Abstract
OBJECTIVE
To determine the association of weight-based insulin dose with hypoglycemia in noncritically ill inpatients with diabetes.
RESEARCH DESIGN AND METHODS
We performed a retrospective, case-control study of 1,990 diabetic patients admitted to hospital wards. Patients with glucose levels <70 mg/dL (case subjects) were matched one to one with nonhypoglycemic control subjects on the basis of the hospital day of hypoglycemia, age, sex, and BMI.
RESULTS
Relative to 24-h insulin doses <0.2 units/kg, the unadjusted odds of hypoglycemia increased with increasing insulin dose. Adjusted for insulin type, sliding-scale insulin use, and albumin, creatinine, and hematocrit levels, the higher odds of hypoglycemia with increasing insulin doses remained (0.6–0.8 units/kg: odds ratio 2.10 [95% CI 1.08–4.09], P = 0.028; >0.8 units/kg: 2.95 [1.54–5.65], P = 0.001). The adjusted odds of hypoglycemia were not greater in patients who received 0.2–0.4 units/kg (1.08 [0.64–1.81], P = 0.78) or 0.4–0.6 units/kg (1.60 [0.90–2.86], P = 0.11). Although the relationship between insulin dose and hypoglycemia did not vary by insulin type, patients who received NPH trended toward greater odds of hypoglycemia compared with those given other insulins.
CONCLUSIONS
Higher weight-based insulin doses are associated with greater odds of hypoglycemia independent of insulin type. However, 0.6 units/kg seems to be a threshold below which the odds of hypoglycemia are relatively low. These findings may help clinicians use insulin more safely.
Treatment with insulin in hospitalized patients is a well-recognized risk factor for hypoglycemia. Although this clearly has been demonstrated in critically ill patients treated with continuous intravenous insulin (1), evidence regarding subcutaneous insulin in non–critical-care settings is lacking. Physicians may underdose insulin in fear of hypoglycemia because there is uncertainty about the dose-response relationship between insulin and hypoglycemia in noncritically ill inpatients with diabetes (2,3).
Current guidelines on inpatient diabetes management call for basal-bolus insulin therapy for hospital-ward patients (4–6), in which one approach to determining insulin dose is based on weight (i.e., total daily units per kilogram of body weight). The initial daily insulin doses of basal-bolus protocols vary widely, from 0.3 to 1.5 units/kg (5,7–10). Rates of hypoglycemia in randomized trials using 0.4–0.5 units/kg/day of insulin range from 3 to 33% of subjects (9,11). Furthermore, these studies excluded patients with known risk factors for hypoglycemia, such as renal impairment. Despite the use of relatively high insulin doses in clinical practice, the risk of hypoglycemia with higher weight-based doses has not been established. A better understanding of the relationship between insulin dose and hypoglycemia may facilitate more effective dosing, thus reducing the risk of hypoglycemia and hyperglycemia.
We performed a retrospective, case-control study of patients with diabetes admitted to general hospital wards to investigate the relationship between insulin dose and hypoglycemia. It was hypothesized that higher weight-based insulin doses are associated with a greater risk of hypoglycemia. Furthermore, we predicted that the association of insulin dose with hypoglycemia varies by type of insulin regimen, with glargine-based regimens being associated with less hypoglycemia than NPH-based regimens.
RESEARCH DESIGN AND METHODS
Study sample
Abstracting data from electronic medical records, we retrospectively selected a sample of adult inpatients who received insulin at Boston Medical Center, most of whom had a diagnosis of diabetes, admitted between 1 July 2005 and 31 December 2009. Diabetes was defined by any one of the following: 1) administration of an oral antidiabetes medication (metformin, a thiazolidinedione, or a sulfonylurea) during hospitalization; 2) a diabetes ICD-9 code of 250.xx; or 3) an A1C >6.5%. Patients were excluded if they were aged <18 years, were admitted to an intensive care unit, had a primary diagnosis of hypoglycemia, had hypoglycemia (any blood glucose <70 mg/dL) within 24 h of admission, or had no point-of-care (POC) capillary glucose values. The sample was restricted to only the first admission per patient, and data subsequent to the first hypoglycemic event after 24 h were excluded. Only POC values were used to measure glucose because of variability among different glucose assays.
