Abstract
OBJECTIVE
To understand the relationships between maternal glycemia during pregnancy and prenatal and early postnatal growth by evaluating cord C-peptide and IGF-I as mediating biomarkers in boys and girls separately.
RESEARCH DESIGN AND METHODS
We evaluated 342 neonates within the EDEN mother-child cohort study born to mothers without diabetes diagnosis before pregnancy. We measured maternal glycemia at 24–28 weeks of gestation and neonates’ cord blood C-peptide (used as a proxy for fetal insulin) and IGF-I at birth. Reported maternal prepregnancy BMI and all measured infant weights and lengths in the 1st year were recorded. Growth modeling was used to obtain an individual growth curve for each infant in the 1st year. Path models, a type of structural equation modeling, were used for statistical analysis. Path analysis is a multivariate method associated with a graphical display that allows evaluation of mediating factors and distinguishes direct, indirect, and total effects.
RESULTS
Cord C-peptide at birth was positively correlated with maternal prepregnancy BMI and maternal glycemia and was higher in girls. In a path model that represented prenatal growth, there was no significant direct effect of maternal glycemia on birth weight, but the effect of maternal glycemia on birth weight was mediated by fetal insulin and IGF-I in both girls and boys. However, in girls only, higher concentrations of cord C-peptide (but not cord IGF-I or maternal glucose) were associated with slower weight growth in the first 3 months of life.
CONCLUSIONS
Our study underlines the role of the fetal insulin–IGF-I axis in the relationship between maternal glycemia during pregnancy and birth weight. We also show for the first time that high insulin concentration in female fetuses is associated with slower early postnatal growth. This slow, early growth pattern may be programmed by fetal hyperinsulinemia, and girls may be more susceptible than boys to its consequences.
A U-shaped relationship has been shown between birth weight and risk of developing type 2 diabetes (1). Catch-up growth and rapid postnatal growth have been associated with obesity and insulin resistance later in life (2,3). However, other mechanisms may also be involved. In Pima Indians, offspring of diabetic mothers had slower ponderal and statural growth in the first 1.5 years of life but were heavier by the age of 7.5 years (4). In offspring of nondiabetic mothers, Eriksson et al. (5–7) have repeatedly shown a specific pattern of growth in individuals who develop type 2 diabetes that involves a lower weight gain in early infancy. The association between short stature in adulthood and type 2 diabetes is also well documented (8). Previous studies have generally focused on maternal hyperglycemia during pregnancy and later diseases in the offspring. However, the specific role of fetal hyperinsulinism itself has seldom been assessed in these relationships.
In fetal life, insulin and the insulin-like growth factors are the two major growth–promoting factors (9). Maternal hyperglycemia stimulates the production of fetal insulin, and fetal hyperinsulinism results in macrosomia (the Pedersen hypothesis) (10) but may also have programming effects that affect postnatal growth and later metabolism (11–14). The international Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study of ∼25,000 women and children recently demonstrated statistically significant linear relationships between maternal glucose and both cord serum C-peptide levels and neonatal adiposity. HAPO’s findings (15) support the Pedersen hypothesis (10).
In addition to its role in glucose homeostasis, insulin enhances tissue accretion via its anabolic effects on fetal metabolism and by stimulating the production of IGF-I (16). Some years ago, Gluckman (17) proposed that the primary endocrine axis regulating fetal growth was the glucose–insulin–IGF-I axis. On the basis of the initial model depicted in this article, the aim of our study was to quantify and test the significance of these pathways in the EDEN cohort, using path analyses (18,19). In addition to the fetal period, we hypothesized that fetal insulin and IGF-I may also affect postnatal growth.
There are some indications in the literature of sex-specific differences in the relationship between fetal insulin and growth. The gender insulin hypothesis, based on the observation that girls have higher concentrations of insulin at birth albeit a lower birth weight than boys, suggests that girls might be more insulin resistant than boys at least to insulin’s growth-promoting effect (20,21).
Our objectives were to analyze the role of cord insulin, measured by C-peptide, and IGF-I in the associations between maternal plasma glucose during pregnancy and 1) anthropometric measures at birth and 2) growth in the 1st year of life. Potential sex-specific associations were investigated.
RESEARCH DESIGN AND METHODS
The EDEN mother-child cohort is an ongoing study that recruited 2,002 pregnant women before 24 weeks of gestation in two French university hospitals, Nancy University Hospital and Poitiers University Hospital, from 2003 to 2006. Exclusion criteria were multiple pregnancies, diabetes diagnosed prior to pregnancy, illiteracy, or moving outside the region planned in the next 3 years. The EDEN study received approval from the ethics committee of Kremlin Bicêtre on 12 December 2002. Written consent was obtained from the mother for herself at the beginning of the study and from both parents for the newborn child after delivery.
Measures.
At 24–28 weeks, mothers self-reported their prepregnancy weight. Maternal height was measured with a wall Seca-206 stadiometer (Hamburg, Germany) to the nearest 0.2 cm. BMI was computed as reported weight (kilogram)/measured height squared (meter squared). Mothers had universal screening for gestational diabetes mellitus (GDM). On the basis of community practice standards, women with a 1-h glucose after a 50-g glucose load ≥7.3 mmol/L in Nancy and ≥7.8 mmol/L in Poitiers were scheduled for a 3-h 100-g glucose diagnostic test. GDM was diagnosed according to the Carpenter and Coustan criteria and retrieved from the obstetrical file (22).
At birth, cord blood samples were collected by research midwives; blood samples were centrifuged, and plasma or serum was stored at −80°C. Intravenous fluid protocols for intrapartum GDM management did not include glucose. Parity, infant’s sex, gestational age at delivery (determined from the date of the last menstrual period and early ultrasound assessment), birth weight (measured with electronic Seca scales, Hamburg, Germany [Seca 737 in Nancy and Seca 335 in Poitiers with a 10-g precision]), and recumbent length at birth measured with a wooden somatometer (Testut, Béthune, France) were collected from obstetrical and pediatric records. On average, 2 days after birth, the neonate’s subscapular and tricipital skinfolds were measured in triplicate using a commercial Holtain caliper (Chasmors Ltd, London, U.K.).
