Abstract
OBJECTIVE
Preferential upper-body fat gain, a typical male pattern, is associated with a greater cardiometabolic risk. Regional differences in lipolysis and meal fat storage cannot explain sex differences in body fat distribution. We examined the potential role of the novel free fatty acid (FFA) storage pathway in determining body fat distribution in postabsorptive humans and whether adipocyte lipogenic proteins (CD36, acyl-CoA synthetases, and diacylglycerol acyltransferase) predict differences in FFA storage.
RESEARCH DESIGN AND METHODS
Rates of postabsorptive FFA (palmitate) storage into upper-body subcutaneous (UBSQ) and lower-body subcutaneous (LBSQ) fat were measured in 28 men and 53 premenopausal women. Stable and radiolabeled palmitate tracers were intravenously infused followed by subcutaneous fat biopsies. Body composition was assessed with a combination of dual-energy X-ray absorptiometry and computed tomography.
RESULTS
Women had greater FFA (palmitate) storage than men in both UBSQ (0.37 ± 0.15 vs. 0.27 ± 0.18 μmol · kg−1 · min−1, P = 0.0001) and LBSQ (0.42 ± 0.19 vs. 0.22 ± 0.11 μmol · kg−1 · min−1, P < 0.0001) fat. Palmitate storage rates were significantly greater in LBSQ than UBSQ fat in women, whereas the opposite was true in men. Plasma palmitate concentration positively predicted palmitate storage in both depots and sexes. Adipocyte CD36 content predicted UBSQ palmitate storage and sex-predicted storage in LBSQ fat. Palmitate storage rates per kilogram fat did not decrease as a function of fat mass, whereas lipolysis did.
CONCLUSIONS
The FFA storage pathway, which had remained undetected in postabsorptive humans until recently, can have considerable, long-term, and sex-specific effects on body fat distribution. It can also offer a way of protecting the body from excessive circulating FFA in obesity.
Obesity is a major contributor to chronic disease (1). Furthermore, a recent large cohort study demonstrated a strong positive association between abdominal fat distribution and all-cause mortality independently of general adiposity (2). Participants in the lowest third of BMI (women <23 kg/m2; men <24.9 kg/m2) and the highest quintile of waist or waist-to-hip ratio had the highest relative risk of death. These findings emphasize the importance of body fat distribution not only in overweight/obese individuals but also in normal-weight individuals.
Men tend to preferentially store fat in their upper-body region and women in their lower-body region (3). Regional differences in fat accumulation must develop from imbalances in fatty acid storage and/or free fatty acid (FFA) release between fat depots. In both sexes, the upper-body subcutaneous (UBSQ) fat depot is lipolytically more active than lower-body subcutaneous (LBSQ) fat (4,5), suggesting that differences in regional lipolysis cannot explain the sex differences in body fat distribution. In addition to the well-known dietary fat storage pathway, an underappreciated storage pathway in adipose tissue also exists (6–8). It involves the uptake and storage of circulating FFAs in the postabsorptive state. The existence of this process under postabsorptive conditions is counterintuitive considering the rapid efflux of fatty acids due to active lipolysis. Interestingly, the pattern of FFA storage between fat depots corresponds to sex differences in body fat distribution (6). However, FFA storage rates in subcutaneous fat regions have not been quantitatively measured, which would allow direct assessment of its quantitative and physiological importance.
Adipose tissue buffers the daily flux of fatty acids in the circulation (9). A remarkable adaptation in obesity is downregulation of lipolysis per unit of fat mass (10–13). This is advantageous because it prevents excessive increases in plasma FFA concentrations. Unfortunately, the enlarged adipose tissue also downregulates dietary fat storage (13). Because adipocytes resist further fat storage, dietary fat may be diverted to nonadipose organs leading to ectopic fat accumulation. It is currently unknown whether postabsorptive FFA storage is also downregulated in obesity or whether the enlarged adipose tissue maintains its ability to store FFA from plasma at rates similar to those in normal-weight individuals.
Fatty acid uptake and storage in adipocytes has been well studied in vitro, but limited information is available in vivo. The initial step in fatty acid uptake involves the transmembrane transport of fatty acids. Both passive diffusion and protein-facilitated transport (e.g., fatty acid translocase/CD36, fatty acid-binding proteins, fatty acid transport proteins) are thought to contribute to the transmembrane movement of fatty acids (14–18). The transmembrane transport is intimately coupled to esterification with CoA via the action of acyl-CoA synthetases (ACSs) (19,20). This reaction decreases the intracellular concentration of fatty acids to favor import and is an essential initial step for triglyceride synthesis. Diacylglycerol acyltransferase (DGAT) catalyses the final and only committed step in triglyceride synthesis, the conversion of diacylglycerol to triglyceride, making this a critical step in vivo (21).
Our goal was to assess the effect of sex and obesity on FFA storage in UBSQ and LBSQ depots under postabsorptive conditions. We examined the potential importance of the direct FFA storage pathway in redistributing fatty acids between fat depots. We also investigated whether obesity downregulates this pathway, as it does for lipolysis and meal fat storage. Lastly, we studied whether CD36, ACS, and DGAT relate to postabsorptive FFA storage and may constitute limiting steps in this process.
RESEARCH DESIGN AND METHODS
The study was approved by the Mayo Clinic Institutional Review Board. Informed written consent was obtained from all volunteers.
Participants.
Healthy participants (28 men, 53 premenopausal women) receiving no medications, including oral contraceptives, participated in the study. They were weight stable for >3 months before the study with a wide range of adiposity (20–36 kg/m2). A complete blood count, chemistry group, and routine urinalysis were documented as normal.
Study protocol.