Case subjects were defined by a POC glucose <70 mg/dL after the first 24 h of admission. The time period examined for each case was 24 h prior to the first hypoglycemic event in order to include all relevant doses of insulin. Control subjects, defined by a POC glucose ≥70 mg/dL, were matched one to one on the basis of the hospital day of hypoglycemia, age (18–30, 31–64, or 65–80 years), sex, and BMI (<18.5, 18.5–25, 25–30, or >30 kg/m2). Matching on hospital day of hypoglycemia provided a control time frame as well as controlled for variation in clinical status during hospitalization.
Variable definitions
The exposure was defined as the total insulin dose per body weight (units/kg) over the 24-h study period. Insulin doses were stratified into nonoverlapping ranges, as follows: 0, <0.2, 0.2–0.4, 0.4–0.6, 0.6–0.8, and ≥0.8 units/kg. The exposure was grouped by insulin regimen type as follows: 1) glargine plus any other insulin, 2) NPH plus any other insulin, 3) lispro and/or regular insulin only, and 4) no insulin. For 11 patients who received both glargine and NPH in 1 day, group assignment was on the basis of whichever insulin dose was greater. For two patients who received the same total 24-h dose of glargine and NPH, group assignment was on the basis of the insulin given closest to the hypoglycemic event. Guidelines for insulin dosing at Boston Medical Center have been published (10).
The following known predictors of hypoglycemia and potential confounders were examined: weight, race, serum creatinine, albumin, liver function (aspartate aminotransferase and alanine aminotransferase), A1C, white blood cell count, hematocrit, use of sliding-scale insulin (SSI), fasting, length of stay, service (medical or surgical), and disease severity defined by the Charlson Comorbidity Index (12). A higher comorbidity index represents a greater burden of comorbid disease. Use of SSI was categorized as only SSI, no SSI, or SSI plus scheduled insulin. Data were collected during hospitalization, except for missing values that were substituted by the closest value up to 3 months prior to admission. BMI values <10 or >100 kg/m2, weight <20 or >400 kg, and height <100 or >300 cm were considered erroneous and were deleted. No height was available for 195 patients (9.8%). For these individuals, the average height of the sample for each sex was imputed (174.2 ± 9.6 cm for male subjects and 160.8 ± 8.5 cm for female subjects). Admission glucose was defined as the first POC glucose value within 24 h of admission.
Statistical analysis
Summaries of categorical variables included counts and percentages, whereas for continuous variables means and SDs were used. Comparability between the case and control groups for categorical variables was determined using the χ2 test for categorical variables and the two-sample t test for continuous variables. γ Regression was used for length of stay because this continuous, positive variable was not normally distributed.
Conditional logistic regression was used to examine the unadjusted and the adjusted association of insulin dose with hypoglycemia. The initial adjusted model included all variables that were associated with hypoglycemia, as well an interaction term for dose by regimen to assess effect modification by insulin regimen type on the relationship between insulin dose and hypoglycemia. We then performed backward selection with the α level to keep variables in the model set at 0.2 (13). A P value < 0.05 was considered statistically significant. All analyses were performed using SAS (version 9.2; SAS Institute, Cary, NC). The Boston University Medical Center Institutional Review Board approved the protocol.
RESULTS
Of 6,376 eligible patients abstracted from hospital records, 1,012 (15.8%) had hypoglycemia. After matching, 995 case subjects (98.3%) and 995 nonhypoglycemic control subjects remained (Supplementary Table 1). Compared with 4,386 unmatched patients, the matched control sample was older (aged 63.3 vs. 60.6 years, P < 0.001), had lower weights (86.1 vs. 91.4 kg, P < 0.001), were less obese (BMI 31.2 vs. 33 kg/m2, P < 0.001), had a higher proportion of African Americans (39.4 vs. 36.6%, P = 0.04 for race), and a had lower proportion of Hispanics (12.7 vs. 16.1%). The matched and unmatched control subjects were similar in terms of sex distribution and mean A1C.
The median time from admission to the first hypoglycemic event in case subjects was 2.7 days (range 1.0–33.7). During the index 24-h period, glargine was the most frequently administered insulin (31.7%), followed by short-acting insulin (28.4%), no insulin (24.0%), and NPH insulin (15.9%) (Table 1). SSI only was less common than SSI plus scheduled insulin and no SSI at all (25.3, 37.0, and 37.7%). Approximately 50% of the patients were given metformin, 33% were given a sulfonylurea, and 13% were given a thiazolidinedione. The sample was racially diverse: 40.6% were African American, 34.1% were white, 13.9% were Hispanic, and 11.4% were of other race/ethnicity. The mean A1C was 8.1 ± 2.2%, and mean serum creatinine was 1.5 ± 1.7 mg/dL. Length of stay averaged 7.6 ± 7.0 days. Of the 1,990-patient sample, 1,418 (71.3%) were not on insulin as an outpatient.
