Abstract
OBJECTIVE
A progressive decline in insulin responses to glucose was noted in individuals before the onset of type 1 diabetes. We determined whether such abnormalities occurred in prediabetic NOD mice—the prototypic model for human type 1 diabetes.
RESEARCH DESIGN AND METHODS
Morning blood glucose was measured every other day in a cohort of NOD females. Glucose tolerance and insulin secretion were measured longitudinally by intraperitoneal glucose tolerance tests in NOD/ShiLtJ and BALB/cJ mice 6 to 14 weeks of age. Arginine-stimulated insulin secretion and insulin sensitivity were assessed during intraperitoneal arginine or intraperitoneal insulin tolerance tests.
RESULTS
During prediabetes, NOD females displayed a progressive increase in glucose levels followed by an acute onset of hyperglycemia. First-phase insulin responses (FPIRs) during the intraperitoneal glucose tolerance test (IPGTT) declined before loss of glucose tolerance in NOD. The failure of FPIR could be detected, with a decline in peak insulin secretion during IPGTT. Arginine-stimulated insulin secretion remained unchanged during the study period. The decline in insulin secretion in NOD mice could not be explained by changes in insulin sensitivity.
CONCLUSIONS
There was an impressive decline in FPIR before changes in glucose tolerance, suggesting that impairment of FPIR is an early in vivo marker of progressive β-cell failure in NOD mice and human type 1 diabetes. We portend that these phenotypes in NOD mice follow a similar pattern to those seen in humans with type 1 diabetes and validate, in a novel way, the importance of this animal model for studies of this disease.
Type 1 diabetes is an autoimmune disease resulting from the destruction of pancreatic insulin-producing β-cells. The NOD mouse is the most commonly used rodent model of the disease. Studies in this mouse strain have led to interventions that have been translated to clinical investigations with human type 1 diabetic patients (1–5). However, intervening at the right therapeutic window is critical to the efficacy of certain therapies. For example, anti-mouse thymocyte globulin can delay or reverse diabetes in NOD only when used in the late prediabetes stage or at onset of the disease (6).
Islet autoimmunity can be identified in the prediabetic stage. Specifically, in NOD mice, insulin autoantibodies can be detected as early as at 3 weeks of age (7). However, as in humans, autoantibody positivity can be transient as well as observed in nonprogressors (8,9). Therefore, markers of β-cell mass and function are needed to identify progression from the onset of islet autoimmunity to and through the stages of prediabetes.
Previous cross-sectional studies have demonstrated decreased first-phase insulin secretion in NOD females at different ages. Using pancreatic perfusion, Kano et al. (10) documented that NOD mice maintain normal fasting glucose even though a significant decrease was measured in first-phase insulin response (FPIR) to glucose. In these studies, increased fasting glucose and loss of FPIR was associated with the degree of insulitis. In cross-sectional studies using intravenous glucose tolerance test, Reddy et al. (11) showed an age-related progressive decline in FPIR to glucose. In another cross-sectional study using in situ pancreas perfusion, Sreenan et al. (12) reported progressive decreases in glucose- and arginine-stimulated insulin secretion in NOD females at 8, 13, and 18 weeks of age. Although single-point analyses were performed, there have not been reports involving sequential analysis of single mice over time to confirm this progression. In this study, we sought to determine whether similar metabolic signatures exist in both humans and in prediabetic NOD mice.
RESEARCH DESIGN AND METHODS
Mice.
NOD/ShiLtJ (NOD) and BALB/cJ (BALB) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice used were maintained in a specific pathogen-free research animal facility at the Children’s Hospital of Pittsburgh or the University of Florida. Mice were allowed free access to acidified drinking water and NIH-31 diet (Purina Mills, Richmond, IN) containing 6% fat. All procedures were approved by the Institutional Animal Use and Care Committees of either the Children’s Hospital of Pittsburgh or the University of Florida and were in compliance with U.S. laws.
Blood glucose measures before onset.
Morning blood glucose concentrations were measured at least every other day in a cohort of 68 female NOD/ShiLtJ mice, starting at 8 weeks of age. Any reading of blood glucose level >250 mg/dL was followed by a test 24 h later. Diabetes was diagnosed by two successive readings of blood glucose >250 mg/dL. The blood glucose levels are reported starting at 22 days before diagnosis.
