Abstract
Objectives
To determine if chronic nicotine exposure blunts angiogenesis.
Background
Cholinergic angiogenesis is mediated by an endothelial nicotinic acetylcholine receptor (EC nAChR). Short-term administration of nicotine stimulates angiogenesis via EC nAChRs. The long-term effects of nicotine upon cholinergic angiogenesis are unknown.
Methods
We exposed C57/Bl6 male mice (n=7 in each group) to nicotine (200μg/ml drinking water) or vehicle for 8 or 16 weeks. Subsequently, hindlimb ischemia was induced by ligation of the left femoral artery. After surgery, animals in the vehicle-treated group were re-randomized to vehicle (Vehicle group) or nicotine for 2 weeks (Acute exposure group); whereas animals that had been previously treated (for 8 or 16 weeks with nicotine) continued to receive nicotine (8WK or 16WK groups). After two weeks, animals were sacrificed for immunohistochemical, gene expression, and angiogenesis studies.
Results
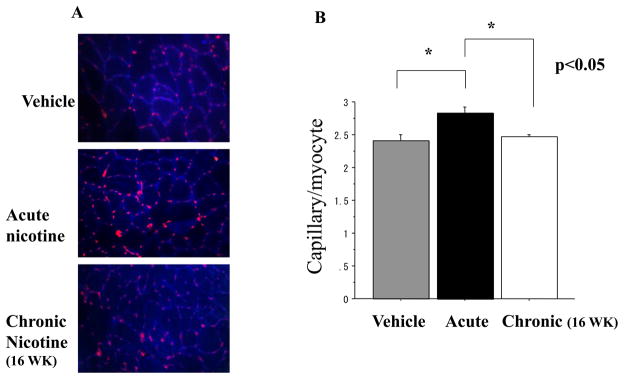
Capillary density of the ischemic hindlimb was increased by nicotine in naïve animals (Vehicle vs Acute exposure: 2.40±0.09 vs 2.82±0.10 capillaries/myocyte, p<0.05). However, prior exposure to nicotine for 16 weeks (16 WK) abolished the effects of nicotine to increase capillary density in the ischemic hindlimb (Acute vs 16 WK : 2.82±0.10 vs 2.47±0.03 capillaries/myocyte; p<0.05). The impairment of cholinergic angiogenesis was associated with a reduction in nAChR expression and plasma VEGF levels. Chronic exposure to nicotine impaired capillary sprouting of aortic segments ex vivo (vehicle vs 16WK :0.303±0.029 vs 0.204±0.017mm2, p<0.05 n=3)
Conclusion
The current study shows for the first time that chronic exposure to nicotine impairs cholinergic angiogenesis, an effect mediated by downregulation of the vascular nAChR, and attenuation of nicotine-induced VEGF release. These studies may explain the impairment in angiogenic processes observed in long-term smokers.
Keywords: Nicotinic acetylcholine receptor, endothelium, tobacco, vascular endothelial growth factor
Introduction
Nicotinic acetylcholine receptors (nAChRs) are expressed in non-neuronal cells, as well as in the central and peripheral nervous system (1–4). Endothelial cells express nAChRs, and also contain the machinery for synthesizing, transporting and metabolizing acetylcholine(5,6). We have observed that stimulation of endothelial nAChRs can induce angiogenesis(7–9).
This endogenous cholinergic pathway for angiogenesis can be hijacked by nicotine (7–9). The effect of nicotine to induce angiogenesis may contribute to several tobacco-related diseases, including atherosclerosis, malignancy and age-related macular degeneration(10). Plaque neovascularization, tumor angiogenesis, and retinal neovascularization are exacerbated by nicotine in animal models of these disorders(7). Antagonists of nAChRs, such as mecamylamine, can block tumor angiogenesis and retinal neovascularization(7,11).
Smoking may thus represent an angiogenic stimulus that participates in pathophysiological processes. We hypothesized that this chronic angiogenic stimulus may ultimately downregulate physiological angiogenesis, as in the normal response to ischemia. This hypothesis is suggested by the fact that chronic exposure to nicotine desensitizes neuronal nAChRs (12). In support of this hypothesis, smokers have reduced numbers and function of endothelial progenitor cells that contribute to angiogenesis(13–15). We therefore hypothesized that chronic exposure to nicotine downregulates nAChR and ultimately may impair angiogenesis. To test this hypothesis we used in vivo and ex vivo angiogenesis models.
