Abstract
OBJECTIVE
Endothelin (ET)-1 is a vasoconstrictor and proinflammatory peptide that may interfere with glucose uptake. Our objective was to investigate whether exogenous ET-1 affects glucose uptake in the forearm of individuals with insulin resistance and in cultured human skeletal muscle cells.
RESEARCH DESIGN AND METHODS
Nine male subjects (aged 61 ± 3 years) with insulin resistance (M value <5.5 mg/kg/min or a homeostasis model assessment of insulin resistance index >2.5) participated in a protocol using saline infusion followed by ET-1 infusion (20 pmol/min) for 2 h into the brachial artery. Forearm blood flow (FBF), endothelium-dependent vasodilatation, and endothelium-independent vasodilatation were assessed. Molecular signaling and glucose uptake were determined in cultured skeletal muscle cells.
RESULTS
ET-1 decreased forearm glucose uptake (FGU) by 39% (P < 0.05) after the 2-h infusion. ET-1 reduced basal FBF by 36% after the 2-h infusion (P < 0.05) and impaired both endothelium-dependent vasodilatation (P < 0.01) and endothelium-independent vasodilatation (P < 0.05). ETA and ETB receptor expression was detected on cultured skeletal muscle cells. One-hour ET-1 incubation increased glucose uptake in cells from healthy control subjects but not from type 2 diabetic patients. Incubation with ET-1 for 24 h reduced glucose uptake in cells from healthy subjects. ET-1 decreased insulin-stimulated Akt phosphorylation and increased phosphorylation of insulin receptor substrate-1 serine 636.
CONCLUSIONS
ET-1 not only induces vascular dysfunction but also acutely impairs FGU in individuals with insulin resistance and in skeletal muscle cells from type 2 diabetic subjects. These findings suggest that ET-1 may contribute to the development of insulin resistance in skeletal muscle in humans.
Endothelial dysfunction, characterized by reduced bioactivity of nitric oxide (NO) and increased activity of the vasoconstrictor and proinflammatory peptide endothelin (ET)-1, is an important factor promoting the development of atherosclerosis (1). Several observations demonstrate that endothelial dysfunction is present in insulin-resistant states, including diabetes, obesity, and the metabolic syndrome (1,2). Insulin exerts important vascular actions via stimulation of NO production in the endothelium, leading to vasodilatation and increased blood flow, which in turn stimulates glucose uptake in skeletal muscle (3). These antiatherogenic effects are mediated via activation of the phosphatidylinositol 3-kinase (PI3-kinase) pathway, resulting in phosphorylation of Ser-Thr kinases, such as Akt, as well as activation of endothelial NO synthase (4). Insulin resistance is associated with reduced activation of this pathway in vascular endothelial cells (5) and in skeletal muscle (6). Instead, the mitogenic-signaling pathway mediated by mitogen-activated protein (MAP) kinase (extracellular signal–related kinase [ERK] MAP) is stimulated. In endothelial cells, this change in intracellular signaling results in the stimulation of cell growth, proinflammatory effects, increased production of ET-1, and reduced bioavailability of NO (2,4). These observations indicate that endothelial dysfunction, including increased activity of ET-1, is of functional importance in insulin-resistant states.
The vascular responses to ET-1 are mediated via the two receptor subtypes, ETA and ETB (7,8). Both types of receptors are located on vascular smooth muscle cells and mediate vasoconstriction. The ETB receptor also is located on endothelial cells and mediates vasodilatation by stimulating the release of NO and prostacyclin (9). Recent studies suggest that ET-1 inhibits insulin-mediated glucose uptake via a plasma membrane–dependent mechanism. ET-1 impairs insulin-stimulated glucose transporter GLUT4 translocation in adipocytes (10,11) and decreases PI3-kinase activity via insulin receptor substrate (IRS)-2 Ser and Tyr phosphorylation in isolated vascular smooth muscle cells (12). Furthermore, ET-1 reduces peripheral glucose utilization (13) and insulin sensitivity in healthy volunteers (14). Selective ETA receptor blockade was shown to augment insulin-mediated glucose uptake in obese but not lean subjects (15). We have demonstrated that the dual ETA/ETB receptor blockade acutely increases total body glucose uptake and insulin sensitivity in obese patients with insulin resistance and coronary artery disease (16). These observations suggest that endogenous ET-1 plays a role in the regulation of glucose uptake. However, it still remains unclear whether ET receptors are expressed on skeletal muscle cells and whether ET-1 affects glucose uptake in the skeletal muscle tissue of subjects with insulin resistance.
The current study was therefore designed to investigate the direct effect of ET-1 on skeletal muscle glucose uptake and blood flow in insulin-resistant individuals in vivo. Furthermore, we aimed to identify ET-1 receptors as well as the effects of ET-1 on basal and insulin-stimulated glucose uptake and signaling in human skeletal muscle cells.