Participants received all their meals from the Mayo Clinic Clinical Research Unit metabolic kitchen for 5 days before the study to ensure stable energy intake and consistent macronutrient composition (50% carbohydrate, 35% fat, and 15% protein). Volunteers were then admitted to the Clinical Research Unit at 1700 h and given a meal at 1800 h. At 0545 h the next day, a forearm vein catheter was inserted and kept patent with a controlled infusion of 0.45% NaCl. Another catheter was placed in a retrograde fashion in a hand vein for collecting arterialized blood using the heated (55°C) hand vein technique. After collecting a baseline blood sample for background palmitate specific activity (SA) and enrichment, a continuous infusion of [U-13C]palmitate (2–4 nmol/kg FFM/min) (Cambridge Isotope Laboratories, Andover, MA) commenced. After 30 min for isotopic equilibration, a series of three blood samples were collected at 10-min intervals and then every 30 min until 0900 h to measure palmitate kinetics. An ∼60 μCi intravenous bolus of [1-14C]palmitate or ∼200 μCi [9,10-3H]palmitate (NEN Life Science Products; PerkinElmer Life and Analytical Sciences, Boston, MA) was given at 0800 h. Abdominal and femoral subcutaneous adipose biopsies were collected at 30 min after the intravenous bolus of the radiolabeled palmitate. The biopsies were timed such that virtually no radiolabeled FFA tracer remained in the circulation and there would be insufficient tracer in VLDL-triglycerides to accumulate in adipose tissue via VLDL (6). Participants were dismissed from the Clinical Research Unit after completing the study.
Body composition measurements.
Total and regional fat masses were assessed with dual-energy X-ray absorptiometry (DXA) (Lunar Radiation, Madison, WI). Leg fat mass was considered LBSQ fat. Visceral fat mass was estimated using a combination of single-slice computed tomography (L2-L3 interspace) and dual-energy X-ray absorptiometry–measured abdominal fat (22). Total body fat minus visceral and LBSQ fat masses was UBSQ fat mass.
Adipose tissue biopsies.
Subcutaneous adipose tissues biopsies were collected from the abdominal (just lateral to the umbilicus) and the femoral region (on the anterior-lateral aspect of the midthigh) as previously described (6–8). The samples were meticulously rinsed with saline through Nitex Nylon Fiber 250/50 and processed for measurement of adipocyte size and lipid SA.
Assays
Measurement of adipocyte size and adipocyte lipid specific activity.
Adipocyte size (23) and adipocyte lipid specific activity (SA) (dpm/g lipid) (6) were assessed as previously described.
Plasma palmitate concentration and SA.
Aliquots of the [1-14C]palmitate and [9,10-3H]palmitate infusates were counted to assess the exact amount of radiotracer administered. Plasma palmitate SA was measured using high-performance liquid chromatography (24). All other metabolic parameters were measured as previously described (25). Plasma palmitate concentration and plasma and [U-13C]palmitate infusate enrichment and concentration were measured using an liquid chromatography/mass spectrometry method (26).
Measurement of adipose tissue CD36 content and ACS and DGAT activities.
We obtained sufficient tissue from a subset of participants to measure adipose tissue CD36 content and ACS and DGAT activities. Approximately 250 mg flash-frozen adipose tissue was homogenized in 2 mL standard homogenization buffer (20 mmol/L Tris-HCl, pH 7.4, 1 mmol/L EDTA, 255 mmol/L sucrose) with antiproteases tablets (Roche, Indianapolis, IN). Supernatant was collected after centrifugation at 2,100 rpm at 4°C for 10 min. The fat cake of the supernatant was removed, and its lipids were extracted (chloroform:methanol) and used to normalize for protein content and enzyme activity per milligram lipid.
CD36 protein content.
We used a sandwich enzyme-linked immunosorbent assay to measure adipose tissue CD36 content (27). In brief, 100 μL of the above-mentioned supernatant was denatured with SDS (final concentration 0.06%) and boiling for 10 min. The CD36 content was compared with that of a calibration standard using a five-point dilution curve for each sample and standard.
ACS activity.
We measured the conversion of [3H]palmitate to its CoA derivative using a modification of the method of Hall et al. (28). All reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise stated. Briefly, the 150-μL reaction mix consisted of 150 mmol/L Tris-HCl, 50 mmol/L MgCl2, 20 mmol/L ATP, 200 μmol/L CoA, 200 μmol/L dithiothreitol, 30 mmol/L NaCl, and 0.1 mmol/L EDTA. A total of 50 μL palmitate with [3H]palmitate (Perkin Elmer NET-043) mixture was added and then 50 μL sample supernatant. At 2 min, the reaction was terminated with 1.25 mL isopropanol:heptane:1 N H2SO4 (40:10:1, vol/vol/vol), and the supernatant was separated with centrifugation. The aqueous phase was counted on a scintillation counter with <2% counting error. The intra- and interassay coefficient of variation (CV) was <10%. Quality control samples were run at the beginning, middle, and end of each assay. Interassay variation was adjusted for using the mean values of the quality control samples relative to the entire set of samples analyzed.
DGAT activity.
We used the method of Coleman et al. (29) modified slightly to use the cytosolic fraction (30) of the adipose tissue homogenate and 20 μL of 10.0 mmol/L (rather than 2.0 mmol/L) 1,2 dioleoyl-sn-glycerol (Sigma-Aldrich) in the reaction mixture. The intra- and interassay CVs were <10%. Interassay variation was adjusted as described above for ACS activity.
Calculations.
Palmitate flux was calculated dividing the [U-13C]palmitate infusion rate by steady-state plasma [U-13C]palmitate enrichment. At steady state, the rate of appearance (Ra) and rate of disappearance (Rd) are equal. The regional adipose lipid SA (dpm/g lipid) was used to calculate the fraction of injected radiolabeled palmitate stored per kilogram adipose tissue lipid (fat). The abdominal biopsy was considered representative of the UBSQ depot, whereas the femoral fat biopsy was considered representative of the LBSQ compartment. Palmitate storage rates into each fat depot were measured as the product of steady-state palmitate Rd ([U-13C]palmitate) and the corresponding fraction of injected radiotracer stored per kilogram fat in each depot. Palmitate storage rates, CD36 content, and ACS and DGAT activities were expressed in μmol · kg fat−1 · min−1 and per 1,000 adipocytes.
