Abstract
OBJECTIVE
In patients with type 2 diabetes, glucagon levels are often increased. Furthermore, pulsatile secretion of insulin is disturbed in such patients. Whether pulsatile glucagon secretion is altered in type 2 diabetes is not known.
RESEARCH DESIGN AND METHODS
Twelve patients with type 2 diabetes and 13 nondiabetic individuals were examined in the fasting state and after mixed meal ingestion. Deconvolution analyses were performed on insulin and glucagon concentration time series sampled at 1-min intervals.
RESULTS
Both insulin and glucagon were secreted in distinct pulses, occurring at ∼5-min intervals. In patients with diabetes, postprandial insulin pulse mass was reduced by 74% (P < 0.001). Glucagon concentrations were increased in the patients during fasting and after meal ingestion (P < 0.05), specifically through an increased glucagon pulse mass (P < 0.01). In healthy subjects, the increase in postprandial insulin levels was inversely related to respective glucagon levels (P < 0.05). This relationship was absent in the fasting state and in patients with diabetes.
CONCLUSIONS
Glucagon and insulin are secreted in a coordinated, pulsatile manner. A plausible model is that the postprandial increase in insulin burst mass represses the corresponding glucagon pulses. Disruption of the insulin–glucagon interaction in patients with type 2 diabetes could potentially contribute to hyperglucagonemia.
The pathogenesis of type 2 diabetes involves multiple metabolic defects, the most important ones likely being β-cell dysfunction and insulin resistance (1,2). In addition, abnormal regulation of glucagon secretion contributes to the hyperglycemia in diabetic patients (3–5), and a number of studies have reported elevated fasting glucagon concentrations in patients with type 2 diabetes as well as in individuals with impaired glucose tolerance (3,6,7). Furthermore, whereas glucagon levels typically decline after oral or intravenous glucose administration in healthy individuals, the glucose-induced suppression of glucagon secretion is markedly impaired in patients with type 2 diabetes, and mixed meal–induced glucagon excursions are typically exaggerated in such patients (3,6). The inappropriately elevated glucagon levels may contribute to the exaggerated hepatic glucose production that characterizes patients with type 2 diabetes (8).
The mechanistic reasons underlying increased glucagon secretion in such patients are less well understood. Thus, some studies have reported increased numbers of α-cells in the diabetic pancreas (9,10). An alternative hypothesis is the lack of α-cell inhibition by insulin in diabetic patients (11,12). Indeed, suppression of glucagon secretion by insulin has been well established in various in vitro and in vivo models (13), and a selective loss of β-cells has been associated with the development of hyperglucagonemia (14). It has also been demonstrated that the glucagon response to hypoglycemia is lost in the absence of insulin (11,15).
Secretion of insulin from pancreatic islets in nondiabetic individuals is regulated in a pulsatile manner, with distinct bursts of insulin release occurring approximately every 5 min (16–18). In contrast, the amplitude and the orderliness of insulin secretion are markedly reduced in patients with type 2 diabetes (19–24). Impaired insulin pulsatility has been suggested to contribute to the development of insulin resistance in such patients (18,20,25).
For glucagon, a pulsatile secretion pattern has been reported in different large animal models (26,27). Based on these studies, a close interaction between insulin and glucagon secretion has been suggested. To examine this relationship in more detail, previous studies have examined insulin and glucagon levels in pigs before and after a selective β-cell reduction induced by the β-cytotoxin alloxan (14). It is noteworthy that there was a significant inverse relationship between postprandial insulin and glucagon secretion in healthy animals, but this pulsatile intra-islet inhibition of glucagon secretion by insulin was lost after reduction of β-cell mass, leading to overt hyperglucagonemia. Such studies have prompted speculation that reduction of intra-islet insulin secretion might also cause insufficient suppression of glucagon in patients with type 2 diabetes (14). However, to date, a pulsatile pattern of glucagon secretion has not been established in humans.
Therefore, in the present studies we addressed the following questions. (1) Is there evidence of a pulsatile pattern of glucagon secretion in humans? (2) Is there an inverse relationship between insulin and glucagon secretion? (3) Are the time patterns of glucagon secretion and its interactions with insulin secretion different in normal subjects and patients with type 2 diabetes?
RESEARCH DESIGN AND METHODS
Study protocol.
The study protocol was approved by the ethics committee of the medical faculty of Ruhr University Bochum prior to the experiments (registration number 2649). Written informed consent was obtained from all participants.
Subjects.
A total of 13 healthy volunteers (7 male and 6 female) without a history of diabetes and 12 patients with type 2 diabetes (6 male and 6 female; diagnosed according to World Health Organization criteria) participated in the study. The groups were matched for age and sex. Patients with diabetes exhibited a higher BMI than controls (P = 0.0078; Table 1). Mean diabetes duration was 8.3 ± 5.6 years. Antidiabetic treatment included diet and exercise only in two cases, metformin only in four cases, glinides in one case, a combination of sulfonylureas and metformin in two cases, and insulin in four cases. All antidiabetic treatment was withdrawn at least 48 h before the start of the study. In insulin-treated patients, short-acting insulin was withheld on the evening preceding the tests, whereas all long–acting insulin preparations were withdrawn at least 24 hours before the experiments to avoid carry-over effects. None of the participants were taking any other medication with a known influence on glucose homoeostasis. Detailed subject characteristics are reported in Table 1.
TABLE 1.
