1. Introduction
Our knowledge about how sleep changes with age has grown significantly over the past few decades. Researchers have shown that there are typical age-related, normal changes that occur in sleep architecture and sleep patterns. However, aging is also accompanied by a variety of sleep complaints and sleep disorders. This chapter will review both normal and abnormal sleep in the elderly.
2. Sleep and Aging
Polysomnography (PSG) has provided objective evidence of the changes in sleep architecture that occur with aging. In general, sleep becomes more fragmented and lighter with an increase in the number of arousals and awakenings. There is a reduction in the amount of slow wave sleep (stage 3 and 4), beginning in middle-age, with some reports that these deeper stages of sleep are completely absent after the age of 90 (Bliwise 1993; Ohayon et al. 2004). There is a compensatory increase in the lighter stages of sleep (stage 1 and 2), and there is a decrease in rapid-eye-movement (REM) sleep, which is proportional to the decrease in total sleep time. Sleep efficiency and total sleep time are reduced with age and there are an increased number of sleep stage shifts. A recent study which included more than 1000 older French adults reported that the mean amount of nightly sleep was approximately seven hours with men sleeping slightly more than women (Ohayon & Vecchierini 2005). Van Cauter et al.(Van Cauter, Leproult, & Plat 2000) found that in men age 16–83, total sleep time decreased on average by 27 minutes per decade from mid-life until the eighth decade. Compared with younger adults, the elderly spend more time in bed but have deterioration in both the quality and quantity of sleep.
All of these changes can lead to excessive daytime sleepiness, which in turn can lead to intentional and unintentional napping. Objective tests of daytime sleepiness performed in the elderly have shown that they are sleepier than younger adults (Carskadon, van den Hoed, & Dement 1980), suggesting that the elderly are not able to obtain an adequate amount of sleep at night (Dement, Seidel, & Carskadon 1982).
Research has suggested that the elderly have a decreased ability to sleep (Ancoli-Israel 1997; Bliwise 1993), which is often reported as insomnia. This decreased ability or insomnia may be due to a variety of factors, each discussed below.
3. Insomnia
Studies have found insomnia, defined as the inability to initiate or maintain sleep resulting in daytime consequences, to be the most common sleep disturbance in older adults (Reid et al. 2006), with up to 40%–50% of those over the age of 60 reporting disturbed sleep (Foley et al. 1995). However, the annual incidence rate is estimated to be 5% in those over the age of 65 (Foley et al. 1999). Complaints range from difficulty falling asleep, to difficulty with sleep maintenance to frequent nighttime awakenings and early morning awakenings. Gender differences exist as well, with women being more likely to complain about insomnia than men (Rediehs, Reis, & Creason 1990).
There are a variety of factors associated with the development of insomnia in the elderly including depression and psychological distress, medical conditions, medications, and circadian rhythm disturbances (Ancoli-Israel 2000). Foley et al. (Foley, Monjan, Simonsick, Wallace, & Blazer 1999) reported that while 28% of older adults suffered from complaints of chronic insomnia, only 7% of the incident cases of insomnia in the elderly occur in the absence of one of these risk factors.
3.1 Depression and Psychological Distress
Psychological distress manifested as daytime anxiety and stress is a common cause of transient insomnia. However, depression, often the result of more serious life events such as divorce or the death of a loved one, can trigger long-lasting, chronic insomnia. It has long been known that depression and insomnia are associated with each other (Ford & Kamerow 1989), as the presence of depressed mood may predict insomnia and, conversely, untreated insomnia may result in depression (Buysse et al. 1994; Cole & Dendukuri 2003; Dryman & Eaton 1991; Livingston, Blizard, & Mann 1993), and having insomnia at baseline is a significant predictor of developing depression one to three years later (Fava 2004; Riemann & Voderholzer 2003). A large study of more than two thousand community-dwelling older men, (the MrOs study), confirmed these associations, finding that those with depression subjectively and objectively had greater sleep disturbances (Paudel et al. 2008). Older women with insomnia seem to be especially susceptible to depression (Breslau et al. 1996; Buysse, Reynolds, Kupfer, Thorpy, Bixler, Manfredi, Kales, Vgontzas, Stepanski, Roth, Hauri, & Mesiano 1994; Cole & Dendukuri 2003; Perlis et al. 2006). Studies in younger adults have suggested that treating the insomnia might also improve depression (Asnis et al. 1999; Nowell & Buysse 2001), but these types of studies have not been conducted in the elderly.
3.2. Sleep and Medical Illness
Older individuals often suffer from multiple medical problems. Pain caused by osteoarthritis, shortness of breath due to chronic obstructive pulmonary disease or congestive heart failure, nocturia due to enlarged prostate, and neurologic deficits related to cerebrovascular accidents or Parkinson’s disease all can lead to difficulty with sleep initiation and maintenance. Additionally, reports of trouble with sleep are strongly correlated with complaints about health and depression (Foley, Monjan, Brown, Simonsick, Wallace, & Blazer 1995). Studies examining the prevalence of sleep disturbances in patients with chronic medical diseases have reported that 31% of arthritis and 66% of chronic pain patients report difficulty falling asleep, while 81% of arthritis, 85% of chronic pain, and 33% of diabetes patients report difficulty staying asleep (Sridhar & Madhu 1994; Wilcox et al. 2000). In a recent National Sleep Foundation survey of adults aged 65 years and over, those with more medical conditions, including cardiac and pulmonary disease and depression, reported significantly more sleep complaints (Foley et al. 2004).
3.3 Sleep and Medications
The medications used to treat these various underlying medical problems can also cause disruptions in sleep. β-blockers, bronchodilators, corticosteroids, decongestants, diuretics as well as other cardiovascular, neurologic, psychiatric, and gastrointestinal medications can all cause sleep disturbances. Furthermore, polypharmacy is increasingly common among older adults, often without consideration of its effect on the patient’s sleep. Whenever feasible, sedating medications should be administered prior to bedtime while stimulating medications and diuretics should be taken during the day.
3.4 Consequences of Insomnia
Insomnia symptoms are associated with overall poor health and mental well being status in older adults (Ohayon & Vecchierini 2005; Reid, Martinovich, Finkel, Statsinger, Golden, Harter, & Zee 2006). In addition to the development of depression, untreated insomnia may result in number of other adverse consequences. Objectively measured sleep disturbances in older adults has been found to be associated with poorer cognition (Blackwell et al. 2006; Ohayon & Vecchierini 2005). In older men, disturbed sleep is associated with poor physical performance (Dam et al. 2008). In a large study of older women, poor sleep (defined as sleep time ≤ 7 hours/night and a sleep efficiency ≤ 65%) was associated with 30–40% increased risk of subsequent falls (Stone et al. 2006).
3.5 Treatment of Insomnia
3.5.1 Behavioral Treatment
Behavioral treatments have been shown to be effective in the treatment of insomnia (Morin et al. 1999b). The concept of sleep hygiene (Morin, Hauri, Espie, Spielman, Buysse, & Bootzin 1999b), which consists of a set of guidelines for the maintenance of healthy sleep and wake habits, should be introduced (see Table 1). Poor sleep hygiene practices can be associated with behavioral patterns that interfere with sleep. Patients should be educated on how to identify specific factors that may be disturbing their own sleep. The use of alcohol, which is widely used as a sleep aid due to its ability to shorten sleep latency, should be discouraged, as it has been shown to induce sleep fragmentation and early morning awakenings (Roehrs & Roth 2001).
Table 1.
Sleep Hygiene Rules
|
Cognitive behavioral therapy (CBT) has been shown to be as effective as medications in the short-run and to have better long-term outcomes in the treatment of insomnia, in both younger and older adults (Morin et al. 1999a). A recent 2005 NIH State-of-the-Science Conference on Insomnia (2005) concluded that CBT is as effective as prescription medications for the treatment of chronic insomnia. Additionally, there are indications that the beneficial effects of CBT, in contrast to those produced by medications, may last well beyond the termination of treatment (Morin, Colecchi, Stone, Sood, & Brink 1999a).
Two specific behavioral therapies, which are often included as part of CBT, are stimulus control therapy and sleep restriction therapy. Stimulus control is based on the belief that insomnia may be the result of maladaptive classical conditioning (Bootzin & Nicassio 1978). Patients are instructed to eliminate all in-bed activities other than sleep, such as reading and television watching. If they are not able to fall asleep within 20 minutes, they are instructed to get out of bed until they feel sufficiently sleepy, when they can return to bed and attempt to again fall asleep. If they are not able to fall asleep within 20 minutes, the pattern of getting out of bed until sleepy repeats itself. This therapy tries to break the association between the bed and wakefulness.
