Abstract
OBJECTIVE
High molecular weight (HMW) adiponectin is a predominant isoform of circulating adiponectin and has been related to type 2 diabetes. Previous linkage studies suggest that different genetic components might be involved in determining HMW and total adiponectin levels.
RESEARCH DESIGN AND METHODS
We performed a genome-wide association study (GWAS) of serum HMW adiponectin levels in individuals of European ancestry drawn from the Nurses’ Health Study (NHS) (N = 1,591). The single nucleotide polymorphisms (SNPs) identified in the GWAS analysis were replicated in an independent cohort of Europeans (N = 626). We examined the associations of the identified variations with diabetes risk and metabolic syndrome.
RESULTS
We identified a novel locus near the FER gene (5q21) at a genome-wide significance level, best represented by SNP rs10447248 (P = 4.69 × 10−8). We also confirmed that variations near the adiponectin-encoding ADIPOQ locus (3q27) were related to serum HMW adiponectin levels. In addition, we found that FER SNP rs10447248 was related to HDL cholesterol levels (P = 0.009); ADIPOQ variation was associated with fasting glucose (P = 0.04), HDL cholesterol (P = 0.04), and a metabolic syndrome score (P = 0.002).
CONCLUSIONS
Our results suggest that different loci may be involved in regulation of circulating HMW adiponectin levels and provide novel insight into the mechanisms that affect HMW adiponectin homeostasis.
Adiponectin is the most abundant adipocyte-secreted hormone in blood (1), which regulates the inflammatory response, enhances insulin action, and affects metabolism of glucose and lipids (2,3). In the circulation, adiponectin is present in multimers of various molecular weights including high (HMW), medium (MMW), and low (LMW) molecular weight, with the predominant form being HMW adiponectin (4), which is thought to represent the biological active form that is better correlated with insulin sensitivity and risk of obesity and diabetes than total adiponectin (5,6).
Family and twin studies indicate that 30–88% variance in adiponectin concentration is potentially accounted for by genetic influence (4,7,8). Candidate gene studies and genome-wide association studies (GWAS) of total adiponectin levels have identified loci near genes ADIPOQ, ARL15, and CDH13 (8–14). Previous linkage studies indicate that different loci might be involved in determining HMW adiponectin (15). We therefore undertook a genome-wide analysis on the serum levels of HMW adiponectin. We describe the identification of a novel locus at genome-wide significance level.
RESEARCH DESIGN AND METHODS
The Nurses’ Health Study (NHS) was established in 1976 when 121,700 female registered nurses aged 30–55 years and residing in 11 large U.S. states completed a mailed questionnaire on their medical history and lifestyle (16). The lifestyle factors have been updated by validated questionnaires every 2 years. The current study was approved by the institutional review board at Brigham and Women’s Hospital. Participants for the current study were a subset of women (N = 1,591) included in a nested case-control study of type 2 diabetes (17,18). To test the association with diabetes risk, we also included a nested case-control study in the Health Professional Follow-up Study (HPFS) (17) (Supplementary Materials).
Replication study.
A total of 626 nondiabetic individuals (240 men and 386 women) from 235 families were recruited in the Gargano area in center-east Italy and examined as previously described. The study and the informed consent procedures were approved by the local research committee. A metabolic syndrome score was calculated according to Adult Treatment Panel III criteria.
Assessment of serum adiponectin..
In NHS, study samples were analyzed in randomly ordered case-control pairs to further reduce systematic bias and interassay variation. Serum HMW adiponectin concentrations were determined by ELISA (Millipore, St. Charles, MO) with a sensitivity of 0.5 µg/mL.
In the Italian sample, serum total adiponectin HMW and MMW plus HMW adiponectin concentrations were measured by ELISA (ALPCO) as previously described (4). MMW values were obtained by subtracting the concentrations of HMW from the combined concentrations of MMW plus HMW. LMW adiponectin fractions were obtained by subtracting the combined concentrations of MMW plus HMW from the total adiponectin concentrations. The intra-assay coefficients of variation were 5.4 and 5.0, 5.2 and 4.9, and 5.0 and 4.8% for total adiponectin, MMW plus HMW adiponectin, and HMW adiponectin, respectively.