Characteristics of patients with type 2 diabetes and healthy control subjects
| Parameter (unit) | Type 2 diabetes | Controls | P value |
|---|---|---|---|
| Age (years) | 62.3 ± 6.7 | 60.5 ± 14.5 | 0.70 |
| Sex (male/female) | 6/6 | 7/6 | 0.85 |
| BMI (kg/m2) | 31.4 ± 4.6 | 26.8 ± 3.3 | 0.0078 |
| HbA1c (%) | 7.2 ± 1.1 | 5.7 ± 0.4 | <0.0001 |
| Total cholesterol (mg/dL) | 209 ± 30.7 | 212 ± 42.5 | 0.86 |
| HDL cholesterol (mg/dL) | 52.5 ± 15.6 | 69.5 ± 16.7 | 0.015 |
| LDL cholesterol (mg/dL) | 139 ± 24.5 | 132 ± 35.3 | 0.61 |
| Triglycerides (mg/dL) | 205 ± 105 | 117 ± 43.6 | 0.011 |
| Creatinine (mg/dL) | 0.98 ± 0.11 | 1.07 ± 0.21 | 0.24 |
| ASAT (units/L) | 22.0 ± 6.3 | 25.0 ± 5.5 | 0.22 |
| ALAT (units/L) | 28.3 ± 13.5 | 26.5 ± 11.1 | 0.72 |
Data are means ± SD unless otherwise indicated. P values were calculated using unpaired Student t test or chi-square test. ASAT, aspartate aminotransferase; ALAT, alanine aminotransferase.
Study design.
At a screening visit, blood was drawn from all participants in the fasting state for measurements of standard hematological and clinical chemistry parameters, and a general clinical examination was performed. In individuals without known diabetes, an oral glucose tolerance test was performed to exclude subjects with diabetes, impaired glucose tolerance, or impaired fasting glucose. Subjects with anemia (hemoglobin <12 g/dL), elevation in liver enzymes (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and γ-glutamyl-transferase) to higher activities than double the respective normal value, or elevated creatinine concentrations (>1.5 mg/dL) were excluded. Body height and weight were determined, and waist and hip circumference were measured to calculate BMI and the waist-to-hip ratio, respectively. Blood pressure was determined according to the Riva-Rocci method.
If subjects met the inclusion criteria, they were studied on two occasions: (1) a fasting experiment over 60 min, with venous blood samples being collected at 1-min intervals; and (2) a mixed meal test, with venous blood samples being collected over 240 min (from 30 to 90 min after meal ingestion, blood samples were collected at 1-min intervals). Both tests were performed in randomized order, separated by an interval of 1–4 weeks to allow for regeneration of the blood pool.
Experimental procedures.
Each test was performed in the morning after an overnight fast, with subjects in a supine position throughout the experiments. A large forearm vein was punctured with a Teflon cannula (Moskito 123, 18 gauge; Vygon, Aachen, Germany), and kept patent using 0.9% NaCl. On visit one, venous blood samples were taken at t = −5 and 0 min (9 mL each) as well as at 1-min intervals from 0 to 60 min (3 mL each). On visit two, a mixed meal (total caloric content 537 kcal; 42% fat, 41% carbohydrates, and 17% protein) was ingested together with 200 mL water over 5 min. Venous blood samples (9 mL each) were drawn frequently, as shown in Fig. 1. In addition, venous blood samples (3 mL each) were collected at 1-min intervals from t = 30 to 90 min after meal ingestion.
FIG. 1.
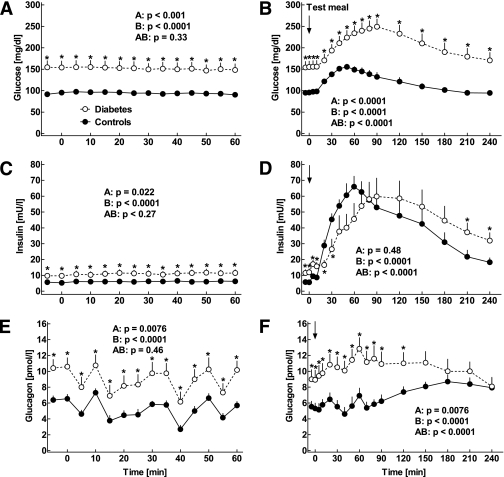
Plasma concentrations of glucose (A and B), insulin (C and D), and glucagon (E and F) in 12 patients with type 2 diabetes (open symbols) and 13 nondiabetic control subjects (filled symbols) studied in the fasting state (A, C, and E) as well as after mixed meal ingestion (B, D, and F). Arrows indicate the time of meal ingestion. Data are presented as means ± SEM. Statistics were carried out using repeated-measures ANOVA and denote (A) differences between the groups, (B) differences over the time course, and (AB) differences due to the interaction of group and time. Asterisks indicate significant differences at individual time points (P < 0.05).
Blood specimens.
Venous blood was drawn into chilled tubes containing EDTA and aprotinin (Trasylol, 20,000 kallikrein inhibitor units [KIU]/mL, 200 μL per 10 mL blood; Bayer AG, Leverkusen, Germany) and kept on ice. After centrifugation at 4°C, plasma for hormone analyses was kept frozen at −28°C.
Laboratory determinations.
Glucose concentrations were measured using a glucose oxidase method as previously described (28). Insulin was measured in duplicate, as previously described (28), using an insulin microparticle enzyme immunoassay (MEIA) (IMx Insulin; Abbott Laboratories, Wiesbaden, Germany). Intra-assay coefficient of variation was ∼4%. C-peptide was measured, as previously described (28), using an enzyme-linked immunoabsorbent assay (ELISA; DAKO Ltd., Cambridgeshire, U.K.).
Glucagon was measured by a radioimmunoassay. The assay uses a polyclonal antiserum (code number 4305) (29,30) that was raised in rabbits against natural porcine glucagon–linked NH2 terminally to albumin. The assay has a detection limit of 1 pmol/L and an intra-assay coefficient of variation of <6%.
Analysis of pulsatile hormone secretion.
