteristics and categories of BMI*
| Ethnic group (rate [95% CI])† |
||||
|---|---|---|---|---|
| White (n = 57,210) | South Asian (n = 1,001) | Chinese (n = 866) | Black (n = 747) | |
| Overall incidence of diabetes | 9.5 (9.1–9.9) | 20.8 (16.1–25.4) | 9.3 (5.8–13.1) | 16.3 (11.8–21.6) |
| Sociodemographic characteristics | ||||
| Age at baseline (years) | ||||
| 30 to <45 | 4.8 (4.4–5.3) | 16.3 (11.7–21.8) | 4.7 (1.7–7.6) | 7.2 (3.8–10.8) |
| 45 to <65 | 12.9 (12.0–13.6) | 28.1 (18.1–40.4) | 13.1 (5.9–21.6) | 30.5 (20.1–42.7) |
| ≥65 | 17.5 (16.1–19.0) | 25.5 (9.8–47.7) | 36.4 (14.0–68.5) | 32.7 (8.2–83.5) |
| Sex | ||||
| Male | 10.8 (10.1–11.4) | 24.0 (16.6–31.8) | 10.3 (5.6–16.1) | 14.1(8.3–20.7) |
| Female | 8.4 (7.8–8.9) | 16.8 (10.9–23.2) | 8.1 (3.5–13.4) | 18.8 (11.3–26.2) |
| Income adequacy | ||||
| 1 (lowest) | 12.9 (11.3–14.5) | 29.4 (14.4–46.9) | 4.4 (0.0–11.7) | 33.6 (18.0–54.9) |
| 2 (lower-mid) | 11.8 (10.8–12.9) | 19.5 (12.2–28.4) | 7.0 (2.8–11.5) | 6.9 (2.2–13.3) |
| 3 (mid-higher) | 9.5 (8.8–10.3) | 16.4 (9.4–24.8) | 10.4 (3.9–18.9) | 21.0 (12.8–31.3) |
| 4 (highest) | 7.9 (7.2–8.7) | 24.4 (14.2–35.6) | 11.1 (5.1–17.1) | 9.9 (3.6–17.7) |
| Highest level of education in household | ||||
| At most secondary school diploma | 13.6 (12.2–14.9) | 25.3 (8.3–40.9) | 19.9 (6.1–36.0) | 22.3 (8.1–41.8) |
| At least some postsecondary | 8.7 (8.0–9.3) | 18.5 (13.0–24.5) | 9.6 (5.2–14.5) | 14.2 (8.2–21.3) |
| Rural or urban dwelling | ||||
| Rural | 9.4 (8.8–10.0) | 18.8 (11.3–28.2) | 4.5 (1.3–9.3) | 17.2 (9.2–26.2) |
| Urban | 9.7 (9.1–10.3) | 21.5 (16.0–27.1) | 10.5 (6.0–15.0) | 15.9 (10.7–21.7) |
| Immigrant status | ||||
| Nonimmigrant | 8.9 (8.5–9.4) | 30.8 (3.4–79.5) | 8.6 (0.9–21.7) | 8.1 (0.7–19.4) |
| Immigrant (born outside of Canada) | 11.7 (10.4–13.0) | 20.5 (15.9–25.1) | 9.4 (5.8–13.5) | 17.2 (12.7–22.8) |
| Number of years in Canada (among immigrants) | ||||
| <10 | 4.0 (2.2–6.4) | 17.5 (11.3–25.5) | 2.6 (0.7–5.0) | 14.3 (5.5–26.2) |
| 10 to <30 | 8.9 (6.8–11.0) | 22.6 (14.8–30.2) | 10.7 (5.4–16.6) | 17.4 (10.7–25.3) |
| ≥30 | 14.9 (13.2–16.7) | 23.8 (10.1–41.8) | 29.9 (8.8–57.4) | 19.4 (8.5–34.3) |
| BMI categories (kg/m2) | ||||
| WHO-defined BMI categories for the general population‡ | ||||
| <18.5, underweight | 3.3 (1.2–5.6) | 1.8 (0.0–7.3) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| 18.5 to <25, normal | 4.1 (3.7–4.5) | 12.1 (7.8–16.9) | 6.8 (3.3–10.6) | 8.4 (3.6–14.6) |
| 25 to <30, overweight | 10.0 (9.3–10.8) | 27.7 (17.1–38.7) | 19.5 (9.3–34.2) | 18.6 (10.6–27.1) |
| ≥30, obese | 25.6 (23.5–27.4) | 76.6 (49.0–110.3) | 79.6 (17.6–157.7) | 38.0 (18.0–61.8) |