Different means of data expression can have a significant impact on data interpretation. We focused on the per-unit fat mass expression, when aiming to understand whether one body fat depot is better at competing for the available FFA than another depot. When examining the factors that may regulate fatty acid storage within a depot, we focused on the per 1,000 adipocytes expression.
Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as fasting serum insulin (μU/mL) × fasting plasma glucose (mmol/L)/22.5. Glucose and insulin were measured as previously described (25,31).
Statistical analysis.
Values are expressed as means ± SD. Nonnormally distributed data were logarithmically transformed to ensure normal distribution. Statistical analyses were performed using unpaired and paired Student t test for sex and depot comparisons, respectively. P values of <0.05 were considered statistically significant. The Pearson test was used to assess bivariate relationships. Multiple linear regression analysis was used to assess independent predictors of regional palmitate storage rates. Variance inflation factor was determined to identify mutually dependent predictors. A variance inflation factor of <10 was accepted.
RESULTS
Subject characteristics.
Table 1 provides the characteristics of the participants. Participants were well matched for age and BMI. We observed the expected differences in body fatness. Specifically, women had significantly greater body fat percentages and subcutaneous fat masses and less visceral fat than men. Women also had significantly larger femoral adipocytes than men. The average plasma palmitate concentrations did not differ between sexes, and concentrations were stable over the study interval. Adipose tissue lipolysis rates (as represented by palmitate Ra) were less in women than in men, but not when differences in resting energy expenditure were taken into account. The time course of plasma palmitate radioactivity was such that there was virtually no remaining counts in plasma palmitate after 30 min and very little in the plasma triglyceride component, similar to previous reports (6) (data not shown).
TABLE 1.
Anthropometrics and fat distribution of study participants
| Women | Men | P | |
|---|---|---|---|
| n | 53 | 28 | |
| Age (years) | 36 ± 8 | 33 ± 9 | * |
| BMI (kg/m2) | 27.7 ± 5.0 | 27.7 ± 5.1 | * |
| Resting energy expenditure (kcal/day) | 1,521 ± 215 | 1,900 ± 305 | <0.0001 |
| Weight (kg) | 75.2 ± 14.3 | 88.9 ± 16.6 | 0.0002 |
| Total body fat (%) | 39 ± 8 | 26 ± 8 | <0.0001 |
| Total body fat (kg) | 29.7 ± 10.0 | 23.2 ± 10.6 | 0.009 |
| UBSQ fat (kg) | 15.8 ± 5.2 | 12.3 ± 5.9 | 0.009 |
| LBSQ fat (kg) | 11.8 ± 4.6† | 7.6 ± 3.1† | <0.0001 |
| Visceral fat (kg) | 2.2 ± 1.8 | 3.3 ± 2.2 | 0.015 |
| Abdominal adipocyte size (μg lipid/cell) | 0.58 ± 0.24 | 0.58 ± 0.33 | 0.90 |
| Femoral adipocyte size (μg lipid/cell) | 0.75 ± 0.20† | 0.64 ± 0.26 | 0.037 |
| HOMA-IR | 1.36 ± 0.72 | 1.42 ± 1.20 | 0.76 |
| Plasma palmitate (μmol/L) | 103 ± 28 | 109 ± 30 | 0.74 |
| Plasma palmitate Ra (μmol/min) | 107 ± 38 | 130 ± 50 | 0.016 |
Data are means ± SD.
*Selected to be similar, not subject to statistical testing.
†P < 0.001 between depots within sex.
Regional palmitate storage rates in adipose tissue.
Plasma palmitate storage rates in both UBSQ and LBSQ fat were significantly greater in women than in men (Table 2), whether expressed per kilogram fat or per 1,000 adipocytes. In addition, storage rates were significantly greater in LBSQ fat than in UBSQ fat in women using either mode of expression. Conversely, adipose tissue palmitate storage rates were greater in UBSQ fat than in LBSQ fat in men.
TABLE 2.
Palmitate storage rates in subcutaneous adipose tissue depots
| Women | Men | P (women vs. men) | |
|---|---|---|---|
| N | 49 | 25 | |
| Palmitate storage in UBSQ fat (μmol · kg fat−1 · min−1) | 0.367 ± 0.153 | 0.267 ± 0.181 | 0.001 |
| Palmitate storage in LBSQ fat (μmol · kg fat−1 · min−1) | 0.418 ± 0.188* | 0.219 ± 0.107† | <0.0001 |
| Palmitate storage in UBSQ fat (pmol · 1,000 adipocytes−1 · min−1) | 0.202 ± 0.114 | 0.153 ± 0.146 | <0.0001 |
| Palmitate storage in LBSQ fat (pmol · 1,000 adipocytes−1 · min−1) | 0.284 ± 0.169‡ | 0.135 ± 0.086 | <0.0001 |
Data are means ± SD. Statistics were performed on log-transformed data.
*†‡ vs. UBSQ fat within sex; *P = 0.01; †P = 0.04; ‡P < 0.0001.
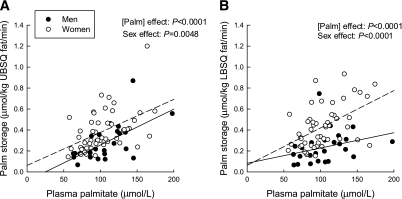
Plasma palmitate concentration was a positive predictor of palmitate storage rates (effect of palmitate concentration: UBSQ, P < 0.0001; LBSQ, P < 0.0001) (Fig. 1). In addition, at a given palmitate concentration, women had greater storage rates than men (sex effect: UBSQ: P = 0.0002; LBSQ: P < 0.0001). Results were similar whether the data were expressed per kilogram fat or per 1,000 adipocytes. Obesity did not significantly affect regional storage rates (effect of body fat mass on UBSQ storage, P = 0.17; LBSQ storage, P = 0.46). HOMA-IR was a significant negative predictor of storage rates, independent of sex and palmitate concentration, in UBSQ (P = 0.025) but not LBSQ fat (P = 0.63).