In vivo glucose-stimulated insulin secretion.
Female NOD/ShiLtJ and BALB/cJ mice were fasted overnight (16–18 h), weighed, and injected with d-glucose (Sigma-Aldrich, St. Louis, MO) at a dose of 1.5 g/kg body weight intraperitoneally (intraperitoneal glucose tolerance test [IPGTT]). Blood (75 μL) was obtained from the retro-orbital sinus before and at 2, 5, 10, 30, 60, and 120 min after glucose injection. Blood was collected in heparinized capillary tubes and analyzed for glucose levels using a portable Freestyle blood glucose meter (Abbott Laboratories, North Chicago, IL) and then centrifuged. Plasma was separated and stored at –20°C until analysis. One milliliter of 0.9% NaCl solution was administered subcutaneously after obtaining the 30-min sample to maintain blood volume.
Insulin tolerance test.
NOD/ShiLtJ and BALB/cJ mice were fasted for 4 h, weighed, and then injected with human insulin (Humulin R; Eli Lilly, Indianapolis, IN) intraperitoneally at a dose of 0.75 units/kg. Blood (75 μL) was obtained from the retro-orbital sinus before and at 15, 30, 45, and 60 min after insulin injection. Glucose levels were measured by a glucose meter as described above.
In vivo l-arginine–stimulated insulin secretion.
A different cohort of female NOD/ShiLtJ and BALB/cJ mice was fasted for 4 h, weighed, and injected with l-arginine at a dose of 1 g/kg for intraperitoneal arginine tolerance tests. Blood (75 μL) was obtained from the retro-orbital sinus before and at 2, 5, 10, and 30 min after the injection of arginine. Blood was collected in heparinized capillary tubes and immediately centrifuged. Plasma was separated and stored at –20°C until analysis.
Insulin assays.
Insulin was determined by radioimmunoanalysis using a guinea pig anti-rat insulin antibody, with 125I-labeled insulin as tracer and rat insulin as standard (Linco Research, St. Charles, MO). The assay was counted using a Wizard 1470 Automated γ Counter (Wallac, Turku, Finland).
Histology.
Pancreata were removed from female mice at the stated ages and fixed in 10% formalin overnight. Paraffin-embedded tissue sections were stained with hematoxylin and eosin and counterstained with Aldehyde Fuschin. Insulitis was scored as previously described (13).
Statistical analysis.
Unless stated otherwise, data are shown as means ± SE. Significance (P < 0.05) was determined by a one-way ANOVA with Bonferroni multiple comparison test or t test for two group comparisons (GraphPad Prism 5.02; GraphPad Software, Inc., San Diego, CA).
RESULTS
Prediabetic NOD/ShiLtJ mice exhibit a steady increase in blood glucose levels before type 1 diabetes onset.
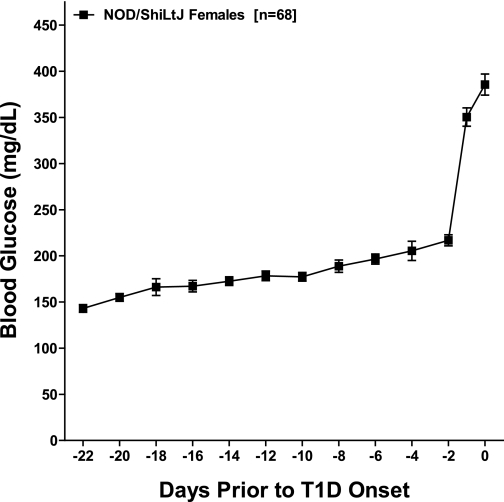
To determine the pattern of development of hyperglycemia, we followed a cohort of NOD/ShiLtJ mice throughout the prediabetic period. These mice developed diabetes between 11 and 16 weeks of age. When the results are synchronized for diabetes onset, blood glucose levels progressively increased over the 3 weeks preceding the onset of diabetes in these 68 NOD/ShiLtJ mice. This trend was followed by an acute onset of hyperglycemia (Fig. 1).
FIG. 1.