| WHO-defined BMI categories for Asian populations‡ | ||||
| 18.5 to <23, increasing but acceptable risk | 3.1 (2.7–3.6) | 11.6 (6.0–17.8) | 3.7 (1.1–6.4) | 7.3 (1.1–16.9) |
| 23 to <27.5, increased risk | 6.9 (6.4–7.6) | 20.2 (13.1–27.8) | 16.8 (8.4–25.2) | 14.1 (8.6–20.2) |
| ≥27.5, high risk | 19.0 (17.9–20.0) | 44.9 (28.1–63.9) | 30.9 (10.9–52.6) | 28.9 (17.0–42.9) |
Data were derived from the Ontario components of Statistics Canada’s NPHS and CCHS, 1996–2005.
*The study cohort included 57,210 white, 1,001 South Asian, 866 Chinese, and 747 black participants living in Ontario. All estimates were weighted by the survey sample weight to allow for estimates to be generalizable to the overall Ontario population.
†Bootstrap methods were used to derive 95% CIs.
‡BMI categories were those defined by the WHO expert panel convened in 2002 (7).
There was a strong gradient in the risk of diabetes with increasing BMI (Table 2). The relative rate of diabetes for people in the obese (BMI ≥30 kg/m2) versus the normal (BMI 18.5 to <25 kg/m2) BMI group varied greatly by ethnicity (black: 4.5, white: 6.2, South Asian: 6.3, and Chinese: 11.7). Moreover, even at BMI ranges that are thought to confer increasing but acceptable risk for Asian populations (7), the incidence rate of diabetes (per 1,000 person-years) was significantly higher among South Asian subjects (11.6 [95% CI 6.0–17.8]) than among white subjects (3.1 [2.7–3.6]).
The cumulative incidence curves for diabetes in each ethnic group are displayed in Supplementary Fig. 2. After controlling for BMI, current smoking, hypertension, age, sex, and other sociodemographic characteristics, all three nonwhite groups had a higher risk of diabetes than the white group.
HRs
The proportional hazards assumption was verified by confirming that ethnicity did not have a significant time-varying covariate effect in the proportional hazards model. HRs adjusted for age, sex, and sociodemographic characteristics revealed a significantly elevated risk of diabetes for South Asian and black subjects compared with white subjects (Table 3). After additionally controlling for BMI, the HRs for all three nonwhite ethnic groups were significantly higher than their white counterparts (South Asian subjects 3.40 [95% CI 2.58–4.24], P < 0.001; black subjects 1.99 [1.39–2.71], P < 0.001; and Chinese subjects 1.87 [1.16–2.60], P = 0.002). Estimates remained significantly elevated after additional adjustment for smoking, hypertension, diet, exercise, alcohol consumption, and psychosocial stress and for all sensitivity analyses (Table 3).
Table 3.