Pulsatile secretion of insulin and glucagon was quantified by use of a deconvolution method developed by Veldhuis and Johnson (31). In brief, the 1-min plasma concentration time series were deconvolved by a multiparameter technique. The method assumes that fluctuating hormone plasma concentrations arise from five determinable and correlated parameters: (1) a finite number of discrete insulin and glucagon secretory bursts occurring at specific times and having (2) individual amplitudes (maximal rate of insulin or glucagon secretion within a burst), (3) a common half-duration (duration of an algebraically Gaussian secretory profile) (19), superimposed on a (4) basal time-invariant secretory rate, and (5) a biexponential insulin disappearance model in the systemic circulation consisting of estimated half-lives of 2.8 and 5.0 min and a fractional slow compartment of 28%, as previously measured (17), as well as a monoexponential glucagon disappearance model in the systemic circulation, with a half-life of 2.9 min, estimated by deconvolution analyses. Assuming the foregoing nominal disappearance values, we estimated the numbers, locations, amplitudes, and half-duration of the respective secretory bursts, as well as a nonnegative basal secretory rate for each data set by nonlinear least squares fitting. Secretory rates are expressed as mass units of insulin or glucagon released per unit of distribution volume (milliliters) per unit of time (minutes). The mean pulse mass of the respective hormones (time integral of the calculated secretory burst) is determined jointly by burst duration and amplitude. The percentage of insulin or glucagon delivered into the circulation in discrete bursts was calculated as described previously (16).
Cross-correlation.
To assess the interrelationship between insulin and glucagon concentration time series, cross-correlation analysis was applied to the paired series, as previously described (14). This procedure consists of linear correlations carried out repeatedly at various time lags between the paired values. Thus, each insulin concentration value is compared with a time-delayed measure (e.g., lag time minus 2 min) of a glucagon concentration value. At a zero time lag, simultaneous values are correlated.
Approximate entropy.
Regularity of insulin and glucagon plasma concentration time series was assessed by the model-independent and scale-invariant statistics approximate entropy (ApEn). ApEn measures the logarithmic likelihood that runs of patterns that are close (within r) for m contiguous observations remain close (within the tolerance width r) on subsequent incremental comparisons. A precise mathematical definition has been given by Pincus (32). A larger absolute value of ApEn indicates a higher degree on process randomness, i.e., less pattern orderliness. ApEn is rather stable to noise that is within tolerances. Detrending the time series by first differencing before ApEn calculation has been used to limit nonstationarity.
Cross-ApEn statistics.
To evaluate relative regularity of glucagon and insulin concentrations, we used the cross-approximate entropy statistic. Cross-ApEn is a bivariate analog of ApEn and quantifies joint pattern synchrony between two separate but linked time series after standardization (z score transformation of the data) (33,34). The interpretation of cross-ApEn as implemented here depends on whether pattern recurrence is assessed in the forward or reverse direction. By way of convention, we computed forward cross-ApEn using serial insulin concentrations as the template to assess pattern reproducibility in glucagon concentrations, and calculated reverse cross-ApEn by using successive glucagon concentrations as the template to evaluate pattern recurrence in insulin concentrations, as previously described (14). Note that, for physiological reasons, the foregoing schema of pairing relates the concentration of an input signal to the secretion rate of the output signal. Individual values for forward cross-ApEn were compared with respective reverse cross-ApEn values in each person to infer the driving force in the interaction between the secretion of both hormones.
Calculations and statistical analyses.
Patient characteristics are reported as mean ± SD. Analytical results are reported as mean ± SEM. All statistical calculations were carried out using repeated-measures analysis of variance (ANOVA) using Statistica version 5.0 (Statsoft Europe, Hamburg, Germany). Values at single time points were compared by one-way ANOVA followed Duncan's post hoc test. A two-sided P value < 0.05 was taken to indicate significant differences. Linear regression analyses were performed using GraphPad Prism, version 4.0 (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Mean fasting glucose concentrations were significantly higher in patients with type 2 diabetes than in controls (151.5 ± 14.8 mg/dL vs. 94.9 ± 1.72 mg/dL, respectively; P < 0.0001; Fig. 1). Corresponding insulin concentrations were 11.1 ± 2.0 mU/L vs. 6.1 ± 0.8 mU/L, respectively (P = 0.024; Fig. 1), and C-peptide (measured at two time points only) was 2.82 ± 0.2 ng/mL vs. 2.40 ± 0.24 ng/mL, respectively (P = 0.20). Mean fasting glucagon concentrations were increased in patients with type 2 diabetes (8.3 ± 1.2 pmol/L vs. 4.8 ± 0.5 pmol/L, respectively; P = 0.012; Fig. 1).
After meal ingestion, plasma glucose concentrations increased in both groups (P < 0.0001), with higher increments in patients than controls (P < 0.0001; Fig. 1). This was accompanied by an increase in insulin concentrations (P < 0.0001; Fig. 1). The postprandial increase in insulin levels was earlier and slightly higher in the control subjects, whereas insulin levels were higher in the patients in the late postprandial period (90–240 min; Fig. 1). Likewise, the early postprandial rise in C-peptide concentrations was higher in control subjects compared with the patients with type 2 diabetes (P < 0.0001; data not shown). Glucagon concentrations increased slightly in both groups and remained higher in the diabetic patients until 120 min after meal ingestion (Fig. 1).
Pulsatile insulin secretion.
