Abstract
Objectives
To investigate the association between the duration of exclusive breast feeding and the development of asthma related outcomes in children at age 6 years.
Design
Prospective cohort study.
Setting
Western Australia.
Subjects
2187 children ascertained through antenatal clinics at the major tertiary obstetric hospital in Perth and followed to age 6 years.
Main outcome measures
Unconditional logistic regression to model the association between duration of exclusive breast feeding and outcomes related to asthma or atopy at 6 years of age, allowing for several important confounders: sex, gestational age, smoking in the household, and early childcare.
Results
After adjustment for confounders, the introduction of milk other than breast milk before 4 months of age was a significant risk factor for all asthma and atopy related outcomes in children aged 6 years: asthma diagnosed by a doctor (odds ratio 1.25, 95% confidence interval 1.02 to 1.52); wheeze three or more times since 1 year of age (1.41, 1.14 to 1.76); wheeze in the past year (1.31, 1.05 to 1.64); sleep disturbance due to wheeze within the past year (1.42, 1.07 to 1.89); age when doctor diagnosed asthma (hazard ratio 1.22, 1.03 to 1.43); age at first wheeze (1.36, 1.17 to 1.59); and positive skin prick test reaction to at least one common aeroallergen (1.30, 1.04 to 1.61).
Conclusion
A significant reduction in the risk of childhood asthma at age 6 years occurs if exclusive breast feeding is continued for at least the 4 months after birth. These findings are important for our understanding of the cause of childhood asthma and suggest that public health interventions to optimise breast feeding may help to reduce the community burden of childhood asthma and its associated traits.
Key messages
Asthma is the leading cause of admission to hospital in Australian children and its prevalence is increasing
Whether breast feeding protects against asthma or atopy, or both, is controversial
Asthma is a complex disease, and the relative risks between breast feeding and asthma or atopy are unlikely to be large; this suggests the need for investigation in a large prospective birth cohort with timely assessment of atopic outcomes and all relevant exposures
Exclusive breast feeding for at least 4 months is associated with a significant reduction in the risk of asthma and atopy at age 6 years and with a significant delay in the age at onset of wheezing and asthma being diagnosed by a doctor
Public health interventions to promote an increased duration of exclusive breast feeding may help to reduce the morbidity and prevalence of childhood asthma and atopy
Introduction
Asthma is the leading cause of admission to hospital in Australian children and its prevalence is increasing.1,2 Susceptibility to asthma may be increased by factors present early in life.3 These include being male, low birth weight, preterm birth, young maternal age, maternal smoking and, possibly, early cessation of exclusive breast feeding.4 Environmental allergens including house dust mite, grasses, or pollens may also cause sensitisation. Conversely, early exposure to respiratory infections may be protective.5,6
Environmental exposures in the early months of life are critical for the development of the immune system but have the potential to predispose to allergy or atopy.7,8 Breast feeding may be an important determinant of the immune response,9,10 but whether breast feeding protects against asthma or atopy, or both, is controversial.11
A prospective study of children from birth to 17 years concluded that exclusive breast feeding protects against atopic disease throughout childhood and into adolescence.4 Although this study was comparatively small, a larger study of children from birth to 7 years reported that the probability of respiratory symptoms (wheeze, breathlessness, or cough) occurring at or before age 7 was reduced in children exclusively breast fed for at least 15 weeks.12 Another study13 showed a protective effect of breast feeding on atopy only when the age of the infant when other milk was introduced was considered.14 Several studies have, however, failed to show an association between breast feeding and either asthma or atopy.15–17 Halpern et al17 reported childhood allergy to be equally common in children fed human, soy, or cows’ milk. Breast feeding has also been reported to have no effect on the incidence of eczema,13 atopic disease, or raised IgE concentrations and may only be protective in non-atopic children.18 In one review, Kramer19 concluded that many studies, both negative and positive, exhibited significant flaws, and that future studies should improve both biological and methodological aspects of design and analysis.