Table 1.
Sample characteristics of unmatched factors by case status
| All patients | Case subjects | Control subjects | P* | |
|---|---|---|---|---|
| n | 1,990 | 995 | 995 | |
| Insulin regimen | ||||
| Glargine | 630 (31.7) | 38.1 | 25.2 | <0.001 |
| NPH | 316 (15.9) | 22.4 | 9.3 | |
| Lispro and/or regular only† | 566 (28.4) | 19.5 | 37.4 | |
| None | 478 (24.0) | 20.0 | 28.0 | |
| Basal-to-total insulin dose ratio | ||||
| 0.00 | 566 (28.4) | 19.5 | 37.4 | <0.001 |
| 0.01–0.39 | 99 (5.0) | 4.5 | 5.4 | |
| 0.40–0.59 | 267 (13.4) | 16.3 | 10.6 | |
| 0.60–1.00 | 580 (29.1) | 39.7 | 18.6 | |
| No insulin | 478 (24.0) | 20.0 | 28.0 | |
| SSI use | ||||
| SSI only | 503 (25.3) | 16.6 | 34.0 | <0.001 |
| SSI plus scheduled insulin | 737 (37.0) | 42.9 | 31.2 | |
| No SSI | 750 (37.7) | 40.5 | 34.9 | |
| Medication use in hospital | ||||
| Metformin | 417 (54.3) | 49.9 | 58.8 | 0.086 |
| Sulfonylureas | 254 (32.8) | 35.7 | 30.3 | |
| Thiazolidinediones | 97 (12.6) | 14.4 | 10.8 | |
| Glucocorticoids | 210 (10.6) | 10.2 | 11.0 | 0.558 |
| Weight (kg) | 85.6 ± 27.0 | 85.2 ± 27.7 | 86.1 ± 26.2 | 0.301 |
| Race | ||||
| African American | 799 (40.6) | 41.8 | 39.4 | 0.167 |
| White | 671 (34.1) | 32.2 | 36.0 | |
| Hispanic | 274 (13.90 | 15.1 | 12.7 | |
| Other | 225 (11.4) | 11.0 | 11.9 | |
| Laboratory studies | ||||
| A1C (%) | 8.1 ± 2.2 | 8.0 ± 2.1 | 8.1 ± 2.3 | 0.438 |
| Albumin (g/dL) | 3.5 ± 0.6 | 3.4 ± 0.6 | 3.5 ± 0.5 | <0.001 |
| Alanine aminotransferase (units/L) | 34.6 ± 99.3 | 34.6 ± 101.2 | 34.5 ± 97.4 | 0.867 |
| Aspartate aminotransferase (units/L) | 41.3 ± 219.9 | 40.1 ± 172.9 | 42.6 ± 260.7 | 0.937 |
| Creatinine (mg/dL) | 1.5 ± 1.7 | 1.7 ± 2.0 | 1.3 ± 1.2 | <0.001 |
| Hematocrit (%) | 32.8 ± 5.2 | 32.1 ± 5.0 | 33.6 ± 5.3 | <0.001 |
| WBC (k/UL) | 8.4 ± 4.1 | 8.4 ± 4.4 | 8.5 ± 3.7 | 0.553 |
| Fasting within prior 24 h‡ | 403 (20.3) | 50.6 | 49.4 | 0.774 |
| Primary service‡ | ||||
| Medicine | 1,404 | 73.5 | 68.1 | 0.008 |
| Surgery | 580 | 26.5 | 31.9 | |
| Comorbidity index | ||||
| 0 | 107 (5.40) | 4.6 | 6.1 | <0.001 |
| 1–2 | 803 (40.4) | 36.4 | 44.3 | |
| 3–4 | 682 (34.3) | 36.4 | 32.2 | |
| 5–6 | 282 (14.2) | 17.3 | 11.1 | |
| >6 | 116 (5.8) | 5.3 | 6.3 | |
| Length of stay (days) | 7.6 ± 7.0 | 8.1 ± 8.2 | 7.1 ± 5.5 | <0.001 |
Data are n (%), percentages, or means ± SD.
