Although autoantibodies (auto-Abs) against β-cell antigens helped in defining type 1 diabetes as an autoimmune disease and are invaluable biomarkers, their pathogenic role is unclear. Studies in nonobese diabetic (NOD) mice devoid of B cells (Igμnull or treated with anti-μ Abs) suggest that B cells are necessary for the disease to develop (1,2). The critical role of B cells in this process is thought to be linked to their antigen-presenting function through major histocompatibility class II molecules, as NOD mice harboring I-Ag7–deficient B cells are also protected from diabetes (3). The capacity of B cells to efficiently uptake β-cell antigens through surface Ig is critical to this function, as inhibiting this Ig-mediated uptake abolishes the β-cell antigen-presenting function of B cells in vitro (4), while transgenic manipulation of the Ig specificity in NOD mice impacts on diabetes incidence (5). Thus, autoreactive B cells may be exquisitely efficient in capturing and presenting self antigens, leading to autoimmune T-cell activation. In a therapeutic perspective, treatment with depleting anti-CD20 Abs delays and reduces diabetes onset in NOD mice and is even capable of reversing established disease (6). These findings have been successfully translated into human clinical trials (7).
In this scenario, the role of B cell–secreted auto-Abs has been controversial. On one hand, NOD embryos implanted into nonautoimmune foster mothers are diabetes-protected compared with embryos implanted into NOD females, suggesting that maternally transmitted factors (but not necessarily Abs) play a role (8). Moreover, passive transfer of Abs against islet-expressed ovalbumin enhances activation of ovalbumin-reactive CD8+ T cells and breaks tolerance (9). On the other hand, Ig infusion from sera of diabetic NOD mice does not restore diabetes susceptibility in Igμnull NOD recipients (10). Moreover, NOD transgenic mice in which B cells express membrane but not secreted IgM display an increased diabetes incidence compared with nontransgenic littermates that lacked B cells altogether, further suggesting that secreted Abs are not required to induce disease (11). Importantly, none of these reports examined the influence of auto-Abs on islet-reactive CD4+ T cells.
A new piece is now added to the puzzle by the study by Silva et al. (12). To address the effect of auto-Abs on islet-reactive CD4+ T cells, these authors used a T-cell receptor (TCR) transgenic mouse harboring high frequencies of CD4+ T cells recognizing the hen egg lysozyme (HEL) “autoantigen” transgenically expressed in β-cells (TCR+HEL+ mice). In a first set of experiments, a mutated Roquin transgene (Roquinsan) was introduced in these mice, causing accumulation of follicular helper T cells and germinal center B cells (13), leading to increased secretion of anti-HEL IgG Abs. These mice rapidly and uniformly developed diabetes, accompanied by accumulation of HEL-specific CD4+ T cells. However, diabetes susceptibility was reduced not only in the absence of B cells (cd79aken transgene), but also in the absence of IgG (IgMDHEL transgene), and passive serum transfer from Roquinsan/san mice was sufficient to confer diabetes susceptibility.
In a second set of experiments, TCR+HEL+ females crossed with nontransgenic males gave rise to diabetes-prone TCR+HEL+ offspring, whereas TCR+HEL+ litters were diabetes-protected when TCR+HEL+ males were crossed with nontransgenic females. The same observation was repeated by crossing TCR+HEL+ fathers with HEL-immunized nontransgenic mothers, in which case diabetes developed in the TCR+HEL+ but not in the TCR−HEL+ offspring, ruling out a direct cytotoxic effect of Abs on HEL-expressing β-cells. TCR+HEL+ neonates receiving anti-HEL IgG also developed diabetes, strongly suggesting that maternally transmitted anti-HEL Abs were at play.
Anti-HEL Abs acted by increasing survival of proliferating islet-reactive CD4+ T cells, and Fcγ receptor (FcγR) blockade delayed and reduced diabetes incidence. Since CD4+ T cells do not express these receptors, the observed activation of T cells is probably achieved through FcγR-bearing antigen-presenting cells. The critical role of FcγRs has been previously proposed (9,14), making them attractive therapeutic targets. Importantly, Harbers et al. (9) further showed some involvement of the complement system in these Ab-mediated mechanisms. Silva et al. conclude that B cells can promote type 1 diabetes by secreting Abs that act in an FcγR-mediated manner to enhance the expansion of islet HEL-reactive CD4+ T cells, thus adding another facet to the multiple roles of B cells in β-cell autoimmunity (Fig. 1).