If exclusive breast feeding isprotective against childhood asthma it requires investigation in a large childhood cohort followed prospectively from birth, with assessment of both exposures and outcomes. We report the results of a unique study in Western Australian of this type.
Subjects and methods
Study population
The Western Australian pregnancy cohort study is a prospective birth cohort initially established (1989-92) as a randomised trial, which showed that pregnancy outcome was not improved in women who had multiple ultrasonography.20 Recruitment to the study was from antenatal clinics at King Edward Memorial Hospital and nearby private practices. A total of 2979 children were enrolled by 18 weeks of gestation. Of 2860 live births, 13 have since died and 121 have been lost to follow up. The parents of 124 children declined follow up or withdrew at a later date. Overall, 2602 (91.0%) of the liveborn children remained available for follow up at 6 years of age.
Questionnaires
At enrolment, parents completed a questionnaire about the general health of the study child. Data recorded at birth included sex, gestational age, birth weight, and smoking within the household. Parents kept a diary of their child’s health in the first year. When the children were 1 year old, the parents completed a standardised questionnaire that included items about feeding: 2411 questionnaires were returned (84.3% of the liveborn cohort, 92.7% of those consenting to follow up), and 2365 children (82.7%, 90.9%) attended for clinical assessment.
Shortly before a child was 6 years old (71 (SD 6.6) months), the parents were contacted and sent another questionnaire, which included items about smoking in the household, family history of respiratory symptoms, and illnesses in the study child. Questionnaires were returned for 2187 children (76.5% of the liveborn cohort, 84.1% of children available for follow up). Results of skin prick tests were available for 1598 (61.4%) children (79.4% of 2012 children examined).
Outcomes
Childhood asthma is a complex phenotype, and several phenotypic definitions were applied in children aged 6 years. These included cumulative incidence measures—asthma diagnosed by a doctor and wheeze three or more times since age 1 year—and point prevalence measures—wheeze in the past year, sleep disturbance due to wheeze in the past year,2 and objective atopy defined by the results of a skin prick test. A child was categorised atopic if the wheal to one or more aeroallergens (house dust mite, ryegrass, cat dander, and aspergillus mould) was ⩾2 mm and larger than a control 10 minutes after testing. A 2 mm cut off point was used rather than 3 mm, which is more usual in studies of adults, because wheal size increases with age.21
Exposures
Exposure to breast feeding was measured in two ways: the duration of breast feeding and the duration of exclusive breast feeding (child’s age when other milk was introduced). These were analysed both as continuous and binary variables and were highly correlated (P<0.001). Over a range of models, the duration of exclusive breast feeding was found to be a better determinant of outcome than the duration of breast feeding, and child’s age at which other milk was introduced became the key exposure variable. Given the statistical power, we elected for conservatism and based the primary analysis on a binary variable. Dichotomisation was at 4 months, the integer cut off point closest to the median; this choice was statistically powerful and bioclinically logical.12
Potential confounding exposures included sex, gestational age, being of Aboriginal descent, and smoking in the household. These were modelled as binary covariates. Smoking in the household was defined as positive if ⩾1 cigarettes a day were smoked inside the house. Maternal education and family income were modelled as categorical factors. Maternal age and height were modelled as continuous covariates.
Childcare or attendance at a playgroup in the first 3 months of life was used as a proxy for early exposure to respiratory infections. Our conclusions pertaining to the effects of breast feeding were, however, similar no matter how respiratory infections were modelled. Parental history of asthma was used as a potential binary confounder, but it was only used in secondary (exploratory) analysis because family history may in part reflect the action of the causal pathway of interest, and naive adjustment could be misleading.
