Abstract
OBJECTIVE
Optimal glycemic control slows diabetic retinopathy (DR) development and progression and is the standard of care for type 1 diabetes. However, these glycemic goals are difficult to achieve and sustain in clinical practice. The Renin Angiotensin System Study (RASS) showed that renin-angiotensin system (RAS) blockade can slow DR progression. In the current study, we evaluate whether glycemic control influenced the benefit of RAS blockade on DR progression in type 1 diabetic patients.
RESEARCH DESIGN AND METHODS
We used RASS data to analyze the relationships between two-steps or more DR progression and baseline glycemic levels in 223 normotensive, normoalbuminuric type 1 diabetic patients randomized to receive 5 years of enalapril or losartan compared with placebo.
RESULTS
A total of 147 of 223 patients (65.9%) had DR at baseline (47 of 74 patients [63.5%] in placebo and 100 of 149 patients [67.1%] in the combined treatment groups [P = 0.67]). Patients with two-steps or more DR progression had higher baseline A1C than those without progression (9.4 vs. 8.2%, P < 0.001). There was no beneficial effect of RAS blockade (P = 0.92) in patients with baseline A1C ≤7.5%. In contrast, 30 of 112 (27%) patients on the active treatment arms with A1C >7.5% had two-steps or more DR progression compared with 26 of 56 patients (46%) in the placebo group (P = 0.03).
CONCLUSIONS
RAS blockade reduces DR progression in normotensive, normoalbuminuric type 1 diabetic patients with A1C >7.5%. Whether this therapy could benefit patients with A1C ≤7.5% will require long-term studies of much larger cohorts.
Diabetic retinopathy (DR) is the most common microvascular complication of diabetes and remains the leading cause of new blindness among adults aged 20–74 years in the U.S. (1). The estimated prevalence of DR and vision-threatening DR among Americans with diabetes who are 40 years or older are 28.5 and 4.4%, respectively (2). Consequent to the ongoing increase in diabetes prevalence, the total number of people older than 40 years with DR is projected to be 16 million by year 2050 (3). The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) and the UK Prospective Diabetes Study (UKPDS) clearly demonstrated the benefit of intensive glycemic control in reducing the incidence and progression of DR in both type 1 and type 2 diabetes (4,5). As a consequence of these studies, guidelines to maintain glycated hemoglobin A1C (A1C) <7.0% are now accepted as a standard of care in diabetes (6). However, achieving and sustaining A1C at these levels remains problematic (7). Thus additional treatment approaches to DR prevention may be helpful.
Tight blood pressure control per se has been shown to reduce progression of retinopathy in hypertensive diabetic patients (8,9). Previous studies have also suggested beneficial effects of ACE inhibitors (ACEI) on progression of DR in both normotensive and hypertensive type 1 diabetic patients (10–12). Recently, the Renin Angiotensin System Study (RASS) (13) documented a beneficial role of renin-angiotensin system (RAS) blockade on DR progression but did not slow nephropathy progression in normotensive normoalbuminuric type 1 diabetic patients. The Diabetic Retinopathy Candesartan Trial (DIRECT-Prevent 1) (14) also reported a reduction in the incidence of DR in type 1 diabetic patients that just failed to achieve statistical significance (P = 0.0508).
Herein we evaluated the role of glycemic control on the treatment benefit of RAS blockade on DR progression in the RASS.
RESEARCH DESIGN AND METHODS
Participants and study design
Patients were enrolled in RASS (ClinicalTrials.gov, NCT00143949), a multicenter randomized, double-blinded, double dummy, placebo-controlled clinical trial comparing the effects of an ACEI, enalapril, and an angiotensin receptor blocker (ARB), losartan, with placebo on the rates of progression of diabetic nephropathy and retinopathy lesions in normotensive, normoalbuminuric type 1 diabetic patients over 5 years. RASS was conducted at the University of Minnesota (Minneapolis, MN), University of Toronto (Toronto, Canada), and McGill University (Montreal, Canada). The primary end point of RASS was a change in the fraction of glomerular volume occupied by mesangium (the mesangial fractional volume). Secondary renal end points included changes in other glomerular, vascular, tubular, and interstitial variables and changes in albumin excretion rate (AER) and glomerular filtration rate (GFR). The retinal primary end point of two-steps or more DR progression was added shortly after RASS began. The study design and protocol (15), as well as the results of the primary endpoint analyses (13), have been detailed elsewhere. In brief, RASS screened 1,065 type 1 diabetic patients; 707 declined participation and 73 were ineligible. Thus, 285 patients were randomly assigned to one of the three study groups with the use of computer-generated blocks of six, stratified according to center and sex, into the following groups: 10 mg daily enalapril, 50 mg daily losartan, or placebo daily (Supplementary Fig. A1). The original doses were doubled during the study as previously detailed (13) because of the new evidence of greater reduction in proteinuria with higher doses of these agents (16). The study patients were ≥16 years old with 2–20 years’ duration of type 1 diabetes and onset before age 45. At baseline, fulfilling the RASS entry criteria, all were normotensive (blood pressure <135/85 mmHg), normoalbuminuric (AER <20 μg/min on at least two out of three timed consecutive overnight urine collections), and had normal or increased GFR (≥90 mL/min/1.73 m2).