RESEARCH DESIGN AND METHODS
In vivo study.
Nine sedentary male subjects (Table 1) with insulin resistance, as determined by either hyperinsulinemic-euglycemic clamp (total body glucose uptake <6 mg/kg/min; n = 6) or homeostasis model assessment of insulin resistance (HOMA-IR >2.5; n = 3) were recruited. Participants were informed of the nature, purpose, and possible risk involved in the study before giving informed consent. The investigation was carried out in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Karolinska Institute.
TABLE 1.
Basal study subject characteristics (n = 9)
| Characteristics | |
|---|---|
| Age (years) | 62 ± 7 |
| BMI (kg/m2) | 27.8 ± 1.9 |
| M value (mg/kg/min) (n = 6) | 4.4 ± 1 |
| HOMA-IR (units) | 3.2 ± 0.2 |
| Smokers (n) | 1 |
| Ex-smokers (n) | 2 |
| Nonsmokers (n) | 6 |
| Hypertension | 4 |
| Prior myocardial infarction | 1 |
| ACE inhibitors or angiotensin receptor blockers | 2 |
| β-Blockers | 1 |
| Aspirin | 1 |
| Fasting venous plasma insulin (pmol/L) | 58.3 ± 7.8 |
| Fasting venous plasma glucose (mmol/L) | 5.4 ± 0.2 |
| Total cholesterol (mmol/L) | 5.1 ± 0.2 |
| LDL (mmol/L) | 3.2 ± 0.1 |
| HDL (mmol/L) | 1.2 ± 0.2 |
| Triglyceride (mmol/L) | 1.5 ± 0.3 |
| HbA1c (%) | 4.7 ± 0.1 |
| High-sensitivity C-reactive protein (mg/L) | 2.2 ± 0.5 |
| ET-1 (pmol/L) | 2.9 ± 0.2 |
Data are means ± SEM, unless otherwise indicated.
Blood flow measurements.
Investigations were performed with subjects in the supine position in a quiet laboratory with controlled temperature. Subjects arrived at the laboratory at 8:00 a.m., having fasted since 10:00 p.m. the night before the investigation. Subjects were instructed not to use caffeine- or nicotine-containing products on the day of the study. A percutaneous catheter was inserted under local anesthesia in the proximal direction into the brachial artery of the nondominant arm for infusions and blood sampling. Another catheter was inserted in the distal direction of a deep cubital vein, draining mainly skeletal muscle tissue (17), of the ipsilateral arm for collection of blood samples. Forearm blood flow (FBF) was measured simultaneously in both arms by venous occlusion plethysmography, using a mercury-in-silastic strain gauge applied around the widest part of the forearm (18). A cuff placed around the upper arm was inflated to 50 mmHg for 10 s to obstruct the venous outflow during the recording of FBF. The circulation of the hands was occluded by inflating a wrist cuff to 30 mmHg above systolic blood pressure.
Study protocol.
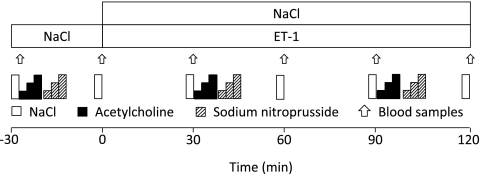
The in vivo protocol is illustrated in Fig. 1. After insertion of the catheters, an infusion of saline (60 mL/h) was started, followed by an infusion of ET-1 (20 pmol/min) for 120 min. Endothelium-dependent vasodilatation was determined by infusion of acetylcholine (3, 10, and 30 μg/min) at a rate of 2.5 mL/min into the brachial artery before and during the ET-1 infusion. This was followed by an infusion of the NO donor sodium nitroprusside (SNP; 1, 3, and 10 μg/min) for determination of endothelium-dependent vasodilatation. All infusions were given into the brachial artery for 2 min at each dose. Arterial and venous blood samples for the determination of glucose and insulin levels were collected repeatedly during the study protocol. The wrist cuff was inflated 2 min before blood sampling to exclude the hand circulation. Blood samples were immediately centrifuged (2,500 rpm for 15 min at +4°C), and plasma was stored at −80°C. Plasma glucose was analyzed by a timed end point method using glucose reagent in conjunction with the Synchron LX System (Beckman Coulter, Fullerton, CA), with a precision corresponding to an SD ±0.11 mmol/L. Plasma insulin was analyzed using an electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany) for the Elecsys analyzer.
FIG. 1.
Study protocols. Arrows indicate periods of blood sampling.
In vitro study
Cell culture.