Statistical analysis
Significance tests for contingency tables were on the basis of the χ2 test for association (without continuity correction). Unconditional logistic regression was used to investigate the multivariate relation between binary response variables and explanatory exposures of interest. Age at asthma diagnosis and onset of wheezing were analysed using Kaplan Meier survival functions and the log rank statistic. We used Cox regression for analysis of multivariate survival. Regression models were subjected to standard tests of goodness of fit including an investigation of the need for additional polynomial or interaction terms, an analysis of Pearson and Martingale residuals, and tests of regression leverage and influence.22
Statistical power
Statistical significance was defined at the two sided P=0.05 level. The final data set generated more than 99% power to detect an odds ratio of 2.0, and more than 95% power to detect an odds ratio of 1.5 for most analyses.
Results
The key characteristics of the cohort are detailed in table 1. A strong association (P<0.001) was found between most measures of asthma and wheeze suggesting that the end point definitions were internally consistent with each other.
Table 1.
Observed prevalence for selected exposure characteristics and asthma and atopy outcomes among 2187 children from the Western Australian pregnancy cohort study
| Exposure characteristics | No (%) |
|---|---|
| Male sex (n=2183) | 1128 (51.7) |
| Gestational age <37 weeks (n=2178) | 235 (10.8) |
| Maternal education more than secondary school (n=2185) | 831 (38.0) |
| Smoking in household (n=2178) | 919 (42.2) |
| Childcare or attendance at playgroup at <3 months (n=2146) | 86 (4.0) |
| Ever parental history of asthma (n=2187) | 991 (45.3) |
| Aboriginal descent (n=1902) | 54 (2.8) |
| Primary exposures | |
| Other milk introduced before (n=2052): | |
| 1 month | 311 (15.2) |
| 3 months | 740 (36.1) |
| 4 months | 965 (47.0) |
| 6 months | 1240 (60.4) |
| Breast feeding stopped by (n=2065): | |
| 1 month | 416 (20.1) |
| 3 months | 735 (35.6) |
| 4 months | 873 (42.3) |
| 6 months | 1087 (52.6) |
| Asthma and atopy outcomes | |
| Asthma diagnosed by doctor (n=2162) | 669 (30.9) |
| Wheezing ⩾3 times since aged 1 year (n=2171) | 488 (22.5) |
| Wheezing in past year (n=2181) | 470 (21.5) |
| Disturbed sleep due to wheeze in past year (n=2185) | 257 (11.8) |
| Positive skin prick test wheal ⩾2 mm (n=1564) | 651 (41.6) |
Binary end points
Table 2 details the results of the logistic regression analyses. Having adjusted for the potential confounding of other risk factors, the introduction of milk other than breast milk before 4 months of age was positively associated with all primary end points at age 6 years. Being male, of gestational age less than 37 weeks, and smoking in the household were also significant risk factors for the development of asthma and wheeze. Early exposure to childcare was negatively associated.
Table 2.