At 4, 8, and 12 months of the newborn’s life, mothers completed mailed questionnaires. They reported weight and length measured every month by the family pediatrician in routine follow-up, which is documented in the infant’s official French personal health record kept by the mother. Mothers were also asked detailed information on the method of feeding between birth and 4 months (exclusive breastfeeding, exclusive formula feeding, or both).
At 1 year of life, research midwives measured the weight of the mothers alone and then holding their infant wearing light clothes using the same Terraillon SL-351 scales as for the mother after birth. Infant weight was obtained by subtraction. A somatometer was used to measure infant length with a precision of 5 mm (NM Medical, Asnières, France). Subscapular and tricipital skinfolds of the infants were also measured using the same protocol as after birth.
Assays.
C-peptide is considered a good estimator of β-cell function. Indeed, proinsulin produced by the β-cell is subsequently cleaved as C-peptide and insulin, which are then secreted in equimolar amounts. We chose to measure C-peptide rather than insulin because C-peptide concentration is not altered by hemolysis, whereas insulin degradation is increased in the presence of even small amounts of hemolysis (23).
We will present our results using the word C-peptide. However, because we used C-peptide as a marker of insulin concentration, we will use the word insulin instead of C-peptide when we discuss the functional significance of our results. C-peptide (nanomole per liter) and IGF-I (nanogram per milliliter) were measured by immunochemiluminescent immunoassays performed on the LIAISON platform manufactured by DiaSorin (Sallugia, Italy). For C-peptide and IGF-I measurement, the detection limit was 0.01 and 15 ng/mL, respectively. Intra- and interassay coefficients of variation were <4.0 and <6.8%, respectively, for C-peptide and <4.6 and <8.5%, respectively, for IGF-I.
Subjects.
Among the 1,893 newborns for whom birth weight was available in the EDEN study, 82% had a cord blood sample available. Neonates without cord blood samples had a lower Apgar score at 1 min and had been more often transferred to another unit and resuscitated than those with cord blood samples, reflecting more difficult deliveries or unhealthy newborns. Our subsample (n = 342) included all neonates born at the end of the EDEN recruitment period (after April 2005) for whom cord blood was available, who had a follow-up of at least 1 year, and for whom the following variables were available: C-peptide and IGF-I concentrations, maternal plasma glucose during pregnancy, and birth weight. We chose the subsample to measure cord blood and C-peptide measurement at the end of the EDEN recruitment period because the subsample also used frozen samples being thawed for another EDEN ancillary study concomitantly, without introducing selection bias concerns.
Statistical analyses.
We used χ2 tests and ANOVA to compare the neonates with and without cord blood sample in the EDEN study and to compare the sample characteristics by offspring’s sex.
Bivariate analyses at birth.
Partial correlations and Student t tests were used to assess the maternal and fetal factors associated with cord C-peptide and IGF-I. These analyses were adjusted for gestational age, time between cord blood sampling and freezing, recruitment center, and sample volume.
Path models at birth and 1 year.
Path models, a type of structural equation modeling, were used for statistical analysis. Path modeling is a multivariate method that is associated with a graphical display. This approach is more mechanistic than the more classical approach that uses regression; it is particularly useful when one studies biological mechanisms (24). A requirement for path analyses is an a priori model based on either the literature (17) and/or previous analyses. This initial model is subsequently refined. This technique makes it possible to evaluate mediating factors and to distinguish direct, indirect, and total effects. Direct causal paths are denoted by single-headed arrows. These relationships are defined through linear equations, and a given variable can appear as explanatory in one or several equations and as the outcome in others. As a consequence, the assumption of normality is critical (24). After log transformation of C-peptide, IGF-I, birth weight, and weight at 1 year, all of the variables in the model were normally distributed. C-peptide and IGF-I were preadjusted for gestational age, the volume of the sample, and time between birth and freezing of the cord sample. Birth weight and birth length were preadjusted for gestational age. Weight and length at 1 year were preadjusted for gestational age and age at examination.
In addition to estimating direct effects, path analysis allows a quantification of the indirect and total effect of one variable on another variable. The indirect effects of a variable are mediated through intervening variables. The total effect of a variable X on Y is the sum of the direct and indirect coefficients of the paths that lead from X to Y respecting Wright’s rules (24). Error terms are associated with all the variables that have at least one arrow pointing toward them and represent the part of the variance that is not explained by these variables (i.e., measurement error along with the effects of variables not measured in the study). To simplify our diagram, these residual variances are not displayed, but they are included in all the computations.
To assess model fit, the hierarchical χ2 test, the goodness of fit index (GFI), and the normal fitted index (NFI) were used (24). A significant χ2 test indicates a difference between the observed and the implied correlation matrix from the set of linear equations that is unlikely to result from sampling error. A GFI and NFI close to 1 indicate a model that fits the data.
Standardized partial coefficients associated with the pathways (all other variables in the path model are held constant) can be interpreted as correlation coefficients and may be tested. Thus, it is possible to simplify the a priori path model by comparing its fit with that of a model with fewer paths, as long as the two models are nested.
As baseline characteristics included in the model differed by sex, we tested a sex interaction in the path analysis. We used a multigroup analysis and provide the coefficients by gender when they were significantly different. Statistical analyses were carried out with SAS (version 9.2., SAS Institute, Cary, NC). More methodological details on path analysis are available in the Supplementary Appendix.
Analyses of growth between birth and 1 year.