Genotyping and quality control.
For the GWAS samples, genotyping was done using the Affymetrix Genome-Wide Human 6.0 array. Genotypic data were checked for quality as described elsewhere (17,18). We used MACH (http://www.sph.umich.edu/csg/abecasis/MACH) to impute 2,543,887 single nucleotide polymorphisms (SNPs) on chromosomes 1–22 with NCBI build 36 of Phase II HapMap CEU data (release 22) as the reference panel. Imputed SNPs with minor allele frequency <0.02 and/or with poor imputation quality scores (MACH r2 ≤0.30) were filtered from analysis. Population structure was investigated by principal component analysis. Adjustment for the top four principal components did not appreciably change the association results.
Replication SNPs were genotyped by Taqman SNP allelic discrimination technique by means of an ABI 7000 (Applied Biosystems, Foster City, CA). Call rate and concordance rate were ≥96 and >99%, respectively. All the SNPs were in Hardy-Weinberg equilibrium (P > 0.05).
Statistical analyses.
GWAS analysis on HMW adiponectin levels in NHS was performed with the linear regression analysis using PLINK software. HMW adiponectin was normally distributed and analyzed without transformation. Associations between adiponectin isoforms and each SNP in the replication sample were tested by a linear mixed-effects model implemented in SOLAR that accounts for within-family correlations. Each SNP was included in a model as a fixed effect with additive coding. We combined study-specific β-estimates from discovery and replication analyses using the inverse of the variance of the study-specific β-estimates to weight the contribution of each study with the fixed-effect model. The associations between SNPs and the risk of type 2 diabetes in NHS and HPFS were analyzed using a logistic regression model. To assess phenotypic correlations between HMW adiponectin and metabolic traits in the Italian samples, we used a mixed-effects model by SOLAR that includes fixed covariate effects. This method could account for the dependence of the family data and provide a more stringent P value. All analyses were adjusted for sex, age, and BMI.
RESULTS
We performed GWAS analyses with serum HMW adiponectin levels in 1,591 women (698 diabetic patients) from the NHS. The characteristics of the participants are presented in Supplementary Table 1. We fit a linear regression model for genotype trend effects (1 df), adjusting for age, BMI, and diabetes status. Quantile-quantile plots (Supplementary Fig. 1) suggest that there was no systemic bias for analyses (genomic inflation factor, λ= 0.998). Further adjustment for fasting status, smoking, alcohol consumption, and physical activity did not change the results.
Supplementary Fig. 2 shows the plots of the –log10 P values for the trend test for serum HMW adiponectin. No associations with HMW adiponectin levels reached genome-wide significance level (P = 5 × 10−8). We took SNPs representing independent loci with P < 5 × 10−6 for further replication (Table 2). In addition, we chose the two best associated but not correlated (r2 =0.05) SNPs at the ADIPOQ (3q27) loci, rs3774261 and rs822354, for replication.
TABLE 2.
Associations with metabolic syndrome components in Italian samples
| Genotype |
P* | |||
|---|---|---|---|---|
| GG | GA | AA | ||
| rs822354 | ||||
| Fasting glucose (mg/dL) | 91.4 | 90.2 | 89 | 0.04 |
| HDL cholesterol (mg/dL) | 52 | 53.5 | 53.9 | 0.04 |
| Triglycerides (mg/dL) | 99.2 | 100.7 | 95.2 | 0.66 |
| Waist (cm) | 85 | 84.6 | 83.8 | 0.11 |
| SBP (mmHg) | 117 | 117 | 115 | 0.22 |
| DBP (mmHg) | 77.2 | 76.7 | 77.3 | 0.73 |
| MS score | 0.97 | 0.9 | 0.73 | 0.002 |
| rs10447248 | ||||
| Fasting glucose (mg/dL) | 89.8 | 91.3 | 90.3 | 0.35 |
| HDL cholesterol (mg/dL) | 54.3 | 52.4 | 51.2 | 0.009 |
| Triglycerides (mg/dL) | 94.9 | 101.9 | 102.9 | 0.21 |
| Waist (cm) | 84.5 | 84.8 | 84.5 | 0.86 |
| SBP (mmHg) | 116.8 | 117.1 | 116.3 | 0.86 |
| DBP (mmHg) | 76.9 | 77.3 | 76.3 | 0.48 |
| MS score | 0.87 | 0.94 | 0.90 | 0.57 |
DBP, diastolic blood pressure; MS, metabolic syndrome; SBP, systolic blood pressure.