Multivariate relation between each primary end point for asthma and atopy in children at age 6 years and possible risk factors on basis of multiple logistic regression model. Each estimated odds ratio adjusted for effect of all other exposure variables in table. Values are odds ratios (95% confidence intervals) unless stated otherwise
| Exposure | Asthma diagnosed by doctor (n=1967) | Wheezing ⩾3 times since aged 1 year (n=1977) | Wheeze in past year (n=1985) | Disturbed sleep due to wheeze in past year (n=1989) | Positive skin prick test wheal ⩾2 mm (n=1441) |
|---|---|---|---|---|---|
| Introduction of other milk at <4 months of age | |||||
| Yes v no | 1.25 (1.02 to 1.52) | 1.41 (1.14 to 1.76) | 1.31 (1.05 to 1.64) | 1.42 (1.07 to 1.89) | 1.30 (1.04 to 1.61) |
| P value | 0.029 | 0.002 | 0.016 | 0.017 | 0.019 |
| Sex | |||||
| Male v female | 1.47 (1.21 to 1.79) | 1.46 (1.18 to 1.82) | 1.37 (1.10 to 1.70) | 1.58 (1.19 to 2.11) | 1.45 (1.17 to 1.80) |
| P value | 0.000 | 0.001 | 0.005 | 0.002 | 0.001 |
| Gestational age <37 weeks | |||||
| Yes v no | 1.75 (1.30 to 2.35) | 1.49 (1.07 to 2.06) | 1.46 (1.05 to 2.02) | 1.10 (0.71 to 1.71) | 0.82 (0.57 to 1.19) |
| P value | 0.000 | 0.017 | 0.024 | 0.670 | 0.301 |
| Smoking in household | |||||
| Any v none | 1.27 (1.04 to 1.55) | 1.22 (0.98 to 1.53) | 1.29 (1.03 to 1.61) | 1.32 (0.99 to 1.76) | 0.83 (0.67 to 1.03) |
| P value | 0.018 | 0.070 | 0.025 | 0.057 | 0.096 |
| Childcare or attendance at playgroup at <3 months | |||||
| Any v none | 0.45 (0.25 to 0.83) | 0.40 (0.19 to 0.83) | 0.54 (0.27 to 1.05) | 0.42 (0.15 to 1.16) | 1.00 (0.61 to 1.66) |
| P value | 0.001 | 0.014 | 0.071 | 0.095 | 0.952 |
Other exposures investigated included maternal age, older siblings at birth, the percentage of expected birth weight, smoking in pregnancy, the age that solids were introduced, maternal education, and family income, and none made a significant contribution to model fit. Substantive conclusions were also unaffected if family history was added to the models.
Conclusions pertaining to asthma and wheezing were robust to the choice of cut off point for the age other milk was introduced (table 3). However, the association between a positive skin prick test result and age at introduction of other milk, which was positive at all cut off points between 3 and 6 months, was significant (P=0.019) only at 4 months. Total duration of breast feeding exhibited a weaker association with the end points although all associations were positive. If duration of breast feeding (cut off point of 6 months) was added to the models in table 2, the age other milk was introduced consistently remained significant whereas duration of breast feeding did not.
Table 3.
Sensitivity to dichotomisation cut off points: relation between each primary end point and infant feeding variables after adjustment for effect of sex, gestational age <37 weeks, smoking in household, and childcare or attendance at playgroup at <3 months of age
| Exposure | Asthma diagnosed by doctor (n=1967) | Wheezing ⩾3 times since aged 1 year (n=1977) | Wheeze in past year (n=1985) | Disturbed sleep due to wheeze in past year (n=1989) | Positive skin prick test wheal ⩾2 mm (n=1441) |