Finally, to better understand the dynamic of the relationships between birth and 1 year, we carried out repeated cross-sectional linear regressions with weight or length. We used C-peptide as a continuous variable, and we also computed tertiles of C-peptide to quantify the effects by comparing the weight and length at 1 year between the lowest and the highest tertile of cord C-peptide. Because weight and length were not measured simultaneously for all the children outside the clinical examinations carried out at birth and at 1 year, we used growth modeling to obtain predictions of weight and length for all the children at 3, 6, and 9 months. Growth modeling in the 1st year was carried out using the Jenss equation, y = a0 + a1x − exp(b0 + b1x), in nonlinear mixed models in R (25,26). We assessed the validity of the growth model by calculating the intraclass correlation coefficient between 1) weight (or length) measured during the clinical examination at 1 year and 2) the predicted weight (or length) calculated at the same age (in days). These coefficients were high: 0.95 (95% CI 0.94–0.95) for weight and 0.91 (95% CI 0.90–0.92) for length.
Sensitivity analyses were carried out to verify whether the exclusion of infants of mothers diagnosed with GDM changed the results and to assess the potential modifications of the results due to feeding mode during the first 3 months of life.
RESULTS
At birth, boys were significantly heavier and taller, whereas girls had a tendency to have a greater amount of adiposity, although this was not significant (P = 0.12). At 1 year, boys were significantly heavier and taller, but adiposity measures were not significantly different (P = 0.85) (Table 1).
TABLE 1.
Maternal, paternal, and infant’s characteristics by sex (N = 342)
| Variable | Boys (n = 192) | Girls (n = 150) | P value |
|---|---|---|---|
| Parental characteristics | |||
| Center (Nancy University Hospital) | 54.2 | 63.3 | 0.09 |
| Maternal age (years) | 29.9 ± 4.5 | 29.8 ± 5.0 | 0.84 |
| Parity (multipara) | 59.4 | 52.7 | 0.22 |
| Maternal BMI | |||
| Thin | 6.9 | 7.3 | 0.11 |
| Normal | 70.9 | 62.7 | |
| Overweight | 17.5 | 20.7 | |
| Obese | 4.8 | 9.3 | |
| Maternal BMI (kg/m2) | 22.9 ± 3.9 | 23.5 ± 4.6 | 0.20 |
| Gestational weight gain (kg) | 9.4 ± 5.0 | 9.6 ± 5.2 | 0.81 |
| GDM | 3.6 | 7.3 | 0.13 |
| Maternal plasma glucose (mg/dL) | 113 ± 26 | 113.5 ± 26 | 0.84 |
| Paternal BMI | |||
| Thin/normal | 48.0 | 53.6 | 0.29 |
| Overweight | 40.8 | 37.7 | |
| Obese | 11.2 | 8.7 | |
| Offspring's characteristics at birth | |||
| Gestational age (weeks) | 39.5 ± 1.5 | 39.5 ± 1.4 | 0.73 |
| Birth weight (g) | 3,427 ± 485 | 3,274 ± 418 | 0.002 |
| Length at birth (cm) | 50.0 ± 2.4 | 49.1 ± 2.2 | <0.001 |
| Sum of skinfolds (mm)* | 8.5 ± 1.5 | 8.8 ± 1.5 | 0.12 |
| C-peptide (mmol/L) | 0.81 ± 0.43 | 0.95 ± 0.49 | 0.01 |
| IGF-I (ng/mL) | 72.5 ± 33.1 | 81.7 ± 36.1 | 0.01 |
| Feeding mode in the first 3 months | |||
| Exclusive breastfeeding | 35.0 | 27.6 | 0.40 |
| Mixed feeding | 39.5 | 47.0 | |
| Exclusive formula feeding | 25.4 | 25.4 | |
| Offspring's characteristics at 1 year | |||
| Weight (kg) | 10.2 ± 1.1 | 9.5 ± 1.0 | <0.0001 |
| Length (cm) | 75.7 ± 2.8 | 73.9 ± 2.3 | <0.0001 |
| Sum of skinfolds (mm)* | 15.1 ± 3.1 | 15.1 ± 2.7 | 0.85 |
Data are percentage or means ± SD. Boldface type represents P ≤ 0.05 or clinically significant.
*Sum of tricipital and subscapular skinfolds.
Bivariate analyses: maternal and fetal factors associated with cord C-peptide and IGF-I (results not shown).
Cord C-peptide and cord IGF-I concentrations were correlated (r = 0.39, P < 0.0001), and the concentrations were significantly higher in girls than in boys (C-peptide, +17%, P = 0.006; IGF-I, +12.7%, P = 0.01). Cord C-peptide, but not IGF-I, was positively correlated with maternal prepregnancy BMI (r = 0.11, P = 0.05) and maternal plasma glucose (r = 0.17, P = 0.002) and was higher in offspring of women with GDM (+40%, P = 0.02). IGF-I was 21% higher in offspring of women with GDM (P = 0.08).
Associations between cord C-peptide or IGF-I and anthropometric measures at birth.
Cord C-peptide and cord IGF-I were significantly associated with birth weight (r = 0.18 and 0.46, respectively), subcutaneous adiposity (r = 0.19 and 0.35, respectively), and ponderal index (r = 0.12 and 0.22, respectively). Birth length and head circumference were associated with IGF-I (respectively, r = 0.25 and 0.20) but not C-peptide. Cord IGF-I, but not C-peptide, was negatively correlated with gestational age (−0.1, P = 0.05).
The association between C-peptide and birth weight disappeared after adjustment for IGF-I, whereas the relation with IGF-I persisted after adjustment for C-peptide, suggesting that IGF-I may be on the causal pathway between C-peptide and birth weight and not the contrary. This was confirmed by the path analyses.
Path model for fetal growth.