Retinal examination
Patients were eligible for this DR study if they had retinal exams performed both at baseline (within 1 year of randomization) and after 5 years in the trial. Patients with baseline proliferative DR (N = 4) or whose baseline fundus photographs were obtained more than 1 year after randomization (N = 28) were excluded from these analyses (Supplementary Fig. A1). Retinal fundus photographs were taken by trained photographers for seven standard fields, according to the Early Treatment Diabetic Retinopathy Study (ETDRS) protocol (17), and graded in a masked fashion at the University of Wisconsin Ocular Epidemiology Reading Center using the modified Airlie House classification and the ETDRS retinopathy severity scale (Supplementary Table A1) (18–20). The maximal grade in any of the standard fields for each eye was used to classify retinopathy severity. If one eye was not gradable, it was assigned the score of the other eye. A concatenated scale for both eyes, with 60–85 collapsed to 60+, have severity scores from 10/10, 21/<21, 21/21, 31/<31, 31/31, 37/<37, 37/37, 43/<43, 43/43, 47/<47, 47/47, 53/<53, 53/53, 60+/<60+, and 60+/60+, creating 15 levels in which a person could be classified. The study end point was two-steps or more DR progression in this concatenated 15-level severity scale, which was previously shown to be clinically meaningful in predicting more severe DR lesions (21).
Other variable measurement
Blood pressure, AER, and A1C were measured before and quarterly after randomization as previously detailed (15). GFR was measured annually by plasma iohexol disappearance (22–24). The study was approved by the institutional review board at each center, and written informed consent or assent as appropriate, was obtained from all participants.
Statistical analysis
Statistical analyses were conducted using SPSS version 18.0 and SAS version 9.1. Baseline characteristics of the study cohort were compared using unpaired t test for quantitative variables and the χ2 test for categorical variables. Logistic regression analysis was used to estimate the odds ratio of two-steps or more of DR progression. Because the beneficial effects of enalapril and losartan on the progression of DR in RASS were nearly identical (13), odds ratios were estimated in combination for these treatment groups, relative to the placebo group, and were adjusted for mean blood pressure during the study, baseline characteristics, center, and baseline grade of DR according to the 15-level severity scale.
RESULTS
There were no statistically significant differences in baseline characteristics between the 223 (78%) of the 285 randomized patients who had both baseline and 5-year gradable fundus photographs and the 62 patients (22%) without these data (Table 1). A total of 147 patients (65.9%) had DR at baseline (13). The large majority of the cohort had absent or mild nonproliferative DR, whereas only 9% had moderate to severe nonproliferative DR; DR severity at baseline was not different between the placebo and treatment groups (Table 2).
Table 1.
Baseline characteristics of the study cohort according to fundus photography status
| Baseline and 5-year fundus photographs |
|||
|---|---|---|---|
| Performed* | Not performed | P value | |
| n | 223 | 62 | |
| Male | 104 (47) | 28 (45) | NS |
| Caucasian (%) | 219 (98) | 60 (97) | NS |
| Age (years) (%) | 29.9 ± 9.7 | 28.8 ± 9.9 | NS |
| Diabetes duration (years) | 11.3 ± 4.7 | 10.7 ± 5.0 | NS |
| BMI (kg/m2) | 25.7 ± 3.8 | 25.8 ± 3.4 | NS |
| Systolic blood pressure (mmHg) | 120.2 ± 11.7 | 117.7 ± 10.7 | NS |
| Diastolic blood pressure (mmHg) | 70.2 ± 8.2 | 70.0 ± 9.0 | NS |
| A1C (%) | 8.5 ± 1.6 | 8.7 ± 1.6 | NS |
| Serum creatinine (μmol/L) | 71.0 ± 12.4 | 71.6 ± 12.4 | NS |
| AER (μg/min) | 5.2 (3.5–7.8) | 4.7 (2.6–6.7) | NS |
| GFR (mL/min/1.73 m2) | 128.9 ± 20.7 | 128.0 ± 18.2 | NS |
Data are number (%) or means ± SD except for AER (median [interquartile range]). NS, not significant.