Sleep restriction therapy limits the time spent in bed to about fifteen minutes beyond the duration of time spent asleep at night (Spielman, Saskin, & Thorpy 1987). As sleep efficiency improves, the amount of time spent in bed gradually increases.
3.5.2. Pharmacological Treatment
A number of different classes of medications have been used to treat insomnia in the elderly including sedative-hypnotics, antihistamines, antidepressants, antipsychotics and anticonvulsants. The recent NIH State-of-the-Science Conference on Insomnia concluded that there is no systematic evidence for the effectiveness of the antihistamines, antidepressants, antipsychotics or anticonvulsants in the treatment of insomnia (2005). The panel also expressed significant concerns about the risks associated with the use of these medications, particularly in the elderly.
Sedative-hypnotic medications are at times appropriate for the management of insomnia, however, studies have shown that pharmacologic treatment should be accompanied by behavioral therapy for the most effective treatment (2005; Lichstein & Reidel 1994; Morin, Colecchi, Stone, Sood, & Brink 1999a). Choosing the sedative-hypnotic that best fits the specific complaint related to insomnia is the key to using this class of medications successfully. For example, agents with a long onset of action would not benefit patients with difficulty falling asleep.
When prescribing sedative-hypnotics, particularly benzodiazepines, the potentially harmful effects must be taken into account. The administration of long-acting hypnotics can cause adverse daytime effects such as excessive daytime sleepiness and poor motor coordination, which may lead to injuries (Roth, Roehrs, & Zorick 1988). In the elderly, the risk of falls, cognitive impairment, and respiratory depression are of particular concern, although recent data suggest that insomnia per se, and not hypnotics might increase the risk of falls (Avidan et al. 2005). Chronic use of long-acting benzodiazepines can lead to tolerance and withdrawal symptoms if abruptly discontinued, and the benefits of these agents for long-term use have not been studied with randomized clinical trials. Additionally, the potential for exacerbating coexisting medical conditions such as hepatic or renal disorders exists when these medications are used.
The newer selective short-acting type-1 GABA benzodiazepines receptor agonists (BzRAs, i.e. zolpidem, zaleplon, eszopiclone) have been shown to be effective with a low propensity for causing clinical residual effects, withdrawal, dependence or tolerance. All three BzRAs, zolpidem, zaleplon and eszopiclone, have been found to be effective in the short-term management of insomnia in the elderly (Ancoli-Israel et al. 1999; Ancoli-Israel et al. 2005; Roger, Attali, & Coquelin 1993; Scharf et al. 1991; Scharf et al. 2005). In addition, eszopiclone has been found to be safe and effective for the treatment of chronic insomnia (Krystal et al. 2003); however these studies have only been conducted in younger adults. Particularly in the elderly, these newer sleep aids should be considered first line agents in the pharmacological treatment of insomnia. Ramelteon, a melatonin agonist, has also recently been approved for the treatment of insomnia (Roth, Stubbs, & Walsh 2005).
4. Circadian Rhythm Disturbances
In humans, many physiological variables such as hormone secretion, blood pressure, immune function, core body temperature, and sleep-wake are regulated by a biological clock that operates over a 24-hour period, termed the circadian rhythm. This rhythm entrains to the 24-hour day by external time cues or zeitgebers, with the light-dark cycle being the most important. Circadian rhythm sleep disturbances typically develop when dysynchrony between the endogenous circadian pacemaker, located in the suprachiasmatic nucleus of the anterior hypothalamus, and exogenous environment demands occur.
In the elderly, several factors likely contribute to circadian rhythm desynchronization. First, the suprachiasmatic nucleus deteriorates with age, which may result in weaker and/or more disrupted rhythms (Swaab, Fliers, & Partiman 1985). Second, other circadian rhythm disturbances known to be involved in the entrainment of the circadian rhythm of sleep may develop. For example, the nocturnal secretion of endogenous melatonin gradually decreases with age (Touitou 2001). As melatonin secretion plays an important role in the sleep-wake cycle, the decline may result in reduced sleep efficiency and an increased incidence of circadian rhythm sleep disturbances. Third, elderly patients may have exogenous cues that are too weak to entrain the circadian rhythm of sleep-wake. For example, light is one of the most powerful zeitgebers yet studies have shown that elderly patients, especially those who are institutionalized, spend too little time in daylight. Daily bright light exposure averaged about 60 minutes for healthy elderly, 30 minutes for Alzheimer’s disease patients living at home, and zero minutes for nursing home patients (Ancoli-Israel et al. 1997; Campbell et al. 1988; Espiritu et al. 1994; Shochat et al. 2000). This reduced level of bright light has been shown to be associated with nighttime sleep fragmentation and circadian rhythm sleep disorders (Shochat, Martin, Marler, & Ancoli-Israel 2000).
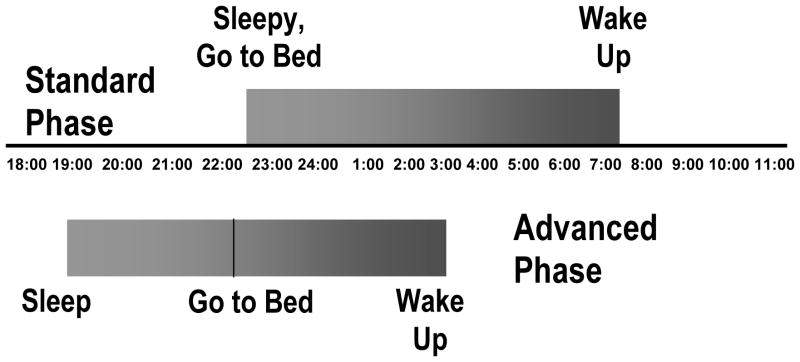
Changes in the phase of the circadian rhythm can develop in the elderly, influencing the timing of the sleep period. Many older patients experience a phase advance in their sleep-wake cycle, causing them to feel sleepy early in the evening. Individuals with advanced sleep phase syndrome (ASPS) will typically fall asleep between 7 to 9 pm and wake up some 8 hours later, at 3 to 5 am (see Fig 1). As a result of societal norms, many older individuals will opt to stay up late, in spite of their sleepiness, yet still awaken early in the morning due to their advanced sleep-wake cycle. Sleep deprivation can ensue which can result in daytime sleepiness and subsequent daytime napping. Finally, the amplitude of the circadian rhythm may also decrease with age. Reductions in rhythm amplitude can increase the frequency of nighttime awakenings and the severity of daytime sleepiness (Vitiello 1996).
Figure 1.
Standard phase of sleep vs. advanced phase of sleep. Reprinted from All I Want is a Good Night’s Sleep, Ancoli-Israel, S. 1996, with permission from Elsevier.
Like insomnia, circadian rhythm changes are considered to be common with age, and presenting symptoms may mimic those of insomnia. Making a distinction between the two disturbances is important, however, as each warrants different treatment approaches. In addition to a careful and detailed sleep history, sleep diaries and activity monitoring with wrist actigraphy can be useful in distinguishing between the two conditions.
Treatments known to strengthen and entrain the sleep-wake cycle are the most appropriate therapies for shifts in the circadian rhythm. The most common treatment for circadian rhythm shifts is bright light therapy, as light is the strongest cue for circadian entrainment. Evening light exposure has been found to delay circadian rhythms and strengthen the sleep-wake cycle in both healthy community living older subjects as well as in nursing home patients (Campbell et al. 1995). Patients with advanced rhythms should spend more time outdoors during the late afternoon or early evening and avoid bright light in the morning hours. If patients are unable to spend enough time outdoors, studies have shown that exposure to artificial light via a bright light-box in the early evening can improve sleep continuity in both healthy and institutionalized elderly patients (Campbell, Terman, Lewy, Dijk, Eastman, & Boulos 1995). In addition, a regular sleep schedule helps to promote a stronger sleep-wake cycle.