Material and methods
Animals
Male C57/Bl6 mice were obtained from Jackson laboratory. Mice were exposed to vehicle or nicotine (200μg/ml) in the drinking water ad libitum for 8 or 16 weeks. Subsequently, animals underwent surgical induction of hindlimb ischemia as described below. After surgery, animals in the vehicle-treated group were re-randomized to vehicle (Vehicle group) or nicotine for 2 weeks (Acute exposure group); whereas animals that had been previously treated (for 8 or 16 weeks with nicotine) continued to receive nicotine (8WK or 16WK groups). In a separate study, animals received nicotine or vehicle in their drinking water for 52 weeks, and the aorta harvested for studies of capillary sprouting. All protocols were approved by the Administrative Panel on Laboratory Animal Care of Stanford University.
Mouse Model of Hindlimb Ischemia
Unilateral hindlimb ischemia was induced in male C57/Bl6 mice (each n=7). Briefly, under sterile conditions, the superficial and deep femoral arteries were ligated and excised as described previously(16). A sham procedure was performed on the contralateral leg. Animals were euthanized 2 weeks after surgery, and the adductor muscles harvested, embedded in OCT, and frozen using isopentane solution and liquid nitrogen.
Histomorphology and Immunohistochemistry
Capillary densities were determined in 10-μm cryostat sections of the adductor and semi-membranous muscles. Capillaries were identified by immunohistochemistry using anti- mouse CD31 antibody (BD Pharmingen) that was probed with a fluorescence-labeled secondary antibody. Myocytes were outlined by use of a laminin antibody (Chemicon) that was probed with a fluorescence-labeled secondary antibody. Capillary density is expressed as a ratio of capillaries to myocytes.
Western Blotting
Segments of soleus muscle from each group were homogenized in ice-cold RIPA buffer. Proteins (20μg) were separated on 10% Tris-glycine, and membranes were probed with polyclonal antibodies against α7-nAChR (Santa Cruz). Immunoreactive bands were visualized by use of an ECL detection kit (Amersham).
Aortic ring assay
To assess chronic exposure to nicotine we used an ex vivo aortic ring assay(17,18). Descending thoracic aortae were isolated from animals treated with vehicle or nicotine (each group n=3). Under a dissecting microscope, multiple 1-mm-thick aortic rings were prepared. Rings were then placed between 2 layers of growth factor–reduced Matrigel (BD) supplemented with Dulbecco’s Modified Eagle Medium (GIBCO), 5% fetal bovine serum, 10 U/mL heparin. Three rings from each animal were used. Four days after embedding the rings into Matrigel, the area of capillary sprouting was quantified(17,18).
RNA isolation and real-time quantitative PCR
To assess mRNA expression of α7-nAChR, quantitative RT-PCR was performed. The RNA from approximately 30 mg of soleus muscle or aorta was isolated by use of an RNeasy mini kits (Qiagen; in the case of soleus muscle, the fibrous tissue mini kit). Real-time RT-PCR reactions were carried out using the cDNA equivalent of 300 ng total RNA for each sample in a total volume of 25 μl of Taqman Universal PCR Master Mix (Applied Biosystems). The thermal cycling program for PCR was 50°C for 2 min, 95°C for 10 min, and 40 cycles of amplification comprising 95°C for 15 s, and 60°C for 1 min. PCR assay was performed using Assays on Demand Gene Expression Products in an ABI Prism 7300 sequence detector (Applied Biosystems). Primer and fluorogenic probe sets for α7-nAChR were designed using Primer Express V2.0 software(Applied Biosystems). The α7-nAChR forward and reverse primers and fluorescent-labeled probe were 5′-TGCTGCTTGTGGCTGAGATC -3′, 5′-CTGGCGAAGTACTGTGCTATCAA -3′, and 5′-TGCCAGCAACATCTGATTCCGTGC- -3′, respectively. The 5′ fluorogenic reporter probe was FAM, and 3′ fluorogenic quencher was TAMRA.
