Abstract
Among Atlantic scleractinian corals, species diversity is highest in the Caribbean, but low diversity and high endemism are observed in various peripheral populations in central and eastern Atlantic islands and along the coasts of Brazil and West Africa. The degree of connectivity between these distantly separated populations is of interest because it provides insight into processes at both evolutionary and ecological time scales, such as speciation, recruitment dynamics and the persistence of coral populations. To assess connectivity in broadly distributed coral species of the Atlantic, DNA sequence data from two nuclear markers were obtained for six coral species spanning their distributional ranges. At basin-wide scales, significant differentiation was generally observed among populations in the Caribbean, Brazil and West Africa. Concordance of patterns in connectivity among co-distributed taxa indicates that extrinsic barriers, such as the Amazon freshwater plume or long stretches of open ocean, restrict dispersal of coral larvae from region to region. Within regions, dispersal ability appears to be influenced by aspects of reproduction and life history. Two broadcasting species, Siderastrea siderea and Montastraea cavernosa, were able to maintain gene flow among populations separated by as much as 1,200 km along the coast of Brazil. In contrast, brooding species, such as Favia gravida and Siderastrea radians, had more restricted gene flow along the Brazilian coast.
Introduction
The ability to disperse over long distances and maintain connectivity over large geographical areas has important repercussions for the population dynamics of a species. Over ecological time scales, connectivity contributes to the persistence of populations [1], [2], [3], as the influx of migrants from neighboring healthy ecosystems can buffer mortality due to local disturbances. Large populations are expected to preserve more genetic variety than smaller populations which are susceptible to loss of genetic diversity through the effects of genetic drift [4]. Populations interconnected by regular dispersal and gene flow can behave as large populations and bypass the negative effects of drift. On the other hand, isolated populations can become locally adapted and the origination of new species can occur over evolutionary time scales [5], [6], contributing to the overall diversity of a region. Peripheral populations, those found towards the edges of a species range, are more likely to have reduced numbers of individuals and lower genetic diversity because they are isolated from the central core and because they are found at the environmental limits of the species range [7]. Understanding connectivity with respect to peripheral populations can provide insight into (1) how these populations are able to persist despite withstanding suboptimal conditions and (2) whether they can contribute evolutionary novelty and distinctiveness to the overall population.
Many marine organisms have broad geographical distributions, but it is unclear whether these distributions reflect the ability to maintain frequent long-distance dispersal or if populations are fragmented across their range. In the Atlantic, the most extensive and diverse tropical reef ecosystems are found in the Caribbean Sea, but reefs with low overall species richness yet high endemism are also found along the coast of Brazil, on mid-Atlantic islands and in West Africa [8], [9], [10]. Species whose distribution spans the Atlantic provide an interesting natural experiment to explore basin-scale dispersal in benthic marine organisms and the role of peripheral populations in the evolution of biodiversity.
Dispersal in benthic marine organisms can be achieved in various ways. Long-lived pelagic larvae are capable of traversing hundreds of kilometers [11] either by feeding in the water column or by relying on nutritional resources imparted by the parents. Rafting of recruits or adults on floating debris [12] also allows organisms to colonize distant locations. Habitat preferences also play a role [13], and species able to survive in a wide range of environments or whose habitat is large and continuous may maintain dispersal over long distances despite shorter larval durations, because dispersal can be achieved as a series of smaller steps. Oceanographic currents can either aid or prevent dispersal of larvae. Physical properties of water masses affect larval survival, and dispersal may be limited by temperature, salinity or nutrient content [14]. Dispersal can also be limited by the absence of suitable substrate or chemical cues for settlement [15], [16], [17] because successful recruitment is dependent not only on reaching a destination, but also the ability to settle and become established. Comparative studies across species with different reproductive strategies and life histories can help to clarify which characteristics contribute to successful long-distance dispersal, and whether dispersal is limited by intrinsic characteristics of a single species or extrinsic barriers affecting multiple species.
Corals support biodiversity in the oceans by providing the substrate and architecture for a host of other marine species. Understanding the processes that limit or promote dispersal in coral species can provide insight into how and why populations persist and evolve. Although connectivity and dispersal have been explored for a number of co-distributed Pacific corals [18], [19], basin-scale connectivity among Atlantic corals has only been explored among populations of one scleractinian species [20] and one species of octocoral [21]. However, it is unclear whether the patterns observed in these two species can be generalized to all corals, or whether they are specific to the dispersal ability of these two organisms. Comparative studies across multiple coral species over similar spatial scales can help elucidate this problem.
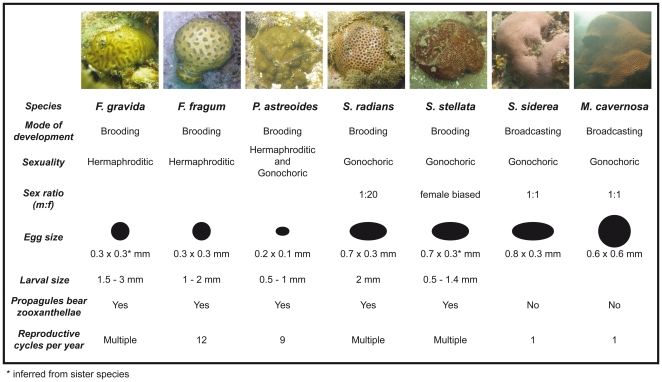
Corals exhibit a variety of reproductive strategies, making them an interesting group for evaluating the role of reproductive traits in determining dispersal potential. Corals reproduce sexually either by internal fertilization and brooding of larvae or by external fertilization of broadcast gametes followed by development in the water column [22]. Various traits aid long-distance dispersal, such as larval longevity, delayed time to competency, and reproductive output. Prolonged longevity allows larvae to be transported greater distances, and the available nutritional resources will affect the larva's ability to remain alive. Large eggs, such as those observed for many broadcasting species [23], [24], or larvae harboring zooxanthellae, such as for many brooded species [25], may have improved chances of survival. Coral larval longevity can reach upwards of two to three months for some species [26], [27], but many larvae will settle upon encountering suitable substrate much sooner. Brooded larvae are more advanced in their development when released and are competent for settling in 24–48 hours [28], [29], [30]. In contrast, larvae produced from broadcast gametes usually require 5–7 days before being capable of settling [31], [32], although shorter times to settlement competency (54–66 hours) have also been observed in some Pacific species [33]. As time to settlement competency increases, so does the probability of long-distance transport of larvae.
The reproductive output of a coral species may increase its ability for long distance dispersal, simply because the chances of successful dispersal events increase as a greater number of propagules are generated. The number of larvae produced by a colony varies according to colony size, the number of eggs produced per polyp, number of reproductive cycles per year, and fertilization success [32]. The number of reproductive cycles per year may be an important difference between brooding versus broadcasting species, since the former are able to produce larvae over several months of the year to year-round, while the latter typically have only one to three spawning events annually [22], [23], [24]. Finally, sexuality and sex ratio can also play a role in fertilization success. Equal sex ratios, self-compatibility and hermaphrodism may improve chances of fertilization by maximizing the possibility of successful matings. These adaptations can be advantageous in locations were colonies are sparse or where environmental conditions may hinder outcrossing, such as may be expected in peripheral populations.
Species richness and regional endemism vary widely across the Atlantic. Among the 81 species of reef-building corals found in the Atlantic, 68 species are found in the Caribbean, 23 in Brazil and 18 in the Eastern Atlantic (Table S1). There are 12 species endemic to either Brazil or the Eastern Atlantic (five in Brazil, five in West Africa, and two species found in both regions) whereas 49 species are endemic to the Caribbean. Twenty species have wide distributions that span two or more biogeographic regions of the Atlantic, but only nine are found across all three regions: Madracis asperula, M. decactis, M. pharensis, Montastraea cavernosa, Porites astreoides, Siderastrea radians, S. siderea, S. stellata and the invasive Tubastraea coccinea. In addition, Favia fragum (Caribbean and West Africa) and F. gravida (Brazil and West Africa) were previously considered synonymous [34] and amphi-Atlantic, but recent work indicates these closely related species can be distinguished on the basis of morphology [9] and genetics [35]. It is important to note that species lists and shared occurrences are a work in progress. The shared occurrence of some species across biogeographic regions, such as for Meandrina braziliensis, Siderastrea stellata, or Scolymia cubensis, is debated among taxonomists and biogeographers (for example, [36]). Future work that uses morphological and molecular data from specimens collected in both regions are required to confirm occurrences and refine estimates of endemism for each region.