Cox proportional HRs for incident diabetes, by ethnicity and sex*
| White |
South Asian |
Chinese | Black |
||||
|---|---|---|---|---|---|---|---|
| HR | HR (95% CI)† | P† | HR (95% CI)† | P† | HR (95% CI)† | P† | |
| Overall | n = 57,210 | n = 1,001 | n = 866 | n = 747 | |||
| Main models | |||||||
| Unadjusted | 1 (reference) | 2.23 (1.72–2.78) | <0.001 | 0.99 (0.62–1.41) | 0.97 | 1.72 (1.27–2.27) | <0.001 |
| Age and sex | 1 (reference) | 2.63 (1.99–3.27) | <0.001 | 1.15 (0.73–1.68) | 0.48 | 2.04 (1.50–2.68) | <0.001 |
| Survey year, income adequacy, urban vs. rural dwelling | 1 (reference) | 2.46 (1.88–3.08) | <0.001 | 1.10 (0.68–1.60) | 0.65 | 1.93 (1.43–2.50) | <0.001 |
| BMI | 1 (reference) | 3.40 (2.58–4.24) | <0.001 | 1.87 (1.16–2.60) | 0.002 | 1.99 (1.39–2.71) | <0.001 |
| Inadequate physical activity, inadequate fruit and vegetable consumption, psychosocial stress, alcohol consumption‡ | 1 (reference) | 3.56 (2.51–4.76) | <0.001 | 2.19 (1.26–3.18) | <0.001 | 1.96 (1.26–2.85) | 0.002 |
| Sensitivity analyses | |||||||
| Highest level of education in household | 1 (reference) | 3.42 (2.39–4.61) | <0.001 | 2.34 (1.33–3.39) | <0.001 | 1.93 (1.22–2.87) | 0.004 |
| Age-sex and age-BMI interactions | 1 (reference) | 3.59 (2.55–4.80) | <0.001 | 2.15 (1.22–3.14) | 0.001 | 1.98 (1.28–2.90) | 0.001 |
| Number of years in Canada | 1 (reference) | 3.86 (2.46–5.57) | <0.001 | 2.47 (1.23–3.89) | 0.001 | 2.06 (1.27–3.23) | 0.003 |
| Individual income, fruit and vegetable consumption, alcohol consumption as continuous variables | 1 (reference) | 3.52 (2.50–4.72) | <0.001 | 2.29 (1.30–3.23) | <0.001 | 2.03 (1.31–2.99) | <0.001 |
| Male subjects | n = 26,395 | n = 546 | n = 420 | n = 375 | |||
| Main models | |||||||
| Unadjusted | 1 (reference) | 2.28 (1.58–3.03) | <0.001 | 0.98 (0.52–1.53) | 0.94 | 1.31 (0.76–1.96) | 0.24 |
| Age | 1 (reference) | 2.73 (1.83–3.69) | <0.001 | 1.11 (0.61–1.78) | 0.69 | 1.53 (0.89–2.23) | 0.06 |
| Survey year, income adequacy, urban vs. rural dwelling | 1 (reference) | 2.60 (1.76–3.49) | <0.001 | 1.06 (0.60–1.71) | 0.84 | 1.51 (0.88–2.23) | 0.08 |
| BMI | 1 (reference) | 3.78 (2.59–5.08) | <0.001 | 1.76 (0.97–2.83) | 0.04 | 1.65 (0.87–2.56) | 0.06 |
| Inadequate physical activity, inadequate fruit and vegetable consumption, psychosocial stress, alcohol consumption‡ | 1 (reference) | 4.02 (2.46–5.98) | <0.001 | 2.05 (1.04–3.51) | 0.02 | 1.51 (0.67–2.59) | 0.23 |
| Sensitivity analyses | |||||||
| Highest level of education in household | 1 (reference) | 3.71 (2.25–5.48) | <0.001 | 2.20 (1.12–3.70) | 0.01 | 1.31 (0.58–2.42) | 0.46 |
| Age-sex and age-BMI interactions | 1 (reference) | 4.05 (2.53–6.11) | <0.001 | 1.99 (1.00–3.47) | 0.03 | 1.51 (0.67–2.59) | 0.23 |
| Number of years in Canada | 1 (reference) | 4.29 (2.38–7.37) | <0.001 | 2.37 (1.10–4.49) | 0.02 | 1.36 (0.50–2.56) | 0.45 |