*P values for categorical variables were generated by χ2 tests. P values for continuous variables were generated by t tests.
†Hypoglycemia prevalence among patients who received lispro was similar to that among patients given regular insulin (31.8 vs. 36.1%).
‡Excludes six patients with missing values.
Hypoglycemic patients were more likely to be given glargine or NPH and less likely to be given only SSI or no insulin than control subjects (P < 0.001). SSI plus scheduled insulin was more common, whereas SSI without scheduled insulin was less common, among case subjects than control subjects (P < 0.001). Hypoglycemic patients had a lower albumin and hematocrit and higher creatinine and length of stay than nonhypoglycemic patients (P < 0.001 for each comparison). Case subjects also had higher comorbidity index scores than control subjects (P < 0.001). There was no difference in mean A1C (8.0 ± 2.1 vs. 8.1 ± 2.3%, P = 0.44) or admission glucose (199.8 ± 109.5 vs. 193.6 ± 96.9 mg/dL, P = 0.20) between case and control subjects. Higher insulin doses were associated with progressively higher blood glucose levels (Supplementary Table 2). Within each dose range, the mean glucose levels of case subjects were lower than the mean glucose levels of control subjects.
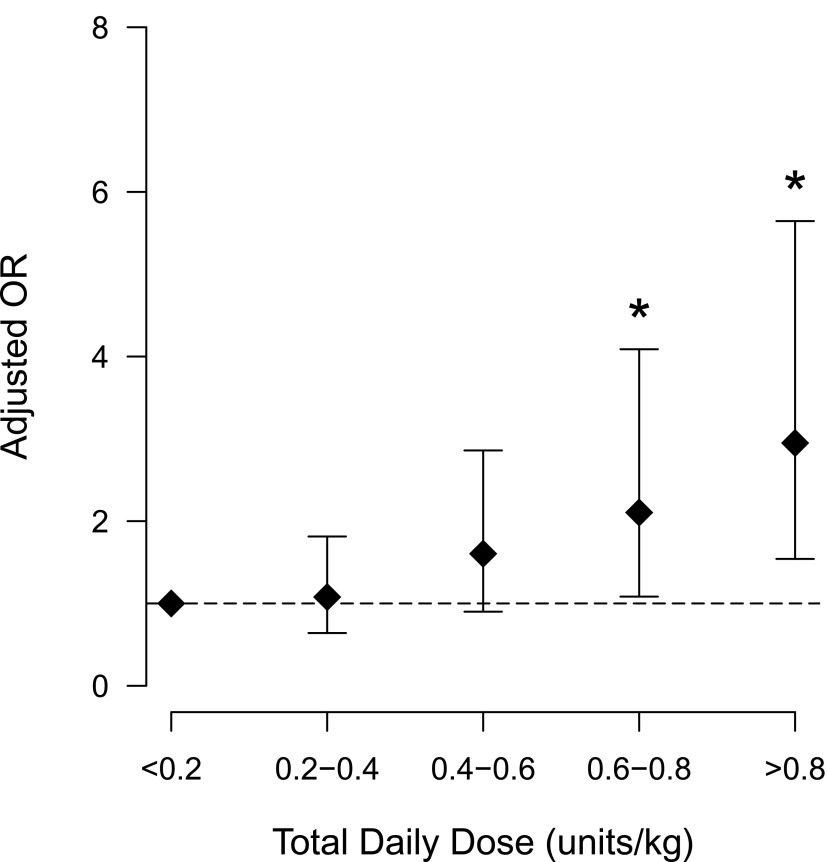
Relative to insulin doses <0.2 units/kg, the unadjusted odds of hypoglycemia increased with increasing insulin dose (Table 2). Adjusted for insulin regimen, SSI use, and albumin, creatinine, and hematocrit levels, the higher odds of hypoglycemia with increasing insulin doses remained (Table 3 and Fig. 1). Patients who received insulin doses of ≥0.6 units/kg were at increased odds of hypoglycemia. In contrast, the adjusted odds of hypoglycemia were not higher in patients who received 0.2–0.6 units/kg. The association of insulin dose with hypoglycemia did not vary by regimen type, as indicated by a statistically nonsignificant interaction term in the multivariate model (P = 0.524).
Table 2.