Here we provide new genetic data from six coral species together with previously published data from a seventh species; these species have broad geographic distributions across the Atlantic and span a range of reproductive strategies (Fig. 1). The aim of this study was to obtain estimates of basin-wide dispersal and connectivity for a number of Atlantic corals having a variety of biological traits and spanning a wide taxonomic range.
Figure 1. Reproductive traits of studied species: Favia fragum, Favia gravida, Porites astreoides, Siderastrea radians, Siderastrea stellata, Siderastrea siderea and Montastraea cavernosa.
Summarized from [23], [24], [25], [32], [67], [68], [69], [70]. Images taken by F.N.
Materials and Methods
DNA extraction, amplification and sequencing
Coral tissue samples were collected from colonies of six coral species: F. fragum, F. gravida, S. siderea, S. radians, S. stellata, and P. astreoides. Sampling took place in Panama in 2005, on the island of São Tomé in West Africa in 2006 and in three populations separated by >500 km in Brazil (Abrolhos, João Pessoa and Fortaleza) in 2002 and 2007. Genomic DNA was preserved and extracted following a protocol described previously [35]. Due to the unusually low levels of genetic variation found in the mitochondrial DNA of corals [37], [38], only nuclear loci were used. The intron and exon of β-tubulin and the intron of the Pax-C gene, which have previously been shown to contain sufficient intraspecific variation in corals for population studies [20], [39], were amplified for all species studied, with the exception of P. astreoides, for which only β-tubulin could be amplified. For P. astreoides, the mitochondrial control region (thought to be one of the most variable regions of the coral mitochondrial genome) was amplified and sequenced for 32 individuals from Panamá and Brazil, but because all individuals contained identical nucleotide sequences at this locus, this marker was not used for further analysis.
Amplification of the two nuclear loci was performed by polymerase chain reaction using published primer sequences for β-tubulin [40] and for Pax-C [39]. The thermal cycler profile had an initial denaturation step at 94°C for 2 min, followed by 38 cycles of 94°C/45 sec, 50–58°C/45 sec for β-tubulin or 50–54°C/45 sec for Pax-C, 72°C/90 sec, with a final extension step at 72°C for 5 min. An additional forward primer was designed for the amplification and sequencing of Pax-C for F. fragum (PaxFsh1: 5′- GGA GGA GCT TGC GAA TAA GA -3′). Multiple bands were amplified in P. astreoides and the Siderastrea species in β-tubulin, and for S. siderea in Pax-C. Bands at ∼750 bp and ∼600 bp were extracted and purified for products of β-tubulin and Pax-C respectively, using the Qiaquick Gel Extraction kit (Qiagen). The remaining PCR products were purified either by gel extraction or by elution through a silica-membrane column using the appropriate Qiaquick purification system (Qiagen).
Sequencing of all loci was performed directly on purified PCR products for both forward and reverse directions on an ABI 3130xl genetic analyzer with the BigDye Terminator v3.1 chemistry (Applied Biosystems). Sequence chromatographs were viewed and edited using the Sequencher v4.5 software (Gene Codes Corp). Heterozygous alleles were identified by double peaks observed in sequences from both directions. Indels were observed in the introns of β-tubulin for S. radians (site 549) and S. siderea (site 549), and in sequences of Pax-C for S. radians (site 134), F. fragum (site 205) and F. gravida (site 205). Because only a small number of individuals were heterozygous for indels within any species and indel positions were straightforward to identify, indel sites were kept and considered in the analysis.
Analysis of sequence data
Haplotypes for heterozygous individuals were reconstructed using PHASE v 2.1.1 [41], [42]. The algorithm was run three times for 100 iterations with ten thinning interval steps and 100 burn in steps. The best pairs of haplotypes for each individual resulting from the run with the highest average value for the goodness of fit were used for each nuclear locus.
Haplotype frequencies, molecular diversity indices, tests of neutrality, analysis of molecular variance (AMOVA) and estimates of population differentiation were calculated using Arlequin v.3.11 [43]. Analysis of molecular variance (AMOVA) was used to estimate levels of genetic differentiation among populations of each coral species. One AMOVA was used to estimate differentiation among all populations sampled in the Caribbean, Brazil and West Africa (ΦST ALL). In this AMOVA, populations of F. fragum (n = 1) and F. gravida (n = 3) were pooled to assess levels of genetic differentiation between these two closely related species. A second AMOVA tested for differentiation among populations within Brazil, the only region for which multiple populations were sampled (ΦST BR). Estimates of pairwise φst used distances between haplotypes. Parsimony haplotype networks were constructed with TCS [44]. Recombination in the two nuclear loci was inferred as reticulations in the haplotype network and tested by the four-gamete test in DnaSP [45]. For clarity, loops were omitted from the haplotype networks and only internal branches are shown. Due to the evidence of recombination, analyses using a coalescent framework (which assume no recombination) were not attempted.
Results
A total of 238 individuals across all species were sequenced for β-tubulin and 191 for Pax-C. Haplotype counts for each species can be found in Table S2 and the genotype for each individual is listed in Table S3. Sequences have been deposited in Genbank (see Table S4 for accession numbers).
Interestingly, individuals identified in the field as S. stellata in Brazil contained alleles that were either (1) identical or had 1–2 mutations difference to alleles of S. siderea from Caribbean or West Africa, or (2) had a combination of haplotypes identified in S. siderea and S. radians at one or both loci. The presence of S. siderea in Brazil has been debated [8], [9], [46], but these results confirm its presence in Brazil, though morphologic differences may exist between regions. Furthermore, the finding that some individuals contain a combination of S. siderea and S. radians haplotypes indicates that S. stellata could be the result of hybridization between these two species. Although most individuals with hybrid genotypes were found in Brazil (n = 20), one hybrid was found in Panamá and four were found in São Tomé. Further investigation is required to confirm the hybrid status of S. stellata. For the purposes of this study, data collected from hybrid individuals were kept in the analysis, but only populations of S. radians and S. siderea were considered. Therefore, haplotypes obtained from S. stellata were included as part of either a S. radians or S. siderea population, depending on similarity/identity with haplotypes from those species.
The number of alleles, unique haplotypes and segregating sites per population are shown in Table 1, as are standard molecular diversity indices and tests of neutrality for each species and each locus. Gene diversity (h), which estimates the probability that two randomly sampled haplotypes in a population are different, ranged from 0–0.863 in Pax-C and 0.235–0.986 in β-tubulin, indicating that while some populations are dominated by a common haplotype, others have a large diversity of haplotypes. Nucleotide diversity (π) ranged from 0.0009–0.0090 in Pax-C and 0.0003–0.0158 in β-tubulin, and the average number of differences between haplotypes of a population (k) ranged from 0.324–3.293 in Pax-C and 0.235–10.505 in β-tubulin. The mean and standard deviation for each molecular diversity index in each population is shown in Table 1. Populations of S. siderea in São Tomé (West Africa) had lower average gene diversity than populations in the Caribbean and Brazil. Reduced gene diversity was also observed for F. gravida in West Africa in the Pax-C locus; however, this trend was not observed in the β-tubulin locus. S. radians and P. astreoides showed overlap in mean values of gene diversity at both loci across Caribbean, Brazilian and West African populations (Table 1). Mean values of nucleotide diversity (π) and average number of differences (k) overlap among populations for nearly all species in both loci (Table 1), with the exception of populations of S. radians in Panamá, which have greater values of π and k in the β-tubulin locus.