*Significance for testing linear trend.
The replication samples include 626 white Italian subjects (240 men and 386 women). Two ADIPOQ SNPs, rs822354 and rs3774261, showed directionally consistent and nominally significant associations with serum HMW adiponectin in the replication sample (Table 1), and the association of rs822354 reached genome-wide significance in meta-analysis of the two studies (P = 3.67 × 10−8). SNP rs822354 accounted for 1.1 and 2.5% of the variance in HMW adiponectin levels in the NHS and Italian samples, respectively. Another SNP, rs10447248 (5q21), also showed directionally consistent associations with HMW adiponectin levels (P = 4.27× 10−3) in the replication samples. In the meta-analysis, the association of rs10447248 reached genome-wide significance level (4.69 × 10−8) (Table 1; Fig. 1). Each A allele of SNP rs10447248 was related to 0.76 and 0.39 µg/mL lower HMW adiponectin and accounted for 1.3 and 2% of the variance in the NHS and Italian samples, respectively.
TABLE 1.
Associations of SNPs with serum concentration of HMW adiponectin
| SNPs | Chromosome | BP | Gene | Effect/reference alleles* | NHS |
Italian |
Combined P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EAF | n | β | P | EAF | n | β | P | ||||||
| Novel loci | |||||||||||||
| rs13358260 |
5 |
78881467 |
PAPD4 |
C/T |
0.93 |
1591 |
1.45 |
8.85E-07 |
0.94 |
625 |
0.04 |
4.51E-01 |
4.96E-06 |
| rs10517133 |
4 |
45059671 |
AC108043.3–1 |
C/G |
0.90 |
1583 |
1.28 |
1.28E-06 |
0.90 |
622 |
0.09 |
3.67E-01 |
4.55E-06 |
| rs10447248 |
5 |
107943635 |
FER |
A/G |
0.34 |
1583 |
−0.76 |
3.23E-06 |
0.38 |
620 |
−0.39 |
4.27E-03 |
4.69E-08 |
| rs2120576 |
12 |
91754320 |
EEA1 |
C/T |
0.89 |
1591 |
1.14 |
3.32E-06 |
0.90 |
623 |
−0.19 |
2.34E-01 |
9.30E-04 |
| rs2468677 |
8 |
140588699 |
KCNK9 |
G/T |
0.52 |
1590 |
0.72 |
3.33E-06 |
0.51 |
622 |
0.24 |
6.07E-02 |
7.95E-07 |
| Known loci | |||||||||||||
| rs822354 |
3 |
187962900 |
ADIPOQ |
A/G |
0.68 |
1590 |
0.71 |
3.17E-05 |
0.64 |
622 |
0.58 |
1.96E-04 |
3.67E-08 |
| rs3774261 | 3 | 188054253 | ADIPOQ | A/G | 0.62 | 1590 | 0.67 | 3.09E-05 | 0.52 | 616 | 0.36 | 9.97E-03 | 9.63E-07 |
BP, base pair physical position; EAF, effect allele frequency.
*Effect alleles represent the alleles associated with serum HMW adiponectin levels.
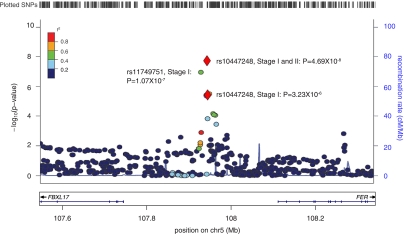
FIG. 1.
Region plot of FER locus. Association signals at chromosome 5q21, across a region centering on SNP rs10447248, with HMW adiponectin levels in NHS. The vertical axis represents the –log10 P values from the linear regression analysis for the genotyped and imputated SNPs.