| Individual income, fruit and vegetable consumption, alcohol consumption as continuous variables | 1 (reference) | 3.90 (2.39–5.93) | <0.001 | 2.10 (1.06–3.53) | 0.02 | 1.53 (0.68–2.65) | 0.22 |
| Female subjects | n = 30,815 | n = 455 | n = 446 | n = 372 | |||
| Main models | |||||||
| Unadjusted | 1 (reference) | 2.04 (1.32–2.85) | <0.001 | 0.99 (0.43–1.63) | 0.98 | 2.26 (1.39–3.24) | <0.001 |
| Age | 1 (reference) | 2.48 (1.62–3.42) | <0.001 | 1.19 (0.53–1.89) | 0.58 | 2.75 (1.71–3.94) | <0.001 |
| Survey year, income adequacy, urban vs. rural dwelling | 1 (reference) | 2.30 (1.51–3.18) | <0.001 | 1.14 (0.49–1.81) | 0.69 | 2.51 (1.56–3.58) | <0.001 |
| BMI | 1 (reference) | 3.01 (1.99–4.20) | <0.001 | 2.00 (0.88–3.18) | 0.03 | 2.40 (1.47–3.52) | <0.001 |
| Inadequate physical activity, inadequate fruit and vegetable consumption, psychosocial stress, alcohol consumption‡ | 1 (reference) | 2.99 (1.68–4.60) | <0.001 | 2.30 (0.91–4.21) | 0.03 | 2.45 (1.26–4.09) | 0.004 |
| Sensitivity analyses | |||||||
| Highest level of education in household | 1 (reference) | 3.08 (1.74–4.69) | <0.001 | 2.39 (0.97–4.24) | 0.02 | 2.55 (1.31–4.30) | 0.003 |
| Age-sex and age-BMI interactions | 1 (reference) | 3.02 (1.72–4.69) | <0.001 | 2.32 (0.93–4.26) | 0.02 | 2.50 (1.28–4.20) | 0.004 |
| Number of years in Canada | 1 (reference) | 3.39 (1.69–5.63) | <0.001 | 2.33 (0.79–4.80) | 0.07 | 2.80 (1.39–5.15) | 0.003 |
| Individual income, fruit and vegetable consumption, alcohol consumption as continuous variables | 1 (reference) | 3.00 (1.69–4.64) | <0.001 | 2.44 (0.99–4.30) | 0.02 | 2.61 (1.38–4.41) | 0.002 |
Data were derived from the Ontario components of Statistics Canada’s NPHS and CCHS, 1996–2005.
*The study cohort included 57,210 white, 1,001 South Asian, 866 Chinese, and 747 black participants living in Ontario. All estimates were weighted by the survey sample weight to allow for estimates to be generalizable to the overall Ontario population.
†Bootstrap methods were used to derive 95% CIs and P values.
‡Definitions: income adequacy, a Statistics Canada measure of socioeconomic status based on annual household income and the number of people in the household; inadequate physical activity, ≤15 min/day; inadequate fruit and vegetable intake, less than three times per day; psychosocial stress, individual feeling “extremely” or “quite a bit” versus “not at all,” “not very,” or “a bit” stressed on most days; nonregular alcohol consumption, less than three drinks per week.
Age at diagnosis
Among those who developed diabetes during the study period, the median age at diagnosis was lowest among South Asian subjects (aged 49 years), followed by Chinese (aged 55 years), black (aged 57 years), and white (aged 58 years) subjects.