Table 1. Molecular diversity indices and tests of neutrality for Pax-C and β-tubulin.
| Na | H | s | h | p | k | Tajima's D | Fu's Fs | |||||||
| Pax-C | ||||||||||||||
| F. fragum | 454 bp | |||||||||||||
| CA1 - Panama | 32 | 3 | 2 | 0.619 | ± | 0.044 | 0.0016 | ± | 0.0014 | 0.724 | ± | 0.555 | 1.535 | 0.886 |
| F. gravida | 454 bp | |||||||||||||
| BR1 - Abrolhos | 26 | 2 | 1 | 0.492 | ± | 0.051 | 0.0011 | ± | 0.0011 | 0.492 | ± | 0.436 | 1.437 | 1.523 |
| BR2 - João Pessoa | 46 | 2 | 1 | 0.394 | ± | 0.063 | 0.0009 | ± | 0.0009 | 0.394 | ± | 0.375 | 1.006 | 1.407 |
| WA1 - São Tome | 40 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| S. radians | 367 bp | |||||||||||||
| CA1 - Panama | 21 | 5 | 5 | 0.491 | ± | 0.127 | 0.0033 | ± | 0.0024 | 1.210 | ± | 0.803 | −0.213 | −0.565 |
| BR1 - Abrolhos | 14 | 2 | 4 | 0.528 | ± | 0.064 | 0.0058 | ± | 0.0038 | 2.110 | ± | 1.250 | 2.232 | 4.844 |
| BR2 - João Pessoa | 27 | 5 | 8 | 0.735 | ± | 0.059 | 0.0090 | ± | 0.0053 | 3.293 | ± | 1.748 | 1.610 | 2.968 |
| BR3 - Fortaleza | 20 | 3 | 5 | 0.653 | ± | 0.065 | 0.0058 | ± | 0.0037 | 2.105 | ± | 1.225 | 1.511 | 3.402 |
| WA1 - São Tome | 44 | 5 | 8 | 0.571 | ± | 0.052 | 0.0061 | ± | 0.0038 | 2.229 | ± | 1.253 | 0.896 | 2.316 |
| S. siderea | 366 bp | |||||||||||||
| CA1 - Panama | 27 | 8 | 13 | 0.863 | ± | 0.035 | 0.0090 | ± | 0.0053 | 3.288 | ± | 1.745 | −0.085 | 0.156 |
| BR2 - João Pessoa | 29 | 3 | 4 | 0.493 | ± | 0.093 | 0.0028 | ± | 0.0022 | 1.034 | ± | 0.710 | 0.041 | 1.676 |
| BR3 - Fortaleza | 20 | 5 | 9 | 0.600 | ± | 0.101 | 0.0042 | ± | 0.0029 | 1.542 | ± | 0.963 | −1.348 | −0.007 |
| WA1 - São Tome | 36 | 2 | 3 | 0.108 | ± | 0.068 | 0.0009 | ± | 0.0010 | 0.324 | ± | 0.335 | −1.237 | 0.939 |
| β-tubulin | ||||||||||||||
| F. fragum | 939 bp | |||||||||||||
| CA1 - Panama | 36 | 4 | 5 | 0.533 | ± | 0.088 | 0.0014 | ± | 0.0010 | 1.321 | ± | 0.842 | 0.248 | 1.340 |
| F. gravida | 939 bp | |||||||||||||
| BR1 - Abrolhos | 38 | 2 | 1 | 0.235 | ± | 0.081 | 0.0003 | ± | 0.0003 | 0.235 | ± | 0.278 | −0.020 | 0.455 |
| BR2 - João Pessoa | 52 | 3 | 3 | 0.604 | ± | 0.034 | 0.0013 | ± | 0.0009 | 1.190 | ± | 0.775 | 1.623 | 2.695 |
| WA1 - São Tome | 40 | 3 | 2 | 0.650 | ± | 0.038 | 0.0008 | ± | 0.0007 | 0.788 | ± | 0.585 | 1.278 | 1.260 |
| S. radians | 665 bp | |||||||||||||
| CA1 - Panama | 20 | 8 | 22 | 0.837 | ± | 0.051 | 0.0158 | ± | 0.0084 | 10.505 | ± | 4.999 | 2.631 | 3.722 |
| BR1 - Abrolhos | 6 | 4 | 6 | 0.867 | ± | 0.129 | 0.0048 | ± | 0.0033 | 3.200 | ± | 1.918 | 1.246 | 0.352 |
| BR2 - João Pessoa | 14 | 4 | 6 | 0.495 | ± | 0.151 | 0.0017 | ± | 0.0013 | 1.099 | ± | 0.765 | −1.499 | −0.214 |
| BR3 - Fortaleza | 14 | 3 | 2 | 0.703 | ± | 0.062 | 0.0013 | ± | 0.0011 | 0.879 | ± | 0.654 | 1.080 | 0.586 |
| WA1 - São Tome | 40 | 5 | 6 | 0.677 | ± | 0.042 | 0.0021 | ± | 0.0014 | 1.363 | ± | 0.860 | −0.090 | 0.531 |
| S. siderea | 665 bp | |||||||||||||
| CA1 - Panama | 38 | 32 | 34 | 0.986 | ± | 0.011 | 0.0119 | ± | 0.0063 | 7.933 | ± | 3.771 | −0.130 | −21.883 |
| BR1 - Abrolhos | 10 | 7 | 15 | 0.933 | ± | 0.062 | 0.0087 | ± | 0.0051 | 5.778 | ± | 3.020 | 0.413 | −0.394 |
| BR2 - João Pessoa | 38 | 19 | 21 | 0.926 | ± | 0.028 | 0.0079 | ± | 0.0043 | 5.266 | ± | 2.602 | 0.180 | −5.479 |
| BR3 - Fortaleza | 24 | 8 | 16 | 0.844 | ± | 0.044 | 0.0062 | ± | 0.0036 | 4.152 | ± | 2.140 | −0.110 | 0.657 |
| WA1 - São Tome | 40 | 5 | 5 | 0.236 | ± | 0.088 | 0.0005 | ± | 0.0006 | 0.342 | ± | 0.346 | −1.804 | −3.315 |
| P. astreoides | 596 bp | |||||||||||||
| CA1 - Panama | 24 | 4 | 10 | 0.728 | ± | 0.045 | 0.0078 | ± | 0.0044 | 4.649 | ± | 2.361 | 2.455 | 5.884 |
| BR2 - João Pessoa | 42 | 4 | 12 | 0.628 | ± | 0.041 | 0.0085 | ± | 0.0047 | 5.087 | ± | 2.518 | 2.515 | 8.829 |
Na is the number of sampled alleles, H is the number of unique haplotypes observed, s is the number of segregating sites, h is the gene diversity, π is the average nucleotide diversity and k is the average number of nucleotide differences. Biogeographic regions are denoted as CA for Caribbean, BR for Brazil and WA for West Africa. Statistically significant values (α = 0.05) are highlighted in bold.
Tajima's D and Fu's Fs suggest that the loci used are evolving according to neutral expectations for most populations. Two populations (S. radians in João Pessoa and S. siderea in São Tomé) had significantly negative values of Tajima's D (Table 1), suggesting deviations from neutral expectations. Negative values of Fu's Fs were observed for three populations of S. siderea for the β-tubulin locus, but only the population of São Tomé also had a significant negative value of Tajima's D (Table 1). Fu's Fs is sensitive to changes in demography, and large negative values in S. siderea are indicative of population expansion. Aside from the exceptions mentioned above, it appears that both loci are evolving according to neutral expectations for the remaining populations.