Muscle cell cultures were established from two separate cohorts of subjects. Muscle biopsies (rectus abdominis) were obtained from 19 subjects without any metabolic disorders during planned abdominal surgery (aged 58 ± 3 years, BMI <25 kg/m2). To ascertain differences between type 2 diabetic and healthy control subjects, muscle biopsies (vastus lateralis) were also obtained from 11 type 2 diabetic male subjects (aged 60 ± 2 years, BMI 29 ± 1 kg/m2, HOMA 4.3 ± 1.9) and 11 male subjects with normal glucose tolerance (NGT) (aged 64 ± 1 years, BMI 28 ± 1 kg/m2, HOMA 2.0 ± 0.5) under local anesthesia (5 mg/mL lidocaine hydrochloride) after an overnight fast. In both cases, muscle biopsies were collected in ice-cold PBS supplemented with 1% PeSt (100 units/mL penicillin and 100 µg/mL streptomycin). Satellite cells were isolated and cultured to form myotubes as described previously (19). Myotubes at passages 4 and 5 were incubated in serum-free medium overnight before each experiment. ET-1 (1, 10, and 100 nmol/L) or vehicle was added for the times indicated in the absence or presence of the ETA/ETB receptor antagonist bosentan (3 μmol/L). The antagonist was always added 30 min before ET-1. Dual blockade was chosen because a previous study demonstrated that dual ETA/ETB but not selective ETA receptor blockade increased insulin sensitivity in obese patients with insulin resistance (16). Control cells were exposed to vehicle for the same length of time. Where indicated, insulin (60 nmol/L) was added for 30 min.
2-Deoxy-glucose uptake in cultured cells.
Glucose uptake in the same cohort of subjects with type 2 diabetes and NGT was determined as described previously (19). Overnight serum-starved myotubes were incubated with 10 nmol/L ET-1 for 1, 2, 6, and 24 h and stimulated with or without 60 nmol/L insulin in serum-free DMEM for 60 min, followed by a 15-min incubation with 2-deoxy-d-glucose [1,2–3H(N)] (0.33 µCi/mL) and 10 nmol/L cold 2-deoxy-d-glucose in glucose-free DMEM. Each experiment was carried out on triplicate wells.
Western blot analysis.
Expression of ETA and ETB receptors, Akt, IRS-1, ERK, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and AMPK, was quantified in lysates from skeletal muscle cell cultures using Western blotting. Aliquots of lysates (20 µg protein) were mixed in Laemmli sample buffer containing β-mercaptoethanol. Proteins were separated by 7.5% SDS-PAGE, transferred to polyvinylidiflroride membrane (Millipore, Bedford, MA), and blocked in 7.5% nonfat dried milk in Tris-buffered saline with 0.02% Tween 20 for 2 h at room temperature. Membranes were incubated overnight at 4°C with antibodies against human ETA and ETB receptors (1:200; Alomone Laboratories, Jerusalem, Israel), phosphospecific antibodies against phospho-IRS-1 Ser636 (1:1,000), phospho-Akt Ser473 (1:1,000), phospho-IRS-1 Tyr612 (1:1,000), phospho-Akt Ser473 (1:1,000), phospho-Akt Thr308 (1:1,000), phospho-AMPK Thr172 (1:1,000), and phospho-ERK1/2 (1:1,000) (all from Cell Signaling Technology, Beverly, MA). Anti-ETA and anti-ETB antibodies, preincubated with control peptide antigen (Alomone Laboratories), were used to assess the specificity of ET receptor bands. After washing in Tris-buffered saline with 0.02% Tween 20, the membranes were incubated with horseradish peroxidase anti-rabbit IgG for all target proteins (1:25,000; Bio-Rad, Hercules, CA) for 1 h at room temperature, followed by additional washing. Proteins were visualized by enhanced chemiluminescence (Amersham, Arlington Heights, IL) and quantified using densitometry and Molecular Analyst Software (Bio-Rad, Richmond, CA).
Substances.
ET-1 (Alexis Biochemicals, Lausanne, Switzerland) was dissolved in sterile 0.9% NaCl, sterile-filtrated through a Millipore filter, tested for bacterial toxins and sterility, and stored frozen at −80°C. Bosentan (courtesy of Dr. Martine Clozel, Actelion Pharmaceuticals, Allschwil, Switzerland) was dissolved in double-distilled water, stored frozen at −80°C, and then diluted to the proper concentration in cell culture media on the day of the experiments. F12-DMEM, FBS, penicillin, streptomycin, and fungizone were obtained from Invitrogen (Stockholm, Sweden). Insulin (Actrapid) was obtained from Novo Nordisk (Bagsværd, Denmark).
Calculations and statistics.