FIG. 1.
Relationship between palmitate storage in UBSQ fat (μmol/kg fat/min) and plasma palmitate (Palm) concentration (μmol/L) (A) and palmitate storage in LBSQ fat (μmol/kg fat/min) and plasma palmitate concentration (μmol/L) (B).
Regional CD36 content, ACS activity, and DGAT activity.
Average values of CD36 content and ACS and DGAT activities for the participants from whom we were able to collect adequate amounts of adipose tissue are provided in Table 3. No significant differences were observed between sexes in the three lipogenic factors examined in UBSQ fat. However, women had significantly greater ACS and DGAT activity in LBSQ fat than men. In addition, women had significantly greater CD36 protein content (per milligram lipid and per 1,000 adipocytes), ACS (per milligram lipid and per 1,000 adipocytes), and DGAT activity (per 1,000 adipocytes) in LBSQ fat than in UBSQ fat. No regional differences were observed in men in any of the three lipogenic factors examined.
TABLE 3.
Regional ACS and DGAT activities and CD36 content in subcutaneous adipose tissue
| Women |
Men |
|||
|---|---|---|---|---|
| UBSQ fat | LBSQ fat | UBSQ fat | LBSQ fat | |
| CD36 (relative units · mg lipid−1) | 17 ± 7 (37) | 20 ± 7 (50)† | 21 ± 9 (14) | 20 ± 11 (15) |
| ACS (pmol · mg lipid–1 · min−1) | 53 ± 22 (37) | 68 ± 23 (50)*† | 61 ± 36 (16) | 51 ± 22 (16) |
| DGAT (pmol · mg lipid–1 · min−1) | 5.1 ± 3.1 (37) | 5.0 ± 2.8 (50)* | 3.7 ± 1.5 (15) | 3.1 ± 1.1 (16) |
| CD36 (relative units · 1,000 adipocytes−1) | 10 ± 9 (34) | 15 ± 6 (44)† | 14 ± 6 (12) | 15 ± 10 (15) |
| ACS (pmol · 1,000 adipocytes−1 · min−1) | 31 ± 18 (34) | 52 ± 22 (44)*† | 35 ± 22 (14) | 36 ± 18 (16) |
| DGAT (pmol · 1,000 adipocytes−1 · min−1) | 2.8 ± 1.6 (34) | 3.6 ± 2.5 (44)*† | 2.3 ± 1.4 (13) | 2.3 ± 1.2 (16) |
Data are means ± SD (n). Statistics were performed on log-transformed data.
*P < 0.05 between sexes;
†P < 0.05 between depots within sex.
We examined whether adipose tissue CD36 content, ACS, and DGAT activities per 1,000 adipocytes were interrelated. We observed significant positive relationships between all of the lipogenic factors in women and between some of them in men (Table 4).
TABLE 4.
Univariate correlations between CD36 content and ACS and DGAT activities in subcutaneous adipose tissue
| Women |
Men |
|||
|---|---|---|---|---|
| CD36 | ACS | CD36 | ACS | |
| UBSQ fat | ||||
| CD36 (relative units · 1,000 adipocytes−1) | ||||
| ACS (pmol · 1,000 adipocytes−1 · min−1) | r = 0.70, P < 0.0001 | r = 0.91, P < 0.0001 | ||
| DGAT (pmol · 1,000 adipocytes−1 · min−1) | r = 0.52, P = 0.0017 | r = 0.77, P < 0.0001 | r = 0.37, P = 0.23 | r = 0.50, P = 0.081 |
| LBSQ fat | ||||
| CD36 (relative units · 1,000 adipocytes−1) | ||||
| ACS (pmol · 1,000 adipocytes−1 · min−1) | r = 0.62, P < 0.0001 | r = 0.72, P = 0.0026 | ||
| DGAT (pmol · 1,000 adipocytes−1 · min−1) | r = 0.31, P = 0.043 | r = 0.62, P < 0.0001 | r = 0.49, P = 0.061 | r = 0.74, P = 0.0009 |
Statistics were performed on log-transformed data.
Relationship between regional lipogenic enzymes/proteins and regional adiposity.
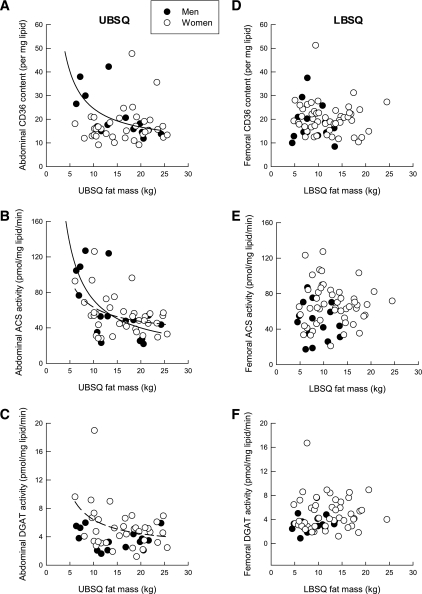
In women, ACS (P = 0.01) and DGAT (P = 0.03) activities per milligram lipid decreased as a function of UBSQ fat mass in a nonlinear fashion (Fig. 2). A similar pattern was observed for CD36 content (P = 0.02) and ACS (P = 0.005) activity in men (Fig. 2). No associations were observed between any of the lipogenic factors studied and LBSQ fat mass in either sex.
FIG. 2.