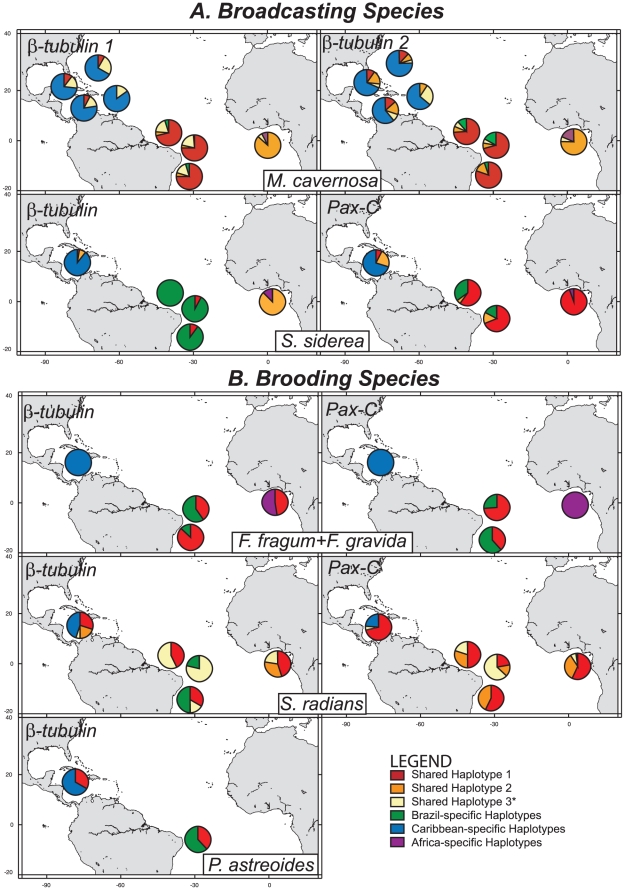
Significant differentiation among populations was observed for nearly all of the studied species at both loci (ΦST ALL = 0.142–0.754, p<0.05), except for P. astreoides (ΦST ALL = 0.026, p>0.05). The strongest levels of differentiation were observed among the pooled populations of F. fragum and F. gravida (Tables 2 and 3), as would be expected for an inter-species comparison. Furthermore, there was significant intra-regional differentiation between populations of the brooders F. gravida and S. radians of Brazil (ΦST BR = 0.148–0.582, p<0.05), but not among populations of the broadcasters S. siderea or M. cavernosa within Brazil (ΦST BR = −0.026–0.018, p>0.05) (Table 2). Significant population differentiation was observed among nearly all pairwise comparisons (Table 3), with only a few exceptions: (1) populations of S. siderea within Brazil were not differentiated at both loci, (2) no significant differentiation was observed between populations of P. astreoides in Brazil and the Caribbean and (3) populations of Abrolhos and Fortaleza (both in Brazil) and São Tomé in S. radians were not significantly different at Pax-C, most likely because similar allele frequencies were observed for two common haplotypes (SRP1 and SRP2). Haplotype frequency pie charts were plotted for each locus and each species on a map to illustrate differences across populations (Fig. 2). Since individuals morphologically identified as F. fragum were observed only in Panamá, haplotype frequencies for this population were plotted in the same figure panel as F. gravida (absent in the Caribbean) so that a comparison of haplotype frequencies could be made between these two species (Fig. 2).
Table 2. Analysis of molecular variance (AMOVA).
| A. AMOVA for all populations (ΦST ALL) | ||||||||||
| BROODERS | ||||||||||
| Species | Favia fragum+Favia gravia | Siderastrea radians | Porites astreoides | |||||||
| Locus | β-tubulin | Pax-C | β-tubulin | Pax-C | β-tubulin | |||||
| Variance components | % of variation | Variance components | % of variation | Variance components | % of variation | Variance components | % of variation | Variance components | % of variation | |
| Among populations | 0.925 | 67.2 | 0.576 | 75.44 | 0.962 | 36.77 | 0.187 | 14.21 | 0.067 | 2.61 |
| Within populations | 0.452 | 32.8 | 0.187 | 24.56 | 1.654 | 63.23 | 1.129 | 85.79 | 2.465 | 97.39 |
| Fixation Index Φst | 0.672 | 0.754 | 0.368 | 0.142 | 0.026 | |||||
AMOVA was used to estimate levels of genetic differentiation among (A) all populations across all biogeographic regions (ΦST ALL) and (B) among populations within Brazil (ΦST BR), the only region for which multiple populations were sampled. Note that in (A) F. fragum and F. gravida have been analyzed together to estimate differentiation across these closely related species. Statistically significant values (α = 0.05) are highlighted in bold.
Table 3. Pairwise φst for each nuclear locus, for each species.
| BROODERS | |||||||||||||||||||
| F. fragum+F. gravida | β-tubulin | F. fragum+F. gravida | Pax-C | ||||||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||||||||||||
| 1 | Abrolhos, Brazil | 1 | Abrolhos, Brazil | ||||||||||||||||
| 2 | João Pessoa, Brazil | 0.479* | 2 | João Pessoa, Brazil | 0.208* | ||||||||||||||
| 3 | Bocas del Toro, Panamá | 0.804* | 0.755* | 3 | Bocas del Toro, Panamá | 0.804* | 0.809* | ||||||||||||
| 4 | Sao Tomé, West Africa | 0.218* | 0.361* | 0.757* | 4 | Sao Tomé, West Africa | 0.660* | 0.230* | 0.871* | ||||||||||
| Siderastrea radians | β-tubulin | Siderastrea radians | Pax-C | ||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | ||||||||||
| 1 | Abrolhos, Brazil | 1 | Abrolhos, Brazil | ||||||||||||||||
| 2 | João Pessoa, Brazil | 0.338* | 2 | João Pessoa, Brazil | 0.193 | ||||||||||||||
| 3 | Fortaleza, Brazil | 0.463* | 0.720* | 3 | Fortaleza, Brazil | −0.010 | 0.209* | ||||||||||||
| 4 | Bocas del Toro, Panamá | 0.17484 | 0.358* | 0.323* | 4 | Bocas del Toro, Panamá | 0.192 | 0.271* | 0.095 | ||||||||||
| 5 | São Tomé, West Africa | 0.277* | 0.521* | 0.167 | 0.393* | 5 | Sao Tomé, West Africa | −0.040 | 0.196* | −0.013 | 0.125 | ||||||||
| Porites astreoides | β-tubulin | ||||||||||||||||||
| 1 | 2 | ||||||||||||||||||
| 1 | João Pessoa, Brazil | 0 | |||||||||||||||||
| 2 | Bocas del Toro, Panamá | 0.02611 | 0 | ||||||||||||||||
Statistically significant p-values (α = 0.05) are highlighted in bold, * denotes significant values after Bonferroni correction.
Figure 2. Haplotype frequency pie charts for coral populations across the Atlantic for (A) broadcasters and (B) brooders.
The three most commonly occurring haplotypes shared between regions for each species and locus have been coded with the same color (see legend). Private alleles for each geographic region have been binned into one category for clarity. *For M. cavernosa, “Shared Haplotype 3” is a sum of the frequencies of all haplotypes shared in two of the three regions.
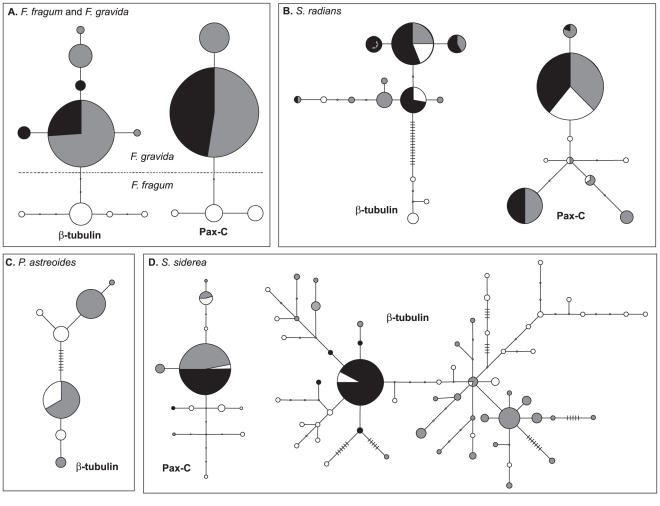
Statistical parsimony haplotype networks for each species and locus are shown in Fig. 3. Networks for F. fragum and F. gravida were plotted together to show levels of divergence among haplotypes of the two species. Haplotype networks were typically composed of a small number of haplotypes (H<15), although the network for β-tubulin in S. siderea had 60 haplotypes. No loops were observed in the haplotype networks of F. fragum and F gravida for both loci, or in the network of P. astreoides for β-tubulin. One loop was observed in the networks of S. radians at each locus. For S. siderea, no loops were observed in Pax-C; however, numerous loops were observed in β-tubulin, suggesting that in this locus, several recombination events have occurred. The four gamete test indicates that only a few recombination events have occurred at each locus for most species (Rm = 0–4), except for β-tubulin of S. siderea, where a minimum of 12 recombination events are inferred. Recombination has also been observed in β-tubulin1 and β-tubulin2 of M. cavernosa [20]. Every haplotype network had 1–5 haplotypes that were shared between two or more regions. Differences between haplotypes from different regions were typically small, on the order of one to a few mutations. There were a relatively high proportion of private alleles (those restricted to a single population) for each locus and species, ranging from 44–87% of the observed haplotypes for a population. The proportion of alleles that were observed only once was low at each locus for most species (0–27%), except for β-tubulin in S.siderea, where 72% of haplotypes were observed only once.