Data are presented as means ± SEM. FBF was calculated as the mean of four to eight inflow recordings during 2 min. During the vasodilator response to acetylcholine and SNP, the four highest flow recordings at the end of the infusion were used for calculations. Because no infusions affected blood pressure, all hemodynamic effects during acetylcholine and SNP are expressed as absolute blood flow changes. Forearm glucose uptake (FGU) was calculated according to the following formula: (arterial − venous glucose conc.) × blood flow × (1 − Htc). Changes in FBF from baseline were assessed by one-way ANOVA for repeated measurements with Dunnett’s post hoc analysis. Differences in FBF change in response to different doses of acetylcholine and SNP were assessed by two-way ANOVA. Changes in FGU and arteriovenous glucose differences were assessed by one-way ANOVA for repeated measurements with the Bonferroni multiple comparison test. Differences in percentage change of phosphorylated proteins were calculated using the Wilcoxon test or the one-sample t test. Differences in protein expression were compared by the Mann-Whitney U test test. A value of P < 0.05 was considered significant.
RESULTS
Study subjects.
The basal characteristics of the study subjects receiving ET-1 infusion are summarized in Table 1. The subjects were overweight and had slightly elevated LDL cholesterol. Four of the subjects had hypertension, and one had a history of previous myocardial infarction.
Blood flow.
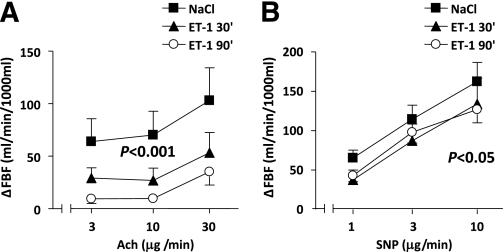
Blood pressure and heart rate did not change significantly during the protocol. During the administration of ET-1, FBF was reduced by 30% (P < 0.01) at 60 min and by 36% (P < 0.05) at 120 min of infusion, compared with basal values. Infusion of ET-1 markedly inhibited acetylcholine-induced vasodilatation (P < 0.001) (Fig. 2A). In addition, the vasodilator response to SNP was slightly but significantly attenuated by ET-1 (P < 0.05) (Fig. 2B).
FIG. 2.
Change in FBF induced by different doses of acetylcholine (Ach) (A) and SNP (B) during NaCl infusion and after 30 and 90 min of ET-1 infusion. Significant differences between the responses to Ach and SNP during saline and after the ET-1 infusion are by two-way ANOVA. Data are means ± SEM (n = 9).
FGU.
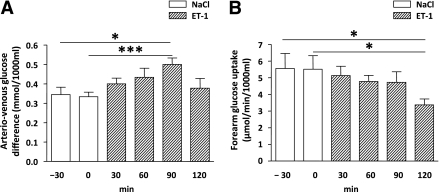
Infusion of NaCl for 30 min did not affect FGU. ET-1 infusion increased the arteriovenous glucose difference by 67% (P < 0.05) at 90 min, followed by a decrease to basal values at 120 min (Fig. 3A). Infusion of ET-1 decreased FGU from 5.8 ± 2.0 μmoL/min × 1,000 mL at baseline (saline) to 3.4 ± 0.8 μmoL/min × 1,000 mL at 120 min (P < 0.05) (Fig. 3B). During the protocol, arterial insulin concentrations decreased from 81.0 ± 11.0 pmol/L at baseline to 65.7 ± 7.5 pmol/L at 120 min of ET-1 infusion (P < 0.05). The arteriovenous concentration difference of insulin remained unchanged. There was no correlation between the reductions in arterial insulin concentration and FGU (r = 0.05, P = 0.91).
FIG. 3.
A: Arteriovenous glucose differences during NaCl infusion and after a 120-min infusion of ET-1. B: FGU during NaCl infusion and after a 120-min infusion of ET-1. *P < 0.05; ***P < 0.01 by one-way ANOVA. Data are means ± SEM (n = 9).
ET-1 effects on cultured skeletal muscle glucose uptake.
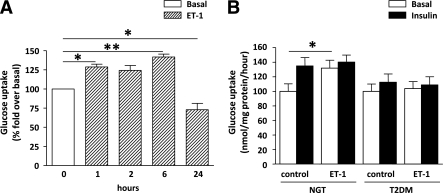
Acute (1 h) exposure to ET-1 increased glucose uptake in skeletal muscle cells established from subjects with NGT (Fig. 4A and B), and this effect persisted at 2 and 6 h (P < 0.05) (Fig. 4A). At 24 h, ET-1 induced a significant reduction in glucose uptake (Fig. 4A). ET-1 did not further increase insulin-stimulated glucose uptake (Fig. 4B). In contrast, ET-1 or insulin did not increase glucose uptake in cells from subjects with type 2 diabetes (Fig. 4B).
FIG. 4.
A: Glucose uptake in skeletal muscle cells at the basal state and after 1-, 2-, 6-, and 24-h treatment with 10 nmol/L ET-1. *P < 0.05; **P < 0.01. Data are means ± SEM (n = 3–5). B: Basal and insulin-stimulated glucose uptake in cells established from subjects with NGT (n = 11) and subjects with type 2 diabetes (T2DM) (n = 11) under basal conditions and after a 1-h ET-1 treatment. *P < 0.05 by Student t test. Data are means ± SEM.