Statistical Analysis
Statistical analysis was facilitated by SPSS 12.0 software (SPSS Inc). All data are given as mean ± SEM. Comparisons between groups were analyzed appropriately by Student’s t test (2 sided), ANOVA, or Tukey’s post-hoc multiple comparisons test. Statistical significance was accepted at a value of P<0.05.
Results
Effect of chronic nicotine exposure on angiogenic response to ischemia
To determine the effect of chronic exposure to nicotine on angiogenesis, we treated animals with vehicle or nicotine (200ug/ml in the drinking water ad libitum) for 8 or 16 weeks prior to induction of hindlimb ischemia. In the vehicle treated animals, acute exposure to nicotine (for two weeks after induction of hindlimb ischemia) increased capillary density (Vehicle vs Acute: 2.40±0.09 vs 2.82±0.10, p<0.05 n=7 each). The angiogenic effect of nicotine was not affected by prior exposure to nicotine for 8 weeks (data not shown). However, prior exposure to nicotine for 16 weeks abolished the effect of nicotine to enhance angiogenesis after induction of hindlimb ischemia (Acute vs 16 WK : 2.82±0.10 vs 2.47±0.03, p<0.05; n=7 each group (Figure 1A and 1B). We did not perform measures of perfusion in this study. However, we and others have previously found that changes in capillary density correlate with changes in perfusion
Figure1.
(a) Microphotographs of cross-sections through the ischemic hindlimb of mice treated with nicotine after prior exposure for 16 weeks to vehicle or nicotine. In the animals receiving vehicle for 16 weeks, the acute treatment with nicotine (Acute nicotine) increases capillary density. The effect of nicotine to increase capillary density in the ischemic hindlimb is abolished in animals with prior long-term exposure to nicotine (Chronic nicotine). Anti-CD31 antibody (red) outlines endothelial cells and laminin (blue) outlines myocytes, for calculation of capillary myocyte ratio.
(b) Histogram showing capillary myocyte ratio in murine hindlimbs two weeks after surgical induction of ischemia. The capillary myocyte ratio is augmented by short-term exposure to nicotine (Acute). The cholinergic augmentation of angiogenesis is abolished by prior long-term exposure (Chronic).
α7-nAChR downregulation in acute ischemia
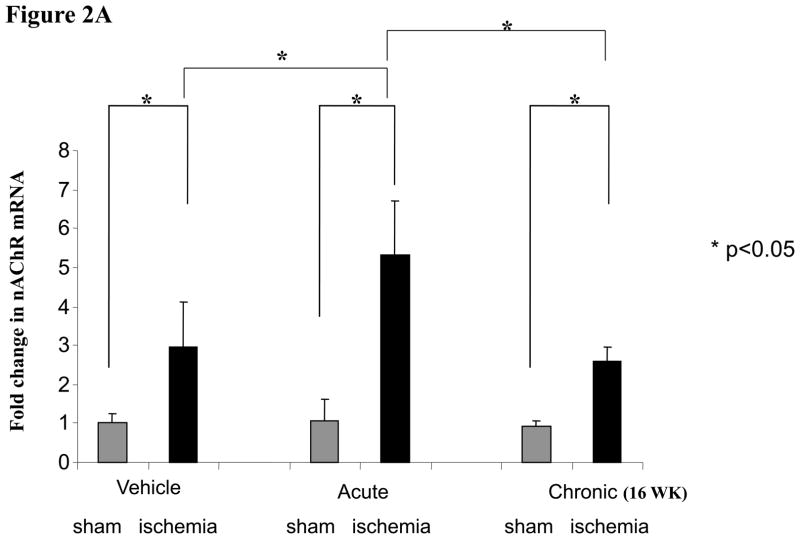
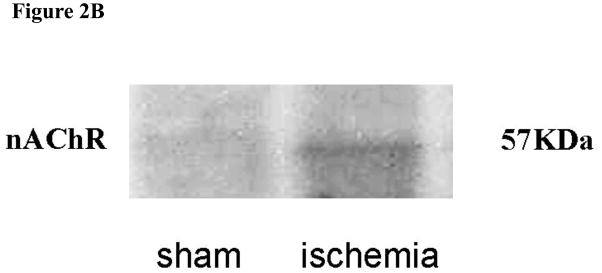
We have previously observed that endothelial α7-nAChR are involved in angiogenic response(7). To address whether chronic exposure to nicotine downregulated α7-nAChR, we assessed mRNA expression using Taqman RT-PCR at 2 weeks after surgery. There was a significant increase in α7-nAChR gene expression in the ischemic limb of animals exposed acutely to nicotine (5.3 fold vs. sham operation, p<0.05) compared to vehicle (2.9 fold vs. sham operation, p<0.05). By contrast, in animals exposed to nicotine for 16 weeks prior to surgery, there was a blunting of α7nAChR gene expression compared to those animals acutely treated with nicotine (2.6 fold vs. 5.3 fold, p<0.05). (Figure2a). To determine if the changes in gene expression were mirrored by alterations in protein, we performed Western analyses. As we have previously observed, ischemia significantly upregulated nAChR protein expression (Figure 2b). However, we were not able to reproducibly document a downregulation of α7-nAChR by chronic nicotine exposure, possibly because of cross-reactivity of our antibody with other nAChRs.