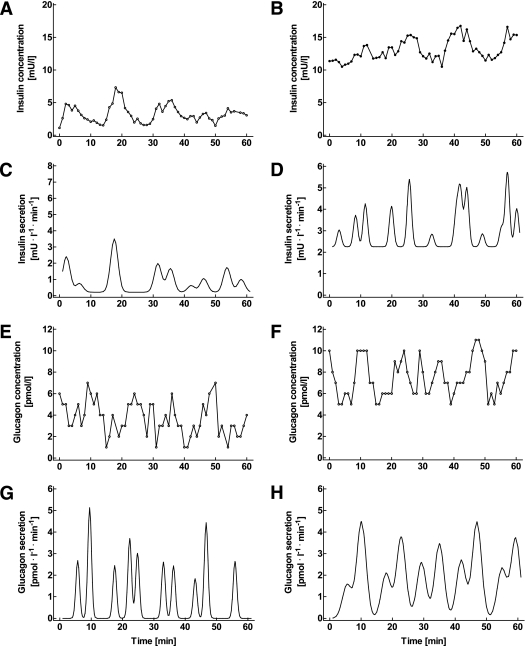
Inspection of the insulin concentration time series clearly revealed the presence of distinct pulses both in the fasting state and after meal ingestion (Figs. 2 and 3). The mean pulse interval during fasting was 5.1 ± 0.2 min in patients with diabetes and 5.2 ± 0.4 min in controls. Values remained unchanged after meal ingestion (5.3 ± 0.5 and 5.1 ± 0.5 min, respectively; P = 0.67 and P = 0.82 vs. fasting values; Tables 2 and 3). In the controls, insulin pulse mass increased from 3.6 ± 0.7 mU ⋅ L−1 ⋅ pulse−1 in the fasting state to 21.5 ± 3.3 mU ⋅ L−1 ⋅ pulse−1 after meal ingestion (P = 0.002). The corresponding values in patients with diabetes were 2.2 ± 0.4 mU ⋅ L−1 ⋅ pulse−1 vs. 7.7 ± 1.0 mU ⋅ L−1 ⋅ pulse−1, respectively (P = 0.0003; Tables 2 and 3). The postprandial insulin pulse mass in the controls was significantly higher than in the patients (P = 0.008; Table 3). In control subjects, basal insulin secretion amounted to 0.84 ± 0.24 mU ⋅ L−1 ⋅ min−1 in the fasting state and 2.99 ± 0.79 mU ⋅ L−1 ⋅ min−1 after meal ingestion (P = 0.0093). In the patients, nonpulsatile insulin secretion was 1.64 ± 0.35 mU ⋅ L−1 ⋅ min−1 at fasting and 2.43 ± 0.40 mU ⋅ L−1 ⋅ min−1 after the test meal (P = 0.15; Tables 2 and 3). Thus, pulsatile secretion accounted for 91 ± 3% of the overall insulin secretion at fasting and 93 ± 3% after the test meal in healthy subjects. In the patients, the proportion of insulin secretion derived from pulses was 76 ± 6% at fasting and 86 ± 3% after meal ingestion (P = 0.035 and P = 0.087 vs. controls, respectively). Approximate entropy, a measure of the orderliness of the insulin secretion time patterns, was 1.32 ± 0.03 in the patients and 1.26 ± 0.03 in controls under fasting conditions (P = 0.11). After meal ingestion, approximate entropy was significantly lower in patients than controls (1.19 ± 0.03 vs. 1.31 ± 0.03, respectively; P = 0.019).
FIG. 2.
Individual concentration time series of insulin (A and B) and glucagon (E and F), as well as the respective insulin secretion rates (C and D) and glucagon secretion rates (G and H), derived from deconvolution analysis, in a representative nondiabetic control subject (left) as well as in a patient with type 2 diabetes (right). The measurements were taken at 1-min intervals over 60 min in the fasting state.
FIG. 3.
Individual concentration time series of insulin (A and B) and glucagon (E and F), as well as the respective insulin secretion rates (C and D) and glucagon secretion rates (G and H), derived from deconvolution analysis, in a representative nondiabetic control subject (left) as well as in a patient with type 2 diabetes (right). The measurements were taken at 1-min intervals from t = 30 to 90 min after mixed meal ingestion.
TABLE 2.
Parameters of pulsatile insulin and glucagon secretion in the fasting state
| Parameter (unit) | Type 2 diabetes | Controls | P value |
|---|---|---|---|
| Insulin | |||
| Pulse interval (min) | 5.1 ± 0.2 | 5.2 ± 0.4 | 0.91 |
| Pulse mass (mU ⋅ L−1 ⋅ pulse−1) | 2.2 ± 0.4 | 3.6 ± 0.7 | 0.91 |
| Nonpulsatile secretion (mU ⋅ L−1 ⋅ min−1) | 1.6 ± 0.4 | 0.8 ± 0.2 | 0.68 |
| ApEn | 1.32 ± 0.03 | 1.26 ± 0.03 | 0.11 |
| Contribution of pulsatile secretion (%) | 75.7 ± 6.0 | 90.6 ± 3.1 | 0.035 |
| Glucagon | |||
| Pulse interval (min) | 5.4 ± 0.4 | 6.3 ± 0.6 | 0.33 |
| Pulse mass (pmol ⋅ L−1 ⋅ pulse−1) | 7.7 ± 0.9 | 5.0 ± 0.4 | 0.011 |
| Nonpulsatile secretion (pmol ⋅ L−1 ⋅ min−1) | 0.58 ± 0.25 | 0.22 ± 0.11 | 0.19 |
| ApEn | 1.27 ± 0.03 | 1.19 ± 0.04 | 0.067 |
| Contribution of pulsatile secretion (%) | 94.7 ± 2.5 | 97.4 ± 1.6 | 0.38 |
Data are means ± SEM. P values were calculated using unpaired Student t test.
TABLE 3.