In NOD/ShiLtJ females, diabetes is preceded by a progressive increase in glucose levels. Morning glucose levels in NOD/ShiLtJ females (n = 68) were recorded every other day starting at 10 weeks of age. Results are synchronized for diabetes onset defined as two consecutive glucose readings >250 mg/dL. The day of onset was set to day 0. Data are presented as mean ± SD. T1D, type 1 diabetes.
NOD mice show evidence of progressive decline of first-phase insulin release before onset of diabetes.
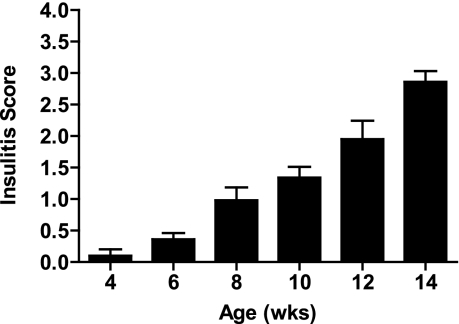
In our animals, the diabetes incidence in NOD/ShiLtJ female mice has consistently been 90% by 30 weeks of age, with an incidence of 60% by 16 weeks of age. A progressive lymphocytic infiltration is evidenced by increasing insulitis scores from 6 to 14 weeks of age (Fig. 2).
FIG. 2.
Progressive lymphocytic infiltration into the pancreatic islets of female NOD/ShiLtJ mice. Formalin-fixed paraffin–embedded pancreata were sectioned, and two sections were mounted from each organ on a single slide that was stained with hematoxylin and eosin and counterstained with Aldehyde Fuschin. An insulitis score was calculated using at least five slides for each age.
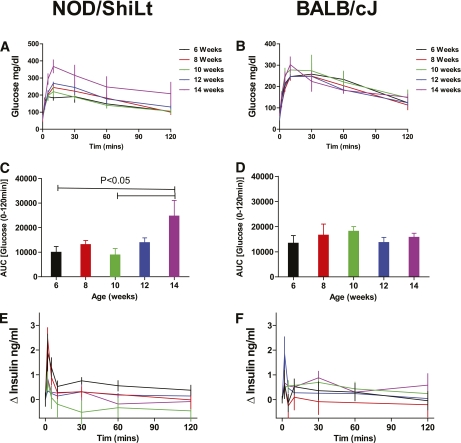
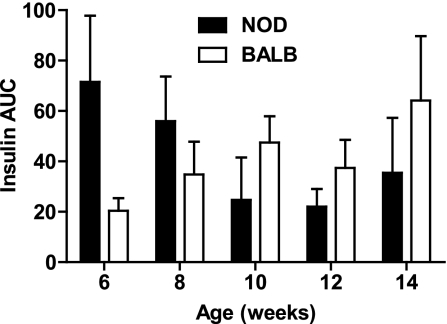
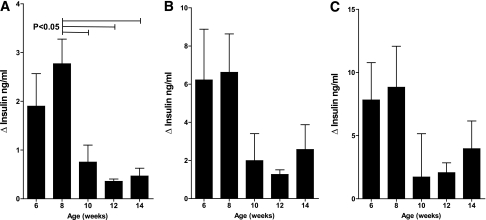
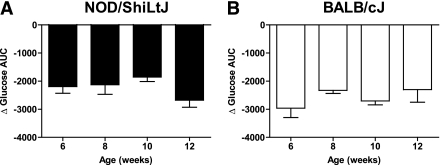
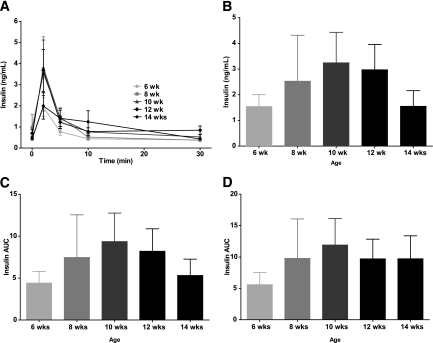
To establish a practical measure for the decline in β-cell function in prediabetic NOD/ShiLtJ mice, we longitudinally measured insulin secretion during IPGTTs as well as intraperitoneal arginine tolerance tests in two groups of NOD/ShiLtJ females (n = 5 for each). During serial IPGTT, NOD/ShiLtJ females maintained similar fasting glucose, glycemic responses, and glucose at 120 min from 6 to 14 weeks of age, with deterioration in glucose area under the curve (AUC) observed by 14 weeks of age. By comparison, BALB/cJ mice maintained stable glucose tolerance throughout the study period (Fig. 3). There was a trend toward lower insulin AUC in NOD/ShiLtJ and increasing insulin AUC in BALB/cJ over time (Fig. 4). It is noteworthy that a loss of first-phase insulin release was evident after 8 weeks of age in all NOD mice tested. In contrast, BALB/cJ mice showed no significant change in first-phase insulin secretion over the same time period (Fig. 3). The decrease in FPIR from 8 to 10 weeks of age was best detected as a reduction of the peak insulin secretion (difference in insulin level from baseline to 2 min) after glucose administration (Fig. 5A). The AUC for insulin from 0 to 5 or 0 to 10 min was less sensitive to the observed change in FPIR (Fig. 5B and C).