SNP rs2468677 (8q24) also showed directionally consistent and marginal association with HMW adiponectin levels in the replication set (P = 0.0607). In the meta-analysis, the association between rs2468677 and HMW adiponectin levels was near genome-wide significance (P = 7.95 × 10−7).
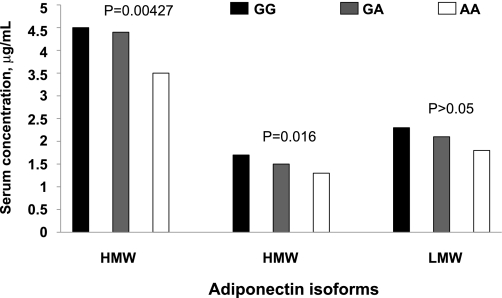
We further examined the impact of the newly identified SNP rs10447248 in the FER locus on the serum levels of other isoforms, MMW and LMW adiponectin, that were measured in the Italian samples. The A allele of SNP rs10447248 was nominally associated with lower MMW (P = 0.016) but was not associated with LMW (P > 0.05) (Fig. 2) adiponectin levels.
FIG. 2.
Associations of SNP rs10447248 with adiponectin isoforms. Levels of circulating HMW, MMW, and LMW adiponectin by the genotypes of SNP rs10447248 in Italian samples. The P values are from the trend test in linear regression model. The analyses were adjusted for age, sex, and BMI.
To get deeper insights about the associations between the newly identify FER locus and HMW adiponectin levels, we performed genotyping imputation in the NHS samples (19). Among the imputed SNPs, rs11749751 in the region near FER showed stronger association with serum HMW adiponectin (P = 1.07 × 10−7) than SNP rs10447248 (Fig. 1). The two SNPs are in moderate linkage disequilibrium (r2 = 0.5). When included in the same regression model, both SNPs rs10447248 and rs11749751 showed independently and nominally significant associations with HMW levels (P = 0.0018 and 0.0044, respectively).
We further examined the associations between the HMW adiponectin–associated SNPs with the risk of type 2 diabetes in NHS and HPFS and with the metabolic traits in the Italian samples. After adjustment for age, BMI, and fasting status, the allele A of ADIPOQ SNP rs822354 was related to a decreased risk of diabetes in the HPFS (odds ratio [OR] 0.87 [95% CI 0.78–0.98]) but not in the NHS (1.00 [0.98–1.03]). FER SNPs were not associated with diabetes risk. In Italian samples, allele A of ADIPOQ SNP rs822354 was associated with a lower metabolic syndrome score (P = 0.002) (Table 2), lower fasting glucose (P = 0.04), and higher HDL cholesterol levels (P = 0.04). In our sample, HMW adiponectin and metabolic syndrome score share a common genetic background as indicated by a significant genetic correlation. Of note, ADIPOQ SNP rs822354 could explain 3% of the genetic correlations between the two variables (ρg = −0.32 ± 0.14) (P = 0.04). FER SNP rs10447248 was not significantly related to metabolic syndrome score but was associated with lower levels of HDL cholesterol (P = 0.009).
DISCUSSION
In this study, we found novel genome-wide significant associations of SNPs on chromosome 5q21 with serum HMW adiponectin levels in Caucasians with European ancestry. SNP rs10447248 is ∼170 kb upstream the FER gene (Fig. 1). Fer protein is a member of the FPS/FES family of nontransmembrane receptor tyrosine kinases. Animal studies suggest that FER has a role in regulating inflammation and innate immunity (20,21). However, there are no available data for the roles of this gene product in regulating adiponectin production.
We confirmed that common SNPs near/in ADIPOQ locus were associated with serum HMW adiponectin levels. The results are consistent with the previous studies (4,11,14). Interestingly, we found that multiple genetic variations in low linkage disequilibrium were related with HMW adiponectin levels, suggesting that allelic heterogeneity may exist at this locus in affecting the homeostasis of the marker. Another locus near the KCNK9 gene on chromosome 8q24 also showed suggestive association with HMW adiponectin levels. Of note, in a previous genome-wide scan this locus was related to the levels of triglycerides (22). SNPs in genes ARL15 (rs4311394) and CDH13 (rs3865188 and rs7195409) were recently associated with total or HMW adiponectin levels (11,12,14). However, neither locus was related to HMW adiponectin in our sample (NHS, P > 0.05) or another GWAS in Europeans (13). It appears that the genetic effects of the CDH13 locus were more significant in Asian populations (11,12).