Parameters of pulsatile insulin and glucagon secretion after ingestion of a mixed test meal
| Parameter (unit) | Type 2 diabetes | Controls | P value |
|---|---|---|---|
| Insulin | |||
| Pulse interval (min) | 5.3 ± 0.5 | 5.1 ± 0.5 | 0.62 |
| Pulse mass (mU ⋅ L−1 ⋅ pulse−1) | 7.7 ± 1.0 | 21.5 ± 3.3 | 0.008 |
| Nonpulsatile secretion (mU ⋅ L−1 ⋅ min−1) | 2.4 ± 0.4 | 3.0 ± 0.8 | 0.54 |
| ApEn | 1.19 ± 0.03 | 1.31 ± 0.03 | 0.019 |
| Contribution of pulsatile secretion (%) | 85.8 ± 2.9 | 93.3 ± 3.0 | 0.087 |
| Glucagon | |||
| Pulse interval (min) | 4.7 ± 0.4 | 6.6 ± 0.6 | 0.11 |
| Pulse mass (pmol ⋅ L−1 ⋅ pulse−1) | 8.1 ± 0.9 | 5.9 ± 0.6 | 0.035 |
| Nonpulsatile secretion (pmol ⋅ L−1 ⋅ min−1) | 1.1 ± 0.29 | 0.53 ± 0.20 | 0.13 |
| ApEn | 1.23 ± 0.03 | 1.27 ± 0.04 | 0.38 |
| Contribution of pulsatile secretion (%) | 94.7 ± 1.6 | 93.0 ± 2.8 | 0.62 |
Data are means ± SEM. P values were calculated using unpaired Student t test.
Pulsatile glucagon secretion.
The individual glucagon concentration time patterns revealed distinct high–amplitude oscillations in all individuals. The pulse interval during fasting was 5.4 ± 0.4 min in patients and 6.3 ± 0.6 min in controls. These values did not differ from those of insulin. The postprandial glucagon pulse interval was similar, both in patients and in controls: 4.7 ± 0.5 min and 6.6 ± 0.6 min, respectively (P = 0.27 and P = 0.71 vs. fasting values). In healthy subjects, glucagon pulse mass was 5.0 ± 0.4 pmol ⋅ L−1 ⋅ pulse−1 at fasting and 5.9 ± 0.6 pmol ⋅ L−1 ⋅ pulse−1 after meal ingestion (P = 0.23). The corresponding numbers in patients were 7.7 ± 0.9 pmol ⋅ L−1 ⋅ pulse−1 vs. 8.1 ± 0.9 pmol ⋅ L−1 ⋅ pulse−1 (P = 0.73). Glucagon pulse mass was higher in patients than controls both fasting and after meal ingestion (P = 0.011 and P = 0.035, respectively). The nonpulsatile component of glucagon secretion amounted to 0.58 ± 0.25 pmol ⋅ L−1 ⋅ min−1 vs. 1.09 ± 0.29 pmol ⋅ L−1 ⋅ min−1 (P = 0.27) in patients and 0.22 ± 0.11 pmol ⋅ L−1 ⋅ min−1 vs. 0.53 ± 0.2 pmol ⋅ L−1 ⋅ min−1 in controls (P = 0.19; each at fasting and after meal ingestion, respectively). Thus, in the fasting state, 95 ± 3 and 97 ± 2% of the total glucagon secretion was derived from pulses in patients with diabetes and controls, respectively, and this was unchanged after meal ingestion (95 ± 2 and 93 ± 3%, respectively). There were no differences with regards to the approximate entropy of glucagon secretion between patients and controls, whether fasting (1.27 ± 0.03 vs. 1.19 ± 0.04, respectively; P = 0.067) or after meal ingestion (1.23 ± 0.03 vs. 1.27 ± 0.04, respectively; P = 0.38).
Relationship between insulin and glucagon secretion.
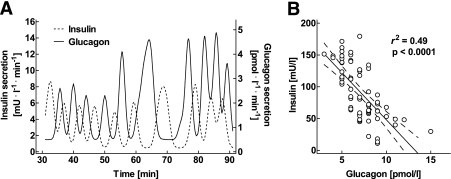
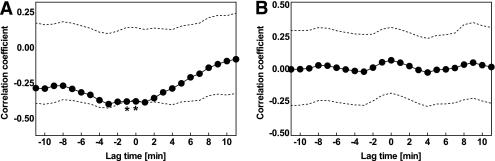
Inspection of the corresponding insulin and glucagon concentration time series identified pulses for both hormones (Figs. 2 and 3). In the fasting state, there was no apparent temporal relationship between the respective concentration time series in patients with type 2 diabetes and controls. In contrast, when postprandial insulin and glucagon concentration time series were analyzed in relation to each other, an inverse association between insulin and glucagon pulses became obvious (Fig. 4). To more specifically assess the timing of the association between insulin and glucagon, paired concentration time series were subjected to a cross-correlation analysis in each subject. Thereby, a significant inverse association of respective time patterns was found in the postprandial state in healthy individuals (P < 0.05; Fig. 5A). The mean lag time between the respective changes in insulin and glucagon levels was between 0 and 1 min. In contrast, the association between the postprandial insulin and glucagon time series was not significant in the patients with diabetes (Fig. 5B). There was no relationship between insulin and glucagon concentration time patterns in the fasting state in either group.
FIG. 4.
A: Individual postprandial secretion rates of insulin (left y-axis) and glucagon (right y-axis), derived from deconvolution analysis, in a representative nondiabetic control subject. B: The corresponding linear regression analysis between the minutely sampled postprandial concentrations of insulin and glucagon in the same individual.
FIG. 5.
Mean cross-correlogram (solid lines, filled symbols) between insulin and glucagon concentration time series in 13 nondiabetic control subjects (A) and 12 patients with type 2 diabetes (B) studied over 60 min after a mixed test meal. Data are presented in relation to the upper and lower 95% confidence intervals (dotted lines). *Significant (P < 0.05) interaction between the insulin and glucagon time series at a given lag period. These analyses demonstrate a significant relationship between the insulin and glucagon concentration time series after meal ingestion in nondiabetic individuals, whereas the time relationship between both hormones is absent in patients with diabetes.
To estimate the driving force in the relationship between insulin and glucagon, cross-ApEn analyses were performed in a forward fashion (by computing cross-ApEn using serial insulin concentrations as the template to assess pattern reproducibility in glucagon concentrations) and in a reverse fashion (using glucagon concentrations as the template to assess pattern reproducibility in insulin concentrations). Forward cross-ApEn was significantly higher (less synchronous) than reverse cross-ApEn (P < 0.001; Fig. 6), indicating that insulin may drive glucagon rather than vice versa. This association was found in both patients and controls under fasting conditions as well as after meal ingestion.