Relationship between abdominal CD36 content (units per mg lipid) (A), abdominal ACS activity (pmol/mg lipid/min) (B), abdominal DGAT activity (pmol/mg lipid/min) (C), and UBSQ fat mass and between femoral CD36 content (units per mg lipid) (D), femoral ACS activity (pmol/mg lipid/min) (E), femoral DGAT activity (pmol/mg lipid/min) (F), and LBSQ fat mass in men (●, solid line) and women (○, dashed line).
Relationship between regional lipogenic enzymes/proteins and regional palmitate storage rates.
When expressed per milligram lipid, no significant correlations were observed between lipogenic factors and regional palmitate storage rates in either UBSQ or LBSQ fat, with the only exception being a weak relationship between ACS activity and palmitate storage rates in UBSQ fat in women (data not shown).
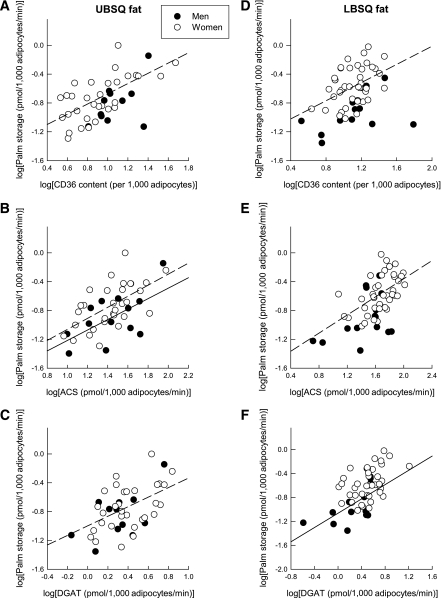
When expressed per 1,000 adipocytes, rates of palmitate UBSQ storage were significantly and positively correlated with CD36 content (women: r = 0.66, P = 0.0001), ACS activity (men: r = 0.61, P = 0.034; women: r = 0.65, P = 0.0002), and DGAT activity (women: r = 0.44, P = 0.02) (Fig. 3). Therefore, in women, palmitate storage rates in UBSQ adipocytes correlated with all three lipogenic factors. In contrast, rates of palmitate storage in UBSQ adipocytes in men correlated with ACS activity only. In the LBSQ depot, rates of palmitate storage per 1,000 adipocytes were significantly and positively correlated with CD36 content (women: r = 0.43, P = 0.006), ACS activity (women: r = 0.57, P = 0.0001), and DGAT activity (men: r = 0.71, P = 0.005) (Fig. 3). Therefore, palmitate storage rates in LBSQ adipocytes correlated significantly with CD36 content and ACS activity in women but with DGAT activity in men.
FIG. 3.
Relationship between palmitate (Palm) storage in UBSQ fat (μmol/1,000 adipocytes/min) and abdominal CD36 content (units per 1,000 adipocytes) (A), abdominal ACS activity (pmol/1,000 adipocytes/min) (B), and abdominal DGAT activity (pmol/1,000 adipocytes/min) (C) and between palmitate storage in LBSQ fat (μmol/1,000 adipocytes/min) and femoral CD36 content (units per 1,000 adipocytes) (D), femoral ACS activity (pmol/1,000 adipocytes/min) (E), and femoral DGAT activity (pmol/1,000 adipocytes/min) (F) in men (●, solid line) and women (○, dashed line). Log values were used to achieve normal distribution.
Predictors of regional palmitate storage rates.
As mentioned, plasma palmitate concentration and sex were independent predictors of palmitate storage rates (Fig. 1). In addition, we assessed whether the lipogenic factors would provide an additional predictive value on palmitate storage above and beyond sex and palmitate concentration. When the three lipogenic factors were included in the multivariate regression analysis, palmitate concentration (P = 0.0002) and CD36 content (P = 0.047) were the only significant independent predictors of palmitate storage in UBSQ adipocytes. In LBSQ adipocytes, both palmitate concentration (P = 0.003) and sex (P = 0.0003) remained significant independent predictors of palmitate storage, whereas none of the lipogenic factors did.
Patterns of palmitate release and storage rates relative to adiposity.
Figure 4 depicts palmitate release (lipolysis) and storage per kilogram fat versus total subcutaneous fat mass. As opposed to the downregulation of lipolysis per kilogram fat that occurred with increasing body fatness, palmitate storage per kilogram fat exhibited an identical range between individuals of low and high adiposity. In individuals with >40 kg subcutaneous fat, palmitate release was on average ∼2.5 μmol · kg fat−1 · min−1, whereas palmitate storage was on average ∼0.8 μmol · kg fat−1 · min−1 (Fig. 4). Therefore, in these cases, palmitate storage was approximately one-third of palmitate release.
FIG. 4.
Palmitate release rate into whole-body subcutaneous fat (μmol/kg fat/min) versus subcutaneous fat mass (kilograms) (A) and palmitate storage rates into whole-body subcutaneous fat (μmol/kg fat/min) versus subcutaneous fat mass (kilograms) (B).
Estimates of body fat redistribution attributable to the direct FFA storage pathway.
Using the palmitate storage data, we can assess whether postabsorptive FFA storage rates in UBSQ and LBSQ fat follow the same pattern as that for postabsorptive regional lipolysis. If an imbalance exists between regional FFA storage and lipolysis, it could result in redistribution of fatty acids from one fat depot to another. We calculated palmitate storage into whole UBSQ and LBSQ fat depots by multiplying the regional storage per kilogram fat (Table 2) by the corresponding regional fat masses (Table 1). In women, palmitate storage was 5.7 ± 2.8 and 5.2 ± 3.6 μmol/min into whole UBSQ and LBSQ fat, respectively. Corresponding values in men were 3.0 ± 2.4 and 1.6 ± 1.1 μmol/min.