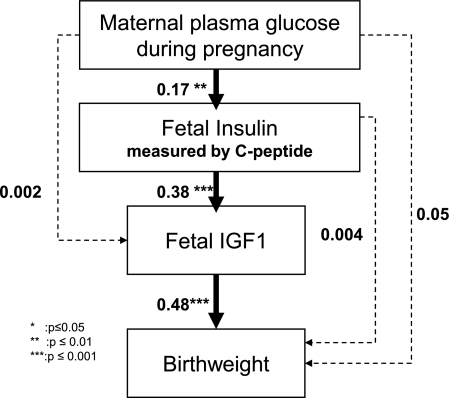
Figure 1 depicts the path diagram that we hypothesized with birth weight, used as a proxy of prenatal growth, as the ultimate factor. At birth, the same model fitted adequately the relationships in boys and girls (no interaction by newborn sex, P = 0.92). As shown in Figure 1 and Table 2, standardized coefficients of the paths between maternal plasma glucose and fetal C-peptide (0.17), as well as between fetal C-peptide and fetal IGF-I (0.38) and between fetal IGF-I and birth weight (0.48), were significant. Maternal plasma glucose was a significant direct contributor to neither IGF-I concentration (0.002, P = 0.97) nor birth weight (0.05, P = 0.3). The effect of fetal C-peptide on birth weight was not direct (0.004, P = 0.93) but indirect through IGF-I (0.18, P < 0.001) (Table 2).
FIG. 1.
Path model for fetal growth showing standardized path coefficients. The dotted paths were not significant, and these paths were not maintained in the second path model for postnatal growth. Coefficients associated with the solid black arrows were significant. Fit indices were P value χ2, 0.99; GFI, 0.99; and NFI, 0.98.
TABLE 2.
Total, direct, and indirect effects of maternal plasma glucose during pregnancy and fetal insulin on birth weight or length (N = 342)
| Birth weight |
Birth length |
|||||
|---|---|---|---|---|---|---|
| Total | Direct | Indirect | Total | Direct | Indirect | |
| Maternal glycemia | ||||||
| Estimate | 0.08 | 0.05 | 0.03 | 0.09 | 0.08 | 0.01 |
| SD | 0.05 | 0.05 | 0.03 | 0.05 | 0.05 | 0.02 |
| P value | 0.13 | 0.30 | 0.24 | 0.10 | 0.15 | 0.46 |
| Fetal C-peptide | ||||||
| Estimate | 0.19 | 0.004 | 0.18 | 0.07 | −0.04 | 0.11 |
| SD | 0.05 | 0.05 | 0.03 | 0.05 | 0.06 | 0.03 |
| P value | <0.001 | 0.93 | <0.001 | 0.17 | 0.53 | <0.001 |
| Fetal IGF-I | ||||||
| Estimate | 0.48 | 0.48 | — | 0.29 | 0.29 | — |
| SD | 0.05 | 0.05 | — | 0.05 | 0.05 | — |
| P value | <0.001 | <0.001 | — | <0.001 | <0.001 | — |
Boldface type represents P ≤ 0.05.
In a model that included birth length instead of birth weight, the estimates were very similar, with the exception of the coefficient of the path between fetal IGF-I and birth length that was lower than the estimate in the birth weight model (0.29, P < 0.0001).
Path model for early postnatal growth.
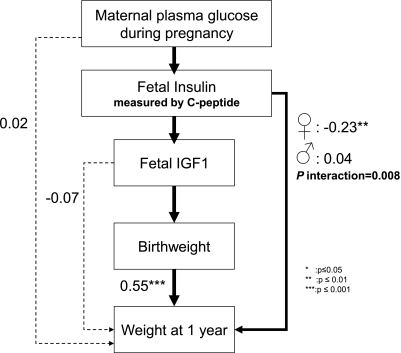
Figure 2 is a path diagram with weight at 1 year as the ultimate factor and was used to study the effects of the insulin–IGF-I axis on postnatal growth. In this model, the coefficients did not differ significantly by sex, except for the association between cord C-peptide and weight at 1 year (P for interaction = 0.008). The association between cord C-peptide and length at 1 year was also significantly different in boys and girls (P for interaction = 0.01). Figure 2 presents the pooled coefficients, except for those associations that are presented by sex. The contribution of the insulin–IGF-I axis on birth weight remained central in this model. Birth weight and weight at 1 year were positively correlated in both boys and girls (0.55, P < 0.0001). There was an indirect significant relationship between fetal IGF-I and weight at 1 year through the effect on birth weight; there was no direct effect of IGF-I on weight at 1 year (Table 3). The relationships were similar in a model that included length at birth and 1 year, although the associations were slightly weaker.
FIG. 2.
Path model for postnatal growth showing standardized path coefficients. The coefficients of the dotted paths were not significant, and these paths were not maintained in the second path model for postnatal growth. Coefficients associated with the solid black arrows were significant. Fit indices were P value χ2, 0.97; GFI, 0.99; and NFI, 0.99 for the boy’s model and the girl’s model.
TABLE 3.