Insulin signaling in cultured skeletal muscle cells.
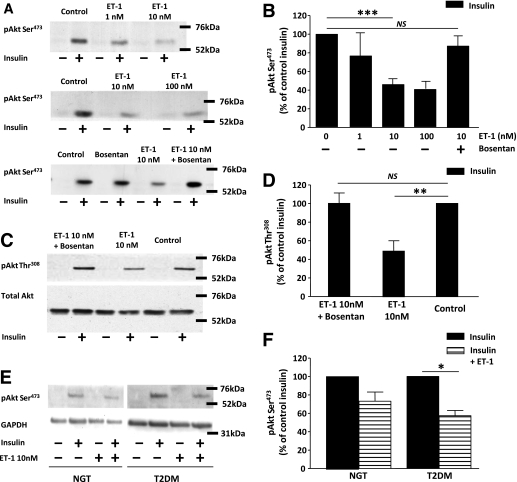
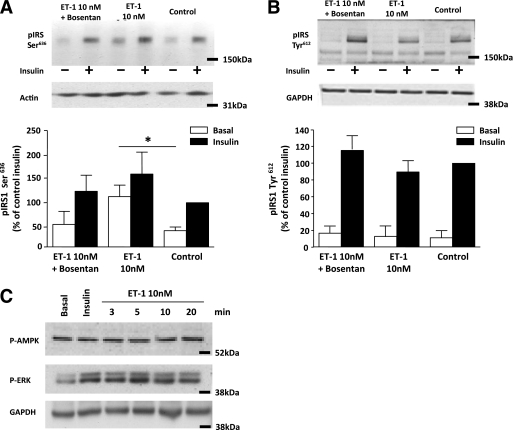
ET-1 decreased insulin-stimulated Akt phosphorylation in a dose-dependent manner (Fig. 5A and B). The inhibitory effect of ET-1 on insulin-induced Akt phosphorylation at both Ser473 (P < 0.001) (Fig. 5B) and Thr308 (P < 0.005) (Fig. 5D) sites was blocked by the ET receptor antagonist bosentan (Fig. 5A–D). This inhibitory effect of ET-1 exposure was evident in cells established from both subjects with NGT and subjects with type 2 diabetes (Fig. 5E and F). ET-1 increased phosphorylation of IRS-1 Ser636, an effect that was blocked by bosentan (Fig. 6A). Insulin increased IRS-1 Tyr612 phosphorylation, and ET-1 treatment did not alter this effect of insulin (Fig. 6B). ET-1 also transiently increased phosphorylation of ERK1/2, peaking at 5 min and returning to baseline by 20 min (Fig. 6C). No effect of ET-1 was noted on phosphorylation of AMPK at time points from 3 to 20 min (Fig. 6C) up to 1 h (data not shown).
FIG. 5.
A and B: Basal and insulin-stimulated expression of phosphorylated Akt at the Ser473 site, under control conditions and after a 1-h exposure to ET-1 (1, 10, and 100 nmol/L) in the absence and presence of the ET receptor antagonist bosentan (3 μmol/L). ***P < 0.001. NS, not significant by Wilcoxon test. Data are means ± SEM (n = 4–19). C and D: Basal and insulin-stimulated expression of phosphorylated Akt at the Thr308 site in the absence and presence of the ET receptor antagonist bosentan (3 μmol/L). Total Akt expression was used as a loading control (n = 8). **P < 0.01 by Wilcoxon test. Data are means ± SEM (n = 7). E and F: Insulin-stimulated expression of phosphorylated Akt under control conditions and after a 1-h exposure to ET-1 (10 nmol/L) in cells established from subjects with NGT (n = 5) and subjects with type 2 diabetes (T2DM) (n = 4). Total expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. *P < 0.05 by one-sample t test. Data are means ± SEM.
FIG. 6.
A: Basal and insulin-stimulated expression of phosphorylated IRS-1 at the Ser636 site, under control conditions and after a 1-h exposure to ET-1 in the absence and presence of the ET receptor antagonist bosentan (3 μmol/L). *P < 0.05 by Mann-Whitney U test. Data are means ± SEM (n = 10). B: Basal and insulin-stimulated expression of phosphorylated IRS-1 at the Tyr612 site, under control conditions and after a 1-h exposure to ET-1 in the absence and presence of the ET receptor antagonist bosentan (3 μmol/L). Data are means ± SEM (n = 8). C: Phosphorylation of ERK1/2 and AMPK under control conditions and 3, 5, 10, and 20 min of ET-1 (10 nmol/L) treatment. Total expression of actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control.
ET receptor expression in cultured skeletal muscle cells.