*n = 222 for A1C and serum creatinine.
Table 2.
Baseline retinopathy status according to treatment group
| Retinopathy status | Placebo | Enalapril/losartan |
|---|---|---|
| n | 74 | 149 |
| None (%) | 27 (36.5) | 49 (32.9) |
| Mild NPDR (%) | 42 (56.8) | 85 (57.0) |
| Moderate to severe NPDR (%) | 5 (6.8) | 15 (10.1) |
NPDR, nonproliferative diabetic retinopathy.
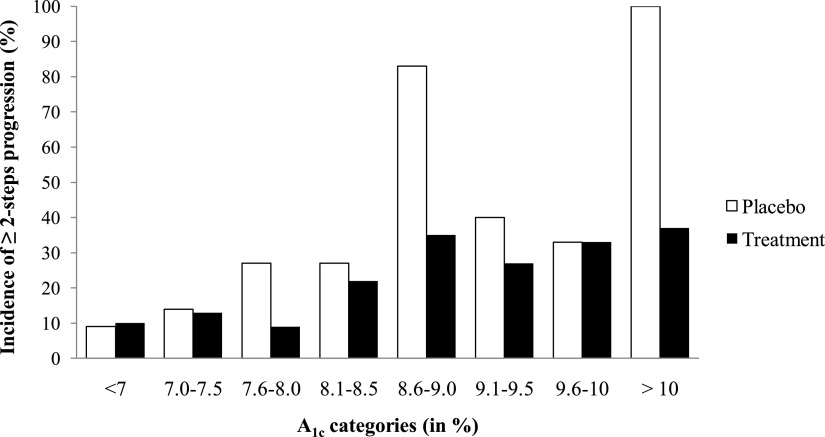
Sixty-two patients (27.8%) had at least two-steps DR progression, and, as reported, RAS blockade significantly reduced this incidence by 65% for enalapril and 70% for losartan (13). There was no statistically significant difference in A1C at baseline between patients randomized to placebo and treatment groups (8.3 vs. 8.7%, P = 0.079), nor were there differences in other baseline characteristics (data not shown). Patients with at least two-steps DR progression had higher A1C at baseline than nonprogressors (P < 0.001; Table 3). A1C (P < 0.000001) and RAS blockade (P = 0.003) were independent predictors of progression. When patients were stratified according to baseline A1C, the overall incidence of at least two-steps DR progression appeared to be increasing with worsening of glycemic control (Fig. 1). There was no detectable effect of RAS blockade on at least two-steps DR progression in patients with baseline A1C ≤7.5% (P = 0.92). In contrast, among patients with A1C greater than 7.5%, 27% on RAS blockade had at least two-steps DR progression versus 46% in the placebo group (P = 0.03), this representing a 60% reduction (Table 4). These results were not altered by adjustment for the mean of all blood pressure measurements obtained during the 5-year study. Results were similar when average A1C during 5 years, instead of baseline A1C, was used (data not shown).
Table 3.
Baseline clinical characteristics according to retinopathy progression
| No progression* | At least two-steps progression | P value | |
|---|---|---|---|
| n | 161 | 62 | |
| Male (%) | 79 (49) | 25 (40) | NS |
| Caucasian (%) | 159 (99) | 60 (97) | NS |
| Age (years) | 30.6 ± 9.8 | 28.0 ± 9.2 | NS |
| Diabetes duration (years) | 11.4 ± 4.7 | 11.1 ± 4.6 | NS |
| BMI (kg/m2) | 25.6 ± 3.6 | 26.0 ± 4.3 | NS |
| Systolic blood pressure (mmHg) | 120.3 ± 11.8 | 120.0 ± 11.3 | NS |
| Diastolic blood pressure (mmHg) | 69.7 ± 8.3 | 71.5 ± 8.0 | NS |
| A1C (%) | 8.2 ± 1.3 | 9.4 ± 1.8 | <0.001 |
| Serum creatinine (μmol/L) | 71.8 ± 12.5 | 68.9 ± 11.7 | NS |
| AER (μg/min) | 5.0 (3.5–7.5) | 5.5 (3.8–8.8) | NS |
| GFR (mL/min/1.73 m2) | 127.6 ± 20.2 | 132.1 ± 21.6 | NS |
Data are number (%) or means ± SD except for AER (median [interquartile range]).