Previous linkage analyses suggest that different loci may be involved in regulating total and HMW adiponectin levels (15,23). It is noteworthy that circulating adiponectin levels may be regulated at different levels including transcription, translation, and posttranslational levels such as protein modification, secretion, oligomerization, degradation, and excretion, as well as clearance (24). It is likely that the genetic variants affecting these various steps may modulate HMW adiponectin levels and that ADIPOQ locus may affect transcription of the gene. The mechanisms underlying the associations for FER locus remain to be clarified in future function studies.
HMW adiponectin levels have been previously related to metabolic traits such as plasma insulin, insulin resistance, and risk of type 2 diabetes (5,6,25). SNP rs822354 was related to a reduced risk of diabetes in HPFS and lower metabolic syndrome score in Italian samples; however, the SNP was not significantly associated with diabetes risk in NHS. No SNP by sex interaction in modulating metabolic syndrome score was observed in the Italian sample (P = 0.48). SNPs at the FER locus were not related to diabetes risk or metabolic syndrome score. Of note, these SNPs only account for a small proportion of the variance in HMW adiponectin levels. This may partly explain the weak relation between the genetic variations and disease risk.
The major strengths of our study included a well-defined cohort, high-quality genotype data, and minimal population stratification (17). We acknowledge several study limitations, including potential errors in biomarker measurements. Nevertheless, these errors more likely bias the association toward null because the measurement errors are uncorrelated with genotyping. The discovery samples include only women while the replication samples include both sexes. However, the previous GWAS did not report sex-specific genetic effects on adiponectin. In addition, the Italian cohort consists only of nondiabetic subjects and may not represent the general population, and all study populations exclusively consist of Caucasians with European ancestry. Therefore, the findings may not be generalizable to other ethnicities.
In conclusion, we found a novel locus at the FER gene (5q21) associated with serum HMW adiponectin levels and provided confirmatory evidence for the ADIPOQ locus with HMW adiponectin levels. Future studies may investigate the potentially additive nature of multiple pathways in regulation of HMW adiponectin concentrations and whether these genetic variants may affect disease risk associated with adiponectin deficiency.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grants R01-HL-071981 and U01-HG-004399, an American Heart Association Scientist Development award, and the Boston Obesity Nutrition Research Center (DK46200). Part of the research described in this article was supported by Accordo Programma Quadro in Materia di Ricerca Scientifica nella Regione Puglia-PST 2006 and by Italian Ministry of Health grants Ricerca Corrente 2009 and 2010 (to C.M.)
No potential conflicts of interest relevant to this article were reported.
L.Q. conceived and designed the experiments; performed the experiments; analyzed data; contributed reagents, materials, and analysis tools; and wrote the manuscript. C.M. performed the experiments; analyzed data; contributed reagents, materials, and analysis tools; and wrote the manuscript. L.S. and C.D.B. critically reviewed the manuscript. V.T. contributed reagents, materials, and analysis tools and critically reviewed the manuscript. F.B.H. conceived and designed the experiments; contributed reagents, materials, and analysis tools; and critically reviewed the manuscript.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1645/-/DC1.