Figure 2.
Figure 2a
Histograms showing relative α7-nAChR expression by Taqman RT-PCR. Ischemia increases the expression of α7-nAChR in the murine hindlimb. The effect of ischemia is augmented by short-term exposure to nicotine (Acute). However long-term exposure to nicotine abolishes the nicotine-induced upregulation of α7-nAChR.
Figure 2b
Western blot showing α7-nAChR expression. Ischemia upregulated α7-nAChR in the murine hindlimb.
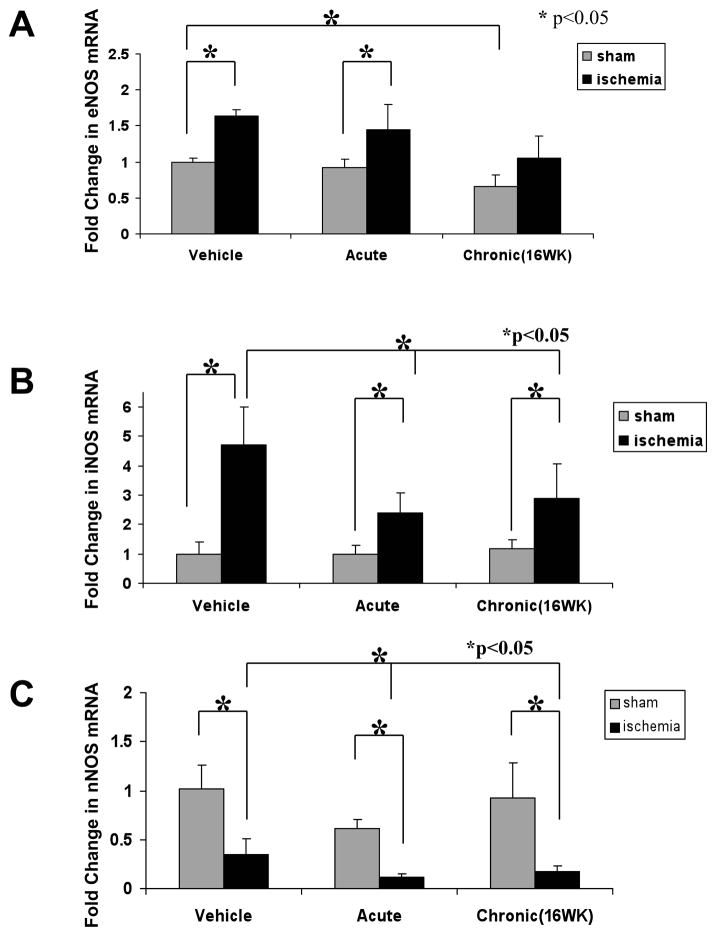
Alterations in nitric oxide synthase (NOS) expression by ischemia and nicotine
In the vehicle treated animals, the expression of eNOS and iNOS were significantly increased by ischemia (by comparison to the sham-operated extremity). By contrast, ischemia induced a significant downregulation of neuronal NOS.