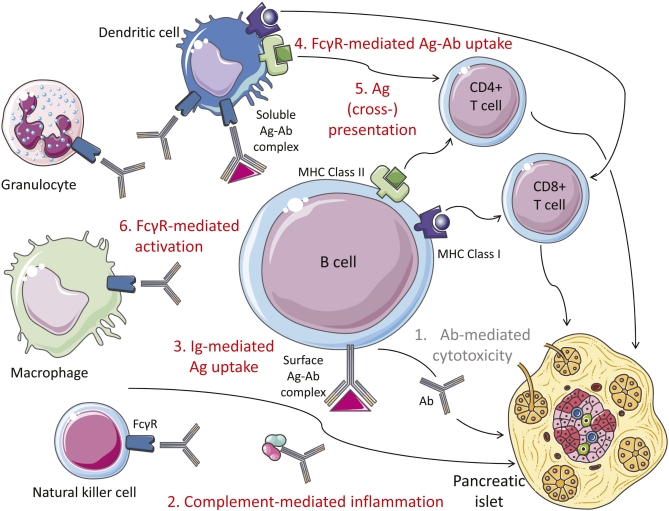
FIG. 1.
Mechanisms of B-cell involvement in β-cell autoimmunity. 1) Ab-mediated cytotoxicity. Available data rule out a direct pathogenic effect of auto-Abs on β-cells. 2) Complement-mediated inflammation. 3) Ig-mediated antigen (Ag) uptake. This mechanism makes autoreactive B cells highly efficient at processing and presenting β-cell antigens through surface Ig binding. 4) Fcγ receptor (FcγR)-mediated antigen-Ab uptake. Soluble antigen-Ab complexes are also efficiently taken up by dendritic cells and other antigen-presenting cells through their surface FcγRs. 5) Antigen (cross-)presentation. β-Cell antigens taken up and processed by B cells and dendritic cells are presented to CD4+ T cells through major histocompatibility complex class II molecules and cross-presented to CD8+ T cells through major histocompatibility complex class I molecules, leading to activation of autoreactive T cells. 6) FcγR-mediated activation. Several FcγR-bearing cells (natural killer, macrophages, granulocytes, and dendritic cells) become activated after binding of the Fc portion of Abs to FcγRs. This triggers secretion of inflammatory cytokines and dendritic cell maturation.
These data are difficult to reconcile with multiple observations. A case report of type 1 diabetes development in a patient suffering from X-linked agammaglobulinemia indicates that B cells are dispensable in disease pathogenesis (15). In line with this interpretation, another B cell–deficient NOD mouse line still developed diabetes in 29% of animals (16). It is possible that the role of auto-Abs in igniting autoreactive T cells may be a facilitating rather than an essential one, as suggested by in vitro human studies (17). However, human type 1 diabetes occurs in children of a type 1 diabetic father twice as frequently as in children of type 1 diabetic mothers (18). These difference may be linked to a protective role of auto-Ab transmission from type 1 diabetic mothers, as auto-Ab− mothers confer a higher type 1 diabetes risk to their progeny compared with auto-Ab+ mothers (19). Thus, vertical auto-Ab transmission seems to be protective rather than harmful in humans.
As learned through many years of clinical trials (20,21), animal models may not suffice to deconvolute the complexity of human type 1 diabetes. Although critical to precisely dissect disease mechanisms as is elegantly done by Silva et al., reductionist transgenic models may further fall short of explanations when confronted by the outbred human species freely wandering in a specific pathogen-rich environment. Although the genetic heterogeneity of human type 1 diabetes casts a first level of complexity, the additional layers of epigenetic (e.g., genomic imprinting) and metagenetic (e.g., microbial colonization) factors are only starting to be dissected both in human and mouse. Comprehensive digging of these multiple layers may offer new solutions to this intriguing conundrum.