Ethnic-specific BMI cutoff values
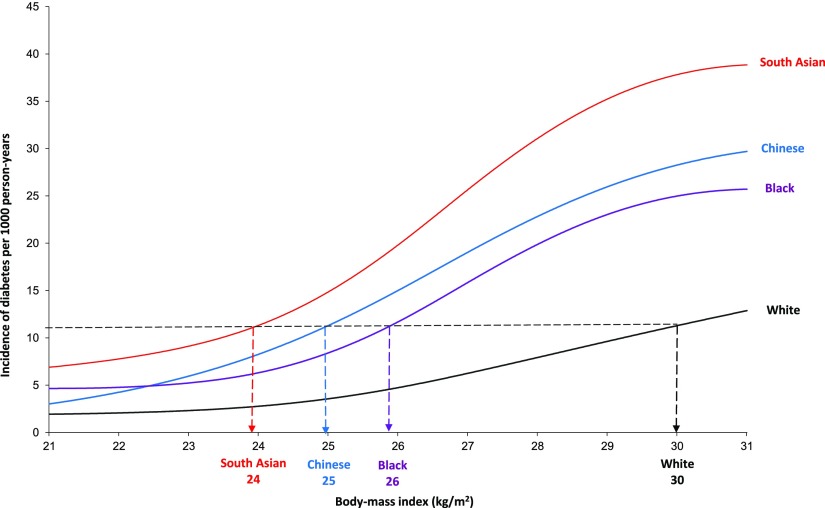
Figure 1 displays the ethnic-specific relationship between BMI and the incidence rate of diabetes, while controlling for age, sex, and other sociodemographic factors. For the equivalent incidence rate of diabetes at BMI 30 kg/m2 in white subjects, the BMI cutoff values were 24 for South Asian, 25 for Chinese, and 26 for black subjects. Similar results were found using five knots for the cubic splines (Supplementary Fig. 3), thus demonstrating the robustness of our findings.
Figure 1.
Association between the incidence rate of diabetes and BMI by ethnic group. The multivariate Poisson regression model included age, sex, BMI, BMI-ethnicity interaction, age-BMI interaction, income adequacy, survey year, and urban versus rural dwelling. Four knots were used to generate the restricted cubic splines. All estimates were weighted by the survey sample weight to allow for estimates to be generalizable to the overall Ontario population. Data were derived from the Ontario components of Statistics Canada’s NPHS and CCHS, 1996–2005.
CONCLUSIONS
In this population-based cohort study in Ontario, Canada, South Asian subjects had the highest crude incidence rate of diabetes, followed by black, white, and Chinese subjects. After adjusting for differences in baseline BMI, age, sex, and other sociodemographic characteristics, South Asian subjects were 3.40 times, black subjects were 1.99 times, and Chinese subjects were 1.87 times more likely than white subjects to develop diabetes. On average, diabetes occurred 9 years earlier among South Asian subjects, 3 years earlier among Chinese subjects, and 1 year earlier among black subjects than among white subjects. The ethnic-specific incidence of diabetes varied markedly across BMI categories. For the equivalent incidence rate of diabetes at BMI 30 kg/m2 in white subjects, we found lower BMI cutoff points for South Asian (24 kg/m2), Chinese (25 kg/m2), and black (26 kg/m2) subjects, thus supporting the need for lower BMI cutoff values for diabetes screening and lower ideal target body weights in nonwhite populations.
The ethnicity-sex patterns of diabetes incidence observed in this study are consistent with results from earlier prevalence studies in Canada (4), the U.K. (2), the U.S. (3), and elsewhere (16). Although most of the earlier studies compared two or three ethnic groups and relied mainly on cross-sectional data, our study is the first to conduct a cohort study to compare the risk of incident diabetes in the world’s four major ethnic groups. An incidence study among individuals from different groups initially free of disease and living in a similar environment is stronger than a prevalence study because it allows for more certainty that ethnicity is a true independent risk factor for the development of diabetes while simultaneously accounting for other confounding factors that might influence the development of diabetes.