FIG. 3.
First-phase insulin release to glucose declines before the onset of glucose intolerance in NOD/ShiLtJ females. NOD/ShiLtJ female mice demonstrated deterioration in glucose tolerance at 14 weeks of age. This result was observed in both the curve for blood glucose (A) as well as after a calculation of AUC for blood glucose for the 2-h period (C). BALB/cJ mice show stable glucose tolerance (B and D). In NOD/ShiLtJ mice, the FPIR significantly declines after 8 weeks of age (E), whereas there is no change in BALB/cJ mice with age (F). n = 5 mice per group. Data presented are the mean ± SEM of the change from baseline during an IPGTT with 1.5 g/kg d-glucose.
FIG. 4.
NOD/ShiLtJ mice show a declining trend in insulin secretion during IPGTT. The AUC for insulin during IPGTT showed a declining trend in NOD/ShiLtJ, whereas BALB/cJ mice displayed the opposite trend. This did not reach significance either for intra-strain insulin AUC over time (NOD/ShiLtJ, P = 0.291; BALB/cJ, P = 0.306) or for inter-strain insulin AUC over time (two-way ANOVA, P = 0.913). Data are shown as mean ± SEM.
FIG. 5.
In NOD/ShiLtJ mice, the change in insulin level from baseline to 2 min during IPGTT provides a simple measurement of loss of FPIR. A: The decline in FPIR from 8 to 10 weeks was readily detected by the change in plasma insulin from 0 to 2 min after intraperitoneal administration of 1.5 mg/kg d-glucose. The insulin AUC for both 0–5 min (B) and 0–10 min (C) was less sensitive to the decline in FPIR. Data are shown as mean ± SEM. n = 5 mice per group.
The decrease in FPIR is not explained by changes in insulin resistance.
We estimated insulin sensitivity as AUC for glucose after intraperitoneal administration of 0.75 units of insulin per kilogram of body weight. There was no significant change in insulin sensitivity from 6 to 12 weeks of age in NOD/ShiLtJ mice, nor a difference in insulin sensitivity when compared with age-matched BALB/cJ mice. This suggests that the decrease in FPIR observed in NOD/ShiLtJ mice between 8 and 12 weeks is primarily due to loss of β-cell function or mass and not the result of increased insulin sensitivity during this period (Fig. 6).
FIG. 6.
Insulin sensitivity as measured by insulin tolerance tests remained stable from 6 to 12 weeks in NOD/ShiLtJ and BALB/cJ females. Both NOD (A) and BALB/cJ (B) mice were administered insulin intraperitoneally (0.75 units/kg body weight). There was no change in insulin tolerance from 6 to 12 weeks of age in NOD/ShiLtJ mice. In addition, NOD and BALB/cJ mice were equally insulin tolerant at the ages tested. Data are shown as mean AUC for change from baseline glucose ± SEM. n = 5 mice per age-group.
Persistent insulin release to l-arginine.
To assess insulin secretory capacity to a nonglucose stimulus, we performed intraperitoneal arginine tolerance tests. In contrast to the insulin response to glucose in NOD/ShiLtJ mice, in which we observed a significant decline in FPIR between 8 and 10 weeks of life, we found no decline in the acute insulin response to intraperitoneal administration of 1 g/kg l-arginine in NOD/ShiLtJ mice. To the contrary, there was a trend toward increased insulin secretion at ∼10 weeks of age, with no significant change from 6 to 14 weeks of life in l-arginine–induced insulin secretion (Fig. 7).