ACKNOWLEDGMENTS
Work performed in the Laboratory is supported by the Juvenile Diabetes Research Foundation (JDRF Grant 1-2008-106), the European Foundation for the Study of Diabetes (EFSD/JDRF/Novo Nordisk European Programme in Type 1 Diabetes Research 2009), and the Ile-de-France CODDIM. The figure was produced using Servier Medical Art. R.M. is an INSERM Avenir Investigator, and V.B. was a fellow of the Ile-de-France CODDIM. No other potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 2102.
REFERENCES
- 1.Serreze DV, Chapman HD, Varnum DS, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J Exp Med 1996;184:2049–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noorchashm H, Noorchashm N, Kern J, Rostami SY, Barker CF, Naji A. B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes 1997;46:941–946 [DOI] [PubMed] [Google Scholar]
- 3.Noorchashm H, Lieu YK, Noorchashm N, et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol 1999;163:743–750 [PubMed] [Google Scholar]
- 4.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol 1998;161:1163–1168 [PubMed] [Google Scholar]
- 5.Hulbert C, Riseili B, Rojas M, Thomas JW. B cell specificity contributes to the outcome of diabetes in nonobese diabetic mice. J Immunol 2001;167:5535–5538 [DOI] [PubMed] [Google Scholar]
- 6.Hu CY, Rodriguez-Pinto D, Du W, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest 2007;117:3857–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. ; Type 1 Diabetes TrialNet Anti-CD20 Study Group Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009;361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greeley SA, Katsumata M, Yu L, et al. Elimination of maternally transmitted autoantibodies prevents diabetes in nonobese diabetic mice. Nat Med 2002;8:399–402 [DOI] [PubMed] [Google Scholar]
- 9.Harbers SO, Crocker A, Catalano G, et al. Antibody-enhanced cross-presentation of self antigen breaks T cell tolerance. J Clin Invest 2007;117:1361–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol 1998;161:3912–3918 [PubMed] [Google Scholar]
- 11.Wong FS, Wen L, Tang M, et al. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes 2004;53:2581–2587 [DOI] [PubMed] [Google Scholar]
- 12.Silva DG, Daley SR, Hogan J, et al. Anti-islet autoantibodies trigger autoimmune diabetes in the presence of an increased frequency of islet-reactive CD4 T cells. Diabetes 2011;60:2102–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinuesa CG, Cook MC, Angelucci C, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 2005;435:452–458 [DOI] [PubMed] [Google Scholar]
- 14.Inoue Y, Kaifu T, Sugahara-Tobinai A, Nakamura A, Miyazaki J, Takai T. Activating Fc gamma receptors participate in the development of autoimmune diabetes in NOD mice. J Immunol 2007;179:764–774 [DOI] [PubMed] [Google Scholar]
- 15.Martin S, Wolf-Eichbaum D, Duinkerken G, et al. Development of type 1 diabetes despite severe hereditary B-lymphocyte deficiency. N Engl J Med 2001;345:1036–1040 [DOI] [PubMed] [Google Scholar]
- 16.Yang M, Charlton B, Gautam AM. Development of insulitis and diabetes in B cell-deficient NOD mice. J Autoimmun 1997;10:257–260 [DOI] [PubMed] [Google Scholar]
- 17.Reijonen H, Daniels TL, Lernmark A, Nepom GT. GAD65-specific autoantibodies enhance the presentation of an immunodominant T-cell epitope from GAD65. Diabetes 2000;49:1621–1626 [DOI] [PubMed] [Google Scholar]
- 18.Warram JH, Krolewski AS, Gottlieb MS, Kahn CR. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med 1984;311:149–152 [DOI] [PubMed] [Google Scholar]
- 19.Koczwara K, Bonifacio E, Ziegler AG. Transmission of maternal islet antibodies and risk of autoimmune diabetes in offspring of mothers with type 1 diabetes. Diabetes 2004;53:1–4 [DOI] [PubMed] [Google Scholar]
- 20.Culina S, Boitard C, Mallone R. Antigen-based immune therapeutics for type 1 diabetes: magic bullets or ordinary blanks? Clin Dev Immunol 2011;2011:286248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brezar V, Carel JC, Boitard C, Mallone R. Beyond the hormone: insulin as an autoimmune target in type 1 diabetes. Endocr Rev. 23 June 2011 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]