Although we did not measure regional lipolysis rates in these volunteers, on average, UBSQ and LBSQ fat contribute ∼75 and ∼18%, respectively, of systemic lipolysis in normal-weight individuals and ∼57 and ∼28%, respectively, in obesity (5). Using these values, we estimate that, if palmitate storage in total subcutaneous fat in women (∼10.8 μmol/min) was distributed into UBSQ and LBSQ regions in proportion to rates of release from these regions (5), ∼7.7 and ∼3.1 μmol/min palmitate would be stored into UBSQ and LBSQ fat, respectively. Compared with the actual storage rates (5.7 and 5.2 μmol/min), the FFA storage pathway could redistribute ∼2.1 μmol/min palmitate from the UBSQ to LBSQ region (∼631 g FFA/year assuming a 12-h postabsorptive state/day) in postabsorptive women. Using the same approach to estimate FFA redistribution in men, if palmitate storage in subcutaneous fat (∼3.6 μmol/min) was distributed in proportion to release rates, ∼3.3 and ∼1.3 μmol/min palmitate would be stored into UBSQ and LBSQ fat, respectively. Compared with the actual storage rates (3.0 and 1.6 μmol/min, respectively), the FFA storage pathway would redistribute only ∼0.3 μmol/min palmitate from UBSQ to LBSQ fat (∼92 g FFA/year).
DISCUSSION
We assessed the quantitative importance of direct FFA storage into subcutaneous adipose tissue in postabsorptive humans and its potential role in body fat distribution. We further examined the effect of obesity and key lipogenic factors involved in distinct steps of FFA handling by the adipose tissue. The major findings are as follows: 1) women stored more circulating FFA (as represented by palmitate) in their subcutaneous fat depots than men; 2) women preferentially stored FFA in their LBSQ region, whereas men preferentially stored FFA in their UBSQ fat depot; 3) circulating FFA concentration was an independent predictor of FFA storage in both depots, and in addition, CD36 predicted storage in UBSQ fat and sex-predicted storage in LBSQ fat; and 4) as opposed to lipolysis and meal fat storage (10–13), obesity did not affect the magnitude of FFA storage per unit fat mass.
The finding that women store greater amounts of circulating FFA in their subcutaneous fat than men agrees with indirect observations from previous work (6). The sex difference was noted in both depots and at any given plasma FFA concentration (Fig. 1). This observation indicates that, as in the case of dietary fat storage (32,33), women store greater amounts of fatty acids in subcutaneous adipose tissue than men. The most striking observation was that palmitate storage (per unit fat mass) exhibited preferential lower body fat accumulation in women and preferential upper body fat accumulation in men. Given that regional in vivo differences in the storage of dietary fat (34,35) or adipose tissue lipolytic rates (36,37) do not seem to account for variations in human body fat distribution under isoenergetic conditions, this is the first direct evidence for a pathway whose pattern matches sex-specific patterns of fat distribution.
By comparing the observed rates of regional FFA storage against what would occur if FFA storage took place in proportion to release, we estimated the postabsorptive FFA storage pathway would redistribute ∼631 g fatty acids/year from the UBSQ to LBSQ fat depot in women but only ∼92 g fatty acids/year in men. These results suggest that under postabsorptive conditions, the FFA storage pathway is quantitatively significant in regulating and/or maintaining the sex-specific body fat distribution patterns by redistributing fatty acids between fat depots. This pathway is easier to detect in the postprandial state, when there is net fat storage into adipose tissue (38,39). FFA uptake into abdominal subcutaneous fat in men during the early and midpostprandial period was 82–183 nmol · 100 g−1 · min−1 (corresponding to ∼0.21–0.46 μmol palmitate · kg−1 · min−1) (38). In addition, in a mixed group of women and men, no difference was observed in postprandial FFA uptake between abdominal and femoral subcutaneous fat (39).
Another approach to evaluate the quantitative significance of the FFA storage pathway in adipose tissue is by comparing its magnitude with that of FFA storage into nonadipose tissues, such as muscle. Muscles rely on the supply of FFA from plasma. It was shown that in the noncontracting skeletal muscle, approximately half of the plasma FFAs entering the muscle are used for esterification into intramyocellular triglycerides (40). The incorporation of plasma palmitate into intramyocellular triglycerides was reported to be 0.28 μmol · kg wet muscle−1 · min−1 in men and 0.50 μmol · kg wet muscle−1 · min−1 in women (41). Palmitate storage in the current study (average of UBSQ and LBSQ fat) was 0.24 μmol · kg fat−1 · min−1 in men and 0.39 μmol · kg fat−1 · min−1 in women. Thus, FFA storage (as represented by palmitate) into adipose tissue lipid appears to be ∼80% of the corresponding rate of FFA incorporation into intramyocellular triglycerides in the resting postabsorptive muscle. Other studies (40) have found somewhat lower rates of palmitate incorporation into intramyocellular triglycerides in men (∼0.18 μmol · kg wet muscle−1 · min−1 calculated using the same precursor pool as in the study by Kanaley et al. [41]). Collectively, these findings indicate that postabsorptive FFA storage into subcutaneous fat is not substantially different in magnitude compared with the incorporation of FFAs into intramyocellular triglycerides.
A surprising discovery was that plasma palmitate concentrations were positively correlated with adipocyte palmitate storage rates in both depots and sexes. Whereas we might logically expect such a relationship in nonadipose tissues such as muscle or liver, this finding for adipose tissue seemed counterintuitive. Because increased FFA concentrations are most often secondary to increased lipolysis, we anticipated the latter would result in an unfavorable concentration gradient, thereby reducing FFA uptake and storage. The implication of this observation is that, as adipose tissue lipolysis increases, the reuptake and storage of circulating FFAs also increases, yet in a depot- and sex-specific manner.