As discussed above, endogenous secretion of melatonin, secreted primarily at night, is known to promote sleep and is reduced in older adults. Some studies suggest that melatonin replacement therapy may improve sleep efficiency in this population (Garfinkel et al. 1995; Haimov & Lavie 1995). However, there is little consensus on the recommended dose or timing of administration. In addition, melatonin is not regulated by the Food and Drug Administration, and therefore, there is no control over the purity and exact drug composition of the various brands currently available. Little is known about the possible drug interactions or side effects related to the administration of melatonin long term. Therefore, clinicians should exercise caution when considering a trial of melatonin replacement therapy in elderly patients. The NIH State-of-the-Science Insomnia Conference concluded that although melatonin appears to be effective for the treatment of circadian rhythm disorders, there is little evidence for efficacy in the treatment of insomnia. They also concluded that there is no definition of an effective dose. While melatonin is thought to be safe in the short term, there is no information about the safety of long-term use (2005). However, as mentioned above, the Federal Drug Administration recently approved the first melatonin agonist, ramelteon, for the treatment of sleep-onset insomnia (Roth, Stubbs, & Walsh 2005).
5. Primary Sleep Disorders
There are three primary sleep disorders that are commonly found in the elderly: sleep disordered breathing (SDB), restless legs syndrome/periodic limb movements in sleep (RLS/PLMS), and REM sleep-behavior disorder (RBD).
5.1. Sleep Disordered Breathing
Sleep-disordered breathing is an umbrella term that includes a spectrum of breathing disorders ranging from benign snoring to obstructive apneas. In general, SDB is characterized by the complete cessation of respiration (apneas) and partial or reduced respiration (hypopneas) during sleep. Each event must last a minimum of 10 seconds and recur throughout the night, resulting in repeated arousals from sleep as well as nocturnal hypoxemia. The total number of apneas plus hypopneas per hour of sleep is called the apnea-hypopnea index (AHI) or respiratory disturbance index (RDI). Depending on the laboratory, an AHI or RDI greater than or equal to 5–10 is required for the diagnosis of SDB.
SDB has been shown to be quite common in the elderly. In the largest series of randomly selected community dwelling elderly, 65 to 95 years of age, Ancoli-Israel et al., (Ancoli-Israel et al. 1991c) reported that 81% of the study subjects had an AHI ≥ 5, with prevalence rates of 62% for an AHI ≥ 10, 44% for an AHI ≥ 20, and 24% for an AHI ≥ 40. The Sleep Heart Health Study (Young et al. 2002), a large cohort of some 6400 patients with a mean age of 63.5 with a range from 40 to 98 years, reported prevalence rates of SDB by 10-year age groups. For those subjects age 60–69, 32% had an AHI 5–14 and 19% had an AHI≥15. For those age 70–79, 33% had an AHI 5–14 and 21% had an AHI≥15. For those age 80–98, 36% had an AHI 5–14 and 20% had an AHI≥15. In contrast, Young et al. (Young et al. 1993) reported the estimated prevalence of SDB among middle-aged adults 30 to 60 years of age, defined by an AHI ≥ 5 and the presence of excessive daytime somnolence (EDS), to be 4% of men and 2% of women.
Longitudinal and cross-sectional studies have both shown that the prevalence of SDB increases or stabilizes with increasing age (Ancoli-Israel, Kripke, Klauber, Mason, Fell, & Kaplan 1991c; Bixler et al. 1998; Bliwise et al. 1984; Hoch et al. 1990). In cross-sectional studies, Hoch et al., (Hoch, Reynolds, Monk, Buysse, Yeager, Houck, & Kupfer 1990) found that the median AHI and prevalence of SDB both increased significantly from age 60 to 90 years, and the Sleep Heart Health Study (Young, Shahar, Nieto, Redline, Newman, Gottlieb, Walsleben, Finn, Enright, & Samet 2002) found a small increase in SDB prevalence with increasing 10-year age groups for those subjects with an AHI≥15. In a longitudinal study where older adults were followed for 18 years, Ancoli-Israel et al. (Ancoli-Israel et al. 2001) found that AHI remained stable and only changed with associated changes in body mass index.
Elderly nursing home patients, in particular those with dementia, have been shown to have higher prevalence rates of SDB than those who live independently with prevalence rates ranging from 33–70% (Ancoli-Israel et al. 1991a; Gehrman et al. 2003). Several studies have also found that the severity of the dementia was positively correlated with the severity of the SDB (Ancoli-Israel, Klauber, Butters, Parker, & Kripke 1991a; Hoch & Reynolds 1991). Despite these findings, several other studies have failed to show a significant difference in the amount of SDB in demented elderly when compared to age-matched controls (Bliwise et al. 1989; Smallwood et al. 1983).
Established risk factors for SDB in the elderly include increasing age, gender, and obesity (Phillips & Ancoli-Israel 2001). Other conditions that increase the risk of developing SDB include the use of sedating medications, alcohol consumption, family history, race, smoking, and upper airway configuration (Phillips & Ancoli-Israel 2001).
Snoring and excessive daytime sleepiness (EDS) are the two principal symptoms of SDB in the elderly. Other less common presentations in the elderly include insomnia, nocturnal confusion, and daytime cognitive impairment including difficulties with concentration and attention, and short-term memory loss. It should be noted, however, that the symptoms and clinical presentations of SDB may not differ from younger adults.
Approximately 50% of patients with habitual snoring have some degree of SDB, and snoring has been identified as an early predictor of SDB (Collop & cassell 2002). In subjects 65 years and older, Enright et al. (Enright et al. 1996) reported that loud snoring was independently associated with BMI, diabetes, and arthritis in elderly women and alcohol use in elderly men. It should be noted, however, that not all patients who snore have SDB and not all patients with SDB snore. As many elderly live alone, this symptom may be difficult to identify.
EDS, resulting from recurrent nighttime arousals and sleep fragmentation is a major feature of SDB in the elderly. The presence of EDS may be manifested as unintentional napping as individuals may fall asleep at inappropriate times during the day such as while watching television or movies, while reading, during conversations, while working, and while driving. EDS can cause reduced vigilance and is associated with cognitive deficits which may be particularly serious in older adults who may already have some cognitive impairment at baseline (Martin, Stepnowsky, & Ancoli-Israel 2002).
The body of literature reporting negative consequences and associated conditions related to SDB, including hypertension, cardiac arrhythmias, congestive heart failure, myocardial infarction, and stroke, continues to grow. However, most of the research to date has focused on younger or middle-aged adults, and therefore, the exact relationship between SDB and these various morbidities in the elderly remains unknown.
Earlier studies have reported a positive association between SDB and hypertension in the elderly (Stoohs et al. 1996). The Sleep Heart Health Study (Haas et al. 2005), has provided some additional insight, although they found no association between SDB and systolic/diastolic hypertension in those aged ≥60 years. They did report a positive association between the severity of SDB (based on overnight polysomnography) and the risk of developing cardiovascular disease including coronary artery disease and stroke (Shahar et al. 2001). This study has also found that the severity of SDB was positively associated with the development of congestive heart failure, and like ischemic disease, even mild to moderate SDB was associated with its development (Shahar, Whitney, Redline, Lee, Newman, Javier, O’Connor, Boland, Schwartz, & Samet 2001).
The negative effect of severe SDB (AHI≥30) on cognitive dysfunction in the healthy elderly is well established, with consistent reports of impairment on attention-based tasks, immediate and delayed recall of verbal and visual material, executive tasks, planning and sequential thinking, and manual dexterity (Aloia et al. 2003). Studies examining the relationship between milder SDB and cognition are less clear-cut, as some studies have found that milder SDB (AHI 10–20) in the absence of sleepiness does not cause cognitive dysfunction (Redline et al. 1997).
In addition to the cognitive deficits that may occur as a result of SDB, there is evidence that many of the progressive dementias including Alzheimer’s disease and Parkinson’s disease may have neuronal degeneration in areas of the brainstem that are responsible for maintaining sleep which may place the patient at an increased risk of developing SDB. For example, Ancoli-Israel et al. (Ancoli-Israel, Klauber, Butters, Parker, & Kripke 1991a) found that those institutionalized elderly with severe dementia had more severe SDB compared to those with mild-moderate or no dementia. Furthermore, those with more severe SDB performed worse on the dementia rating scales, suggesting that more severe SDB was associated with more severe dementia.