FIG. 7.
NOD/ShiLtJ mice showed no decline in arginine-stimulated insulin secretion from 6 to 14 weeks of age. There was no significant change in insulin levels over time (A) (P = 0.32), change from baseline to 2 min (B) (P = 0.73), or change in AUC from 0 to 5 min (C) (P = 0.79) or from 0 to 10 min (D) (P = 0.89). n = 5 mice per group. Data are presented as mean ± SEM after intraperitoneal administration of l-arginine (1 g/kg body weight).
DISCUSSION
In our studies, NOD/ShiLtJ mice show a progressive decline in first-phase insulin release in the weeks preceding the onset of hyperglycemia. This result emulates what has been documented in humans during the progression to type 1 diabetes (14–16). As in human type 1 diabetes, our results demonstrate that a loss of first-phase insulin response to glucose precedes the defect in insulin secretion to l-arginine stimulation (17,18). This finding contrasts with a similar decrease in glucose and arginine-stimulated insulin secretion in perfused pancreata (12), and it is possible that differential insulin clearance rates during glucose and arginine stimulation could explain this difference. The longitudinal nature of our data does not allow us to assess whether the decline in FPIR is associated with peri-islet inflammation or if it develops once a loss of β-cell mass occurs. Data from human studies have indicated that the fall in FPIR is consequent to the observation of autoantibodies (19). Antibodies, however, appear to be less consistent as predictors of type 1 diabetes in mouse than in humans (8). In contrast, heightened levels of autoreactive T-cells, both CD4+ and CD8+, have been measured in the circulation at approximately the window (9–11 weeks) where we have measured the decline in FIPR, in mice ≥10 weeks of age (Fig. 3) (20,21). Therefore, we propose that the decline in FPIR likely results from a change in the autoaggressive nature of type 1 diabetes and may be a molecular signature of the transition from stable insulitis toward diabetes (22).
The decrease in FPIR early in prediabetes is reminiscent of that described in young children (i.e., 1 to 5 years in age) enrolled in the Diabetes Prediction and Prevention Project, where low FPIR was documented in 42% of genetically at-risk children shortly after seroconversion to islet cell cytoplasmic autoantibodies (islet cell antibodies). During follow-up, some children had developed diabetes ∼2 years after FPIR determination; but one-half of the autoantibody-positive children with low FPIR were disease free 2 to 4 years later (23).
In serial oral glucose tolerance tests performed in islet cell antibody–positive relatives of patients with type 1 diabetes studied as part of the Diabetes Prevention Trial–Type 1 (DPT-1), Ferrannini et al. (24) demonstrated that glucose tolerance declined late in the progression to diabetes. As in NOD/ShiLtJ mice, insulin sensitivity remained stable during prediabetes. The trajectory of the 2-h plasma glucose time series in that DPT-1 population was remarkably similar to the fed glucose levels we describe in NOD/ShiLtJ mice. Our results confirm that glucose tolerance or fed glucose levels have limited sensitivity for early defects in insulin secretion and glucose homeostasis in NOD/ShiLtJ mice. It is possible that other parameters such as β-cell glucose sensitivity could provide an even earlier predictor of progression to diabetes; unfortunately, models for the study of such measures have not been described in mice.
In humans, the relationship between β-cell function and glucose tolerance has been shown to be nonlinear (25). This curvilinear relationship could explain the persistence of glucose tolerance, whereas the FPIR decreases until the final stages of diabetes development. Increased hepatic insulin sensitivity, which might not be detectable via insulin tolerance tests, could also contribute to the persistence of glucose tolerance with decreased FPIR.