Figure 3. Parsimony haplotype network for (A) F. fragum and F. gravida, (B) S. radians, (C) P. astreoides and (D) S. siderea.
Haplotypes observed in the Caribbean, Brazil and West Africa are shown as white, grey and black circles, respectively. The size of each circle reflects the frequency that a haplotype is observed. Notches symbolize intermediate haplotypes not observed.
Discussion
Genetic differentiation across regions of the Atlantic
The data collected for six coral species plus an existing dataset for Montastraea cavernosa [20] suggest that significant differentiation is present between the Caribbean, Brazil and West Africa for most sampled coral species. Concordance in the pattern of regional isolation occurs at the scale of genes and species. Both nuclear markers show consistent patterns in differentiation, and regional differentiation across multiple species indicates that barriers to gene flow between biogeographic regions in the Atlantic are effective for most corals.
The most likely biogeographic barrier separating Caribbean and Brazilian coral populations are the deltas and low salinity waters of the Amazon, Orinoco and numerous rivers along the coast of northern South America. The Amazon is the largest among them, and accounts for 16% of the annual freshwater discharge into the world's oceans [47]. Its plume extends 200–500 km in width [48] and is recognizable 24–32 m below the surface [49]. The Amazon and Orinoco Rivers attained their current drainage configuration around the Late Miocene [50], indicating that low salinity and high sedimentation may have been a feature of this coastal region for ∼10 Ma. Approximately 2,300 km of coastline rich in soft sediment bottoms and low salinity coastal waters between Caribbean and Brazilian reefs may pose substantial barriers to dispersal, because the substrate near river deltas is inadequate for coral settlement and coral larvae are very sensitive to the changes in salinity [51] that these large river plumes create.
Levels of genetic differentiation between between Caribbean F. fragum and South Atlantic F. gravida were the most pronounced among all of the studied species (Table 2A and 3). This finding, combined with the observation that the species do not co-occur [9], supports the hypothesis that F. fragum and F. gravida are distinct [35]. Only a small number of mutations separate F. fragum and F. gravida (Fig. 3), but this may be due to the recent divergence of the two species coupled with the slow rates of mutation observed in corals [38].
The only species for which no significant differentiation was observed between Caribbean and Brazilian populations was P. astreoides. For β-tubulin (the only marker amplified for this species, see Methods), P. astreoides has one common haplotype that is observed both in Brazil and Panamá at similar frequencies, with all other haplotypes being unique to each respective population (see Fig. 3). This common haplotype may be an ancestral haplotype that has been maintained over time in the two populations without continued gene flow. Sequences of β-tubulin for P. astreoides have lower variation compared to the other species, perhaps insufficient to detect differences among populations. Additional support from faster-evolving markers such as microsatellites is required to determine whether continued gene flow is maintained between the Caribbean and Brazil in this species. Alternatively, P. astreoides, a brooder, may be able to raft or be more tolerant to freshwater and high sedimentation.
Significant regional differentiation was also observed with respect to West Africa (Table 3), suggesting that long distances of open ocean may be impassable for coral larvae. The easternmost point of Brazil and the island of São Tomé are separated by 4,800 km. Some mid-Atlantic islands could serve as stepping-stones for dispersal, decreasing dispersal distances by about one half. However, it appears that even these distances are too great to maintain gene flow or that the populations on these islands (such as the St. Peter and Paul rocks) are too small or ephemeral to be significant in trans-Atlantic dispersal. Interestingly, the population of M. cavernosa on the island of Bermuda is able to maintain connectivity with Caribbean populations despite being separated by at least 1,000 km [20]. Presumably the fast-moving currents of the Gulf Stream are able to maintain the influx of larvae to Bermuda. The combined effects of distance and physical oceanography are likely important isolating factors for West African coral populations.
Concordance in patterns of regional connectivity in the Atlantic is not observed in most other species of marine organisms. Four species of sea urchins sharing similar life histories and pelagic larval durations show markedly different patterns of differentiation across the Atlantic. The Amazon appears to be a significant barrier to gene flow for most species [52], [53], [54], but not all [55]. Multiple diagnostic mutations separate eastern and western Atlantic populations of some urchin species [53], [54], while others maintain continued exchange across the South Atlantic [52], [55]. The discordant patterns observed among sea urchins with similar traits in reproduction and dispersal indicate that barriers to gene flow in the Atlantic may be permeable depending on aspects other than reproduction and pelagic larval duration.
Among reef fish, similar discordance has been observed across species. Some species achieve high gene flow throughout the Atlantic (Myripristis jacobus [56]), or throughout their range (Halichoeres garnoti [57]). Others are highly differentiated [58], [59], while some display intermediate levels of gene flow between these two extremes. Interestingly, broad adult habitat preferences among surgeonfish of the genus Acanthurus correlates well with dispersal ability across the Amazon, suggesting that among reef fish, both ecology and reproduction play important roles in dispersal potential [13].
In sum, the similarities in patterns of connectivity observed among various coral species indicate that barriers such as the Amazon or stretches of open ocean are likely impassible for most coral larvae, leading to concordance of patterns. These barriers may be more permeable, however, for other organisms whose ecology and life history permit dispersal at greater distances than corals, resulting in more variable patterns of connectivity observed in other amphi-Atlantic marine organisms.
Genetic differentiation within regions
Although patterns in inter-regional differentiation are concordant among most coral species, different estimates of gene flow are observed within regions across coral species, and the extent of gene flow is correlated with reproductive traits. In this study, multiple populations within a region were sampled only in Brazil. For each species, two or three populations each separated by >500 km across a total of 2,000 km along the coast of Brazil were sampled for all species except F. fragum (present only in the Caribbean) and P. astreoides, for which specimens were only taken in João Pessoa (see Fig. 2). Strong differentiation observed at both loci between populations of the brooder F. gravida in Abrolhos and João Pessoa (Table 3) indicates that little exchange is occurring between populations of this species along the coast of Brazil. Intermediate levels of differentiation were observed for the brooder S. radians, where AMOVA indicates that differentiation is significant between populations within regions for both loci (Table 2), but not all pairwise population comparisons showed significant differentiation (Table 3). In contrast, gene flow appears to be maintained between three populations spanning 2,000 km of Brazilian coast of the two broadcasting corals studied, S. siderea and M. cavernosa (Table 2).
The two broadcasting species that are able to maintain gene flow along the coast of Brazil are both gonochoric with one reproductive cycle per year, while the two brooding species with fragmented populations are hermaphroditic and spawn multiple times per year (Fig. 1). Egg sizes for broadcasting M. cavernosa and S. siderea are larger than for the brooder F. gravida, but only marginally larger than brooding S. radians. However, the dispersing propagules for brooders, the planula larvae, are larger than broadcast eggs and bear zooxanthellae (Fig. 1). The findings of this study indicate that neither the frequency of spawning nor the size of the dispersing propagules (and associated energy reserves) appear to provide an advantage for long distance dispersal. Brooded larvae may allocate much of their energy reserves towards settlement and growth rather than dispersal. Because broadcast gametes must spend time in the water column before being competent for settlement, their chances of being entrained in currents that take them away from their natal reef are greater, resulting in greater dispersal distances.