In our attempts to identify regulatory factors that may play a role in FFA storage within a fat depot, we examined three proteins/enzymes involved in FFA handling by the adipocyte: CD36 content, ACS activity, and DGAT activity (collectively termed “lipogenic factors”). Although significant univariate correlations were observed between certain lipogenic factors and palmitate storage rates (Fig. 3), only CD36 (along with plasma palmitate concentration) independently predicted FFA storage in UBSQ fat. None of the three proteins/enzymes predicted FFA storage in LBSQ fat independently of sex and palmitate concentration. These results suggest that transmembrane transport of FFA (as represented by CD36 content) may be a limiting step in FFA storage in UBSQ fat. The findings are in line with previous work where, compared with wild-type adipocytes, the incorporation of radiolabeled fatty acid into triacylglycerol was decreased in adipocytes from FAT/CD36-null mice (42). We were unable to identify any of the lipogenic factors that we assessed as rate-limiting for the storage of FFAs in the LBSQ depot. Therefore, women exhibit greater FFA storage rates in LBSQ fat than men (Fig. 1); however, none of the three lipogenic factors were implicated in that effect. A limitation that has to be noted is that because of the limited CD36, ACS, and DGAT data in men compared with women, our ability to detect significant relationships was probably greater in women than in men. We also note that CD36 is expressed on adipose tissue macrophages as well as adipocytes. It seems unlikely that this source of CD36 affects our conclusions, however. Macrophages represent a small fraction (≤5%) of stromovascular cells in adipose tissue of individuals with the BMI range of the current study, and the proportions do not differ between UBSQ and LBSQ fat (43). Stromovascular cells represent ∼64% of total adipose tissue cells (44); thus, macrophages contribute only ∼3% (0.64 × 0.05) of adipose tissue cells. Furthermore, CD36 mRNA is more highly expressed in adipocytes than SV cells in humans (45). Collectively, these findings suggest that macrophage CD36 protein does not substantially affect the relationships we observed.
In the context of obesity, a fascinating observation was that obese individuals maintained the ability to store FFAs from plasma to the same extent as normal-weight individuals (Fig. 4). This is in stark contrast with the well-known downregulation that occurs in obesity in lipolysis (10–13) and dietary fat storage (13). This is a biologically remarkable observation, because it allows obese people to dispose more fatty acids in their subcutaneous fat than lean individuals. In individuals with >40 kg subcutaneous fat, one-third of the fatty acids that were released in the postabsorptive state were stored back in subcutaneous fat.
In summary, this is the first study to directly show that the FFA storage pathway, which had remained undetected in postabsorptive humans until recently (6–8), can have considerable, long-term, and sex-specific effects on body fat distribution. Furthermore, as opposed to the “inappropriate” downregulation that occurs in meal fat storage (13), the subcutaneous adipose tissue of obese individuals maintains its ability to store FFA from plasma to the same degree as normal-weight individuals. This result suggests that, along with reduced lipolysis (10–13), the FFA storage pathway offers another way of protecting the body from excessive amounts of circulating FFAs in obesity.
ACKNOWLEDGMENTS
This work was supported by grants DK40484, DK45343, DK50456, and R00585 from the U.S. Public Health Service and by the Mayo Foundation.
No potential conflicts of interest relevant to this article were reported.
C.K. researched the data and wrote the manuscript. A.H.A. and M.S.M. researched the data and reviewed and edited the manuscript. M.D.J. designed the study and reviewed and edited the manuscript.
The authors are indebted to the research volunteers for their participation. They are also grateful to Barbara Norby, Carley Vrieze, Christy Allred, Debra Harteneck, Darlene Lucas, Lendia Zhou, Maksym Karpyak, Rebekah Herrmann, Jessica Eastman, and Carol Siverling, as well as the Mayo Clinic Clinical Research Unit nursing staff and the Center for Translational Science Activities Mass Spectrometry Core Laboratory for technical assistance and help with data collection.
REFERENCES
- 1.Kim S, Popkin BM. Commentary: understanding the epidemiology of overweight and obesity: a real global public health concern (discussion 81–82). Int J Epidemiol 2006;35:60–67 [DOI] [PubMed]
- 2.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008;359:2105–2120 [DOI] [PubMed] [Google Scholar]
- 3.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr 1956;4:20–34 [DOI] [PubMed] [Google Scholar]
- 4.Basu A, Basu R, Shah P, Vella A, Rizza RA, Jensen MD. Systemic and regional free fatty acid metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab 2001;280:E1000–E1006 [DOI] [PubMed] [Google Scholar]
- 5.Nielsen S, Guo ZK, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 2004;113:1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes 2007;56:1369–1375 [DOI] [PubMed] [Google Scholar]
- 7.Koutsari C, Dumesic DA, Patterson BW, Votruba SB, Jensen MD. Plasma free fatty acid storage in subcutaneous and visceral adipose tissue in postabsorptive women. Diabetes 2008;57:1186–1194 [DOI] [PubMed] [Google Scholar]
- 8.Hannukainen JC, Kalliokoski KK, Borra RJ, et al. Higher free fatty acid uptake in visceral than in abdominal subcutaneous fat tissue in men. Obesity (Silver Spring) 2010;18:261–265 [DOI] [PubMed] [Google Scholar]
- 9.Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia 2002;45:1201–1210 [DOI] [PubMed] [Google Scholar]
- 10.Birkenhäger JC, Tjabbes T. Turnover rate of plasma FFA and rate of esterification of plasma FFA to plasma triglycerides in obese humans before and after weight reduction. Metabolism 1969;18:18–32 [DOI] [PubMed] [Google Scholar]
- 11.Campbell PJ, Carlson MG, Nurjhan N. Fat metabolism in human obesity. Am J Physiol 1994;266:E600–E605 [DOI] [PubMed] [Google Scholar]