The development of an intervention that could prevent progression to diabetes in at-risk individuals, children in particular, continues to be one of the predominant goals of type 1 diabetes research. The potential harm to minors with existing therapies has limited advancement in the field. Another limitation has been the difficulty in the translation of prediabetes metabolic analyses in NOD mice to assess the time course of effective mouse interventions in designing human trials seeking to prevent and/or reverse type 1 diabetes. There are clear data documenting that β-cell functional defects precede glucose alterations during the development of type 1 diabetes in humans (24,26,27), and β-cell function has become a key factor in human therapeutic interventions (5,28). However, these factors have not been considered in the preclinical stages of disease or even after onset in rodent models. Having a practical measure of early β-cell failure in prediabetic NOD mice could help identify critical periods for intervention. Therapies to prevent the development of autoimmunity, to avoid further loss of β-cell function once FPIR decreases, or to control the existing autoimmune process with restoration of β-cell mass could be tailored in a stepwise fashion. In this study, the change in insulin from 0 to 2 min during intraperitoneal glucose tolerance testing was the most sensitive measurement of insulitis-related β-cell dysfunction. This determination could be used as an early marker of β-cell failure in preclinical trials. In conclusion, these studies suggest metabolic parameters added to the existing immunologic markers can better delineate the timeline of progression to diabetes in NOD mice and should be considered in preclinical trials.
ACKNOWLEDGMENTS
This work was supported by grants from the Cochrane-Weber Endowed Fund in Diabetes Research (D.I.-L.), the David Nicholas Fellowship Fund (D.I.-L.), The Brehm Coalition for Type 1 Diabetes Research (M.A.A.), and the National Institutes of Health (DK078863 [M.J.H.], DK0204021 [D.J.B.], AI056374 [C.E.M.], and DK074656 [C.E.M.]).
No potential conflicts of interest relevant to this article were reported.
D.I.-L. designed research, performed research, analyzed data, and wrote the manuscript. Y.L.L. performed research and reviewed and edited the manuscript. M.P., S.X., and C.W. performed research. M.J.H. performed research and edited the manuscript. D.S. contributed to hypothesis generation as well as editing of the manuscript. D.J.B. contributed to the planning discussions for this work and editing of the manuscript. M.A.A. designed research, contributed to discussion, and co-wrote, reviewed, and edited the manuscript. C.E.M. designed research, performed research, analyzed data, and co-wrote, reviewed, and edited the manuscript.
Parts of the study were presented previously in abstract form at the 2009 Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009, and published in Diabetes [2009;58 (Suppl. 1):A327].
Footnotes
Current address for D.I.-L.: Department of Pediatrics, Division of Pediatric Endocrinology, University of Illinois at Chicago, Chicago, Illinois.
REFERENCES
- 1.Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005;54:1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 [DOI] [PubMed] [Google Scholar]
- 3.Ludvigsson J, Faresjö M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–1920 [DOI] [PubMed] [Google Scholar]
- 4.Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2009;301:1573–1579 [DOI] [PubMed] [Google Scholar]
- 5.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009;361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon G, Parker M, Ramiya V, et al. Murine antithymocyte globulin therapy alters disease progression in NOD mice by a time-dependent induction of immunoregulation. Diabetes 2008;57:405–414 [DOI] [PubMed] [Google Scholar]
- 7.Melanitou E, Devendra D, Liu E, Miao D, Eisenbarth GS. Early and quantal (by litter) expression of insulin autoantibodies in the nonobese diabetic mice predict early diabetes onset. J Immunol 2004;173:6603–6610 [DOI] [PubMed] [Google Scholar]
- 8.Abiru N, Yu L, Miao D, et al. Transient insulin autoantibody expression independent of development of diabetes: comparison of NOD and NOR strains. J Autoimmun 2001;17:1–6 [DOI] [PubMed] [Google Scholar]