The effect of ischemia to increase eNOS expression was unaffected by acute exposure to nicotine, but was abolished by chronic exposure. The effect of ischemia to increase iNOS expression was reduced by acute or chronic exposure to nicotine. Furthermore, the effect of ischemia to reduce nNOS expression was aggravated by acute or chronic expression to nicotine (Figure 6).
Figure 6.
Histogram showing fold change in skeletal muscle mRNA levels for A. eNOS, B. iNOS, and C. nNOS, in the sham-operated (Sham) or ischemic hindlimb (ischemia) of vehicle-treated animals (Vehicle), or animals exposed to 2 weeks (Acute) or 16 weeks (Chronic) administration of nicotine. Ischemia increased eNOS and iNOS expression, but reduced nNOS expression. Chronic exposure to nicotine abolished the ischemia-induced upregulation of eNOS. Acute and chronic exposure to nicotine suppressed the ischemia-induced upregulation of iNOS, and accentuated the ischemia-induced downregulation of nNOS.
Chronic exposure to nicotine abolished plasma VEGF increase
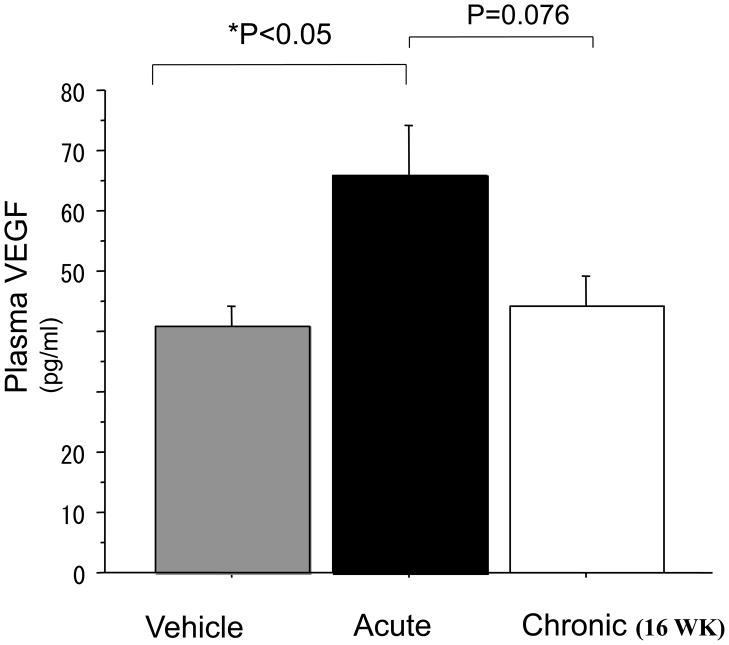
Five days after induction of hindlimb ischemia, plasma VEGF levels were increased to a greater degree in those animals acutely exposed to nicotine (vehicle vs nicotine: 40.8±3.2 vs 65.7±8.6 pg/ml p<0.05 n=4). However, in animals chronically exposed to nicotine for 16 weeks, the augmentation of VEGF levels by nicotine in ischemic animals was abolished (acute vs chronic exposure to nicotine: 65.7±8.6 vs 44.1±5.0 pg/ml, p=0.07 n=4) (Figure3)
Figure 3.