Distribution of insulin dose by case status
| All patients | Case subjects | Control subjects | P* | |
|---|---|---|---|---|
| n | 1,990 | 995 | 995 | |
| Insulin dose (units/kg) | ||||
| 0 | 478 (24.0) | 20.0 | 28.0 | <0.001 |
| <0.2 | 595 (29.9) | 22.2 | 37.6 | |
| 0.2–0.4 | 285 (14.4) | 15.0 | 13.7 | |
| 0.4–0.6 | 263 (13.2) | 16.5 | 9.9 | |
| 0.6–0.8 | 161 (8.1) | 11.4 | 4.8 | |
| ≥0.8 | 208 (10.5) | 15.0 | 5.9 |
Data are n (%) or percentages.
*P value by χ2 test.
Table 3.
Predictors of hypoglycemia in the multivariate conditional logistic regression model
| Odds ratio (95% CI) | P | |
|---|---|---|
| Insulin dose | 0.005 | |
| 0.2–0.4 vs. <0.2 | 1.08 (0.64–1.82) | 0.777 |
| 0.4–0.6 vs. <0.2 | 1.60 (0.90–2.86) | 0.109 |
| 0.6–0.8 vs. <0.2 | 2.10 (1.08–4.09) | 0.028 |
| >0.8 vs. <0.2 | 2.95 (1.54–5.65) | 0.001 |
| Insulin regimen | 0.062 | |
| Glargine vs. lispro/regular | 1.58 (0.67–3.74) | 0.297 |
| NPH vs. lispro/regular | 2.38 (0.97–5.87) | 0.059 |
| NPH vs. glargine | 1.51 (1.00– 2.27) | 0.050 |
| SSI | <0.001 | |
| No SSI vs. SSI only | 3.04 (1.23–7.51) | 0.016 |
| SSI plus scheduled insulin vs. SSI only | 0.83 (0.36–1.88) | 0.648 |
| No SSI vs. SSI plus scheduled insulin | 3.68 (2.25–6.03) | <0.001 |
| Albumin level | 0.80 (0.60–1.08) | 0.143 |
| Creatinine level | 1.14 (1.02–1.28) | 0.018 |
| Hematocrit level | 0.96 (0.93–0.99) | 0.013 |
Figure 1.
Multivariate-adjusted odds of hypoglycemia at different insulin doses in 995 case subjects vs. 995 matched control subjects. Error bars represent 95% CIs. OR, odds ratio. *P < 0.05 by conditional logistic regression.
In addition to insulin dose, there were several significant predictors of hypoglycemia, including creatinine and hematocrit. The odds of hypoglycemia were threefold greater among those who did not receive SSI relative to those who did. Patients who received SSI plus scheduled insulin or SSI alone had higher blood glucose levels than patients who did not receive SSI (203 ± 64, 185 ± 52, and 128 ± 33 mg/dL, P < 0.001). There was a trend toward higher odds of hypoglycemia among patients who received NPH compared with patients given glargine or short-acting insulin.
To investigate whether the risk of hypoglycemia depends on the ratio of basal insulin (NPH or glargine) relative to the total daily dose, a post hoc analysis revealed that basal ratios <0.4 confer lower odds of hypoglycemia than 0.4–0.6 (odds ratio 0.50 [95% CI 0.25–0.97], P = 0.040). In contrast, there was no difference in the odds of hypoglycemia between basal ratios >0.6 or 0 and 0.4–0.6. Furthermore, there was no interaction of basal ratio with the insulin dose–hypoglycemia relationship.
Hypoglycemia was not more common among patients given insulin with an oral diabetes medication compared with those on insulin alone (49.4 vs. 54.6%, P = 0.13). However, of the 201 patients who did not receive insulin but did receive an oral agent, sulfonylureas were more common among hypoglycemic patients than in control subjects (27.4 vs. 16.4%, P < 0.001). Patients who received only SSI were more likely to be given an oral agent than those on SSI plus scheduled insulin and those not given SSI (54.5, 28.1, and 38.3%, P < 0.001).
Although hypoglycemia was proportionately more common on medical services than on surgical services (Table 1, P = 0.008), this variable was not significant in the multivariate model. There was no difference in mean blood glucose in medical versus surgical patients (173 ± 62 vs. 168 ± 59, P = 0.12). However, medical patients received more insulin than surgical patients (0.35 ± 0.88 vs. 0.26 ± 0.38 units/kg, P = 0.013). In addition, SSI only was less common among medical patients (20.9 vs. 36.0%, P < 0.001). The sample included 77 patients (3.9%) without a diagnosis of diabetes who had an inpatient A1C >6.5%. A sensitivity analysis excluding these patients yielded similar results.