The relatively low crude rate of diabetes in the Chinese population living in Ontario might be partly attributed to the relatively low average BMI observed in this population. Our data suggest that a population shift from the normal BMI range to the obese BMI range would result in an alarming 11.7-fold–increased rate of diabetes in Chinese subjects compared with a 4.5–6.3-fold increased rate of diabetes in the other ethnic groups. Likewise, a recent trend toward urbanization and the associated rise in obesogenic behaviors already has resulted in a rapid rise in prediabetes and diabetes in the Chinese population in China (17).
Several hypotheses have been proposed to explain why non-Europeans have a higher risk of diabetes than people of European descent. Researchers suggest that non-European ethnic groups are more likely to have inherited the thrifty gene because their ancestors were more likely than Europeans to have been exposed to extended periods of starvation. The thrifty gene was advantageous during feast or famine cycles because it enabled individuals to store calories more efficiently during times of food shortages. In the present day, however, where there is an abundance of high-fat and high-calorie foods, the thrifty gene makes it difficult for individuals to control their weight. Other hypotheses for the higher risk of diabetes in nonwhite versus white subjects include a genetic susceptibility to insulin resistance, particularly in South Asian subjects (18,19); a higher likelihood of intrauterine deprivation coupled with weight gain and physical inactivity later in life; and higher central adiposity at similar BMI levels (20).
Our findings suggest that the current definition of obesity may provide a false sense of security for South Asian, Chinese, and black populations and that, even at a BMI range thought to be acceptable, the risk of diabetes may be markedly underestimated in nonwhite ethnic groups who appear to be particularly sensitive to weight gain in terms of diabetes risk. To better reflect the risk of incident diabetes, our data suggest that the current BMI cutoff value for obesity should be lowered in South Asian, Chinese, and black groups. Most of the earlier studies that attempted to redefine BMI cutoff points in Asian populations relied on prevalence data, in which BMI and obesity-related conditions were ascertained at the same point in time. This is potentially problematic because having diabetes could influence metabolic and lifestyle changes, which in turn could influence body weight. A number of previous studies also were impeded by a lack of data on specific clinical outcomes (20,21). Nevertheless, our findings are consistent with earlier reports (6,22,23) on Asian populations that recommended BMI cutoff values between 22 and 27 kg/m2 for predicting the risk of diabetes, as well as of hypertension, cardiovascular disease, and mortality.
A major strength of this study was our ability to compare diabetes risk across diverse ethnic groups living in the same geographic region. This was especially important in identifying ethnic-specific BMI cutoff points for assessing diabetes risk because body composition and other determinants of obesity and diabetes, such as the built environment and availability of healthy foods, can vary widely by geographic location (24,25). Another strength of this study was our ability to achieve complete follow-up of all study participants using a highly sensitive and specific validated source of incident diabetes and several linked administrative databases of Canada’s single-payer universal health care system.
We recognize the following limitations of our study. 1) Our baseline variables were based on self-reported data; however, wherever possible, survey data were augmented using information from administrative sources. 2) BMI was calculated from self-reported height and weight and may be influenced by ethnic differences in reporting. However, an independent analysis of self-reported and measured BMI collected on a representative sample of participants of the CCHS cycle 3.1 found very high concordance between self-reported and measured BMI, irrespective of ethnicity (Supplementary Table A1) (M. Shields, Statistics Canada, unpublished data). 3) The number of nonwhite subjects was relatively smaller than the number of white subjects; however, with 2,614 nonwhite participants (who represented >1.8 million nonwhite people in Ontario), our study represents the largest multiethnic cohort study of its kind. 4) We did not have data on waist-to-hip ratio, data on family history of diabetes, or detailed information about diet. 5) We were unable to account for undiagnosed cases of diabetes or the possibility of the proportion of undiagnosed diabetes being different in the different ethnic groups. 6) We were unable to identify whether incident diabetes cases were type 1 or type 2; however, we greatly reduced the likelihood of identifying type 1 diabetes cases by limiting our cohort to individuals aged ≥30 years.