Total, direct, and indirect effects of maternal plasma glucose during pregnancy, fetal C-peptide, and fetal IGF-I on weight or length at 1 year (N = 339)
| Weight at 1 year |
Length at 1 year |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Girls (n = 150) |
Boys (n = 189)* |
Girls (n = 150) |
Boys (n = 189)* |
|||||||||
| Total | Direct | Indirect | Total | Direct | Indirect | Total | Direct | Indirect | Total | Direct | Indirect | |
| Maternal glycemia | ||||||||||||
| Estimate | −0.03 | 0.001 | −0.03 | 0.06 | 0.04 | 0.01 | −0.08 | −0.06 | −0.02 | −0.04 | −0.07 | 0.02 |
| SD | 0.07 | 0.07 | 0.02 | 0.07 | 0.07 | 0.01 | 0.08 | 0.08 | 0.02 | 0.07 | 0.07 | 0.02 |
| P value | 0.70 | 0.98 | 0.14 | 0.39 | 0.52 | 0.26 | 0.30 | 0.43 | 0.29 | 0.50 | 0.29 | 0.11 |
| Fetal C-peptide | ||||||||||||
| Estimate | −0.14 | −0.23 | 0.10 | 0.09 | 0.04 | 0.05 | −0.09 | −0.15 | 0.06 | 0.16 | 0.13 | 0.03 |
| SD | 0.07 | 0.07 | 0.03 | 0.07 | 0.07 | 0.04 | 0.08 | 0.08 | 0.03 | 0.07 | 0.07 | 0.03 |
| P value | 0.06 | <0.001 | <0.001 | 0.19 | 0.56 | 0.20 | 0.25 | 0.06 | 0.05 | 0.02 | 0.07 | 0.44 |
| Fetal IGF-I | ||||||||||||
| Estimate | 0.31 | 0.04 | 0.27 | 0.10 | −0.12 | 0.22 | 0.18 | 0.09 | 0.09 | 0.06 | −0.07 | 0.13 |
| SD | 0.08 | 0.08 | 0.05 | 0.08 | 0.08 | 0.04 | 0.08 | 0.08 | 0.03 | 0.08 | 0.08 | 0.04 |
| P value | <0.001 | 0.57 | <0.001 | 0.19 | 0.14 | <0.001 | 0.03 | 0.25 | 0.01 | 0.44 | 0.35 | <0.001 |
Boldface type represents P ≤ 0.05 or clinically significant.
*Three boys had missing weight or length at 1 year.
In girls only (Fig. 2), C-peptide was directly and negatively associated with weight at 1 year (−0.23, P < 0.01). The direct effect of C-peptide on height at 1 year was negative and was partially counterbalanced by a positive indirect effect (Table 3).
In boys, there was no association between C-peptide and weight at 1 year. There was an indication of a positive direct effect on length at 1 year, but this effect was not significant (0.13, P = 0.07) (Table 3).
In sensitivity analyses, the results were not modified by either exclusion of the offspring of mothers with GDM (n = 18) or adjustment or stratification of the feeding mode in the first 3 months of life.
Relationships between cord C-peptide, weight, and length growth in the 1st year.
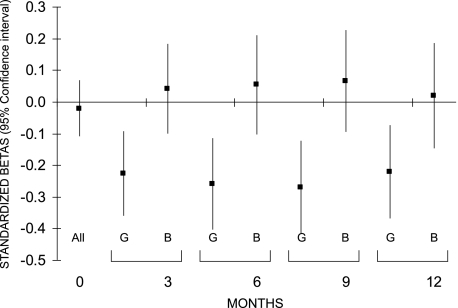
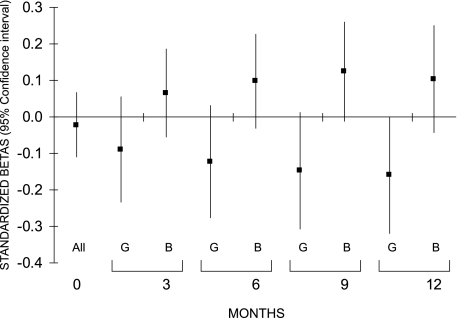
In girls, the negative relationship between cord C-peptide and weight was significant as soon as 3 months (adjusted standardized β, −0.23, P = 0.001) and seemed to persist up to 1 year (Fig. 3). A similar result was seen for length in the 1st year in girls (at 1 year: adjusted standardized β, −0.16, P = 0.05) (Fig. 4).
FIG. 3.
Associations between fetal insulin measured in cord blood and weight in the 1st year of life in girls (G) and boys (B). Because there was no interaction at birth, a pooled estimate is given for boys and girls together. Models were adjusted for IGF-I, maternal plasma glucose during pregnancy, maternal prepregnancy BMI, gestational age, time between cord blood sampling and freezing, sample volume, recruitment center, birth weight, and feeding mode in the first 3 months of life (except for the relationship at birth).
FIG. 4.
Associations between fetal insulin measured in cord blood and length in the 1st year of life in girls (G) and boys (B). Because there was no interaction at birth, a pooled estimate is given for boys and girls together. Models were adjusted for IGF-I, maternal plasma glucose during pregnancy, maternal prepregnancy BMI, gestational age, time between cord blood sampling and freezing, sample volume, recruitment center, length at birth, and feeding mode in the first 3 months of life (except for the relationship at birth).
Quantification of the association between cord C-peptide and anthropometric measures at 1 year.
In girls, at 1 year, those in the highest tertile of cord C-peptide compared with the lowest were 490 g lighter (P = 0.02) and 1 cm shorter (P = 0.04) after adjustment for birth weight or length, gestational age, maternal plasma glucose during pregnancy, maternal prepregnancy BMI, feeding mode in the first 3 months, and age at examination. There was no significant association between cord C-peptide and offspring’s BMI (17.4 vs. 17.1 kg/m2, P = 0.32), but there was a negative trend with adiposity estimated at 1 year as the sum of the subscapular and tricipital skinfolds (15.5 vs. 14.3 mm, P = 0.05).
In boys, the C-peptide associations were not significant. Length at 1 year was greater in the highest tertile of cord C-peptide (76 vs. 75.4 cm) but this was not significant (P = 0.21).
DISCUSSION
Our data show that the effect of maternal plasma glucose on birth weight and length is mediated by fetal insulin and IGF-I. The role of the glucose–insulin–IGF-I axis has been suggested previously (17). However, to our knowledge this is the first time that this mechanism has been described and quantified using path analysis. Fetal insulin is a growth-promoting hormone that acts as a signal of nutrient availability and is a major regulatory factor of IGF-I concentration (16). Our results confirm the Pedersen hypothesis (10). In the prenatal period, higher maternal plasma glucose and the associated higher insulin concentrations were associated with accelerated growth in both boys and girls (10).