The species with the greatest population differentiation, F. gravida, is a hermaphroditic brooder, possibly capable of self-fertilization like its sibling species F. fragum [60]. Self-fertilization may ensure reproductive success when population densities are low and sperm limitation reduces chances of fertilization, but with the disadvantage that populations may become inbred and dominated by clonemates. Self-fertilization will also result in apparent reduced gene flow, so at least part of the high differentiation among populations of F. gravida could reflect inbreeding.
Some Caribbean coral species are able to maintain gene flow throughout the Caribbean and Bermuda, such as M. cavernosa [20] and M. faveolata [61], but dispersal within the Caribbean is more restricted for other species. Among the seven Caribbean corals studied to date, gene flow is restricted to <500 km for most species [62]. Acropora palmata [63] and A. cervicornis [62] are able to disperse widely, but their populations are subdivided between the eastern and western Caribbean. Gene flow appears to be restricted to even shorter distances for species such as Agaricia agaricites [64] and some members of the M. annularis species complex [61], [65]. All of the aforementioned Caribbean corals are broadcasters with the exception of A. agaricites. In agreement with findings for Brazilian brooders, A. agaricites in the Caribbean has a more fragmented population than broadcasting species, although studies on additional Caribbean brooders are required to confirm the generality of this trend.
Among Caribbean broadcasters, there are no clear explanations for differences in dispersal ability. Dispersal ability in A. palmata and A. cervicornis appears to be more restricted than for M. cavernosa and M. faveolata, even though Caribbean Acropora eggs are at least 1.4 times greater in size [23]. Egg size may not be a good predictor of dispersal ability, as nutritional reserves may be allocated for settlement rather than survival in the water column. Most striking is the difference in dispersal ability among closely related species with similar reproductive traits, such as short-ranging M. annularis and Caribbean-wide M. faveolata [61].
In the Indo-Pacific, trends in dispersal ability show even less association with reproductive mode. A survey of nine co-distributed species along 1200 km of the Great Barrier Reef showed no correlation between connectivity and reproductive mode [18]. Likewise, reproductive mode was not a good predictor of dispersal ability among several coral populations along the GBR and the peripheral populations on Lord Howe Island [19]. Paradoxically, species with highly different larval longevity estimates and life history characteristics have been observed to have similar levels of fine to meso-scale population subdivision [66].
Determining which life history traits impart greater success in long-distance dispersal is not straightforward. In Atlantic corals, reproductive mode appears to provide an advantage for long-distance dispersal, but this trend is not observed for corals in the Indo-Pacific. These results indicate that single aspects of coral life history, such as reproductive mode, propagule size or larval longevity, cannot alone predict dispersal ability, and that complex interactions of multiple factors likely play a role in determining connectivity. In other organisms, aspects of reproduction and life history, when taken alone, have at times also not been good predictors of dispersal ability and connectivity. In reef fish of the Atlantic, for example, pelagic larval duration is not a good predictor of population connectivity [56].
While dispersal ability is an important factor for maintaining connectivity, environmental factors also play an important role, as migrants must also be able to adapt to the environment at a distant reef in order to become established and contribute to gene flow. Interactions with other organisms, such as competition for space, can also affect successful recruitment. In sum, patterns in connectivity are difficult to predict based on reproductive traits alone and are likely influenced by multiple biotic and abiotic factors.
Patterns of genetic diversity
Mean values of molecular diversity indices overlap among regions for most coral species, although for some comparisons, diversity appears to be lower in the peripheral populations, such as gene diversity in S. siderea of West Africa for both loci and F. gravida in West Africa for Pax-C (Table 1). On average, however, there does not appear to be a strong trend in decreasing genetic diversity towards the edges of the species range for most species.
This finding is at odds with patterns observed in populations of the coral M. cavernosa, where genetic diversity was found to be lower in Brazil and West Africa compared to the Caribbean [20]. Similarly, reduced allozyme allelic diversity and heterozygosity was also found for several coral species on the isolated Lord Howe Island relative to populations on the Great Barrier Reef [19] as would be predicted by small population size and founder effects. Peripheral populations that are isolated from the center of species range or center of diversity may have smaller effective population size (Ne) and can suffer loss of diversity via genetic drift. In addition, peripheral populations colonized by a small group of founders may only contain a subset of the full diversity of alleles observed in the main population. However, loss of genetic diversity in Brazil and West Africa does not appear to be a consistent trend among the coral species studied here, or if differences in genetic diversity exist among regions, they have not been captured with the markers used here.
Overall, levels of polymorphism and the number of haplotypes for the brooding species F. fragum, F. gravida, P. astreoides and S. radians were much lower than for the broadcasters S. siderea and M. cavernosa. For example, >40 haplotypes were observed for M. cavernosa and S. radians, while the number of haplotypes observed for the four brooding species ranged from 6–12. High levels of polymorphism in M. cavernosa may have made the patterns of reduced variation in the peripheral populations more pronounced and readily detected. In S. siderea, decreasing trends in gene diversity (h) are observed, but there is overlap in the mean values of other diversity indices among the three biogeographic regions (Table 1).
Regional differences in genetic diversity may have gone undetected, but another reasonable alternative is that loss of variation has occurred in peripheral populations of M. cavernosa, but not in other species. Although regional isolation is observed for nearly all species, the mechanisms that lead to reduced variation may be less effective in some species relative to others. Loss of variation resulting from genetic drift is more pronounced in populations with small effective size (Ne). Differences in Ne between the species may result in different rates in the loss of genetic diversity. Founder effects may also be less pronounced if peripheral populations have received multiple founding events. Some of the brooding species studied have multiple mating cycles per year and reproductive strategies that may allow them to establish larger populations over shorter time periods, thereby increasing Ne and buffering the loss of genetic diversity. Another possible scenario is that historically some of the other species were able to maintain gene flow up until more recently, while gene flow for M. cavernosa ended a longer time in the past – subjecting the species to a longer period of time for drift to act upon.
Conclusions
Patterns of connectivity across broad regions of the Atlantic are congruent across multiple species of corals, suggesting that barriers to dispersal such as the Amazon freshwater plume and the long distances that separate the east and west South Atlantic are effective for most coral species. At shorter, regional scales, it appears that some aspects of reproduction such as mode of larval development can influence gene flow, although this pattern cannot be generalized to all scenarios. Broadcasters have greater dispersal ability in the Atlantic, but several exceptions exist in the Indo-Pacific, suggesting that complex interactions between biotic and abiotic factors can limit connectivity rather than single traits of a species. Loss of genetic diversity in peripheral populations of corals is also not a general trend observed among all amphi-Atlantic corals, likely due to differences in population size and timing of isolation across species. Regional isolation may mean that the persistence of peripheral populations relies primarily on local to regional recruitment since migration from other regions must occur rarely. These populations may be more vulnerable to disturbance on ecological time scales as a result of their isolation. On the other hand, isolated peripheral populations with small effective population size may become the sites for local adaptation and allopatric speciation. In both scenarios, these regions require special attention for conservation as a result of their potential vulnerability to environmental change, but also their importance in generating diversity.
Supporting Information
Coral species list for the Atlantic. Species occurrence in each biogeographic region is indicated by an X. Total species count for each region is found at the bottom of each column.
(PDF)
Haplotype count for each species, locus and population.
(PDF)
Two-locus genotypes for sampled individuals.
(PDF)
Genbank accession number for all sequenced haplotypes.