FIG. 6.
Cross-ApEn statistics for the interaction of insulin and glucagon concentration time series in 13 nondiabetic control subjects (A and C) and 12 patients with type 2 diabetes (B and D) studied over 60 min in the fasting state (A and B) as well as over 60 min after mixed meal ingestion (C and D). Analyses were carried out by entering either glucagon (forward) or insulin (reverse) first. P values were calculated using paired Student t test.
DISCUSSION
The present study was undertaken to examine ultradian time patterns of glucagon secretion in humans. We report that (1) glucagon is secreted in a coordinated, pulsatile manner, with distinct secretory bursts occurring at ∼5-min intervals; (2) circulating glucagon and insulin levels are inversely related, with insulin patterns possibly driving glucagon patterns; and (3) in patients with type 2 diabetes, the postprandial association between insulin and glucagon secretion is significantly disturbed.
The inverse association between both islet hormones was evident from various analyses. First, an inverse association between postprandial insulin and glucagon levels was already apparent by linear regression analysis of respective concentrations. Second, cross-correlation analyses of insulin and glucagon concentration time series revealed a close inverse relationship between both hormones in the postprandial state at the 0- and 1-min lag times. It is noteworthy that this association was significant only in healthy control subjects, indicating that the inverse interaction between each insulin and glucagon concentration wavefront was perturbed in patients with diabetes. Third, forward and reverse cross-ApEn statistics identified insulin as the putative primary signal.
Given that the association between the peptide hormones was apparent only after mixed meal ingestion, but not in the fasting state when the size of the insulin pulses was relatively small, one could postulate that the postprandial increase in insulin burst mass contributes to the glucose-induced decline in glucagon secretion. In the present experiments, postprandial glucagon concentrations were not lowered, because a mixed meal rather than a pure glucose load was administered.
A number of previous studies have examined the interrelationship between α-cells and β-cells in different experimental models. In this regard, earlier studies by Samols et al. (35) suggested centripetal blood flow from the β-cell–enriched core of the islets toward α-cells in the islet periphery. This concept of a paracrine interaction has recently been extended by the notion that, in addition to insulin itself, other β-cell secretory products, such as zinc, might contribute to intra-islet inhibition of glucagon secretion (36).
A specific reduction in insulin burst mass has been reported previously in patients with type 2 diabetes, whereas insulin pulse frequency was typically unaltered in such patients (19,24). The present results are in good agreement with these studies by showing a 74% reduction in postprandial insulin pulse mass in patients with diabetes. Of note, to our knowledge, this is the largest study to intensively analyze the patterns of pulsatile insulin secretion in humans in vivo.
One of the key challenges in analyzing pulsatile insulin secretion under in vivo conditions lies in the fact that the amplitude of insulin pulses in the peripheral circulation is relatively small, because the liver selectively extracts insulin pulses during its first passage (18). In contrast, when insulin concentrations are determined directly in the portal venous circulation, the amplitude of the insulin pulses far exceeds those in the periphery (37). Therefore, to properly identify the insulin pulses in the peripheral circulation, deconvolution analysis has been employed, which takes into account the elimination kinetics of endogenous insulin to compute the respective insulin secretion rates (20). For glucagon, there is little hepatic elimination (38,39). Accordingly, glucagon secretory bursts can be already identified even from the serial concentrations in the peripheral blood.
The results of the present study in humans are in good agreement with previous data in pigs and in baboons (14,26,27), in which an oscillatory secretion pattern of insulin and glucagon has been described. Furthermore, the inverse association between postprandial insulin and glucagon pulses in mini-pigs was found to be absent after a selective ∼50% reduction of β-cell mass using alloxan (14). It is noteworthy that in the present experiments, cross-correlation analysis also revealed disruption of the inverse relationship between the two peptides in patients with type 2 diabetes. Comparable outcomes in the alloxan-pig model and the presently examined patients with type 2 diabetes might, therefore, be interpreted to emphasize the importance of endogenous β-cell function in the pathogenesis of type 2 diabetes, although clearly the clinical phenotype of type 2 diabetes is far more complex and heterogeneous than that of animal models.
The reasons underlying the insufficient suppression of glucagon levels by glucose are still debated, and some previous studies have suggested an intrinsic alteration of the α-cell phenotype in type 2 diabetes (40). However, normal glucagon responses in first-degree relatives of patients with type 2 diabetes favor the hypothesis that this defect develops secondary to other metabolic alterations in diabetic patients (41), consistent with animal studies showing a loss of glucose-induced glucagon suppression following an experimental reduction in β-cell mass (14,27,42). Furthermore, the present clinical study suggests that loss of pulsatile insulin secretion goes along with an increase in glucagon secretion. The tight time relationship between the peptide concentration time series indicates that this interaction takes place primarily in a paracrine fashion at the level of individual islets. However, because exogenous insulin can lower glucagon levels in diabetic patients as well (43), a classical endocrine effect of circulating insulin may contribute to the control of glucagon secretion as well.
Assuming that the hyperglucagonemia in patients with diabetes is largely attributable to attenuation of pulsatile insulin secretion, a logical implication would be to enhance pulsatile release of insulin in such patients. In support of such reasoning, several previous studies examining pulsatile insulin delivery regimens in patients with diabetes have demonstrated a greater suppression of hepatic glucose production by this approach (25,44). However, from a practical point of view, it is difficult to restore pulsatile insulin secretion by therapeutic means, because the typical route of endogenous insulin delivery from the β-cells toward the α-cells and, subsequently, to the liver via the portal vein cannot readily be mimicked by intravenous or subcutaneous insulin administration.