CONCLUSIONS
This retrospective, matched, case-control study of 1,990 hospital-ward patients with diagnosed or probable diabetes shows that higher weight-based insulin doses are associated with greater odds of hypoglycemia, independent of the types of insulin used. Adjusted for insulin regimen, SSI use, and albumin, creatinine, and hematocrit levels, patients given at least 0.8 units/kg within a 24-h period are at threefold-higher odds of hypoglycemia than patients who receive <0.2 units/kg. However, 0.6 units/kg seems to be a threshold below which the odds of hypoglycemia are relatively low. In addition, patients who do not receive SSI are at threefold-greater odds of hypoglycemia than patients who receive SSI with or without scheduled insulin. Because SSI typically is administered in response to a glucose value above a predetermined threshold, patients given SSI are hyperglycemic by design. Patients who did not receive SSI may have been given excessive scheduled insulin, resulting in more frequent hypoglycemia. Together, these data suggest that the insulin program with the lowest risk of hypoglycemia consists of scheduled insulin plus SSI at total daily doses <0.6 units/kg.
Our findings are broadly consistent with other studies of insulin use in non–intensive care unit inpatients that show that insulin is a risk factor for hypoglycemia (14,15). These studies, however, did not examine how the risk of hypoglycemia varies by insulin dose or type. The lack of a clear difference in the odds of hypoglycemia between glargine and NPH is similar to data from a randomized trial comparing weight-based analog insulins with NPH and regular insulins that found no difference in hypoglycemia rates between the treatment groups (11). Also consistent with other studies are our findings that high creatinine and low hematocrit levels are independently associated with hypoglycemia (16,17).
The frequent use of oral agents was surprising given that our medication guideline for hospitalized patients with diabetes encourages the discontinuation of oral agents. However, we support the judicious use of oral diabetes medications in patients who are eating regularly, who are no longer acutely ill, and/or who preparing for discharge, consistent with some expert opinions (1,18). Our data provide evidence for the safety of this approach in terms of hypoglycemia.
A number of limitations must be acknowledged. Generalizability is limited by the inclusion of only one medical center. Also, bias cannot be completely accounted for by matching and multivariate analysis of retrospective data. The use of POC glucose values at the exclusion of serum glucose values may underestimate the occurrence of hypoglycemia. This effect, however, is likely to be small because almost all inpatients with diabetes are ordered POC testing four times daily, and a POC glucose is more likely to be checked on a patient with hypoglycemic symptoms than a serum glucose. Another limitation is that a case-control design precludes the calculation of hypoglycemia rates; however, the odds ratio provides a reasonable estimate of relative risk. An additional limitation is our inability to classify the sample by diabetes type because of unavailable data. However, at least 90% of the sample is estimated to have type 2 diabetes, in keeping with national estimates (19). Finally, the focus of our study was hypoglycemia, and we did not examine the association of insulin dose with hyperglycemia.
Although lower-dose insulin regimens may confer a lower risk of hypoglycemia, they may be associated with a higher risk of hyperglycemia. The weight-based insulin dose that minimizes both hypoglycemia and hyperglycemia remains unclear. These data, however, suggest that 0.4–0.6 units/kg, a dose range tested in randomized controlled trials and recommended by the American Diabetes Association (5,9,11), may be the highest dose at which the risk of hypoglycemia remains low. To determine a starting dose of insulin within this range for a specific patient, clinicians must balance the presence of hypoglycemia risk factors with the benefits of achieving glycemic control.
Despite these limitations, strengths of our study include a large sample size spanning 4.5 years. Because the case and control subjects had similar admission glucose values and because we adjusted for numerous potential confounders, it is likely that we have isolated the effect of inpatient insulin dose on hypoglycemia. Matching case subjects on the basis of hospital day, age, sex, and BMI created a comparable control group. Furthermore, no published study has investigated the association of weight-based insulin with inpatient hypoglycemia across a range of doses and insulin types.
Our research leads to additional questions. One is whether the risk of hypoglycemia depends on the ratio of basal insulin relative to the total daily dose, which is an important component of insulin protocols (9–11). Post hoc analysis of our data revealed that basal ratios <0.4 confer a lower odds of hypoglycemia than basal ratios of 0.4–0.6. This may reflect a higher proportion of patients who received SSI, who are by definition hyperglycemic as discussed above. Prospective studies are necessary to determine the effect of basal ratio in scheduled insulin doses across heterogeneous inpatient populations. Another question is whether higher insulin doses cause hypoglycemia more quickly than lower insulin doses.