Western blot analysis revealed expression of ETA and ETB receptors in skeletal muscle cell cultures (Fig. 7A and B). ETA and ETB receptor expression did not significantly differ between cells established from subjects with type 2 diabetes or NGT (Fig. 7A and B).
FIG. 7.
Western blot analysis of ETA (A) and ETB (B) receptor expression in cells established from subjects with NGT (n = 8) and subjects with type 2 diabetes (T2DM) (n = 8). Data are means ± SEM.
DISCUSSION
Insulin resistance is associated with endothelial dysfunction and increased plasma levels of ET-1 (20). We recently demonstrated that dual ETA/ETB receptor blockade increases total-body glucose uptake and insulin sensitivity in obese patients with insulin resistance and coronary artery disease (16). This is supported by the observation that selective ETA receptor blockade increased glucose uptake during a hyperinsulinemic-euglycemic clamp in obese individuals (17). The current study tested the hypothesis that exogenous ET-1 reduces glucose uptake in the skeletal muscle tissue of subjects with insulin resistance. Exogenous infusion of ET-1 resulted in a significant reduction in FGU after 2 h of infusion. However, arteriovenous glucose concentrations were significantly increased during the first 90 min of ET-1 infusion and then returned to basal levels at 2 h. This change in arteriovenous glucose concentrations may be independent of the decrease in blood flow, because similar reductions in FBF were observed at 90 and 120 min of ET-1 infusion (30 and 36%, respectively). This could indicate an initial ET-1–dependent stimulation of glucose uptake into skeletal muscle, which may be obscured by the parallel reduction in blood flow, and thereby the overall FGU was unchanged.
During the study protocol, we observed a reduction in arterial insulin levels by 19%. The underlying cause for this change is unknown, but this could contribute to the reduction in glucose uptake. The lack of constant insulin levels during the experiment is a limitation of the current study. We did not include constant intra-arterial insulin infusion into the study protocol because this is known to increase blood flow in an NO-dependent manner and to stimulate glucose uptake in skeletal muscle (21). We recently demonstrated that infusion of ET-1 in combination with insulin did not affect FGU in the forearm of patients with insulin resistance (22). The reduction in arterial insulin concentrations did not result in a decrease of arteriovenous glucose concentrations below the basal level observed during the initial 30 min of saline infusion. This observation suggests that the moderate fall in insulin levels did not significantly affect local FGU, per se. Furthermore, in the current study, there was no correlation between the reduction in insulin levels and the ~40% reduction noted in FGU. Thus, the reduction in FGU may be related to other factors, such as the direct effects of ET-1 on insulin signaling and blood flow. From the present data, it cannot be determined whether the effect of ET-1 on FGU in vivo is the result of a direct effect on skeletal muscle cells or secondary to the reduction in blood flow.
To gain additional insight into the complex nature of these changes in vivo, we examined the effect of ET-1 in cultured human skeletal muscle, giving us an opportunity to determine any direct and flow-independent effects of ET-1 on glucose uptake. In skeletal muscle cells from healthy subjects with NGT, acute ET-1 exposure (up to a 6-h exposure) increased glucose uptake in agreement with previous observations in rodent muscle (23). At 24 h, however, ET-1 significantly reduced glucose uptake. This implies that prolonged exposure to ET-1 reduces glucose uptake, which is in agreement with the present in vivo findings and our previous observations that the ET receptor blockade increased basal glucose uptake in insulin-resistant individuals in vivo (22) when sustained increased levels of ET-1 have been noted. Twenty-four-hour ET-1 exposure reduced both basal and insulin-stimulated glucose uptake in cultured muscle cells while maintaining an insulin response, suggesting that at this time point there is an overall reduction in basal glucose transport (22). Of interest, ET-1 did not affect glucose uptake in cells cultured from individuals with type 2 diabetes, indicating a quantitative difference regarding the effect of ET-1 on glucose metabolism in insulin-sensitive and insulin-resistant states. We observed that both ETA and ETB receptors are expressed in skeletal muscle cells, suggesting a direct effect of ET-1 that could be mediated by both subtypes of receptor.