|---|---|---|---|---|---|
| Age at introduction of other milk | |||||
| 3 months | 1.20 (0.98 to 1.48) | 1.32 (1.06 to 1.65) | 1.19 (0.95 to 1.49) | 1.33 (0.99 to 1.77) | 1.19 (0.95 to 1.48) |
| P value | 0.084 | 0.015 | 0.135 | 0.056 | 0.138 |
| 4 months | 1.25 (1.02 to 1.52) | 1.41 (1.14 to 1.76) | 1.31 (1.05 to 1.64) | 1.42 (1.07 to 1.89) | 1.30 (1.04 to 1.61) |
| P value | 0.029 | 0.002 | 0.016 | 0.017 | 0.019 |
| 5 months | 1.21 (0.99 to 1.47) | 1.51 (1.20 to 1.88) | 1.31 (1.05 to 1.64) | 1.51 (1.12 to 2.04) | 1.19 (0.96 to 1.48) |
| P value | 0.063 | 0.000 | 0.018 | 0.006 | 0.114 |
| 6 months | 1.26 (1.02 to 1.54) | 1.49 (1.18 to 1.88) | 1.26 (1.00 to 1.59) | 1.38 (1.02 to 1.88) | 1.11 (0.89 to 1.38) |
| P value | 0.023 | 0.001 | 0.046 | 0.037 | 0.356 |
| Breast feeding stopped by | |||||
| 3 months | 1.12 (0.91 to 1.34) | 1.10 (0.88 to 1.38) | 1.12 (0.89 to 1.41) | 1.23 (0.92 to 1.64) | 1.26 (1.01 to 1.59) |
| P value | 0.273 | 0.410 | 0.329 | 0.171 | 0.044 |
| 4 months | 1.14 (0.94 to 1.40) | 1.21 (0.97 to 1.50) | 1.08 (0.87 to 1.36) | 1.23 (0.92 to 1.64) | 1.15 (0.92 to 1.43) |
| P value | 0.187 | 0.095 | 0.478 | 0.159 | 0.218 |
| 5 months | 1.20 (0.98 to 1.47) | 1.29 (1.03 to 1.60) | 1.12 (0.90 to 1.40) | 1.19 (0.89 to 2.59) | 1.07 (0.86 to 1.33) |
| P value | 0.073 | 0.025 | 0.305 | 0.240 | 0.540 |
| 6 months | 1.18 (0.97 to 1.45) | 1.35 (1.08 to 1.69) | 1.14 (0.91 to 1.42) | 1.17 (0.87 to 1.56) | 1.07 (0.86 to 1.34) |
| P value | 0.101 | 0.008 | 0.261 | 0.293 | 0.523 |
In Australia, Aboriginal children have high rates of respiratory illness. There were few Aboriginal children in our study (54 of 1902 (2.8%) children) but they were non-significantly more likely to be given milk other than breast milk before 4 months (odds ratio 1.42, 95% confidence interval 0.73 to 2.78), and they were at significantly greater risk of asthma being diagnosed by a doctor (1.84, 1.07 to 3.17; P=0.029), current wheeze (2.05, 1.16 to 3.62; P=.0014), and sleep disturbance due to wheeze (3.28, 1.80 to 6.00; P<0.001) but not a positive skin prick test result (0.95, 0.50 to 1.80; P=0.87). The exclusion of all Aboriginal children from primary analyses made no substantial difference to our conclusions.
Age at onset of asthma and wheeze
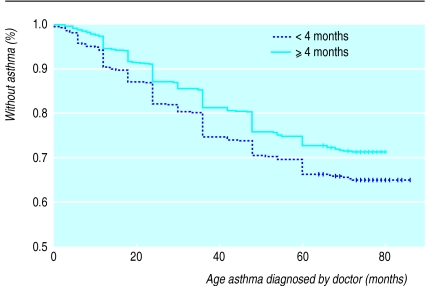
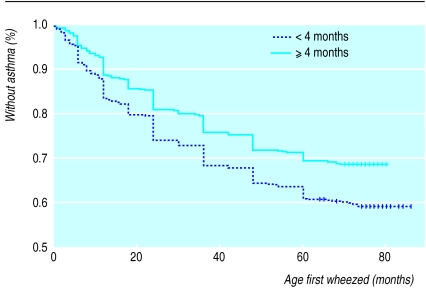
Figures 1 and 2 detail the Kaplan Meier survival functions indicating age at diagnosis of asthma by a doctor, and age at onset of wheezing, stratified by duration of exclusive breast feeding. The cumulative incidence of both asthma (P=0.001) and wheeze (P<0.001) was higher if other milk was introduced before 4 months. Cox regression showed that age at asthma diagnosis (hazard ratio 1.22, 1.03 to 1.43; P=0.02) and age at first wheeze (1.36, 1.17 to 1.59; P=0.0002) were both earlier if other milk was introduced before 4 months, after controlling for confounders.
Figure 1.
Kaplan Meier survival functions for age at diagnosis of asthma stratified by duration of exclusive breast feeding (log rank statistic 10.70, df=1, P=0.001). Vertical bars denote censoring events
Figure 2.