Our other novel finding pertains to the association between fetal insulin and growth in the 1st year of life. Contrary to what happens during fetal life, in the postnatal period, higher insulin concentrations were associated with slower ponderal and statural growth in girls only. This opposite association of high-cord insulin concentration with prenatal and early postnatal growth in girls suggests a phenomenon related to the abrupt change from the intra- to the extrauterine environment rather than to an intrinsic fetal defect. The slower rate of postnatal growth that we observed in girls may reflect a relative long-lasting peripheral insulin resistance that could have been induced by a peripheral effect of hyperinsulinism during fetal life. However, a central effect of fetal hyperinsulinism is also plausible. Indeed, the fetal and early postnatal period is critical for the development of the brain. A number of animal studies have shown that modifying this environment results in noticeable changes in the brain (27). For example, modifications of the concentration of leptin as a result of experimental nutritional modifications seem to affect the maturation of the appetite regulatory system in the hypothalamus (28). Fetal hyperinsulinism may affect the structure or the function of the hypothalamus (29,30) and, thus, intervene in the programming of satiety (31). The clinical relevance of our results is important. They suggest that high insulin concentrations during fetal life may affect not only prenatal growth but also postnatal growth, with a weight difference close to 500 g at 1 year between the infants who belonged to the extreme tertiles of cord C-peptide at birth.
The negative association between cord insulin and postnatal growth was seen only in girls. This is consistent with the gender insulin hypothesis that states girls may be more resistant than boys to insulin’s growth-promoting effect (20,21). In addition to insulin’s effects on growth, insulin resistance may also involve metabolic pathways. One study has shown that girls were more insulin-resistant than boys at age 5, even after accounting for the difference in fat mass (32). In addition, the incidence of type 2 diabetes in adolescence is higher in girls than in boys (21). However, with puberty, the sex difference seems to reverse (33).
The lack of association between insulin and postnatal growth in boys may be explained by a dose effect of hyperinsulinism. We and others have shown that girls have higher insulin concentrations in the cord blood. It is possible that girls are exposed to higher concentrations of insulin in fetal life than boys and that only those high insulin concentrations result in slower growth in the early postnatal period. These sex-specific associations might also be explained by a biological interaction of insulin with sex-steroid hormones. Boys experience a surge in plasma testosterone concentration in the first months of life (34). This may mask the association between cord C-peptide and postnatal ponderal growth and may explain their different growth pattern at this age compared with girls. In addition, in the 1st year of life, growth hormone starts to control IGF-I production (9), and growth hormone is itself regulated by a number of factors, including sex-steroid hormones (35). Other sex-specific mechanisms are increasingly revealed, such as sex-specific programming or epigenetics (36,37). Unfortunately, animal studies of perinatal plasticity and early development are often carried out in males only, to avoid confounding by sex, yet males and females have different growth patterns as early as during fetal life (38). More studies taking sex into account are needed.
Our path model failed to show a significant direct effect of maternal plasma glucose at the screening test on offspring’s birth weight. We believe that this nonsignificant total effect can be explained by a lack of power and the low prevalence of glucose intolerance in our population. Indeed, this association has been clearly shown in previous studies, in particular in the high-risk Pima population (39) and by the HAPO study in ∼25,000 women (15). In addition, in the full sample of the EDEN women with 1-h glycemia available (n = 1,804), the correlation between 1-h maternal glycemia and birth weight was 0.07, and this association was significant (P = 0.008). This correlation in the entire cohort is similar to the total effect of maternal 1-h glycemia on birth weight in the path analysis for our subsample (Table 2) (total effect = 0.08, P = 0.13).
There was no significant total or direct effect of maternal plasma glucose on weight or length at 1 year for both girls and boys. This is consistent with the transitory disappearance of the association between maternal glycemia and offspring’s anthropometric measurements in the first months of life that we and others have previously shown (40–43).
Strengths of our study include the prospective measurement of maternal plasma glucose during pregnancy, cord blood C-peptide and IGF-I at birth, and infant growth during the 1st year of life. We also used an original approach (path analysis) that allowed us to better understand and to quantify the mediating relationships involving insulin and IGF-I. Path analyses remain, however, a schematic approach of complex biological phenomena that should be verified in other studies. Although our models had good fit, there may be equivalent models that fit the data equally well (24).
A limitation of our work is that maternal plasma glucose was measured in the study only once during pregnancy—on average, 3 months before the cord blood sample was taken. Whether this is a good proxy of the mean fetal glucose concentration during gestation is a legitimate question, especially because our sample included women diagnosed for GDM who had been treated. Even though GDM management aims at lowering fetal insulin, cord C-peptide and IGF-I of infants of GDM mothers were still among the highest values of the distribution. It is important to note that a sensitivity analysis showed that GDM exclusion did not significantly alter the results. Another potential limitation is that the two EDEN centers used different thresholds for the 50-g glucose challenge test, which could have led to an increased number of GDM cases detected in Nancy compared with Poitiers. However, our results did not differ according to center, suggesting that the difference in diabetes screening procedure did not significantly affect the relationship between maternal glycemia, the IGF-I–insulin axis, and fetal growth as assessed over the whole range of maternal screening blood glucose concentrations.
Conclusion.