We also aimed to dissect molecular signaling events underlying the ET-1 effects on glucose uptake. ET-1 decreased insulin-stimulated Akt phosphorylation in a dose-dependent manner. This finding is supported by previous observations in rat skeletal muscle and vascular smooth muscle cells, suggesting that ET-1 inhibits activation of the PI3-kinase pathway and insulin-stimulated Akt phosphorylation (12,23). In primary human skeletal muscle cells, Akt Ser473 phosphorylation primarily is a reflection of phosphorylation on Akt2, whereas Thr308 represents both Akt1 and Akt2 (24). Because ET-1 exerts similar effects on both Akt phosphorylation sites, this implies that the effect of ET-1 to reduce insulin-stimulated Akt phosphorylation is evident for both Akt1/Akt2, with Akt2 making the most significant contribution. ET-1 increased in IRS-1 Ser phosphorylation, which could be prevented by the ET receptor antagonist bosentan. Phosphorylation of IRS-1 on Ser636 is a negative signal, which acts as a negative-feedback control mechanism decreasing IRS-1 tyrosine phosphorylation after prolonged insulin exposure (25). Insulin also induces phosphorylation of IRS-1 at several different tyrosine sites (25), with Tyr612 being important for subsequent activation of PI3-kinase and activation of Akt. However, ET-1 treatment had no effect on insulin-induced IRS-1 Tyr612 phosphorylation, suggesting that the ET-1–mediated impairment noted on Akt phosphorylation and glucose transport is independent of PI3-kinase activation. This is in agreement with results in adipocytes, where ET-induced impairment in glucose transport has been shown to be mediated via PI3-kinase–independent mechanisms (11,26). Furthermore, in skeletal muscle cells, a reduction in glucose uptake was only evident after longer ET-1 exposure (24 h) in cells derived from subjects with NGT. Thus, although reduced Akt phosphorylation may contribute to the observed reduction in glucose uptake, it is likely that deregulation of additional pathways are required for ET-induced impairment in skeletal muscle glucose uptake.
We hypothesized that the acute ET-1–mediated increase in glucose uptake noted in cultured skeletal muscle would be a result of a transient stress response because ET-1 alone did not increase Akt phosphorylation and thus is unlikely to use an insulin-like signaling cascade. However, ET-1 exposure of skeletal muscle to ET-1 did not increase phosphorylation of AMPK, and the molecular signal mediating the increased glucose uptake remains to be determined. Acute exposure of skeletal muscle cells to ET-1 increased phosphorylation of ERK MAP kinase, which is known to be associated with cell proliferation and migration as well as excessive formation of reactive oxygen species (27).
In the current study, we show that ET-1 impairs both endothelium-dependent and endothelium-independent vasodilatation in subjects with insulin resistance. This result extends our previous findings that exogenous ET-1 markedly reduces endothelium-dependent vasodilatation in healthy subjects (18). This effect of ET-1 is independent of its vasoconstrictor effects (18). Furthermore, ET receptor blockade acutely improves endothelium-dependent vasodilatation in subjects with insulin resistance (28) and atherosclerosis (18,29). Altogether, these observations suggest that elevated levels of ET-1, in addition to alterations in glucose uptake, contribute to endothelial dysfunction in subjects with insulin resistance.
In conclusion, the current study demonstrates that prolonged exposure to ET-1 impairs glucose uptake both in vivo and in cultured skeletal muscle cells. In cultured human muscle established from healthy subjects, ET-1 induces a biphasic response, with an initial stimulation, followed by a reduction, in glucose uptake. ET-1 does not elicit an increase in glucose uptake in muscle cells established from type 2 diabetic subjects. Furthermore, ET-1 interferes with insulin signaling in cultured skeletal muscle cells, reducing Akt phosphorylation and increasing phosphorylation of IRS-1 Ser636. These observations indicate that ET-1 signaling may exacerbate metabolic dysregulation in insulin resistance.
ACKNOWLEDGMENTS
This study was supported by the Swedish Research Council (10857 and 12669); the Swedish Heart and Lung Foundation; the Novo Nordisk Foundation; the Hedlund Foundation; the State of Sao Paulo Research Foundation, Brazil; the Swedish Diabetes Association; the King Gustav Vth and Queen Victoria Foundation; the Johan and Jakob Söderbergs Foundation; the Cardiovascular Program at the Karolinska Institute, and the Stockholm County Council. No other potential conflicts of interest relevant to this article were reported.
A.S. and F.S. wrote the manuscript, researched the data, and contributed to the discussion. D.E.D.-G. researched the data. F.B., T.G., J.P., and A.K. contributed to the discussion and reviewed and edited the manuscript. E.R. researched the data and contributed to the discussion.