Although the differences in pulsatile insulin and glucagon secretion between patients with type 2 diabetes and healthy individuals are most likely due to the reduction in β-cell mass and function in type 2 diabetes; other factors such as obesity might contribute as well. Because the presently examined group of patients with diabetes was significantly more obese, such confounding effects cannot be excluded completely.
Physiological implications of pulsatile glucagon secretion have been examined largely under in vitro conditions. In isolated rat hepatocytes, induction of glucose production was significantly greater when the same amount of glucagon was delivered in a pulsatile rather than a continuous fashion (45). Likewise, suppression of hepatic glucose production in humans was found to be closely related to the amplitude and mass of prehepatic insulin secretion (18). Taken together, these findings suggest that pulsatile secretion of insulin and glucagon enhances the actions of these hormones at the level of the hepatocytes.
In conclusion, the present study demonstrates that, in healthy individuals, insulin and glucagon secretion arises from distinct secretory bursts, occurring in a reciprocal fashion at ∼5-min intervals. In patients with type 2 diabetes, the postprandial mass of the insulin pulses is significantly diminished, and the interaction between both hormones is disturbed. These alterations in the β–α-cell cross-talk might contribute to the hyperglucagonemia in patients with type 2 diabetes.
ACKNOWLEDGMENTS
This study was supported by unrestricted grants from Ruhr-University Bochum (FoRUM Grant F282/2003 to J.J.M.), The Danish Medical Research Council (to J.J.H.), the Deutsche Forschungsgemeinschaft (Grant Me 2096/6-1 to J.J.M.), and GlaxoSmithKline (to J.J.M.). No other potential conflicts of interest relevant to this article were reported.
B.A.M. analyzed the data and contributed to the experiments and manuscript preparation. L.G. contributed to the experiments. S.M.J. contributed to the hormone measurements. C.F.D. contributed to the hormone measurements, data interpretation, and manuscript preparation. J.D.V. contributed to the data analyses and interpretation and manuscript preparation. W.E.S. contributed to the manuscript review. J.J.H. contributed to the hormone measurements, data interpretation, and manuscript preparation. J.J.M. contributed to the study design, data collection, and interpretation and wrote the manuscript.
The excellent technical assistance of Birgit Baller and Kirsten Mros (Department of Medicine, St. Josef-Hospital, Ruhr-University Bochum) is greatly acknowledged.
REFERENCES
- 1.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988;37:667–687 [DOI] [PubMed] [Google Scholar]
- 3.Unger RH, Aguilar-Parada E, Müller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest 1970;49:837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia 1985;28:574–578 [DOI] [PubMed] [Google Scholar]
- 5.Lefèbvre PJ. Glucagon and its family revisited. Diabetes Care 1995;18:715–730 [DOI] [PubMed] [Google Scholar]
- 6.Sherwin RS, Fisher M, Hendler R, Felig P. Hyperglucagonemia and blood glucose regulation in normal, obese and diabetic subjects. N Engl J Med 1976;294:455–461 [DOI] [PubMed] [Google Scholar]
- 7.Mitrakou A, Kelley D, Mokan M, et al. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 1992;326:22–29 [DOI] [PubMed] [Google Scholar]
- 8.Rizza RA, Gerich JE. Persistent effect of sustained hyperglucagonemia on glucose production in man. J Clin Endocrinol Metab 1979;48:352–355 [DOI] [PubMed] [Google Scholar]
- 9.Clark A, Wells CA, Buley ID, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res 1988;9:151–159 [PubMed] [Google Scholar]
- 10.Yoon KH, Ko SH, Cho JH, et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 2003;88:2300–2308 [DOI] [PubMed] [Google Scholar]
- 11.Raju B, Cryer PE. Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. Diabetes 2005;54:757–764 [DOI] [PubMed] [Google Scholar]
- 12.Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci USA 2010;107:16009–16012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravier MA, Rutter GA. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes 2005;54:1789–1797 [DOI] [PubMed] [Google Scholar]
- 14.Meier JJ, Kjems LL, Veldhuis JD, Lefèbvre P, Butler PC. Postprandial suppression of glucagon secretion depends on intact pulsatile insulin secretion: further evidence for the intraislet insulin hypothesis. Diabetes 2006;55:1051–1056 [DOI] [PubMed] [Google Scholar]
- 15.Zhou H, Tran PO, Yang S, et al. Regulation of alpha-cell function by the beta-cell during hypoglycemia in Wistar rats: the “switch-off” hypothesis. Diabetes 2004;53:1482–1487 [DOI] [PubMed] [Google Scholar]
- 16.Pørksen N, Munn S, Steers J, Vore S, Veldhuis JD, Butler PC. Pulsatile insulin secretion accounts for 70% of total insulin secretion during fasting. Am J Physiol 1995;269:E478–E488 [DOI] [PubMed] [Google Scholar]
- 17.Pørksen N, Nyholm B, Veldhuis JD, Butler PC, Schmitz O. In humans at least 75% of insulin secretion arises from punctuated insulin secretory bursts. Am J Physiol 1997;273:E908–E914 [DOI] [PubMed] [Google Scholar]
- 18.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes 2005;54:1649–1656 [DOI] [PubMed] [Google Scholar]
- 19.Hollingdal M, Juhl CB, Pincus SM, et al. Failure of physiological plasma glucose excursions to entrain high-frequency pulsatile insulin secretion in type 2 diabetes. Diabetes 2000;49:1334–1340 [DOI] [PubMed] [Google Scholar]