Our study shows for the first time that high insulin concentration in female fetuses is associated with slower early postnatal growth. Growth in the early postnatal period is critical for the risk of later diabetes (4–7,44,45). Given our findings that maternal glucose mediates its effect on both sexes’ birth weight via insulin and IGF-I, we propose that this slow, early growth pattern in girls may be programmed by fetal hyperinsulinemia and that girls are more susceptible than boys to its consequences. The association of slow, early growth with later diabetes suggests that both the mitogenic and the metabolic effects of insulin are involved. Additional studies evaluating males and females separately are warranted to better understand potential interactions between fetal insulin and chromosomal sex or sex-steroid hormones on early postnatal growth.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge all of the funding sources for the EDEN study: Fondation pour la Recherche Médicale (FRM), French Ministry of Research: IFR Program, INSERM Human Nutrition National Research Program, Diabetes National Research Program (via a collaboration with the Association for Diabetes Research), French Ministry of Health Perinatality Program, French Agency for Environment Security (AFFSET), French National Institute for Population Health Surveillance (INVS), Paris–Sud University, French National Institute for Health Education (INPES), Nestlé, Mutuelle Générale de l’Education Nationale (MGEN), French-Speaking Association for the Study of Diabetes and Metabolism (ALFEDIAM), National Agency for Research (ANR nonthematic program), and the National Institute for Research in Public Health (IRESP: TGIR cohorte santé 2008 program). Statistical analyses were carried out in complete independence from funders. Sponsors were not involved in the study design, collection, or data analysis.
No potential conflicts of interest relevant to this article were reported.
N.R. performed the statistical analyses and wrote the first draft of the article. J.B. and B.H. provided statistical insight. A.F. participated in data management and analysis. R.H., B.F., and T.A.H. revised the manuscript. J.-C.S. supervised the assays and revised the manuscript. P.D.-M. revised the manuscript. M.-A.C. coordinated the EDEN study, is the guarantor of the study, and supervised the writing of the first draft of the article.
Parts of this study were published in abstract form in Diabetes Metab 2010;36:A16–A17.
The authors are indebted to the participating families; to midwife research assistants L. Douhaud, B. Lortholary, and M. Rogeon (Poitiers University Hospital) and S. Bedel, S. Gabriel, and M. Malinbaum (Nancy University Hospital) for data collection; and to P. Lavoine, J. Sahuquillo, and G. Debotte (INSERM, U1018) for checking, coding, and data entry.
APPENDIX
Members of the EDEN Mother-Child Cohort Study Group: M.A. Charles, M. de Agostini, A. Forhan, and B. Heude (INSERM, U1018); P. Ducimetière, M. Kaminski, M.J. Saurel-Cubizolles, P. Dargent-Molina, X. Fritel, B. Larroque, N. Lelong, L. Marchand, and C. Nabet (INSERM, U953); I. Annesi-Maesano (INSERM, U707); R. Slama (INSERM, U823); V. Goua, G. Magnin, and R. Hankard (Poitiers University Hospital); O. Thiebaugeorges, M. Schweitzer, and B. Foliguet (Nancy University Hospital); and N. Job-Spira (Agence Nationale de Recherche sur le Sida).
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1189/-/DC1.
*A complete list of the members of the EDEN Mother-Child Cohort Study Group can be found in the appendix.
REFERENCES
- 1.Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol 2007;165:849–857 [DOI] [PubMed] [Google Scholar]
- 2.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 2005;331:929–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong KK, Dunger DB. Birth weight, infant growth and insulin resistance. Eur J Endocrinol 2004;151(Suppl 3):U131–U139 [DOI] [PubMed] [Google Scholar]
- 4.Touger L, Looker HC, Krakoff J, Lindsay RS, Cook V, Knowler WC. Early growth in offspring of diabetic mothers. Diabetes Care 2005;28:585–589 [DOI] [PubMed] [Google Scholar]
- 5.Eriksson JG, Forsen TJ, Osmond C, Barker DJ. Pathways of infant and childhood growth that lead to type 2 diabetes. Diabetes Care 2003;26:3006–3010 [DOI] [PubMed] [Google Scholar]
- 6.Salonen MK, Kajantie E, Osmond C, et al. Childhood growth and future risk of the metabolic syndrome in normal-weight men and women. Diabetes Metab 2009;35:143–150 [DOI] [PubMed] [Google Scholar]
- 7.Salonen MK, Kajantie E, Osmond C, et al. Role of childhood growth on the risk of metabolic syndrome in obese men and women. Diabetes Metab 2009;35:94–100 [DOI] [PubMed] [Google Scholar]
- 8.Asao K, Kao WHL, Baptiste-Roberts K, Bandeen-Roche K, Erlinger TP, Brancati FL. Short stature and the risk of adiposity, insulin resistance, and type 2 diabetes in middle age: the Third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. Diabetes Care 2006;29:1632–1637 [DOI] [PubMed] [Google Scholar]
- 9.Gicquel C, Le Bouc Y. Hormonal regulation of fetal growth. Horm Res 2006;65(Suppl 3):28–33 [DOI] [PubMed] [Google Scholar]
- 10.Pedersen J. Diabetes and Pregnancy: Blood Sugar of Newborn Infants. Copenhagen, Denmark, Danish Science Press, 1952 [Google Scholar]
- 11.Plagemann A. A matter of insulin: developmental programming of body weight regulation. J Matern Fetal Neonatal Med 2008;21:143–148 [DOI] [PubMed] [Google Scholar]
- 12.Dörner G, Plagemann A. Perinatal hyperinsulinism as possible predisposing factor for diabetes mellitus, obesity and enhanced cardiovascular risk in later life. Horm Metab Res 1994;26:213–221 [DOI] [PubMed] [Google Scholar]