Kaplan Meier analysis of age at first symptomatic wheeze stratified by duration of exclusive breast feeding (log rank statistic 18.36, df=1, P=0.000). Vertical bars denote censoring events
Discussion
Validity
The Western Australian pregnancy cohort study has a high response rate. Nevertheless, random non-response may have reduced statistical power. Our reported power calculations are on the basis of thefinal sample size and take account of non-response, therefore our positive conclusions remain valid. Any systematic non-response is most likely to be determined by disease status and social class and this may have biased estimated effects in either direction. But, the addition of social class covariates to our models made little difference to point estimates. Mothers were enrolled in midpregnancy, and selection bias was unlikely in relation to key outcomes. Most dropout occurred early in the study and was unlikely to have been associated with the later development of asthma or atopy. The study is often viewed as representative of the general Western Australian population.20 Nevertheless, recruitment was mainly through a tertiary obstetric hospital and included a small excess of mothers with preterm babies. Our models, however, include a covariate reflecting preterm delivery, and confounding due to pregnancies at risk should not have distorted our conclusions.
Measurement of outcome data was based on validated methodologies, and the study was powerful enough to detect the risk ratios of only moderate size that were likely to be found in the study of a complex disease such as asthma. Standard regression diagnostics showed that our models fitted well.22
Protective effect of exclusive breast feeding
Our study provides evidence consistent with others4,12,14 of a protective effect of exclusive breast feeding (⩾4 months) against a range of end points reflecting asthma and atopy. This protective effect may operate through several mechanisms. These include the exclusion of milk other than breast milk (and its potentially allergenic components) from the infant’s diet; and the provision of immunomodulatory, anti-inflammatory, nutritional, or other components in human milk.9,23,24 Like others,14 we found that it was the age that other milk was introduced rather than the duration of breast feeding that was more closely associated with asthma or atopy at age 6 years. This favours “exclusion” mechanisms. The two variables are, however, strongly correlated, and we cannot definitively reject the possibility that it is breast feeding itself that is of prime importance.
Conclusions
Delaying the introduction of milk other than breast milk until at least 4 months of age may protect against asthma and atopy later in childhood. These findings are relevant to our understanding of the cause of childhood asthma and also to public health. Although further studies and analyses are required to confirm these benefits and to understand better the mechanisms concerned, public health interventions promoting an increased duration of exclusive breast feeding may help to reduce the morbidity and prevalence of childhood asthma.
Footnotes
Funding: WHO was supported by a research award from the Western Australian Health Promotion Foundation. The Western Australian pregnancy cohort study is funded by project and programme grants from the National Health and Medical Research Council of Australia, and GlaxoWellcome.
Competing interests: None declared.
References
- 1.Peat JK, Li J. Reversing the trend: reducing the prevalence of asthma. J Allergy Clin Immunol. 1999;103:1–10. doi: 10.1016/s0091-6749(99)70517-8. [DOI] [PubMed] [Google Scholar]
- 2.Robertson CF, Dalton MF, Peat JK, Haby MM, Bauman A, Kennedy JD, et al. Asthma and other atopic diseases in Australian children: Australian arm of the international study of asthma and allergy in childhood. Med J Australia. 1998;168:434–438. [PubMed] [Google Scholar]
- 3.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. New Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 4.Saarinen UM, Kajosaari M. Breastfeeding as prophylaxis against atopic disease: prospective follow-up study until 17 years old. Lancet. 1995;346:1065–1069. doi: 10.1016/s0140-6736(95)91742-x. [DOI] [PubMed] [Google Scholar]