In conclusion, we found that ethnicity was an independent predictor of incident diabetes; that South Asian, Chinese, and black individuals presented with diabetes at younger ages than white individuals and that the current definition of obesity is inadequate for assessing diabetes risk in these nonwhite groups. The diabetes epidemic is expected to worsen with the ageing of the population, with increasing urbanization, and with growing obesity rates in Canada and most other parts of the world. Our findings highlight the urgent need for ethnically appropriate diabetes education and screening programs targeted toward the South Asian, Chinese, and black populations and health services planning that aims to reduce the risk of diabetes in these high-risk populations.
Supplementary Material
Acknowledgments
This study was supported by an operating grant from the Heart and Stroke Foundation of Ontario (HSFO) to the Institute for Clinical Evaluative Sciences (ICES), a Canadian Institutes of Health Research (CIHR) Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award (to M.C.), and a CIHR Team Grant in Cardiovascular Outcomes Research. ICES is funded by the Ontario Ministry of Health and Long-Term Care. The study results and conclusions are those of the authors and should not be attributed to any of the funding or sponsoring agencies. No endorsement by ICES or the Ontario Ministry of Health and Long-Term Care is intended or should be inferred. Funding for this project has been made possible through a contribution from the Public Health Agency of Canada. The views expressed herein do not necessarily represent the views of the Public Health Agency of Canada. All decisions regarding study design, publication, and data analysis were made independent of the funding agencies. P.C.A. was supported by a Career Investigator Award from the HSFO. D.G.M. was supported by a CIHR/Public Health Agency of Canada Chair in Applied Public Health. B.R.S. was supported by a Canadian Diabetes Association Clinician-Scientist Award. J.V.T. was supported by a Canada Research Chair in Health Services Research and a Career Investigator Award from the HSFO.
No potential conflicts of interest relevant to this article were reported.
The study sponsors had no role whatsoever in the study design; in the collection, analyses, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
M.C. conceived the study, obtained funding, performed the literature search, performed statistical analyses, prepared the figures, and prepared drafts of the manuscript. M.C., P.C.A., D.G.M., B.R.S., and J.V.T. interpreted the data, critically revised the manuscript for important intellectual content, and approved the final version of the manuscript. J.V.T. obtained funding and provided overall supervision for the study. M.C., P.C.A., D.G.M., B.R.S., and J.V.T. provided administrative, technical, and logistic support. M.C. and J.V.T. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The content of this manuscript has not been published elsewhere and is not being considered for publication elsewhere.
Parts of this work were presented at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–27 June 2011; as an oral presentation at the 13th Annual Canadian Diabetes Association/Canadian Society of Endocrinology and Metabolism Professional Conference and Annual Meetings, Edmonton, Alberta, Canada, October 2010; and as a poster presentation at the 46th Annual Meeting of the European Association for the Study of Diabetes, Stockholm, Sweden, September 2010.