- 13.Botton J, Heude B, Maccario J, Ducimetière P, Charles M-A; FLVS Study Group Postnatal weight and height growth velocities at different ages between birth and 5 y and body composition in adolescent boys and girls. Am J Clin Nutr 2008;87:1760–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzoulaki I, Sovio U, Pillas D, et al. Relation of immediate postnatal growth with obesity and related metabolic risk factors in adulthood: the northern Finland birth cohort 1966 study. Am J Epidemiol 2010;171:989–998 [DOI] [PubMed] [Google Scholar]
- 15.HAPO Study Cooperative Research Group Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes 2009;58:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction 2004;127:515–526 [DOI] [PubMed] [Google Scholar]
- 17.Gluckman PD. Endocrine and nutritional regulation of prenatal growth. Acta Paediatr Suppl 1997;423:153–157; discussion 158 [DOI] [PubMed] [Google Scholar]
- 18.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol 2002;31:285–293 [PubMed] [Google Scholar]
- 19.Tu YK. Commentary: Is structural equation modelling a step forward for epidemiologists? Int J Epidemiol 2009;38:549–551 [DOI] [PubMed] [Google Scholar]
- 20.Shields BM, Knight B, Hopper H, et al. Measurement of cord insulin and insulin-related peptides suggests that girls are more insulin resistant than boys at birth. Diabetes Care 2007;30:2661–2666 [DOI] [PubMed] [Google Scholar]
- 21.Wilkin TJ, Murphy MJ. The gender insulin hypothesis: why girls are born lighter than boys, and the implications for insulin resistance. Int J Obes (Lond) 2006;30:1056–1061 [DOI] [PubMed] [Google Scholar]
- 22.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982;144:768–773 [DOI] [PubMed] [Google Scholar]
- 23.O’Rahilly S, Burnett MA, Smith RF, Darley JH, Turner RC. Haemolysis affects insulin but not C-peptide immunoassay. Diabetologia 1987;30:394–396 [DOI] [PubMed] [Google Scholar]
- 24.Loehlin JC. Latent Variable Models: An Introduction to Factor, Path, and Structural Analysis. Hillsdale, NJ, Erlbaum, 2004 [Google Scholar]
- 25.R Development Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Ed. Vienna, Austria, 2009 [Google Scholar]
- 26.Pinheiro J, Bates D, DebRoy S, Sarkar D; R Development Core Team. nlme: Linear and Nonlinear Mixed Effects Models, R Package Version 3.1-96, 2009
- 27.Bouret SG. Early life origins of obesity: role of hypothalamic programming. J Pediatr Gastroenterol Nutr 2009;48(Suppl 1):S31–S38 [DOI] [PubMed] [Google Scholar]
- 28.Lustig RH. Childhood obesity: behavioral aberration or biochemical drive? Reinterpreting the First Law of Thermodynamics. Nat Clin Pract Endocrinol Metab 2006;2:447–458 [DOI] [PubMed] [Google Scholar]
- 29.Plagemann A, Harder T, Janert U, et al. Malformations of hypothalamic nuclei in hyperinsulinemic offspring of rats with gestational diabetes. Dev Neurosci 1999;21:58–67 [DOI] [PubMed] [Google Scholar]
- 30.Leloup C, Magnan C, Alquier T, et al. Intrauterine hyperglycemia increases insulin binding sites but not glucose transporter expression in discrete brain areas in term rat fetuses. Pediatr Res 2004;56:263–267 [DOI] [PubMed] [Google Scholar]
- 31.Plagemann A, Harder T. Hormonal programming in perinatal life: leptin and beyond. Br J Nutr 2009;101:151–152 [DOI] [PubMed] [Google Scholar]
- 32.Murphy MJ, Metcalf BS, Voss LD, et al. ; EarlyBird Study (EarlyBird 6) Girls at five are intrinsically more insulin resistant than boys: The Programming Hypotheses Revisited—The EarlyBird Study (EarlyBird 6). Pediatrics 2004;113:82–86 [DOI] [PubMed] [Google Scholar]
- 33.Moran A, Jacobs DR, Jr, Steinberger J, et al. Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation 2008;117:2361–2368 [DOI] [PubMed] [Google Scholar]
- 34.Bidlingmaier F, Dörr HG, Eisenmenger W, Kuhnle U, Knorr D. Testosterone and androstenedione concentrations in human testis and epididymis during the first two years of life. J Clin Endocrinol Metab 1983;57:311–315 [DOI] [PubMed] [Google Scholar]
- 35.Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev 2006;27:101–140 [DOI] [PubMed] [Google Scholar]
- 36.Vigé A, Gallou-Kabani C, Junien C. Sexual dimorphism in non-Mendelian inheritance. Pediatr Res 2008;63:340–347 [DOI] [PubMed] [Google Scholar]
- 37.McCarthy MM, Auger AP, Bale TL, et al. The epigenetics of sex differences in the brain. J Neurosci 2009;29:12815–12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta 2010;31(Suppl):S33–S39 [DOI] [PubMed] [Google Scholar]
- 39.Pettitt DJ, Knowler WC, Baird HR, Bennett PH. Gestational diabetes: infant and maternal complications of pregnancy in relation to third-trimester glucose tolerance in the Pima Indians. Diabetes Care 1980;3:458–464 [DOI] [PubMed] [Google Scholar]
- 40.Regnault N, Botton J, Forhan A, et al. Determinants of early ponderal and statural growth in full-term infants in the EDEN mother-child cohort study. Am J Clin Nutr 2010;92:594–602 [DOI] [PubMed] [Google Scholar]
- 41.Pettitt DJ, McKenna S, McLaughlin C, Patterson CC, Hadden DR, McCance DR. Maternal glucose at 28 weeks of gestation is not associated with obesity in two-year-old offspring: the Belfast Hyperglycemia and Adverse Pregnancy Outcome (HAPO) family study. Diabetes Care 2010;33;1219–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knight B, Shields BM, Hill A, Powell RJ, Wright D, Hattersley AT. The impact of maternal glycemia and obesity on early postnatal growth in a nondiabetic Caucasian population. Diabetes Care 2007;30:777–783 [DOI] [PubMed] [Google Scholar]
- 43.Ong KK, Diderholm B, Salzano G, et al. Pregnancy insulin, glucose, and BMI contribute to birth outcomes in nondiabetic mothers. Diabetes Care 2008;31:2193–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barker DJ, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med 2005;353:1802–1809 [DOI] [PubMed] [Google Scholar]
- 45.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med 2004;350:865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.