*n = 160 for A1C and serum creatinine.
Figure 1.
Incidence of at least two-steps progression of DR in the combined treatment group vs. placebo group, according to A1C categories at baseline.
Table 4.
Effect of enalapril and losartan on DR progression according to A1C levels
| At least two-steps progression (n/total n [%]) | Adjusted odds ratio (95% CI)* | P value | |
|---|---|---|---|
| Baseline A1C ≤7.5% | |||
| Placebo | 2/18 (11) | Reference | |
| Treatment (enalapril/losartan) | 4/36 (11) | 1.16 (0.06–22.5) | 0.92 |
| Baseline A1C >7.5% | |||
| Placebo | 26/56 (46) | Reference | |
| Treatment (enalapril/losartan) | 30/112 (27) | 0.40 (0.17–0.93) | 0.03 |
*The odds ratio was adjusted for mean blood pressure during the study, baseline characteristics, center, and baseline grade on the 15-level DR severity scale.
CONCLUSIONS
Although RASS found no beneficial effects of early RAS blockade on diabetic nephropathy-related structural or functional end points, there was an approximately two-thirds reduction in two-steps or more DR progression in normoalbuminuric, normotensive type 1 diabetic patients receiving either an ACEI (enalapril) or an ARB (losartan), and this was independent of blood pressure levels during the trial (13). These analyses have now been extended to demonstrate that the detectable beneficial effects of these drugs on DR are largely dependent on glycemic control. When compared with placebo, enalapril and losartan reduced at least two-steps DR progression only in the subset of patients whose baseline A1C was greater than 7.5%. This protective effect might be specially pronounced in patients with worse glycemic control.
There is little question that improving glycemic control has a major beneficial effect on DR progression and development (4,5), and glycemia was an independent predictor of DR progression in the current study. However, in type 1 diabetes, as reported by the DCCT, maintaining an A1C less than 7% required a major effort from a dedicated research staff and highly motivated volunteer participants. After an average of 6.5 years of intensive glycemic control in the DCCT, when patients returned to their usual care as reported in the EDIC component of the study, the long-term follow-up of the DCCT cohort, the A1C approached 8%, whereas in the former DCCT conventional therapy group A1C decreased from ∼9 to 8% with the initiation of intensive insulin therapy (7). This illustrates the challenges of implementing and maintaining the recommended glycemic targets in patients with type 1 diabetes despite the development of newer types of insulin, insulin pumps, and better glucose monitoring systems. Thus, development of alternative strategies to prevent progression of DR remains relevant. The current study demonstrated that reduction in the incidence of at least two-steps DR progression with RAS blockade was greater in patients with poorer glycemic control. The risk of at least two-steps DR progression could be reduced by 60% by RAS blockade in patients whose baseline A1C was >7.5%, and this effect is independent of achieved blood pressure during the 5 years of the study and remained true if average A1C during the 5 years of the study, rather than baseline A1C, was used for the analyses.
Although our results, demonstrating protective effects of RAS blockade in patients with worse glycemic control (A1C >7.5%), appear to contrast with those previously reported in the EURODIAB controlled trial of lisinopril in insulin-dependent diabetes (EUCLID) study (12), where the patients with better glycemic control (A1C < 7%) benefited most from ACEI treatment, the two studies are not fully comparable. Patients in EUCLID were followed for a shorter time period compared with RASS (2 vs. 5 years). Moreover, in EUCLID, A1C levels at baseline and throughout the study were significantly lower in the ACEI than in the placebo group, making it difficult to evaluate any association between A1C and ACEI therapy. In fact, adjusted for baseline A1C, there was no beneficial effect of ACEI therapy in the progression of retinopathy in the EUCLID study.
RASS results (13) were consistent with those of ∼5 years DIRECT-Prevent 1 (14) where there was a strong trend (P = 0.0508) for a reduction in the primary end point, time to two-steps or more DR progression, in type 1 diabetic patients with no DR at baseline who were randomized to candesartan. There was a preventative effect of this ARB on the secondary end point, time to three-steps or more DR progression, which remained significant after adjustment for baseline A1C; however, DIRECT-Protect 1 found no protective effect of ARB on three-steps or more DR progression in patients with DR at baseline (14). The smaller numbers of patients in RASS preclude such subanalyses.