(PDF)
Acknowledgments
We would like to thank A. Solé-Cava for the use of his laboratory facilities at the Federal University of Rio de Janeiro for molecular work on Brazilian corals and N. Le Dantec, K.J. Nichols, B.M. Feitoza, and J.E.P. Freitas for assistance in sample collection. Permission to collect coral samples was approved by IBAMA and SEMACE in Brazil (permit IBAMA/MMA 02001.005656/2005-57 No. 114), by Autoridad Nacional del Ambiente in Panamá (CITES SEX/A-25-06) and Direcção das Pescas in São Tomé & Príncipe (CITES 139/DP/ME/05).
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the John Dove Isaacs Chair in Natural Philosophy to NK, by an anonymous private donation to the SIO Graduate Department to FN, and by a Graduate Fellowship from the Center of Marine Biodiversity and Conservation to FN. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fahrig L, Merriam G. Habitat patch connectivity and population survival. Ecology. 1985;66:1762–1768. [Google Scholar]
- 2.Gonzalez A, Lawton JH, Gilbert FS, Blackburn TM, Evans-Freke I. Metapopulation dynamics, abundance, and distribution in a microecosystem. Science. 1998;281:2045–2047. doi: 10.1126/science.281.5385.2045. [DOI] [PubMed] [Google Scholar]
- 3.Richards CM. Inbreeding depression and genetic rescue in a plant metapopulation. American Naturalist. 2000;155:383–394. doi: 10.1086/303324. [DOI] [PubMed] [Google Scholar]
- 4.Wright S. Evolution in Mendelian populations. Genetics. 1931;16:0097–0159. doi: 10.1093/genetics/16.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer Associates Inc; 2004. 545 [Google Scholar]
- 6.Gavrilets S. Perspective: Models of speciation: What have we learned in 40 years? Evolution. 2003;57:2197–2215. doi: 10.1111/j.0014-3820.2003.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 7.Kawecki TJ. Adaptation to marginal habitats. Annual Review of Ecology Evolution and Systematics. 2008;39:321–342. [Google Scholar]
- 8.Laborel J. Les peuplements de madréporaires des côtes tropicales du Brésil. Annales de l'Université d'Abidjan Serie E. 1969:260. [Google Scholar]
- 9.Laborel J. Madréporaires et hydrocoralliaires récifaux des côtes brésiliennes. Systematique, écologie, répartition verticale et géographique. Annales De L Institut Oceanographique de Paris. 1969;47:171–229. [Google Scholar]
- 10.Laborel J. West African reefs corals: An hypothesis on their origin. Proceedings of the 2nd International Coral Reef Symposium. 1974;1:425–443. [Google Scholar]
- 11.Kinlan BP, Gaines SD. Propagule dispersal in marine and terrestrial environments: A community perspective. Ecology. 2003;84:2007–2020. [Google Scholar]
- 12.Thiel M, Gutow L. The ecology of rafting in the marine environment. II. The rafting organisms and community. Oceanography and Marine Biology - an Annual Review. 2005;43:279–418. [Google Scholar]
- 13.Rocha LA, Bass AL, Robertson DR, Bowen BW. Adult habitat preferences, larval dispersal, and the comparative phylogeography of three Atlantic surgeonfishes (Teleostei : Acanthuridae). Molecular Ecology. 2002;11:243–252. doi: 10.1046/j.0962-1083.2001.01431.x. [DOI] [PubMed] [Google Scholar]
- 14.Palumbi SR. Genetic divergence, reproductive isolation, and marine speciation. Annual Review of Ecology and Systematics. 1994;25:547–572. [Google Scholar]
- 15.Morse DE, Hooker N, Morse ANC, Jensen RA. Control of larval metamorphosis and recruitment in sympatric Agariciid corals. Journal of Experimental Marine Biology and Ecology. 1988;116:193–217. [Google Scholar]
- 16.Pawlik JR. Chemical ecology of the settlement of benthic marine invertebrates. Oceanography and Marine Biology. 1992;30:273–335. [Google Scholar]
- 17.Heyward AJ, Negri AP. Natural inducers for coral larval metamorphosis. Coral Reefs. 1999;18:273–279. [Google Scholar]
- 18.Ayre DJ, Hughes TP. Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution. 2000;54:1590–1605. doi: 10.1111/j.0014-3820.2000.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 19.Ayre DJ, Hughes TP. Climate change, genotypic diversity and gene flow in reef-building corals. Ecology Letters. 2004;7:273–278. [Google Scholar]
- 20.Nunes F, Norris RD, Knowlton N. Implications of isolation and low genetic diversity in peripheral populations of an amphi-Atlantic coral. Molecular Ecology. 2009;18:4283–4297. doi: 10.1111/j.1365-294X.2009.04347.x. [DOI] [PubMed] [Google Scholar]
- 21.Concepcion GT, Kahng SE, Crepeau MW, Franklin EC, Coles SL, et al. Resolving natural ranges and marine invasions in a globally distributed octocoral (genus Carijoa). Marine Ecology Progress Series. 2010;401:113–127. [Google Scholar]
- 22.Fadlallah YH. Sexual reproduction, development and larval biology in scleractinian corals: a review. Coral Reefs. 1983;2:129–150. [Google Scholar]
- 23.Soong K. Sexual reproductive patterns of shallow-water reef corals in Panama. Bulletin of Marine Science. 1991;49:832–846. [Google Scholar]
- 24.Szmant AM. Reproductive ecology of Caribbean reef corals. Coral Reefs. 1986;5:43–53. [Google Scholar]
- 25.Baird AH, Guest JR, Willis BL. Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annual Review of Ecology Evolution and Systematics. 2009;40:551–571. [Google Scholar]
- 26.Graham EM, Baird AH, Connolly SR. Survival dynamics of scleractinian coral larvae and implications for dispersal. Coral Reefs. 2008;27:529–539. [Google Scholar]
- 27.Richmond RH. Energetics, competence, and long-distance dispersal of planula larvae of the coral Pocillopora damicornis. Marine Biology. 1987;93:527–533. [Google Scholar]
- 28.Atoda K. The larva and postlarval development of some reef-building corals. I. Pocillopora damicornis cespitosa (Dana). Science Reports of the Tohoku Imperial University, 4th Series (Biology) 1947;18:24–47. [Google Scholar]
- 29.Atoda K. The larva and postlarval development of some reef-building corals. II. Stylophora pistillata (Esper). Science Reports of the Tohoku Imperial University, 4th Series (Biology) 1947;18:48–64. [Google Scholar]
- 30.Atoda K. The larva and postlarval development of some reef-building corals. V. Seriatopora hystrix Dana. Reports of the Tohoku University, 4th Series (Biology) 1951;19:33–39. [Google Scholar]
- 31.Baird A. The length of the larval phase in corals: new insights into patterns of reef connectivity. Australian Coral Reef Society Newsletter. 1998;27:6–8. [Google Scholar]
- 32.Harrison PL, Wallace C. Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z, editor. Ecosystems of the World vol 25, Coral Reefs. Amsterdam: Elsevier; 1990. pp. 133–208. [Google Scholar]
- 33.Miller K, Mundy C. Rapid settlement in broadcast spawning corals: implications for larval dispersal. Coral Reefs. 2003;22:99–106. [Google Scholar]
- 34.Veron JEN, Stafford-Smith M. Corals of the World: Australian Institute of Marine Science 2000 [Google Scholar]
- 35.Nunes F, Fukami H, Vollmer SV, Norris RD, Knowlton N. Re-evaluation of the systematics of the endemic corals of Brazil by molecular data. Coral Reefs. 2008;27:423–432. [Google Scholar]
- 36.Neves E, Johnsson R, Sampaio C, Pichon M. The occurrence of Scolymia cubensis in Brazil: revising the problem of the Caribbean solitary mussids. Zootaxa. 2006:45–54. [Google Scholar]