In regards to mortality, in general, rates from all causes increase 30% during the night, and for those aged 65 and over, the excess deaths typically occur between the hours of 2 am and 8 am (Mitler et al. 1987). The presence of unrecognized or untreated SDB may partially account for these findings as several studies have found an association between SDB in the elderly and increased mortality rates (Bliwise et al. 1988; He et al. 1988), although some studies of community dwelling, nondemented elderly subjects have not found AHI to be an independent predictor of mortality (Ancoli-Israel et al. 1996; Mant et al. 1995). Rather than directly causing an increased mortality, these studies have found that SDB may be one of several predisposing factors for cardiopulmonary disease, which, in combination, leads to increased mortality. This hypothesis is strengthened by a study by Ancoli-Israel et al. (Ancoli-Israel et al. 2003b) which reported that elderly men with congestive heart failure (CHF) had more severe SDB than those with no heart disease. Further, men with both heart failure and SDB, had shortened life spans compared to those with just CHF, just SDB or neither. More studies are needed to further elicit the exact nature of the relationship of SDB and mortality in the elderly, specifically in older women as most of the studies completed in this age category have involved predominantly men.
To accurately assess the presence of SDB in the elderly, a step-wise process should be employed. A complete sleep history should be obtained, focusing on symptoms of SDB such as EDS, unintentional napping, and snoring as well as symptoms of other sleep disorders (i.e restless leg syndrome), and sleep-related habits and routines, in the presence of a bedpartner, roommate or caregiver if possible. The patient’s medical and psychiatric history should be thoroughly reviewed, paying particular attention to associated medical conditions and medications, the use of alcohol, and evidence of cognitive impairment. Lastly, when the evaluation is suggestive of SDB, an overnight sleep recording should be obtained.
Treatment of SDB in the elderly should be guided by the significance of the patient’s symptoms and the severity of the SDB (Ancoli-Israel & Coy 1994). Patients with more severe SDB (AHI > 20) deserve a trial of treatment. For those with milder SDB (AHI < 20), treatment should be considered if co-morbid conditions are present, such as hypertension, cognitive dysfunction, or EDS. Age alone should never be a reason to withhold treatment, nor should assumed noncompliance.
There are a number of effective treatments for SDB. Continuous positive airway pressure (CPAP) is the gold standard treatment of SDB. This device provides continuous positive pressure via the nasal passages or oral airway, creating a pneumatic splint to keep the airway open during inspiration. If used appropriately, CPAP has been shown to safely and effectively manage SDB at night with minimal side effects and is generally well-tolerated. Three months of compliant CPAP use in older adults has been reported to improve cognition, particularly in the areas of attention, psychomotor speed, executive functioning, and non-verbal delayed recall (Aloia, Ilniczky, Di Dio, Perlis, Greenblatt, & Giles 2003).
CPAP compliance can be an issue for any adult with SDB, and clinicians should not assume that elderly patients would be noncompliant simply because of more advanced age. Our laboratory has found that patients with mild-moderate Alzheimer’s disease and SDB tolerate CPAP treatment (Ayalon et al. 2006), and the only factor associated with poor compliance was the presence of depression – not age, severity of dementia, or severity of SDB (Ayalon, Ancoli-Israel, Stepnowsky, Palmer, Liu, Loredo, Corey-Bloom, Greenfield, & Cooke 2006).
Alternatives to CPAP include oral appliances and surgery, however neither has been shown to be as effective as CPAP. All patients should be counseled on weight loss and smoking cessation if indicated. Longer-acting benzodiazepines should generally be avoided in the elderly with SDB as most of these medications are respiratory depressants and may actually increase the number and duration of apneas. Elderly patients with SDB should be encouraged to abstain completely from alcohol consumption, as even small amounts can exacerbate SDB.
While there is a growing body of literature exploring SDB in the elderly, there is also an ongoing debate in the field as to what the presence of SDB in the elderly means and whether it represents an entity which is different than that found in younger or middle-aged adults. Some propose that a distinction should be made between age-dependent conditions, in which aging causes the pathology, and age-related conditions, in which the disease only occurs during a particular age period (Young 1996). Whether SDB is an age-dependent or an age-related condition remains unknown and as more research is focused in this area, answering this question may help target new therapies. However, from a clinical standpoint, as Ancoli-Israel (Ancoli-Israel 2007) and others (Launois, Pepin, & Levy 2006) have opined, the answer may not be as relevant as elderly patients with symptoms and/or related-consequences of SDB (i.e. EDS, cognitive dysfunction, stroke etc.) should be treated, regardless of age. This recommendation has recently been substantiated further by a review of the literature which concluded that data suggest that in the elderly, CPAP improves daytime sleepiness, improves vascular resistance, platelet coagulability and other factors affecting cardiac function, improves some aspects of memory and cognitive functioning, improves nocturia, improves self-reported snoring and improves sleep architecture (Weaver & Chasens 2006).
5.2. Periodic Limb Movement Disorder in Sleep/Restless Legs Syndrome
Periodic limb movement disorder in sleep is a disorder characterized by clusters of repetitive leg movements during sleep, typically accompanied by nighttime arousals and sleep fragmentation. These leg jerks or kicks occur typically every 20 to 40 seconds, and may recur several hundred times over the course of the night, with each jerk potentially causing a brief awakening. Patients with PLMS may complain of EDS and/or insomnia due the frequent arousals from sleep. As PLMS can also interfere with sleep onset, patients may have shorter total sleep times at night. The number of limb movements per hour of sleep is called the periodic limb movement index (PLMI). Clinically, the diagnosis of PLMS requires a PLMI greater than or equal to 5. A diagnosis of PLMS therefore can only be made with an overnight sleep recording. The etiology of PLMS is unknown. PLMS can be seen in patients with fibromyalgia and in conjunction with other primary sleep disorders including SDB and narcolepsy.
In adults, the prevalence of PLMS is estimated at 5–6% (Bixler et al. 1982). This rate increases dramatically with age however, with reported prevalence rates of up to 45% in community dwelling elderly over the age of 65 (Ancoli-Israel et al. 1991b; Ohayon & Roth 2002). While the prevalence of PLMS increases with age, its severity remains stable and does not appear to worsen with advancing age (Gehrman et al. 2002).
Restless leg syndrome (RLS) is a condition strongly linked to PLMS. This disorder is characterized by leg dysesthesia, often described as “a creepy crawling” or “restless” sensation, which occurs while in a relaxed awake state and can only be relieved by movement (Walters et al. 1995). The diagnosis of RLS can be made on the basis of history alone, often with one question: “When you try to relax in the evening or sleep at night, do you ever have unpleasant, restless feelings in your legs that can be relieved by walking or movement?” Patients may have no knowledge that they kick and therefore, interviewing the patient’s bed partner may be helpful in elucidating the history. If symptoms of RLS are present, clinicians should also assess the patient for iron deficiency states including pregnancy, uremia, and peripheral neuropathy as each of these conditions can cause or exacerbate RLS.
Like PLMS, the prevalence of RLS increases significantly with age, with rates in older adults reported to range from 9% to 20% (Allen et al. 2003; Hornyak & Trenkwalder 2004; Ohayon & Roth 2002). Women have been reported to be affected twice as often as men (Allen, Picchietti, Hening, Trenkwalder, Walters, Montplaisir, Restless Legs Syndrome Diagnosis and Epidemiology workshop at the National Institutes of Health, & International Restless Legs Syndrome Study Group 2003).
Pharmacologic intervention is typically required to manage RLS/PLMS. Dopamine agonists are effective in both reducing the number of kicks and associated arousals and are therefore considered the preferred therapy for RLS/PLMS in the elderly (Hening et al. 2004; Littner et al. 2004). Both ropinirole and pramipexole are currently FDA approved for the treatment of RLS although the off-label use of other dopamine agonists (e.g., pergolide and carbidopa-levodopa) may be effective alternatives (see Table 2). Clinicians should be aware that carbidopa-levodopa (sinemet) may shift the leg movements from the nighttime to the early morning.
Table 2.
Dopamine Agonists for Restless Leg Syndrome/Periodic Limb Movements in Sleep
| Recommended Doses: | |
|---|---|
| Ergot-Dopamine Agonists: | |
| Bromocriptine* | 7.5mg qhs |
| Pergolide* | 0.05mg–0.65mg qhs |
| Cabergoline* | 2 mg qhs |
| Non-Ergot Dopamine Agonists: | |
| Pramipexole | 0.25–0.75mg qhs |
| Ropinirole | 2 mg qhs |
off-label use
5.3. Rapid Eye Movement Sleep-Behavior Disorder
REM sleep-behavior disorder (RBD) is characterized by the intermittent absence of normal skeletal muscle atonia during REM sleep, associated with excessive motor activity while dreaming. This disorder typically occurs during the second half of the night when REM is more common. Patients may walk, talk, eat, or appear to be acting out their dreams, which can result in violent movements that are potentially harmful to themselves and their bed partner. Vivid dreams, consistent with the patient’s aggressive and/or violent behavior may be recalled upon waking.