- 20.Pørksen N, Hollingdal M, Juhl C, Butler P, Veldhuis JD, Schmitz O. Pulsatile insulin secretion: detection, regulation, and role in diabetes. Diabetes 2002;51(Suppl 1):S245–S254 [DOI] [PubMed] [Google Scholar]
- 21.Polonsky KS, Given BD, Hirsch LJ, et al. Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N Engl J Med 1988;318:1231–1239 [DOI] [PubMed] [Google Scholar]
- 22.Sturis J, Polonsky KS, Shapiro ET, Blackman JD, O’Meara NM, van Cauter E. Abnormalities in the ultradian oscillations of insulin secretion and glucose levels in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1992;35:681–689 [DOI] [PubMed] [Google Scholar]
- 23.Lang DA, Matthews DR, Burnett M, Turner RC. Brief, irregular oscillations of basal plasma insulin and glucose concentrations in diabetic man. Diabetes 1981;30:435–439 [DOI] [PubMed] [Google Scholar]
- 24.Ritzel R, Schulte M, Nauck M, März W, Porksen N, Schmiegel W, Nauck MA. GLP-1 increases secretory burst mass of pulsatile insulin secretion in patients with impaired glucose tolerance and Type 2 diabetes (Abstract). Diabetologia 1998;41(Suppl. 1):A182 [DOI] [PubMed]
- 25.Bratusch-Marrain PR, Komjati M, Waldhäusl WK. Efficacy of pulsatile versus continuous insulin administration on hepatic glucose production and glucose utilization in type I diabetic humans. Diabetes 1986;35:922–926 [DOI] [PubMed] [Google Scholar]
- 26.Goodner CJ, Walike BC, Koerker DJ, et al. Insulin, glucagon, and glucose exhibit synchronous, sustained oscillations in fasting monkeys. Science 1977;195:177–179 [DOI] [PubMed] [Google Scholar]
- 27.Goodner CJ, Koerker DJ, Weigle DS, McCulloch DK. Decreased insulin- and glucagon-pulse amplitude accompanying beta-cell deficiency induced by streptozocin in baboons. Diabetes 1989;38:925–931 [DOI] [PubMed] [Google Scholar]
- 28.Meier JJ, Hücking K, Holst JJ, Deacon CF, Schmiegel WH, Nauck MA. Reduced insulinotropic effect of gastric inhibitory polypeptide in first-degree relatives of patients with type 2 diabetes. Diabetes 2001;50:2497–2504 [DOI] [PubMed] [Google Scholar]
- 29.Holst JJ. Evidence that glicentin contains the entire sequence of glucagon. Biochem J 1980;187:337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holst JJ. Evidence that enteroglucagon (II) is identical with the C-terminal sequence (residues 33-69) of glicentin. Biochem J 1982;207:381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veldhuis JD, Johnson ML. Deconvolution analysis of hormone data. Methods Enzymol 1992;210:539–575 [DOI] [PubMed] [Google Scholar]
- 32.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA 1991;88:2297–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu PY, Pincus SM, Keenan DM, Roelfsema F, Veldhuis JD. Analysis of bidirectional pattern synchrony of concentration-secretion pairs: implementation in the human testicular and adrenal axes. Am J Physiol Regul Integr Comp Physiol 2005;288:R440–R446 [DOI] [PubMed]
- 34.Liu PY, Pincus SM, Keenan DM, Roelfsema F, Veldhuis JD. Joint synchrony of reciprocal hormonal signaling in human paradigms of both ACTH excess and cortisol depletion. Am J Physiol Endocrinol Metab 2005;289:E160–E165 [DOI] [PubMed]
- 35.Samols E, Stagner JI, Ewart RB, Marks V. The order of islet microvascular cellular perfusion is B----A----D in the perfused rat pancreas. J Clin Invest 1988;82:350–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 2005;54:1808–1815 [DOI] [PubMed] [Google Scholar]
- 37.Song SH, McIntyre SS, Shah H, Veldhuis JD, Hayes PC, Butler PC. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J Clin Endocrinol Metab 2000;85:4491–4499 [DOI] [PubMed] [Google Scholar]
- 38.Deacon CF, Kelstrup M, Trebbien R, Klarskov L, Olesen M, Holst JJ. Differential regional metabolism of glucagon in anesthetized pigs. Am J Physiol Endocrinol Metab 2003;285:E552–E560 [DOI] [PubMed] [Google Scholar]
- 39.Holst JJ. Degradation of pancreatic peptides: glucagon. In Degradation of Bioactive Substances, Physiology and Pathophysiology. Henriksen JH, Ed. Boca Raton, Florida, CRC press, Inc., 1991, p. 167–180 [Google Scholar]
- 40.Gerich JE. Abnormal glucagon secretion in type 2 (noninsulin-dependent) diabetes mellitus: causes and consequences. In Diabetes Mellitus: Pathophysiology and Therapy. Creutzfeldt W, Lefèbvre P, Eds. Berlin, Germany, Springer Verlag, 1989, p. 127–133 [Google Scholar]
- 41.Meier JJ, Deacon CF, Schmidt WE, Holst JJ, Nauck MA. Suppression of glucagon secretion is lower after oral glucose administration than during intravenous glucose administration in human subjects. Diabetologia 2007;50:806–813 [DOI] [PubMed]
- 42.Meier JJ, Ueberberg S, Korbas S, Schneider S. Diminished glucagon suppression after β-cell reduction is due to impaired α-cell function rather than an expansion of α-cell mass. Am J Physiol Endocrinol Metab 2011;300:E717–E723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raskin P, Fujita Y, Unger RH. Effect of insulin-glucose infusions on plasma glucagon levels in fasting diabetics and nondiabetics. J Clin Invest 1975;56:1132–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paolisso G, Scheen AJ, Giugliano D, et al. Pulsatile insulin delivery has greater metabolic effects than continuous hormone administration in man: importance of pulse frequency. J Clin Endocrinol Metab 1991;72:607–615 [DOI] [PubMed] [Google Scholar]
- 45.Weigle DS, Koerker DJ, Goodner CJ. Pulsatile glucagon delivery enhances glucose production by perifused rat hepatocytes. Am J Physiol 1984;247:E564–E568 [DOI] [PubMed] [Google Scholar]