REFERENCES
- 1.Böhm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res 2007;76:8–18 [DOI] [PubMed] [Google Scholar]
- 2.Jansson PA. Endothelial dysfunction in insulin resistance and type 2 diabetes. J Intern Med 2007;262:173–183 [DOI] [PubMed] [Google Scholar]
- 3.Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diab Rep 2003;3:279–288 [DOI] [PubMed] [Google Scholar]
- 4.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 2007;28:463–491 [DOI] [PubMed] [Google Scholar]
- 5.Zeng G, Nystrom FH, Ravichandran LV, et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 2000;101:1539–1545 [DOI] [PubMed] [Google Scholar]
- 6.Krook A, Björnholm M, Galuska D, et al. Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes 2000;49:284–292 [DOI] [PubMed] [Google Scholar]
- 7.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature 1990;348:730–732 [DOI] [PubMed] [Google Scholar]
- 8.Sakurai T, Yanagisawa M, Takuwa Y, et al. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature 1990;348:732–735 [DOI] [PubMed] [Google Scholar]
- 9.de Nucci G, Thomas R, D’Orleans-Juste P, et al. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci USA 1988;85:9797–9800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strawbridge AB, Elmendorf JS. Phosphatidylinositol 4,5-bisphosphate reverses endothelin-1-induced insulin resistance via an actin-dependent mechanism. Diabetes 2005;54:1698–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strawbridge AB, Elmendorf JS. Endothelin-1 impairs glucose transporter trafficking via a membrane-based mechanism. J Cell Biochem 2006;97:849–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang ZY, Zhou QL, Chatterjee A, et al. Endothelin-1 modulates insulin signaling through phosphatidylinositol 3-kinase pathway in vascular smooth muscle cells. Diabetes 1999;48:1120–1130 [DOI] [PubMed] [Google Scholar]
- 13.Ahlborg G, Weitzberg E, Lundberg JM. Endothelin-1 infusion reduces splanchnic glucose production in humans. J Appl Physiol 1994;77:121–126 [DOI] [PubMed] [Google Scholar]
- 14.Ottosson-Seeberger A, Lundberg JM, Alvestrand A, Ahlborg G. Exogenous endothelin-1 causes peripheral insulin resistance in healthy humans. Acta Physiol Scand 1997;161:211–220 [DOI] [PubMed] [Google Scholar]
- 15.Lteif A, Vaishnava P, Baron AD, Mather KJ. Endothelin limits insulin action in obese/insulin-resistant humans. Diabetes 2007;56:728–734 [DOI] [PubMed] [Google Scholar]
- 16.Ahlborg G, Shemyakin A, Böhm F, Gonon A, Pernow J. Dual endothelin receptor blockade acutely improves insulin sensitivity in obese patients with insulin resistance and coronary artery disease. Diabetes Care 2007;30:591–596 [DOI] [PubMed] [Google Scholar]
- 17.Rabinowitz D, Zierler KL. Forearm metabolism in obesity and its response to intra-arterial insulin: evidence for adaptive hyperinsulinism. Lancet 1961;2:690–692 [DOI] [PubMed] [Google Scholar]
- 18.Böhm F, Ahlborg G, Pernow J. Endothelin-1 inhibits endothelium-dependent vasodilatation in the human forearm: reversal by ETA receptor blockade in patients with atherosclerosis. Clin Sci (Lond) 2002;102:321–327 [PubMed] [Google Scholar]
- 19.Al-Khalili L, Chibalin AV, Kannisto K, et al. Insulin action in cultured human skeletal muscle cells during differentiation: assessment of cell surface GLUT4 and GLUT1 content. Cell Mol Life Sci 2003;60:991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheatcroft SB, Williams IL, Shah AM, Kearney MT. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med 2003;20:255–268 [DOI] [PubMed] [Google Scholar]
- 21.Vincent MA, Clerk LH, Lindner JR, et al. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 2004;53:1418–1423 [DOI] [PubMed] [Google Scholar]
- 22.Shemyakin A, Salehzadeh F, Böhm F, et al. Regulation of glucose uptake by endothelin-1 in human skeletal muscle in vivo and in vitro. J Clin Endocrinol Metab 2010;95:2359–2366 [DOI] [PubMed] [Google Scholar]
- 23.Wilkes JJ, Hevener A, Olefsky J. Chronic endothelin-1 treatment leads to insulin resistance in vivo. Diabetes 2003;52:1904–1909 [DOI] [PubMed] [Google Scholar]
- 24.Bouzakri K, Zachrisson A, Al-Khalili L, et al. siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab 2006;4:89–96 [DOI] [PubMed] [Google Scholar]
- 25.Gual P, Le Marchand-Brustel Y, Tanti J-F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 2005;87:99–109 [DOI] [PubMed] [Google Scholar]
- 26.Elmendorf JS. Signals that regulate GLUT4 translocation. J Membr Biol 2002;190:167–174 [DOI] [PubMed] [Google Scholar]
- 27.Avogaro A, de Kreutzenberg SV, Fadini GP. Oxidative stress and vascular disease in diabetes: is the dichotomization of insulin signaling still valid? Free Radic Biol Med 2008;44:1209–1215 [DOI] [PubMed] [Google Scholar]
- 28.Shemyakin A, Böhm F, Wagner H, Efendic S, Båvenholm P, Pernow J. Enhanced endothelium-dependent vasodilatation by dual endothelin receptor blockade in individuals with insulin resistance. J Cardiovasc Pharmacol 2006;47:385–390 [DOI] [PubMed] [Google Scholar]
- 29.Böhm F, Beltran E, Pernow J. Endothelin receptor blockade improves endothelial function in atherosclerotic patients on angiotensin converting enzyme inhibition. J Intern Med 2005;257:263–271 [DOI] [PubMed] [Google Scholar]