The estimated prevalence of RBD in the elderly is reported to be 0.5% (Ohayon, Caulet, & Priest 1997) with the highest incident occurring after the age of 50 years in elderly men (Olson, Boeve, & Silber 2000a; Olson, Boeve, & Silber 2000b; Schenck, Hurwitz, & Mahowald 1993). Although the etiology of RBD remains unknown, there appears to be a strong association between idiopathic RBD and degenerative neurologic diseases, including Parkinson’s disease, multiple system atrophy, and Lewy Body Dementia (Boeve et al. 1998; Montplaisir 2004; Olson, Boeve, & Silber 2000b). Additionally, in many cases of neurodegenerative disease, RBD may precede other symptoms of the neurodegenerative disorder by years (Boeve, Silber, Ferman, Kokmen, Smith, Ivnik, Parisi, Olson, & Petersen 1998; Olson, Boeve, & Silber 2000b; Schenck, Bundlie, & Mahowald 1996). Olson et al. (Olson, Boeve, & Silber 2000b) reported that 50% of patients diagnosed with idiopathic RBD developed Parkinson’s disease or multiple system atrophy within 3–4 years. Schenck et al. (Schenck, Bundlie, & Mahowald 1996) found that Parkinsonism developed in 38% of men, a mean of 3.7 years after an initial diagnosis of idiopathic RBD. Withdrawal of REM suppressing agents such as alcohol, tricyclic antidepressants, amphetamines, and cocaine have been strongly linked to the onset of acute RBD (Olson, Boeve, & Silber 2000b; Sforza, Krieger, & Petiau 1997). Other medications and conditions reported to induce acute RBD include monoamine oxidase inhibitors, fluoxetine, and stress disorders (Olson, Boeve, & Silber 2000b; Sforza, Krieger, & Petiau 1997).
As with the other primary sleep disorder, the diagnosis of RBD requires a thorough sleep history in the presence of the bed partner if possible. A screening questionnaire has been developed and validated and may become useful in the clinical evaluation of RBD (Stiasny-Kolster et al. 2007). In order to confirm a relationship between REM sleep and the patient’s complex motor behaviors, an overnight polysomnogram with video recording of the nighttime behavior should be performed. Clinicians should pay close attention to intermittent elevations in muscle tone or limb movements on the electromyelogram channel during REM sleep.
Clonazepam, a long-acting benzodiazepine, is the treatment of choice for RBD. It has been shown to result in partial or complete cessation of abnormal nocturnal motor movements in 90% of patients (Schenck & Mahowald 1990). However, patients may complain of residual sleepiness due to its long half-life. Clonazepam is contraindicated in patients with co-existing SBD. Several alternative medications have shown some positive effects in RBD including carbamazepine (Schenck et al. 1987), melatonin (Boeve, Silber, & Ferman 2003), and dopaminergics agents (Bamford 1993), although none has been shown to be as effective as clonazepam.
In addition to pharmacologic treatment, sleep hygiene changes and education of the patient and the bed partner are important aspects of RBD treatment. Efforts to make the bedroom safer such as removing heavy or breakable or potentially injurious objects from the bed’s vicinity should be employed. Heavy curtains should be placed on bedroom windows and doors and windows should be locked at night. Finally, to avoid falling out of bed, patients may consider sleeping on a mattress on the floor.
6.0 Sleep in Dementia
There is considerable evidence that dementia affects sleep differently from the normal aging process (Bliwise 1993). This is not surprising considering that dementing illnesses such as Alzheimer’s disease, Parkinson’s disease, multi-infarct dementia, Lewy Body dementia, etc may result in irreversible damage to the brain in areas responsible for regulating sleep. In general, patients with dementia have disturbed sleep at night, and laboratory sleep studies of demented patients have found increased sleep fragmentation and sleep onset latency, and decreased sleep efficiency, total sleep time, and slow wave sleep (Vitiello 1996). Furthermore, the severity of dementia appears to be associated with the severity of the sleep disruption (Pat-Horenczyk et al. 1998).
Due to these sleep architecture changes, patients with dementia may have excessive daytime sleepiness, nighttime wandering, confusion and agitation (sundowning). Such nighttime behavior and disruptions may eventually lead to institutionalization (Pollak & Perlick 1991). Therefore, addressing issues related to sleep disturbances in the community-dwelling demented elderly is especially important, as it may potentially postpone institutionalization.
It may be difficult to determine the exact nature of the sleep disturbance in patients with dementia although caregivers can be a valuable source of information. The same causes of sleep disruption in the non-demented older adult will also be found in the patient with dementia. Pain from medical illnesses, medications, circadian rhythm changes, and depression are all potential causes of sleep disturbances in this population. It is also important to inquire about treatable primary sleep disorders such as sleep disordered breathing, restless leg syndrome, or periodic limb movements in sleep. Depending on the severity of the dementia, overnight sleep studies may not be feasible and therefore, actigraphy may serve as a useful method to assess sleep and circadian rhythms in these patients (Ancoli-Israel et al. 2003a).
Treatment of specific sleep disturbances in the elderly with dementia should be guided by that specific sleep disorder. SBD should be treated with continuous positive airway pressure if appropriate, RLS/PLMS should be treated with a dopamine agonist, and circadian rhythm disturbances should be treated with bright light therapy. Maintenance of regular physical activity and social interaction can also promote a more robust sleep/wake cycle. Sedating medications including benzodiazepines, tricyclic antidepressants, antihistamines, anticonvulsants and antipsychotics are frequently prescribed for the nighttime restlessness associated with dementia. However, attempting to enhance sleep continuity with these medications may paradoxically result in increased sleep disturbance and daytime sleepiness (“hang over effect”) which may result in impaired motor and cognitive functioning. Therefore, in general, nonpharmacologic interventions are preferred.
6.1 Sleep in Institutionalized Elderly
Institutionalized elderly experience extremely fragmented sleep (Ancoli-Israel & Kripke 1989). Middelkoop et al. (Middelkoop et al. 1994) reported that patients living in nursing homes had poorer sleep quality, more disturbed sleep onset, more phase-advanced sleep periods, and higher use of sedative-hypnotics when compared with those elderly living in the community or in assisted living environments. Studies have found that for older adults living in nursing homes, not a single hour in a 24 hour day was spent fully awake or fully asleep (Ancoli-Israel & Kripke 1989; Jacobs et al. 1989; Pat-Horenczyk, Klauber, Shochat, & Ancoli-Israel 1998).
There are a variety of environmental factors that contribute to the reduction in sleep quality. Noise and light exposure occur intermittently throughout the 24-hour day. Schnelle et al. (Schnelle et al. 1998) demonstrated that both ambient light and nighttime noise contributed significantly to sleep disruption in nursing home patients. This study also found that those patients living in nursing homes where nighttime noise and light were kept to a minimum had better sleep. Ancoli-Israel and Kripke (Ancoli-Israel & Kripke 1989) reported that the nursing home patients were exposed to less than 10 minutes of bright light per day and those with more light exposure had fewer sleep disruptions (Shochat, Martin, Marler, & Ancoli-Israel 2000). Chronic bed rest is known to disrupt circadian rhythms yet institutionalized patients typically spend large amounts of the 24-hour day in bed (Ancoli-Israel & Kripke 1989). Changes in sleep hygiene and the sleep environment may greatly improve the sleep quality of nursing home inhabitants. Strategies to reduce nighttime disturbances and to promote stronger sleep/wake cycles are listed in Table 3.
Table 3.
Tips for Improving Sleep in the Nursing Home
|
Modified from: Ancoli-Israel, et al, Sleep Disorders. In Quality Care for the Nursing Home, Morris et al (Eds), 1997, pp. 64–73.
7. Summary
Significant changes in sleep accompany aging for most adults. There are a variety of potential causes including SDB, circadian rhythm disturbances, RLS/PLMS, RBD, depression and other psychiatric disorders, medical illness, and medications. The diagnosis requires a good sleep history and at times, a sleep study. Treatment should address the primary problem rather than the complaint itself and may result in significant improvement in quality of life and daytime functioning in the elderly.
Acknowledgments
Supported by NIA AG08415 and NIH M01 RR00827 and The VA Center of Excellence for Stress and Mental Health (CESAMH) which is supported by Department of Veterans Affairs.