Although enalapril and losartan showed equal benefits in slowing DR progression, the normotensive, normoalbuminuric type 1 diabetic patients randomized to losartan had a higher incidence of microalbuminuria than those in the ACEI or placebo groups (13). This may be a drug-specific effect, since no differences in the rates of microalbuminuria onset between the candesartan and the placebo groups were found in the DIRECT study (25). RASS also found greater progression in some renal structural variables in losartan- versus enalapril-treated patients (13). These observations may inform the selection of RAS blocking agents to be used for DR prevention in normoalbuminuric normotensive type 1 diabetic patients.
One limitation of this study is that the rate of two-steps or more DR progression is relatively low in patients with A1C less than 7% and that RASS was underpowered to detect an effect of RAS blockade in these patients. Thus, although RASS cannot address the issue of whether there is an A1C threshold in relation to DR, it does support the addition of RAS blockade in patients whose A1C may be difficult to maintain below 7.5%.
In summary, RASS demonstrated a protective effect of renin-angiotensin blockade on the progression of DR only in the normotensive normoalbuminuric type 1 diabetic patients whose baseline A1C was greater than 7.5%. This finding may inform clinical decisions on an appropriate therapeutic approach to slowing the progression of DR.
Supplementary Material
Acknowledgments
This study was funded by research grants from the National Institutes of Health (NIH), the National Institute of Diabetes and Digestive and Kidney Diseases (grant DK-51975), Merck (U.S.), Merck Frosst (Canada), and the Canadian Institutes of Health Research (CIHR; grant DCT 14281). RASS was also supported in part by a grant from the National Center for Research Resources of the NIH, to the University of Minnesota General Clinical Research Center (GCRC) (M01-RR-00400). M.L.C. is a current recipient of a Career Development Award from the Juvenile Diabetes Research Foundation (JDRF).
M.M. reports receiving research grants from Merck and Genzyme and consulting fees from Genzyme, sanofi-aventis, and Boehringer Ingelheim. B.Z. has received research grants and consulting fees from Merck. R.K. reports being a consultant for AstraZeneca, Novartis, Takeda, GlaxoSmithKline, Pfizer, and Lilly. No other potential conflicts of interest relevant to this article were reported.
T.H. wrote the first draft of the manuscript and performed the statistical analyses. M.M. contributed to the first draft of the manuscript and revised and reviewed the final manuscript. R.K., B.Z., and A.S. revised and reviewed the final manuscript. M.L.C. contributed to the first draft of the manuscript, revised and reviewed the final manuscript, and performed the statistical analyses. All authors contributed to data review, interpretation, and the writing process. All authors reviewed and approved the report.
RASS co-investigators, in addition to the authors of this study, included Dr. Robert Gardiner and Dr. Keith Drummond, who directed the Montreal center; Dr. Sandra Donnelly (Toronto Center) and Dr. Paul Goodyer (Montreal Center), who were the center nephrologists; Dr. Samy Suissa, who was the data center director; Trudy Strand, RN (Minneapolis Center), the lead study coordinator; and Dr. Marie Claire Gubler, Paris Light Microscopy Center.
The authors thank the dedicated staff of the RASS trial in Minneapolis: J. Basgen (morphometry laboratory supervisor), J. Bucksa (central biochemistry laboratory manager), B. Chavers (central albumin laboratory director), M. Cohen and P. Stanaitis (fundus photographers), K. Johnson (pharmacist), S. Kupcho (central albumin laboratory supervisor), B. Lohr (pharmacy clinical specialist), D. Luke (pharmacy coordinator), M. Nowicki (central laboratory lead technician), K. Sawyer (central albumin laboratory junior scientist), S. Sisson-Ross (light-microscopy morphometrist), J. Stein (assistant project manager), and the GCRC staff; in Montreal: B. Maruca (trial coordinator), G. Carro-Ciampi (pharmacy coordinator), L. Marcon (fundus photographer), A. Roy (research nurse), and the GCRC staff; in Toronto: A. Barnie (trial coordinator), A. Roode and E. Vivero (research nurses), and Drs. Hertzel Gerstein and Ronnie Aronson (physicians); the Madison Ocular Epidemiology Reading Center staff: S. Meuer (grader), T. Jan (coordinator), and S. Moss (biostatistician); and the Montreal Data Center staff: D. Gaudreau (administrative assistant), V. Lucas (data entry technician), C. Delaney, S. Vahey, and S. Dell'Aniello (statisticians), and Dr. Michael Kramer (advisor); and especially the patients who volunteered for these demanding studies. The authors also thank Dr. Samy Suissa for his help with the statistical analyses in this study.