- 5.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez FD. Role of viral infections in the inception of asthma and allergies during childhood: could they be protective? Thorax. 1994;49:1189–1191. doi: 10.1136/thx.49.12.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wold AE, Adlerberth I. Does breastfeeding affect the infant’s immune responsiveness? Acta Paediatr. 1998;87:19–22. doi: 10.1080/08035259850157804. [DOI] [PubMed] [Google Scholar]
- 8.Holt PG, Macaubas C. Development of long term tolerance versus sensitisation to environmental allergens during the perinatal period. Curr Opin Immunol. 1997;9:782–787. doi: 10.1016/s0952-7915(97)80178-1. [DOI] [PubMed] [Google Scholar]
- 9.Hanson LA. Breastfeeding provides passive and likely longlasting active immunity. [Review.] Ann Allergy, Asthma Immunol. 1998;81:523–537. doi: 10.1016/S1081-1206(10)62704-4. [DOI] [PubMed] [Google Scholar]
- 10.Pabst HF. Immunomodulation by breast-feeding. Pediatr Infect Dis J. 1997;16:991–995. doi: 10.1097/00006454-199710000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Golding J, Emmett PM, Rogers IS. Eczema, asthma and allergy. Early Hum Dev. 1997;49:121–30S. doi: 10.1016/s0378-3782(97)00058-3. [DOI] [PubMed] [Google Scholar]
- 12.Wilson AC, Stewart Forsyth J, Greene SA, Irvine L, Hau C, Howie PW. Relation of infant diet to childhood health: seven year follow up of cohort of children in Dundee infant feeding study. BMJ. 1998;316:21–25. doi: 10.1136/bmj.316.7124.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hide DW. The clinical expression of allergy in breastfed infants. Adv Exp Med Biol. 1991;310:475–480. doi: 10.1007/978-1-4615-3838-7_61. [DOI] [PubMed] [Google Scholar]
- 14.Tariq SM, Matthews SM, Hakim EA, Stevens M, Arshad SH, Hide DW. The prevalence of and risk factors for atopy in early childhood: a whole population birth cohort study. J Allergy Clin Immunol. 1998;101:587–593. doi: 10.1016/S0091-6749(98)70164-2. [DOI] [PubMed] [Google Scholar]
- 15.Juvonen P, Månsson M, Andersson C, Jakobsson I. Allergy development and macromolecular absorption in infants with different feeding regimens during the first three days of life. A three-year prospective follow-up. Acta Paediatr. 1996;85:1047–1052. doi: 10.1111/j.1651-2227.1996.tb14215.x. [DOI] [PubMed] [Google Scholar]
- 16.Strachan DP, Anderson HR, Johnston IDA. Breastfeeding as prophylaxis against atopic disease. [Letter.] Lancet. 1995;346:1714. doi: 10.1016/s0140-6736(95)92884-7. [DOI] [PubMed] [Google Scholar]
- 17.Halpern SR, Sellars WA, Johnson RB, Anderson DW, Saperstein S, Reisch JS. Development of childhood allergy in infants fed breast, soy, or cow milk. J Allergy Clin Immunol. 1973;51:139–151. doi: 10.1016/0091-6749(73)90019-5. [DOI] [PubMed] [Google Scholar]
- 18.Wright AL, Holberg CJ, Taussig LM, Martinez FD. Relationship of infant feeding to recurrent wheezing at age 6 years. Arch Pediatr Adolesc Med. 1995;149:758–763. doi: 10.1001/archpedi.1995.02170200048006. [DOI] [PubMed] [Google Scholar]
- 19.Kramer MS. Does breastfeeding help protect against atopic disease? Biology, methodology, and a golden jubilee of controversy. J Pediatr. 1988;112:181–190. doi: 10.1016/s0022-3476(88)80054-4. [DOI] [PubMed] [Google Scholar]
- 20.Newnham JP, Evans SF, Michael CA, Stanley FJ, Landau LI. Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet. 1993;342:887–891. doi: 10.1016/0140-6736(93)91944-h. [DOI] [PubMed] [Google Scholar]
- 21.Skassa-Brociek W, Manderscheid JC, Michel FB, Bousquet J. Skin test reactivity to histamine from infancy to old age. J Allergy Clin Immunol. 1987;80:711–716. doi: 10.1016/0091-6749(87)90292-2. [DOI] [PubMed] [Google Scholar]
- 22.McCullagh P, Nelder JA. Generalized linear models, 2nd ed. Oxford: Chapman and Hall; 1989. [Google Scholar]
- 23.Hamosh M. Protective functions of proteins and lipids in human milk. [Review.] Biol Neonate. 1998;74:163–176. doi: 10.1159/000014021. [DOI] [PubMed] [Google Scholar]
- 24.Xanthou M. Immune protection of human milk. Biol Neonate. 1998;74:121–133. doi: 10.1159/000014018. [DOI] [PubMed] [Google Scholar]