7.0. References
- National Institutes of Health State of the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults, June 13–15, 2005. Sleep. 2005;28(9):1049–1057. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- Allen RP, Picchietti DL, Hening WA, Trenkwalder C, Walters AS, Montplaisir J Restless Legs Syndrome Diagnosis and Epidemiology workshop at the National Institutes of Health, & International Restless Legs Syndrome Study Group. Restless Legs Syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the Restless Legs Syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Medicine. 2003;4:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- Aloia MS, Ilniczky N, Di Dio P, Perlis ML, Greenblatt DW, Giles DE. Neuropsychological changes and treatment compliance in older adults with sleep apnea. Journal of Psychosomatic Research. 2003;54:71–76. doi: 10.1016/s0022-3999(02)00548-2. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S. Sleep problems in older adults: Putting myths to bed. Geriatrics. 1997;52(1):20–30. [PubMed] [Google Scholar]
- Ancoli-Israel S. Insomnia in the elderly: A review for the primary care practitioner. Sleep. 2000;23(suppl 1):S23–S30. [PubMed] [Google Scholar]
- Ancoli-Israel S. Guest Editorial: Sleep apnea in older adults - is it real and should age be the determining factor in the treatment decision matrix? Sleep Med Rev. 2007;11:83–85. doi: 10.1016/j.smrv.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi CA, Chambers M, Moorcroft WH, Pollak C. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003a;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Coy TV. Are breathing disturbances in elderly equivalent to sleep apnea syndrome? Sleep. 1994;17:77–83. doi: 10.1093/sleep/17.1.77. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, DuHamel ER, Stepnowsky C, Engler R, Cohen-Zion M, Marler M. The relationship between congestive heart failure, sleep disordered breathing and mortality in older men. Chest. 2003b;124(4):1400–1405. doi: 10.1378/chest.124.4.1400. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Gehrman P, Kripke DF, Stepnowsky C, Mason W, Cohen-Zion M, Marler M. Long-term follow-up of sleep disordered breathing in older adults. Sleep Medicine. 2001;2(6):511–516. doi: 10.1016/s1389-9457(00)00096-4. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Klauber MR, Butters N, Parker L, Kripke DF. Dementia in institutionalized elderly: Relation to sleep apnea. Journal of the American Geriatrics Society. 1991a;39(3):258–263. doi: 10.1111/j.1532-5415.1991.tb01647.x. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Klauber MR, Jones DW, Kripke DF, Martin J, Mason W, Pat-Horenczyk R, Fell R. Variations in circadian rhythms of activity, sleep and light exposure related to dementia in nursing home patients. Sleep. 1997;20(1):18–23. [PubMed] [Google Scholar]
- Ancoli-Israel S, Kripke DF. Now I lay me down to sleep: The problem of sleep fragmentation in elderly and demented residents of nursing homes. Bulletin of Clinical Neurosciences. 1989;54:127–132. [Google Scholar]
- Ancoli-Israel S, Kripke DF, Klauber MR, Fell R, Stepnowsky C, Estline E, Khazeni N, Chinn A. Morbidity, mortality and sleep disordered breathing in community dwelling elderly. Sleep. 1996;19(4):277–282. doi: 10.1093/sleep/19.4.277. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Periodic limb movements in sleep in community-dwelling elderly. Sleep. 1991b;14(6):496–500. doi: 10.1093/sleep/14.6.496. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep disordered breathing in community-dwelling elderly. Sleep. 1991c;14(6):486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Richardson GS, Mangano R, Jenkins L, Hall P, Jones WS. Long-term use of sedative hypnotics in older patients with insomnia. Sleep Medicine. 2005;6:107–113. doi: 10.1016/j.sleep.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Walsh JK, Mangano RM, Fujimori M Zaleplon Clinical Study Group. Zaleplon, a novel nonbenzodiazepine hypnotic, effectively treats insomnia in elderly patients without causing rebound effects. Primary Care Companion to Journal of Clinical Psychiatry. 1999;1(4):114–120. doi: 10.4088/pcc.v01n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnis GM, Chakraburrty A, Duboff EA, Krystal AD, Londborg PD, Rosenberg R, Roth-Schechter B, Scharf MB, Walsh JK. Zolpidem for persistent insomnia in SSRI-treated depressed patients. J Clin Psychiat. 1999;60:668–676. doi: 10.4088/jcp.v60n1005. [DOI] [PubMed] [Google Scholar]
- Avidan AY, Fries BE, James MC, Szafara KL, Wright GT, Chervin RD. Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes. Journal of the American Geriatrics Society. 2005;53(6):955–962. doi: 10.1111/j.1532-5415.2005.53304.x. [DOI] [PubMed] [Google Scholar]
- Ayalon L, Ancoli-Israel S, Stepnowsky C, Palmer BW, Liu L, Loredo JS, Corey-Bloom J, Greenfield D, Cooke JR. Adherence to continuous positive airway pressure treatment in patients with Alzheimer’s disease and obstructive sleep apnea. American Journal of Geriatric Psychiatry. 2006;14(2):176–180. doi: 10.1097/01.JGP.0000192484.12684.cd. [DOI] [PubMed] [Google Scholar]
- Bamford CR. Carbamazepine in REM sleep behavior disorder. Sleep. 1993;16:33–34. [PubMed] [Google Scholar]
- Bixler EO, Kales A, Vela-Bueno A, Jacoby JA, Scarone S, Soldatos CR. Nocturnal myoclonus and nocturnal myoclonic activity in a normal population. Res Commun Psychol Psychiat. 1982;36:129–140. [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men. Am J Res Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, Fink HA, Stone KL. Poor sleep is associated with impaired cognitive function in older women: the Study of Osteoporotic Fractures. J Gerontol: Med Sci. 2006;61(4):405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Review: Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Bliwise DL, Bliwise NG, Partinen M, Pursley AM, Dement WC. Sleep apnea and mortality in an aged cohort. American Journal of Public Health. 1988;78:544–547. doi: 10.2105/ajph.78.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL, Carskadon MA, Carey E, Dement WC. Longitudinal development of sleep-related respiratory disturbance in adult humans. Journal of Gerontology. 1984;39:290–293. doi: 10.1093/geronj/39.3.290. [DOI] [PubMed] [Google Scholar]
- Bliwise DL, Yesavage JA, Tinklenberg JR, Dement WC. Sleep apnea in Alzheimer’s disease. Neurobiology of Aging. 1989;10:343–346. doi: 10.1016/0197-4580(89)90046-8. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Medicine. 2003;4(4):281–284. doi: 10.1016/s1389-9457(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Silber MH, Ferman TJ, Kokmen E, Smith GE, Ivnik RJ, Parisi JE, Olson EJ, Petersen RC. REM sleep behavior disorder and degenerative dementia: An association likely reflecting Lewy body disease. Neurology. 1998;51(2):363–370. doi: 10.1212/wnl.51.2.363. [DOI] [PubMed] [Google Scholar]
- Bootzin RR, Nicassio PM. Behavioral treatments for insomnia. In: Hersen M, Eisler RM, Miller PM, editors. Progress in Behavior Modification. Vol. 6. Academic Press, Inc; New York: 1978. pp. 1–45. [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Sociaty of Biological Psychiatriy. 1996;39(6):411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Kupfer DJ, Thorpy MJ, Bixler E, Manfredi R, Kales A, Vgontzas A, Stepanski E, Roth T, Hauri P, Mesiano D. Clinical diagnoses in 216 insomnia patients using the international classification of sleep disorders (ICSD), DSM-IV and ICD-10 categories: A report from the APA/NIMH DSM-IV field trial. Sleep. 1994;17(7):630–637. doi: 10.1093/sleep/17.7.630. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Kripke DF, Gillin JC, Hrubovcak JC. Exposure to light in healthy elderly subjects and Alzheimer’s patients. Physiology and Behavior. 1988;42:141–144. doi: 10.1016/0031-9384(88)90289-2. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Terman M, Lewy AJ, Dijk DJ, Eastman CI, Boulos Z. Light Treatment for Sleep Disorders: Consensus report. V. Age-related disturbances. Journal of Biological Rhythms. 1995;10(2):151–154. doi: 10.1177/074873049501000207. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, van den Hoed J, Dement WC. Sleep and daytime sleepiness in the elderly. Journal of Geriatric Psychiatry. 1980;13:135–151. [PubMed] [Google Scholar]
- Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147–1156. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- Collop NA, Cassell DK. Snoring and Sleep-Disordered Breathing. In: Lee-Chiong TL, Sateia MJ, Carskadon MA, editors. Sleep Medicine. Hanley & Belfus; Philadelphia: 2002. pp. 349–355. [Google Scholar]
- Dam TL, Ewing SK, Ancoli-Israel S, Ensrud K, Redline SS, Stone KL. Is there an association between objective measures of sleep and physical function in older men? The MrOS Sleep Study. Journal of the American Geriatrics Society. 2008 doi: 10.1111/j.1532-5415.2008.01846.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dement WC, Seidel W, Carskadon MA. Daytime alertness, insomnia and benzodiazepines. Sleep. 1982;5:S28–S45. doi: 10.1093/sleep/5.suppl_1.s28. [DOI] [PubMed] [Google Scholar]
- Dryman A, Eaton WW. Affetive symptoms associatd with the onset of major depression in the communiyt: findings from the US National Institute of Mental Health Epidemiologic Catchment Area Program. Acta Psychiatr Scand. 1991;84(1):1–5. doi: 10.1111/j.1600-0447.1991.tb01410.x. [DOI] [PubMed] [Google Scholar]
- Enright PL, Newman AB, Wahl PW, Manolio TA, Haponik EF, Boyle P. Prevalence and correlates of snoring and observed apneas in 5,201 Older Adults. Sleep. 1996;19(7):531–538. doi: 10.1093/sleep/19.7.531. [DOI] [PubMed] [Google Scholar]
- Espiritu RC, Kripke DF, Ancoli-Israel S, Mowen MA, Mason WJ, Fell RL, Klauber MR, Kaplan OJ. Low illumination by San Diego adults: Association with atypical depressive symptoms. Biological Psychiatry. 1994;35:403–407. doi: 10.1016/0006-3223(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Fava M. Daytime sleepiness and insomnia as correlates of depression. J Clin Psychiatry. 2004;65(Supple 16):27–32. [PubMed] [Google Scholar]
- Foley DJ, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation. Sleep in America Survey. Journal of Psychosomatic Research. 2004;56(5):497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22(Suppl 2):S366–S372. [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: An opportunity for prevention? Journal of the American Medical Association. 1989;262(11):1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Garfinkel D, Laudon M, Nof D, Zisapel N. Improvement of sleep quality in elderly people by controlled-release melatonin. The Lancet. 1995;346:541–544. doi: 10.1016/s0140-6736(95)91382-3. [DOI] [PubMed] [Google Scholar]
- Gehrman PR, Martin JL, Shochat T, Nolan S, Corey-Bloom J, Ancoli-Israel S. Sleep disordered breathing and agitation in institutionalized adults with Alzheimer’s disease. American Journal of Geriatric Psychiatry. 2003;11(4):426–433. [PubMed] [Google Scholar]
- Gehrman PR, Stepnowsky C, Cohen-Zion M, Marler M, Kripke DF, Ancoli-Israel S. Long-term follow-up of periodic limb movements in sleep in older adults. Sleep. 2002;25(3):340–346. doi: 10.1093/sleep/25.3.340. [DOI] [PubMed] [Google Scholar]
- Haas DC, Foster GL, Nieto FJ, Redline S, Resnick HE, Robbins JA, Young T, Pickering TG. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111:614–621. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- Haimov I, Lavie P. Potential of melatonin replacement therapy in older patients with sleep disorders. Drugs and Aging. 1995;7(2):75–78. doi: 10.2165/00002512-199507020-00001. [DOI] [PubMed] [Google Scholar]
- He J, Kryger MH, Zorick FJ, Conway W, Roth T. Mortality and apnea index in obstructive sleep apnea: experience in 385 male patients. Chest. 1988;94:9–14. [PubMed] [Google Scholar]
- Hening W, Allen RP, Picchietti DL, Silber MH Restless Legs Syndrome Task Force of the Standards of Practice Committee of the American Academy of Sleep Medicine. An update on the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004;27(3):560–583. doi: 10.1093/sleep/27.3.560. [DOI] [PubMed] [Google Scholar]
- Hoch CC, Reynolds CFI. Cognitive Function and Sleep Disordered Breathing in Dementia: The Pittsburg Experience. In: Kuna ST, Suratt PM, Remmers JE, editors. Sleep and Respiration in Aging Adults. Elsevier; New York: 1991. pp. 245–250. [Google Scholar]
- Hoch CC, Reynolds CFI, Monk TH, Buysse DJ, Yeager AL, Houck PR, Kupfer DJ. Comparison of sleep-disordered breathing among healthy elderly in the seventh, eighth, and ninth decades of life. Sleep. 1990;13(6):502–511. doi: 10.1093/sleep/13.6.502. [DOI] [PubMed] [Google Scholar]
- Hornyak M, Trenkwalder C. Restless legs syndrome and periodic limb movement disorder in the elderly. Journal of Psychosomatic Research. 2004;56(5):543–548. doi: 10.1016/S0022-3999(04)00020-0. [DOI] [PubMed] [Google Scholar]
- Jacobs D, Ancoli-Israel S, Parker L, Kripke DF. Twenty-four hour sleep-wake patterns in a nursing home population. Psychology and Aging. 1989;4(3):352–356. doi: 10.1037//0882-7974.4.3.352. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Walsh JK, Laska E, Canon J, Amato DA, Wessel TC, Roth T. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26(7):793–799. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- Launois SH, Pepin JL, Levy P. Sleep apnea in the eldelry: A specific entity? Sleep Med Rev. 2006 doi: 10.1016/j.smrv.2006.08.005. in press. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Reidel BW. Behavioral assessment and treatment of insomnia: a review with an emphasis on clinical application. Behavior Therapy. 1994;25:659–688. [Google Scholar]
- Littner M, Kushida C, Anderson WM, Bailey D, Berry RB, Hirshkowitz M, Kapen S, Kramer M, Lee-Chiong TL, Li KK, Loube D, Morgenthaler T, Wise M Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004;27(3):557–559. doi: 10.1093/sleep/27.3.557. [DOI] [PubMed] [Google Scholar]
- Livingston G, Blizard B, Mann A. Does sleep disturbance predict depression in elderly people? A study in inner London. Brit J Gen Pract. 1993;43:445–448. [PMC free article] [PubMed] [Google Scholar]
- Mant A, King M, Saunders NA, Pond CD, Goode E, Hewitt H. Four-year follow-up of mortality and sleep-related respiratory disturbance in non-demented seniors. Sleep. 1995;18(6):433–438. doi: 10.1093/sleep/18.6.433. [DOI] [PubMed] [Google Scholar]
- Martin J, Stepnowsky C, Ancoli-Israel S. Sleep Apnea in the Elderly. In: McNicholas WT, Phillipson EA, editors. Breathing Disorders During Sleep. W.B. Saunders Company, Lmt; London: 2002. pp. 278–287. [Google Scholar]
- Middelkoop HA, Kerkhof GA, Smilde-van den Doel DA, Ligthart GJ, Kamphuisen HA. Sleep and ageing: the effect of institutionalization on subjective and objective characteristics of sleep. Age and Ageing. 1994;23(5):411–417. doi: 10.1093/ageing/23.5.411. [DOI] [PubMed] [Google Scholar]
- Mitler MM, Hajdukovic RM, Shafor R, Hahn PM, Kripke DF. When people die. Cause of death versus time of death. American Journal of Medicine. 1987;82:266–74. doi: 10.1016/0002-9343(87)90067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montplaisir J. Abnormal motor behavior during sleep. Sleep Medicine. 2004;5(Suppl 1):S31–S34. doi: 10.1016/s1389-9457(04)90005-6. [DOI] [PubMed] [Google Scholar]
- Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late life insomnia. Journal of the American Medical Association. 1999a;281(11):991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review. Sleep. 1999b;22(8):1134–56. doi: 10.1093/sleep/22.8.1134. [DOI] [PubMed] [Google Scholar]
- Nowell PD, Buysse DJ. Treatment of insomnia in patients with mood disorders. Depression and Anxiety. 2001;14(1):7–18. doi: 10.1002/da.1042. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Caulet M, Priest RG. Violent behavior during sleep. Journal of Clinical Psychiatry. 1997;58(8):369–376. [PubMed] [Google Scholar]
- Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. Journal of Psychosomatic Research. 2002;53:547–554. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28(8):981–989. [PubMed] [Google Scholar]
- Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000a;123(Pt 2):331–339. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000b;123:331–339. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- Pat-Horenczyk R, Klauber MR, Shochat T, Ancoli-Israel S. Hourly profiles of sleep and wakefulness in severely versus mild-moderately demented nursing home patients. Aging Clin Exp Res. 1998;10:308–315. doi: 10.1007/BF03339793. [DOI] [PubMed] [Google Scholar]