In conclusion, we found that higher weight-based insulin doses are associated with greater odds of hypoglycemia, and this association does not differ by the type of insulin used. Insulin doses <0.6 units/kg confer a lower odds of hypoglycemia, whereas doses >0.6 units/kg are associated with higher odds of hypoglycemia. These data imply that 0.4–0.6 units/kg may be the optimal dose range. Given that insulin is recommended for most hospitalized patients with diabetes (4), and hypoglycemia is associated with increased length of stay and mortality (15), strategies to reduce hypoglycemia are of critical importance. Our findings provide additional information to help clinicians dose insulin more safely for inpatients with diabetes.
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
D.J.R. designed the study, researched data, and wrote the manuscript. D.R. designed the study, researched data, and reviewed and edited the manuscript. G.D. and M.E.M. designed the study and reviewed and edited the manuscript.
Parts of this study were presented in abstract form at the 93rd Annual Meeting of The Endocrine Society, Boston, Massachusetts, 4–7 June 2011.
The authors thank Linda Rosen of the Boston Medical Center for her help with data collection.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-2434/-/DC1.
References
- 1.Inzucchi SE. Clinical practice: management of hyperglycemia in the hospital setting. N Engl J Med 2006;355:1903–1911 [DOI] [PubMed] [Google Scholar]
- 2.Trujillo JM, Barsky EE, Greenwood BC, et al. Improving glycemic control in medical inpatients: a pilot study. J Hosp Med 2008;3:55–63 [DOI] [PubMed] [Google Scholar]
- 3.Matheny ME, Shubina M, Kimmel ZM, Pendergrass ML, Turchin A. Treatment intensification and blood glucose control among hospitalized diabetic patients. J Gen Intern Med 2008;23:184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moghissi ES, Korytkowski MT, Dinardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009;32:1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clement S, Braithwaite SS, Magee MF, et al. ; American Diabetes Association Diabetes in Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004;27:553–591 [DOI] [PubMed] [Google Scholar]
- 6.King AB, Armstrong DU. Basal bolus dosing: a clinical experience. Curr Diabetes Rev 2005;1:215–220 [DOI] [PubMed] [Google Scholar]
- 7.Chen HJ, Steinke DT, Karounos DG, Lane MT, Matson AW. Intensive insulin protocol implementation and outcomes in the medical and surgical wards at a Veterans Affairs Medical Center. Ann Pharmacother 2010;44:249–256 [DOI] [PubMed] [Google Scholar]
- 8.Maynard G, Lee J, Phillips G, Fink E, Renvall M. Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm. J Hosp Med 2009;4:3–15 [DOI] [PubMed] [Google Scholar]
- 9.Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007;30:2181–2186 [DOI] [PubMed] [Google Scholar]
- 10.Pietras SM, Hanrahan P, Arnold LM, Sternthal E, McDonnell ME. State-of-the-art inpatient diabetes care: the evolution of an academic hospital. Endocr Pract 2010;16:512–521 [DOI] [PubMed] [Google Scholar]
- 11.Umpierrez GE, Hor T, Smiley D, et al. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab 2009;94:564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol 1996;49:1429–1433 [DOI] [PubMed] [Google Scholar]
- 13.Budtz-Jørgensen E, Keiding N, Grandjean P, Weihe P. Confounder selection in environmental epidemiology: assessment of health effects of prenatal mercury exposure. Ann Epidemiol 2007;17:27–35 [DOI] [PubMed] [Google Scholar]
- 14.Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care 2007;30:367–369 [DOI] [PubMed] [Google Scholar]
- 15.Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care 2009;32:1153–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosiborod M, Inzucchi SE, Goyal A, et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA 2009;301:1556–1564 [DOI] [PubMed] [Google Scholar]
- 17.Kagansky N, Levy S, Rimon E, et al. Hypoglycemia as a predictor of mortality in hospitalized elderly patients. Arch Intern Med 2003;163:1825–1829 [DOI] [PubMed] [Google Scholar]
- 18.Korytkowski MT. Treatment options for safely achieving glycemic targets in the hospital. ACP Hospitalist, 2009;(Suppl.) Chapter 3:15–23
- 19.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2008 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.