- 12.Horowitz JF, Coppack SW, Paramore D, Cryer PE, Zhao G, Klein S. Effect of short-term fasting on lipid kinetics in lean and obese women. Am J Physiol 1999;276:E278–E284 [DOI] [PubMed] [Google Scholar]
- 13.McQuaid SE, Hodson L, Neville MJ, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 2011;60:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton JA, Kamp F. How are free fatty acids transported in membranes? Is it by proteins or by free diffusion through the lipids? Diabetes 1999;48:2255–2269 [DOI] [PubMed] [Google Scholar]
- 15.Frohnert BI, Bernlohr DA. Regulation of fatty acid transporters in mammalian cells. Prog Lipid Res 2000;39:83–107 [DOI] [PubMed] [Google Scholar]
- 16.Hajri T, Abumrad NA. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu Rev Nutr 2002;22:383–415 [DOI] [PubMed] [Google Scholar]
- 17.Doege H, Stahl A. Protein-mediated fatty acid uptake: novel insights from in vivo models. Physiology (Bethesda) 2006;21:259–268 [DOI] [PubMed] [Google Scholar]
- 18.Kampf JP, Parmley D, Kleinfeld AM. Free fatty acid transport across adipocytes is mediated by an unknown membrane protein pump. Am J Physiol Endocrinol Metab 2007;293:E1207–E1214 [DOI] [PubMed] [Google Scholar]
- 19.Gargiulo CE, Stuhlsatz-Krouper SM, Schaffer JE. Localization of adipocyte long-chain fatty acyl-CoA synthetase at the plasma membrane. J Lipid Res 1999;40:881–892 [PubMed] [Google Scholar]
- 20.Lobo S, Wiczer BM, Smith AJ, Hall AM, Bernlohr DA. Fatty acid metabolism in adipocytes: functional analysis of fatty acid transport proteins 1 and 4. J Lipid Res 2007;48:609–620 [DOI] [PubMed] [Google Scholar]
- 21.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res 2004;43:134–176 [DOI] [PubMed] [Google Scholar]
- 22.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 1995;61:274–278 [DOI] [PubMed] [Google Scholar]
- 23.Tchoukalova YD, Harteneck DA, Karwoski RA, Tarara J, Jensen MD. A quick, reliable, and automated method for fat cell sizing. J Lipid Res 2003;44:1795–1801 [DOI] [PubMed] [Google Scholar]
- 24.Miles JM, Ellman MG, McClean KL, Jensen MD. Validation of a new method for determination of free fatty acid turnover. Am J Physiol 1987;252:E431–E438 [DOI] [PubMed] [Google Scholar]
- 25.Nielsen S, Guo Z, Albu JB, Klein S, O’Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest 2003;111:981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persson X-MT, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res 2010;51:2761–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allred CC, Krennmayr T, Koutsari C, Zhou L, Ali AH, Jensen MD. A novel ELISA for measuring CD36 protein in human adipose tissue. J Lipid Res 2011;52:408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall AM, Smith AJ, Bernlohr DA. Characterization of the acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J Biol Chem 2003;278:43008–43013 [DOI] [PubMed] [Google Scholar]
- 29.Coleman RA. Diacylglycerol acyltransferase and monoacylglycerol acyltransferase from liver and intestine. Methods Enzymol 1992;209:98–104 [DOI] [PubMed] [Google Scholar]
- 30.Hou XG, Moser S, Sarr MG, Thompson GB, Que FG, Jensen MD. Visceral and subcutaneous adipose tissue diacylglycerol acyltransferase activity in humans. Obesity (Silver Spring) 2009;17:1129–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koutsari C, Basu R, Rizza RA, Nair KS, Khosla S, Jensen MD. Nonoxidative free fatty acid disposal is greater in young women than men. J Clin Endocrinol Metab 2011;96:541–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in adipose tissue: gender effects in nonobese humans. Am J Physiol Endocrinol Metab 2000;279:E455–E462 [DOI] [PubMed] [Google Scholar]
- 33.Votruba SB, Jensen MD. Sex-specific differences in leg fat uptake are revealed with a high-fat meal. Am J Physiol Endocrinol Metab 2006;291:E1115–E1123 [DOI] [PubMed] [Google Scholar]
- 34.Mårin P, Rebuffé-Scrive M, Björntorp P. Uptake of triglyceride fatty acids in adipose tissue in vivo in man. Eur J Clin Invest 1990;20:158–165 [DOI] [PubMed] [Google Scholar]
- 35.Mårin P, Lönn L, Andersson B, et al. Assimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosterone. J Clin Endocrinol Metab 1996;81:1018–1022 [DOI] [PubMed] [Google Scholar]
- 36.Martin ML, Jensen MD. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest 1991;88:609–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo ZK, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes 1999;48:1586–1592 [DOI] [PubMed] [Google Scholar]
- 38.Bickerton AS, Roberts R, Fielding BA, et al. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes 2007;56:168–176 [DOI] [PubMed] [Google Scholar]
- 39.McQuaid SE, Humphreys SM, Hodson L, Fielding BA, Karpe F, Frayn KN. Femoral adipose tissue may accumulate the fat that has been recycled as VLDL and nonesterified fatty acids. Diabetes 2010;59:2465–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sacchetti M, Saltin B, Osada T, van Hall G. Intramuscular fatty acid metabolism in contracting and non-contracting human skeletal muscle. J Physiol 2002;540:387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanaley JA, Shadid S, Sheehan MT, Guo ZK, Jensen MD. Relationship between plasma FFA, intramyocellular triglycerides and long-chain acylcarnitines in resting humans. J Physiol 2009;587:5939–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Febbraio M, Abumrad NA, Hajjar DP, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 1999;274:19055–19062 [DOI] [PubMed] [Google Scholar]
- 43.Tchoukalova Y, Koutsari C, Jensen M. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia 2007;50:151–157 [DOI] [PubMed] [Google Scholar]
- 44.Tchoukalova YD, Sarr MG, Jensen MD. Measuring committed preadipocytes in human adipose tissue from severely obese patients by using adipocyte fatty acid binding protein. Am J Physiol Regul Integr Comp Physiol 2004;287:R1132–R1140 [DOI] [PubMed] [Google Scholar]
- 45.Rasouli N, Yao-Borengasser A, Varma V, et al. Association of scavenger receptors in adipose tissue with insulin resistance in nondiabetic humans. Arterioscler Thromb Vasc Biol 2009;29:1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]