Parts of this work were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, 24–28 June 2011, San Diego, California.
Footnotes
Clinical trial reg. no. NCT00143949, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0476/-/DC1.
References
- 1.Fong DS, Aiello L, Gardner TW, et al. ; American Diabetes Association. Retinopathy in diabetes. Diabetes Care 2004;27(Suppl. 1):S84–S87 [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 2010;304:649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saaddine JB, Honeycutt AA, Narayan KM, Zhang X, Klein R, Boyle JP. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005-2050. Arch Ophthalmol 2008;126:1740–1747 [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan DM, Zinman B, Cleary PA, et al. ; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005). Arch Intern Med 2009;169:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM; UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 2004;122:1631–1640 [DOI] [PubMed] [Google Scholar]
- 9.Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002;61:1086–1097 [DOI] [PubMed] [Google Scholar]
- 10.Jackson WE, Holmes DL, Garg SK, Harris S, Chase HP. Angiotensin-converting enzyme inhibitor therapy and diabetic retinopathy. Ann Ophthalmol 1992;24:99–103 [PubMed] [Google Scholar]
- 11.Chase HP, Garg SK, Harris S, Hoops S, Jackson WE, Holmes DL. Angiotensin-converting enzyme inhibitor treatment for young normotensive diabetic subjects: a two-year trial. Ann Ophthalmol 1993;25:284–289 [PubMed] [Google Scholar]
- 12.Chaturvedi N, Sjolie AK, Stephenson JM, et al. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet 1998;351:28–31 [DOI] [PubMed] [Google Scholar]
- 13.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 2009;361:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaturvedi N, Porta M, Klein R, et al. ; DIRECT Programme Study Group. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet 2008;372:1394–1402 [DOI] [PubMed] [Google Scholar]
- 15.Mauer M, Zinman B, Gardiner R, et al. ACE-I and ARBs in early diabetic nephropathy. J Renin Angiotensin Aldosterone Syst 2002;3:262–269 [DOI] [PubMed] [Google Scholar]
- 16.Andersen S, Tarnow L, Rossing P, Hansen BV, Parving HH. Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int 2000;57:601–606 [DOI] [PubMed] [Google Scholar]
- 17.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 1991;98(Suppl.):786–806 [PubMed] [Google Scholar]
- 18.Klein R, Zinman B, Gardiner R, et al. ; Renin-Angiotensin System Study. The relationship of diabetic retinopathy to preclinical diabetic glomerulopathy lesions in type 1 diabetic patients: the Renin-Angiotensin System Study. Diabetes 2005;54:527–533 [DOI] [PubMed] [Google Scholar]
- 19.Klein BE, Davis MD, Segal P, et al. Diabetic retinopathy. Assessment of severity and progression. Ophthalmology 1984;91:10–17 [DOI] [PubMed] [Google Scholar]
- 20.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98(Suppl.):823–833 [PubMed] [Google Scholar]
- 21.Klein R, Klein BE, Moss SE. How many steps of progression of diabetic retinopathy are meaningful? The Wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthalmol 2001;119:547–553 [DOI] [PubMed] [Google Scholar]
- 22.Bröchner-Mortensen J, Giese J, Rossing N. Renal inulin clearance versus total plasma clearance of 51Cr-EDTA. Scand J Clin Lab Invest 1969;23:301–305 [DOI] [PubMed] [Google Scholar]
- 23.Gaspari F, Perico N, Remuzzi G. Application of newer clearance techniques for the determination of glomerular filtration rate. Curr Opin Nephrol Hypertens 1998;7:675–680 [DOI] [PubMed] [Google Scholar]
- 24.Gaspari F, Perico N, Matalone M, et al. Precision of plasma clearance of iohexol for estimation of GFR in patients with renal disease. J Am Soc Nephrol 1998;9:310–313 [DOI] [PubMed] [Google Scholar]
- 25.Bilous R, Chaturvedi N, Sjølie AK, et al. Effect of candesartan on microalbumiuria and albumin excretion rate in diabetes: three randomized trials. Ann Intern Med 2009;151:11–20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.