The authors acknowledge that the data used in this publication are from Statistics Canada’s NPHS and CCHS. The authors thank all of the participants of the NPHS and CCHS and the staff from Statistics Canada who assisted in the survey data collection and management. The authors thank Dr. Elizabeth Barrett-Connor, Distinguished Professor and Chief of the Division of Epidemiology, University of California, San Diego, and Linda Donovan from the Institute for Clinical Evaluative Sciences, Toronto, Canada, for providing comments on an earlier version of the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-2300/-/DC1.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas [Internet], 2009. 4th Ed., Brussels, Belgium. Available from www.diabetesatlas.org. Accessed 31 March 2011 [Google Scholar]
- 2.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 1991;337:382–386 [DOI] [PubMed] [Google Scholar]
- 3.McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care 2004;27:66–69 [DOI] [PubMed] [Google Scholar]
- 4.Chiu M, Austin PC, Manuel DG, Tu JV. Comparison of cardiovascular risk profiles among ethnic groups using population health surveys between 1996 and 2007. CMAJ 2010;182:E301–E310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flegal KM, Williamson DF, Pamuk ER, Rosenberg HM. Estimating deaths attributable to obesity in the United States. Am J Public Health 2004;94:1486–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen CP, David Cheng TY, Tsai SP, et al. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr 2009;12:497–506 [DOI] [PubMed] [Google Scholar]
- 7.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163 [DOI] [PubMed] [Google Scholar]
- 8.Low S, Chin MC, Ma S, Heng D, Deurenberg-Yap M. Rationale for redefining obesity in Asians. Ann Acad Med Singapore 2009;38:66–69 [PubMed] [Google Scholar]
- 9.Béland Y. Canadian community health survey: methodological overview. Health Rep 2002;13:9–14 [PubMed] [Google Scholar]
- 10.Tu K, Campbell NR, Chen ZL, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med 2007;1:e18–e26 [PMC free article] [PubMed] [Google Scholar]
- 11.Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512–516 [DOI] [PubMed] [Google Scholar]
- 12.Austin PC. Absolute risk reductions and numbers needed to treat can be obtained from adjusted survival models for time-to-event outcomes. J Clin Epidemiol 2010;63:46–55 [DOI] [PubMed] [Google Scholar]
- 13.Harrell FE., Jr Regression Modeling Strategies. New York, Springer-Verlag, 2001 [Google Scholar]
- 14.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, FL, Chapman & Hall/CRC, 1993 [Google Scholar]
- 15.Creatore MI, Moineddin R, Booth G, et al. Age- and sex-related prevalence of diabetes mellitus among immigrants to Ontario, Canada. CMAJ 2010;182:781–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Söderberg S, Zimmet P, Tuomilehto J, et al. Increasing prevalence of type 2 diabetes mellitus in all ethnic groups in Mauritius. Diabet Med 2005;22:61–68 [DOI] [PubMed] [Google Scholar]
- 17.Yang W, Lu J, Weng J, et al. ; China National Diabetes and Metabolic Disorders Study Group. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–1101 [DOI] [PubMed] [Google Scholar]
- 18.Whincup PH, Gilg JA, Papacosta O, et al. Early evidence of ethnic differences in cardiovascular risk: cross sectional comparison of British South Asian and white children. BMJ 2002;324:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yajnik CS, Lubree HG, Rege SS, et al. Adiposity and hyperinsulinemia in Indians are present at birth. J Clin Endocrinol Metab 2002;87:5575–5580 [DOI] [PubMed] [Google Scholar]
- 20.Razak F, Anand SS, Shannon H, et al. Defining obesity cut points in a multiethnic population. Circulation 2007;115:2111–2118 [DOI] [PubMed] [Google Scholar]
- 21.Gu D, He J, Duan X, et al. Body weight and mortality among men and women in China. JAMA 2006;295:776–783 [DOI] [PubMed] [Google Scholar]
- 22.Wildman RP, Gu D, Reynolds K, Duan X, He J. Appropriate body mass index and waist circumference cutoffs for categorization of overweight and central adiposity among Chinese adults. Am J Clin Nutr 2004;80:1129–1136 [DOI] [PubMed] [Google Scholar]
- 23.Mohan V, Deepa M, Farooq S, Narayan KM, Datta M, Deepa R. Anthropometric cut points for identification of cardiometabolic risk factors in an urban Asian Indian population. Metabolism 2007;56:961–968 [DOI] [PubMed] [Google Scholar]
- 24.Nakagami T, Qiao Q, Carstensen B, et al. ; DECODE-DECODA Study Group. Age, body mass index and type 2 diabetes-associations modified by ethnicity. Diabetologia 2003;46:1063–1070 [DOI] [PubMed] [Google Scholar]
- 25.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 2002;3:141–146 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.