- Paudel M, Taylor B, Diem S, Stone KL, Ancoli-Israel S, Redline SS, Ensrud KE for the Osteoporotic Fractures in Men (MrOS) Study Group. Association between Depressive Symptoms and Sleep Disturbances among Community-Dwelling Older Men. Journal of the American Geriatrics Society. 2008 doi: 10.1111/j.1532-5415.2008.01753.x. in press May 14 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Smith LJ, Lyness JM, Matteson SR, Pigeon WR, Jungquist CR, Tu X. Insomnia as a risk factor for onset of depression in the elderly. Behavioral Sleep Medicine. 2006;4(2):104–113. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- Phillips B, Ancoli-Israel S. Sleep disorders in the elderly. Sleep Medicine. 2001;2(2):99–114. doi: 10.1016/s1389-9457(00)00083-6. [DOI] [PubMed] [Google Scholar]
- Pollak CP, Perlick D. Sleep problems and institutionalization of the elderly. Journal of Geriatric Psychiatry and Neurology. 1991;4:204–210. doi: 10.1177/089198879100400405. [DOI] [PubMed] [Google Scholar]
- Rediehs MH, Reis JS, Creason NS. Sleep in old age: Focus on gender differences. Sleep. 1990;13(5):410–424. [PubMed] [Google Scholar]
- Redline S, Strauss ME, Adams N, Winters M, Roebuck T, Spry K, Rosenberg C, Adams K. Neuropsychological function in mild sleep-disordered breathing. Sleep. 1997;20(2):160–167. doi: 10.1093/sleep/20.2.160. [DOI] [PubMed] [Google Scholar]
- Reid KJ, Martinovich Z, Finkel S, Statsinger J, Golden R, Harter K, Zee PC. Sleep: A Marker of Physical and Mental Health in the Elderly. American Journal of Geriatric Psychiatry. 2006;14:860–866. doi: 10.1097/01.JGP.0000206164.56404.ba. [DOI] [PubMed] [Google Scholar]
- Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disorder. 2003;76:255–259. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5(4):287–297. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- Roger M, Attali P, Coquelin JP. Multicenter, double-blind, controlled comparison of zolpidem and triazolam in elderly patients with insomnia. Clinical Therapeutics. 1993;15(1):127–136. [PubMed] [Google Scholar]
- Roth T, Roehrs T, Zorick F. Pharmacological treatment of sleep disorders. In: Williams RL, Karacan I, Moore CA, editors. Sleep Disorders: Diagnosis and treatment. John Wiley & Sons; New York: 1988. pp. 373–395. [Google Scholar]
- Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep. 2005;28(3):303–307. [PubMed] [Google Scholar]
- Scharf MB, Erman M, Rosenberg R, Seiden D, McCall WV, Amato D, Wessel TC. A 2-week efficacy and safety study of eszopiclone in elderly patients with primary insomnia. Sleep. 2005;28(6):720–727. doi: 10.1093/sleep/28.6.720. [DOI] [PubMed] [Google Scholar]
- Scharf MB, Mayleben DW, Kaffeman M, Krall R, Ochs R. Dose response effects of zolpidem in normal geriatric subjects. J Clin Psychiatry. 1991;52(2):77–83. [PubMed] [Google Scholar]
- Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathoc rapid eye movement sleep behavior disorder. Neurology. 1996;46:388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Bundlie SR, Patterson AL, Mahowald MW. Rapid eye movement sleep bahavior disorder. A treatable parasomnia affecting older adults. Journal of the American Medical Association. 1987;257:1786–1789. [PubMed] [Google Scholar]
- Schenck CH, Hurwitz TD, Mahowald MW. Symposium: Normal and abnormal REM sleep regulation: REM sleep behaviour disorder: an update on a series of 96 patients and a review of the world literature. J Sleep Res. 1993;2(4):224–231. doi: 10.1111/j.1365-2869.1993.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Mahowald MW. Polysomnographic, neurologic, psychiatric, and clinical outcome report on 70 consecutive cases with the REM sleep behavior disorder (RBD): sustained clonazepam efficacy in 89.5% of 57 treated patients. Cleveland Clinic Journal of Medicine. 1990;57:S10–24. [Google Scholar]
- Schnelle JF, Cruise PA, Alessi CA, Al-Samarrai N, Ouslander JG. Sleep hygiene in physically dependent nursing home residents. Sleep. 1998;21(5):515–523. [PubMed] [Google Scholar]
- Sforza E, Krieger J, Petiau C. REM sleep behavior: clinical and physiopathological findings. Sleep Medicine Reviews. 1997;1(1):57–69. doi: 10.1016/s1087-0792(97)90006-x. [DOI] [PubMed] [Google Scholar]
- Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier NF, O’Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- Shochat T, Martin J, Marler M, Ancoli-Israel S. Illumination levels in nursing home patients: Effects on sleep and activity rhythms. J Sleep Res. 2000;9(4):373–380. doi: 10.1046/j.1365-2869.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- Smallwood RG, Vitiello MV, Giblin EC, Prinz P. Sleep apnea: relationship to age, sex, and Alzheimer’s dementia. Sleep. 1983;6:16–22. doi: 10.1093/sleep/6.1.16. [DOI] [PubMed] [Google Scholar]
- Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45–56. [PubMed] [Google Scholar]
- Sridhar GR, Madhu K. Prevalence of sleep disturbance in diabetes mellitus. Diabetes Res Clin Pract. 1994;23(3):183–186. doi: 10.1016/0168-8227(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Stiasny-Kolster K, Mayer G, Schafer S, Carsten Moller J, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire - a new diagnostic instrument. Movement Disorders. 2007;22(16):2386–2393. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- Stone KL, Ewing SK, Lui LY, Ensrud KE, Ancoli-Israel S, Bauer DC, Cauley JA, Hillier TA, Cummings SR. Self-reported sleep and nap habits and risk of falls and fractures in older women: the study of osteoporotic fractures. Journal of the American Geriatrics Society. 2006;54(8):1177–1183. doi: 10.1111/j.1532-5415.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- Stoohs RA, Gingold J, Cohrs S, Harter R, Finlayson E, Guilleminault C. Sleep-disordered breathing and systemic hypertension in the older male. Journal of the American Geriatrics Society. 1996;44(11):1295–1300. doi: 10.1111/j.1532-5415.1996.tb01398.x. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Research. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Touitou Y. Human aging and melatonin. Clinical relevance. Exp Gerontol. 2001;36(7):1083–1100. doi: 10.1016/s0531-5565(01)00120-6. [DOI] [PubMed] [Google Scholar]
- Van Cauter EV, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. Journal of the American Medical Association. 2000;284(7):861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- Vitiello MV. Sleep disorders and aging. Current Opinion in Psychiatry. 1996;9(4):284–289. [Google Scholar]
- Walters AS, Aldrich MS, Allen R, Ancoli-Israel S, Buchholz DW, Chokroverty S, Coccagna G, Earley C, Ehrenberg B, Feest TG, Hening W, Kavey N, Lavigne G, Lipinski J, Lugaresi E, Montagna P, Montplaisir J, Mosko SS, Oertel W, Picchietti D, Pollmacher T, Shafor R, Smith RC, Telstad W, Trenkwalder C, von Scheele C, Ware JC, Zucconi M. Toward a better definition of the restless legs syndrome. Movement Disorders. 1995;10(5):634–642. doi: 10.1002/mds.870100517. [DOI] [PubMed] [Google Scholar]
- Weaver TE, Chasens ER. Continuous Positive Airway Pressure Treatment for Sleep Apnea in Older Adults. Sleep Med Rev. 2006 doi: 10.1016/j.smrv.2006.08.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox S, Brenes GA, Levine D, Sevick MA, Shumaker SA, Craven T. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. Journal of the American Geriatrics Society. 2000;48(10):1241–1251. doi: 10.1111/j.1532-5415.2000.tb02597.x. [DOI] [PubMed] [Google Scholar]
- Young T. Sleep-disordered breathing in older adults: Is it a condition distinct from that of middle-aged adults? Sleep. 1996;19(7):529–530. doi: 10.1093/sleep/19.7.529. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep disordered breathing among middle-aged adults. New England Journal of Medicine. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, Walsleben JA, Finn L, Enright P, Samet JM. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Archives of Internal Medicine. 2002;162(8):893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]