- 37.Shearer TL, Van Oppen MJH, Romano SL, Worheide G. Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria). Molecular Ecology. 2002;11:2475–2487. doi: 10.1046/j.1365-294x.2002.01652.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen IP, Tang CY, Chiou CY, Hsu JH, Wei NV, et al. Comparative analyses of coding and noncoding DNA regions indicate that Acropora (Anthozoa: Scleractina) possesses a similar evolutionary tempo of nuclear vs. mitochondrial genomes as in plants. Marine Biotechnology. 2009;11:141–152. doi: 10.1007/s10126-008-9129-2. [DOI] [PubMed] [Google Scholar]
- 39.Van Oppen MJH, Willis BL, Van Vugt H, Miller DJ. Examination of species boundaries in the Acropora cervicornis group (Scleractinia, Cnidaria) using nuclear DNA sequence analyses. Molecular Ecology. 2000;9:1363–1373. doi: 10.1046/j.1365-294x.2000.01010.x. [DOI] [PubMed] [Google Scholar]
- 40.Fukami H, Budd AF, Paulay G, Solé-Cava A, Chen CLA, et al. Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. Nature. 2004;427:832–835. doi: 10.1038/nature02339. [DOI] [PubMed] [Google Scholar]
- 41.Stephens M, Scheet P. Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. American Journal of Human Genetics. 2005;76:449–462. doi: 10.1086/428594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Excoffier L, Laval G, Schneider S. Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online. 2005;1 [PMC free article] [PubMed] [Google Scholar]
- 44.Clement M, Posada D, Crandall KA. TCS: a computer program to estimate gene genealogies. Molecular Ecology. 2000;9:1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 45.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 46.Neves EG, Silveira FL, Pichon M, Johnsson R. Cnidaria, Scleractinia, Siderastreidae, Siderastrea siderea (Ellis and Solander, 1786): Hartt Expedition and the first record of a Caribbean siderastreid in tropical Southwestern Atlantic. Check List. 2010;6:505–510. [Google Scholar]
- 47.Muller-Karger FE, McClain CR, Richardson PL. The dispersal of the Amazon's Water. Nature. 1988;333:56–59. [Google Scholar]
- 48.Lentz SJ. Seasonal variations in the horizontal structure of the Amazon Plume inferred from historical hydrographic data. Journal of Geophysical Research-Oceans. 1995;100:2391–2400. [Google Scholar]
- 49.Nikiema O, Devenon JL, Baklouti M. Numerical modeling of the Amazon River plume. Continental Shelf Research. 2007;27:873–899. [Google Scholar]
- 50.Hoorn C, Guerrero J, Sarmiento GA, Lorente MA. Andean tectonics as a cause for changing drainage patterns in Miocene northern South America. Geology. 1995;23:237–240. [Google Scholar]
- 51.Vermeij MJA, Fogarty ND, Miller MW. Pelagic conditions affect larval behavior, survival, and settlement patterns in the Caribbean coral Montastraea faveolata. Marine Ecology Progress Series. 2006;310:119–128. [Google Scholar]
- 52.McCartney MA, Keller G, Lessios HA. Dispersal barriers in tropical oceans and speciation in Atlantic and eastern Pacific sea urchins of the genus Echinometra. Molecular Ecology. 2000;9:1391–1400. doi: 10.1046/j.1365-294x.2000.01022.x. [DOI] [PubMed] [Google Scholar]
- 53.Lessios HA, Kane J, Robertson DR. Phylogeography of the pantropical sea urchin Tripneustes: Contrasting patterns of population structure between oceans. Evolution. 2003;57:2026–2036. doi: 10.1111/j.0014-3820.2003.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 54.Lessios HA, Kessing BD, Pearse JS. Population structure and speciation in tropical seas: Global phylogeography of the sea urchin Diadema. Evolution. 2001;55:955–975. doi: 10.1554/0014-3820(2001)055[0955:psasit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 55.Lessios HA, Kessing BD, Robertson DR, Paulay G. Phylogeography of the pantropical sea urchin Eucidaris in relation to land barriers and ocean currents. Evolution. 1999;53:806–817. doi: 10.1111/j.1558-5646.1999.tb05374.x. [DOI] [PubMed] [Google Scholar]
- 56.Bowen BW, Bass AL, Muss A, Carlin JL, Robertson DR. Phylogeography of two Atlantic squirrelfishes (Family Holocentridae): exploring links between pelagic larval duration and population connectivity. Marine Biology. 2006;149:899–913. [Google Scholar]
- 57.Rocha LA. Mitochondrial DNA and color pattern variation in three western Atlantic Halichoeres (labridae), with the revalidation of two species. Copeia. 2004:770–782. [Google Scholar]
- 58.Muss A, Robertson DR, Stepien CA, Wirtz P, Bowen BW. Phylogeography of Ophioblennius: The role of ocean currents and geography in reef fish evolution. Evolution. 2001;55:561–572. doi: 10.1554/0014-3820(2001)055[0561:pootro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 59.Taylor MS, Hellberg ME. Genetic evidence for local retention of pelagic larvae in a Caribbean reef fish. Science. 2003;299:107–109. doi: 10.1126/science.1079365. [DOI] [PubMed] [Google Scholar]
- 60.Brazeau DA, Gleason DF, Morgan ME. Self-fertilization in brooding hermaphroditic Caribbean corals: Evidence from molecular markers. Journal of Experimental Marine Biology and Ecology. 1998;231:225–238. [Google Scholar]
- 61.Severance EG, Karl SA. Contrasting population genetic structures of sympatric, mass-spawning Caribbean corals. Marine Biology. 2006;150:57–68. [Google Scholar]
- 62.Vollmer SV, Palumbi SR. Restricted gene flow in the Caribbean staghorn coral Acropora cervicomis: Implications for the recovery of endangered reefs. Journal of Heredity. 2007;98:40–50. doi: 10.1093/jhered/esl057. [DOI] [PubMed] [Google Scholar]
- 63.Baums IB, Miller MW, Hellberg ME. Regionally isolated populations of an imperiled Caribbean coral, Acropora palmata. Molecular Ecology. 2005;14:1377–1390. doi: 10.1111/j.1365-294X.2005.02489.x. [DOI] [PubMed] [Google Scholar]
- 64.Brazeau DA, Sammarco PW, Gleason DF. A multi-locus genetic assignment technique to assess sources of Agaricia agaricites larvae on coral reefs. Marine Biology. 2005;147:1141–1148. [Google Scholar]
- 65.Fukami H, Budd AF, Levitan DR, Jara J, Kersanach R, et al. Geographic differences in species boundaries among members of the Montastraea annularis complex based on molecular and morphological markers. Evolution. 2004;58:324–337. [PubMed] [Google Scholar]
- 66.Miller KJ, Ayre DJ. Population structure is not a simple function of reproductive mode and larval type: insights from tropical corals. Journal of Animal Ecology. 2008;77:713–724. doi: 10.1111/j.1365-2656.2008.01387.x. [DOI] [PubMed] [Google Scholar]
- 67.Neves EG, Andrade SCS, da Silveira FL, Solferini VN. Genetic variation and population structuring in two brooding coral species (Siderastrea stellata and Siderastrea radians) from Brazil. Genetica. 2008;132:243–254. doi: 10.1007/s10709-007-9168-z. [DOI] [PubMed] [Google Scholar]
- 68.Calderon EN, Castro CB, Pires DO. Natação, assentamento e metamorfose de plânulas do coral Favia gravida Verrill 1868 (Cnidaria, Scleractinia) Boletim do Museu Nacional, Zoologia. 2000;429:1–12. [Google Scholar]
- 69.Neves EG, da Silveira FL. Release of planula larvae, settlement and development of Siderastrea stellata Verrill, 1868 (Anthozoa, Scleractinia). Hydrobiologia. 2003;501:139–147. [Google Scholar]
- 70.Edmunds PJ, Gates RD, Gleason DF. The biology of larvae from the reef coral Porites astreoides, and their response to temperature disturbances. Marine Biology. 2001;139:981–989. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coral species list for the Atlantic. Species occurrence in each biogeographic region is indicated by an X. Total species count for each region is found at the bottom of each column.
(PDF)
Haplotype count for each species, locus and population.
(PDF)
Two-locus genotypes for sampled individuals.
(PDF)
Genbank accession number for all sequenced haplotypes.
(PDF)