REFERENCES
- 1.Pajvani UB, Du X, Combs TP, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem 2003;278:9073–9085 [DOI] [PubMed] [Google Scholar]
- 2.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002;8:1288–1295 [DOI] [PubMed] [Google Scholar]
- 3.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 2001;7:947–953 [DOI] [PubMed] [Google Scholar]
- 4.Menzaghi C, Salvemini L, Paroni G, et al. Circulating high molecular weight adiponectin isoform is heritable and shares a common genetic background with insulin resistance in nondiabetic white Caucasians from Italy: evidence from a family-based study. J Intern Med 2010;267:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher FF, Trujillo ME, Hanif W, et al. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia 2005;48:1084–1087 [DOI] [PubMed] [Google Scholar]
- 6.Hara K, Horikoshi M, Yamauchi T, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care 2006;29:1357–1362 [DOI] [PubMed] [Google Scholar]
- 7.Cesari M, Narkiewicz K, De Toni R, Aldighieri E, Williams CJ, Rossi GP. Heritability of plasma adiponectin levels and body mass index in twins. J Clin Endocrinol Metab 2007;92:3082–3088 [DOI] [PubMed] [Google Scholar]
- 8.Menzaghi C, Trischitta V, Doria A. Genetic influences of adiponectin on insulin resistance, type 2 diabetes, and cardiovascular disease. Diabetes 2007;56:1198–1209 [DOI] [PubMed] [Google Scholar]
- 9.Qi L, Li T, Rimm E, et al. The +276 polymorphism of the APM1 gene, plasma adiponectin concentration, and cardiovascular risk in diabetic men. Diabetes 2005;54:1607–1610 [DOI] [PubMed] [Google Scholar]
- 10.Richards JB, Waterworth D, O’Rahilly S, et al. ; GIANT Consortium A genome-wide association study reveals variants in ARL15 that influence adiponectin levels. PLoS Genet 2009;5:e1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jee SH, Sull JW, Lee JE, et al. Adiponectin concentrations: a genome-wide association study. Am J Hum Genet 2010;87:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Li Y, Lange EM, et al. Genome-wide association study for adiponectin levels in Filipino women identifies CDH13 and a novel uncommon haplotype at KNG1-ADIPOQ. Hum Mol Genet 2010;19:4955–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heid IM, Henneman P, Hicks A, et al. Clear detection of ADIPOQ locus as the major gene for plasma adiponectin: results of genome-wide association analyses including 4659 European individuals. Atherosclerosis 2010;208:412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling H, Waterworth DM, Stirnadel HA, et al. Genome-wide linkage and association analyses to identify genes influencing adiponectin levels: the GEMS Study. Obesity (Silver Spring) 2009;17:737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kangas-Kontio T, Huotari A, Ruotsalainen H, et al. Genetic and environmental determinants of total and high-molecular weight adiponectin in families with low HDL-cholesterol and early onset coronary heart disease. Atherosclerosis 2010;210:479–485 [DOI] [PubMed] [Google Scholar]
- 16.Qi L, Kang K, Zhang C, et al. Fat mass-and obesity-associated (FTO) gene variant is associated with obesity: longitudinal analyses in two cohort studies and functional test. Diabetes 2008;57:3145–3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi L, Cornelis MC, Kraft P, et al. ; Meta-Analysis of Glucose and Insulin-related traits Consortium (MAGIC); Diabetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium Genetic variants at 2q24 are associated with susceptibility to type 2 diabetes. Hum Mol Genet 2010;19:2706–2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi L, Cornelis MC, Kraft P, et al. Genetic variants in ABO blood group region, plasma soluble E-selectin levels and risk of type 2 diabetes. Hum Mol Genet 2010;19:1856–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Willer C, Sanna S, Abecasis G. Genotype imputation. Annu Rev Genomics Hum Genet 2009;10:387–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCafferty DM, Craig AW, Senis YA, Greer PA. Absence of Fer protein-tyrosine kinase exacerbates leukocyte recruitment in response to endotoxin. J Immunol 2002;168:4930–4935 [DOI] [PubMed] [Google Scholar]
- 21.Greer P. Closing in on the biological functions of Fps/Fes and Fer. Nat Rev Mol Cell Biol 2002;3:278–289 [DOI] [PubMed] [Google Scholar]
- 22.Middelberg RP, Gordon SD, Zhu G, et al. Linkage and association analyses of longitudinally measured lipid phenotypes in adolescence. Twin Res Hum Genet 2008;11:603–620 [DOI] [PubMed] [Google Scholar]
- 23.Menzaghi C, Ercolino T, Salvemini L, et al. Multigenic control of serum adiponectin levels: evidence for a role of the APM1 gene and a locus on 14q13. Physiol Genomics 2004;19:170–174 [DOI] [PubMed] [Google Scholar]
- 24.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev 2005;6:13–21 [DOI] [PubMed] [Google Scholar]
- 25.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes 2006;55:249–259 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.