- 9.Robles DT, Eisenbarth GS, Dailey NJ, Peterson LB, Wicker LS. Insulin autoantibodies are associated with islet inflammation but not always related to diabetes progression in NOD congenic mice. Diabetes 2003;52:882–886 [DOI] [PubMed] [Google Scholar]
- 10.Kano Y, Kanatsuna T, Nakamura N, et al. Defect of the first-phase insulin secretion to glucose stimulation in the perfused pancreas of the nonobese diabetic (NOD) mouse. Diabetes 1986;35:486–490 [DOI] [PubMed] [Google Scholar]
- 11.Reddy S, Liu W, Thompson JM, Bibby NJ, Elliott RB. First phase insulin release in the non-obese diabetic mouse: correlation with insulitis, beta cell number and autoantibodies. Diabetes Res Clin Pract 1992;17:17–25 [DOI] [PubMed] [Google Scholar]
- 12.Sreenan S, Pick AJ, Levisetti M, Baldwin AC, Pugh W, Polonsky KS. Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes 1999;48:989–996 [DOI] [PubMed] [Google Scholar]
- 13.Mathews CE, Graser RT, Savinov A, Serreze DV, Leiter EH. Unusual resistance of ALR/Lt mouse beta cells to autoimmune destruction: role for beta cell-expressed resistance determinants. Proc Natl Acad Sci U S A 2001;98:235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srikanta S, Ganda OP, Gleason RE, Jackson RA, Soeldner JS, Eisenbarth GS. Pre-type I diabetes: linear loss of beta cell response to intravenous glucose. Diabetes 1984;33:717–720 [DOI] [PubMed] [Google Scholar]
- 15.Barker JM, McFann K, Harrison LC, et al. Pre-type 1 diabetes dysmetabolism: maximal sensitivity achieved with both oral and intravenous glucose tolerance testing. J Pediatr 2007;150:31–36.e36 [DOI] [PMC free article] [PubMed]
- 16.Srikanta S, Ganda OP, Jackson RA, et al. Type I diabetes mellitus in monozygotic twins: chronic progressive beta cell dysfunction. Ann Intern Med 1983;99:320–326 [DOI] [PubMed] [Google Scholar]
- 17.Chaillous L, Rohmer V, Maugendre D, et al. Differential beta-cell response to glucose, glucagon, and arginine during progression to type I (insulin-dependent) diabetes mellitus. Metabolism 1996;45:306–314 [DOI] [PubMed] [Google Scholar]
- 18.Ganda OP, Srikanta S, Brink SJ, et al. Differential sensitivity to beta-cell secretagogues in “early,” type I diabetes mellitus. Diabetes 1984;33:516–521 [DOI] [PubMed] [Google Scholar]
- 19.Verge CF, Gianani R, Yu L, et al. Late progression to diabetes and evidence for chronic beta-cell autoimmunity in identical twins of patients with type I diabetes. Diabetes 1995;44:1176–1179 [DOI] [PubMed] [Google Scholar]
- 20.Trudeau JD, Kelly-Smith C, Verchere CB, et al. Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J Clin Invest 2003;111:217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, He Q, Garland A, et al. Beta cell-specific CD4+ T cell clonotypes in peripheral blood and the pancreatic islets are distinct. J Immunol 2009;183:7585–7591 [DOI] [PubMed] [Google Scholar]
- 22.André I, Gonzalez A, Wang B, Katz J, Benoist C, Mathis D. Checkpoints in the progression of autoimmune disease: lessons from diabetes models. Proc Natl Acad Sci U S A 1996;93:2260–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keskinen P, Korhonen S, Kupila A, et al. First-phase insulin response in young healthy children at genetic and immunological risk for type I diabetes. Diabetologia 2002;45:1639–1648 [DOI] [PubMed] [Google Scholar]
- 24.Ferrannini E, Mari A, Nofrate V, Sosenko JM, Skyler JS; DPT-1 Study Group Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes 2010;59:679–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn SE, Verchere CB, Andrikopoulos S, et al. Reduced amylin release is a characteristic of impaired glucose tolerance and type 2 diabetes in Japanese Americans. Diabetes 1998;47:640–645 [DOI] [PubMed] [Google Scholar]
- 26.Lo SS, Hawa M, Beer SF, Pyke DA, Leslie RD. Altered islet beta-cell function before the onset of type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1992;35:277–282 [DOI] [PubMed] [Google Scholar]
- 27.Kibirige M, Metcalf B, Renuka R, Wilkin TJ. Testing the accelerator hypothesis: the relationship between body mass and age at diagnosis of type 1 diabetes. Diabetes Care 2003;26:2865–2870 [DOI] [PubMed] [Google Scholar]
- 28.Gottlieb PA, Quinlan S, Krause-Steinrauf H, et al. Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new-onset type 1 diabetes. Diabetes Care 2010;33:826–832 [DOI] [PMC free article] [PubMed] [Google Scholar]