Plasma VEGF levels 5 days after surgical induction of hindlimb ischemia. Short-term exposure to nicotine (Acute) increased plasma VEGF levels (Vehicle vs Acute: 40.8±3.2 vs 65.7±8.6 pg/ml p<0.05 n=4 each ) The augmentation by nicotine of VEGF was abolished by prior long-term nicotine exposure. (Acute vs Chronic: 65.7±8.6 vs 44.1±5.0 pg/ml, p=0.07 n=4 each)
Chronic exposure to nicotine decreases capillary sprouting
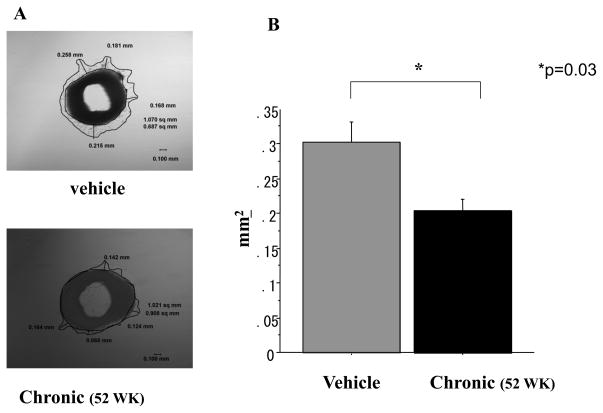
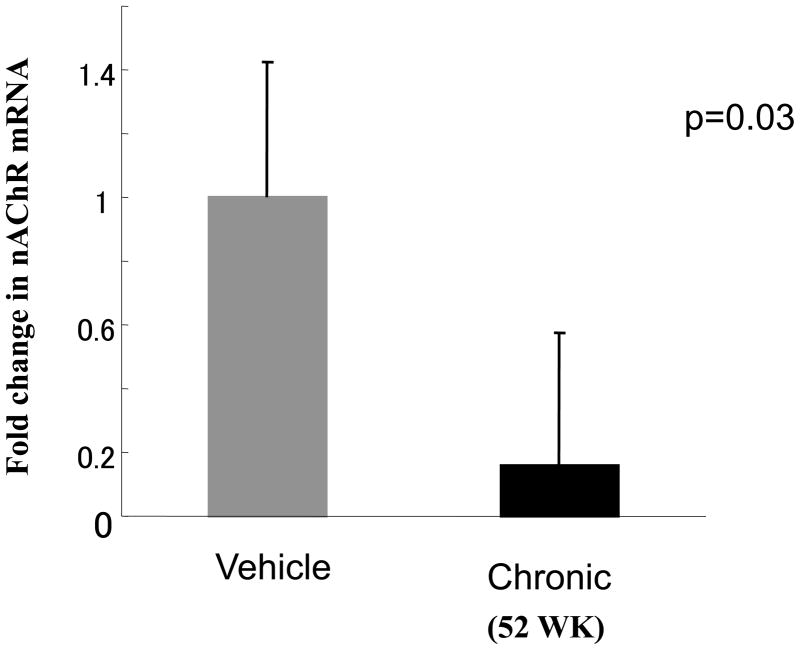
To confirm the effect of chronic nicotine exposure on endothelial processes involved in angiogenesis, we assessed the sprouting of capillaries from aortic segments placed in matrigel (Figure 4A). Vessel sprouting from vascular rings placed in Matrigel was impaired in those aortae obtained from animals chronically exposed to nicotine (for 52 weeks). The area encompassed by vascular sprouts from animals exposed for 52 weeks to vehicle vs nicotine was reduced by 33% from 0.303±0.029 vs 0.204±0.017mm2, p<0.05 n=3; Figure4B). In adjacent vascular segments we assessed expression of α7-nAChR using quantitative RT-PCR. We found chronic exposure to nicotine (52 weeks) markedly downregulated aortic α7-nAChR expression, to only 16% of levels observed in vehicle treated animals (Figure5)
Figure 4.
A. Aortic ring after 4d in Matrigel. Capillary sprouting is observed, and the area of sprouting outlined.
B. Histogram showing average area of capillary sprouting in vitro. Prior chronic exposure (52 weeks) to nicotine decreased sprouting area compared to vehicle. (Vehicle vs Chronic niotine exposure: 0.303±0.029 vs 0.200±0.017 mm2:p<0.05 n=3 each)
Figure 5.
Histogram showing aortic α7-nAChR expression by Taq-man RT-PCR. Long-term exposure to nicotine markedly downregulated receptor expression (0.23±0.11 fold vs vehicle p<0.05 n=3 each)
Discussion
The salient observation of this study is that chronic exposure to nicotine abolished cholinergic augmentation of the angiogenic response to ischemia. We have previously shown that short-term exposure to nicotine enhances angiogenesis. In the current study, prior and prolonged exposure to nicotine (for 16 weeks, about 1/8 the lifespan of a mouse) abolished the acute angiogenic effect of nicotine in the setting of ischemia. The current study may reconcile our previous observations (regarding the acute angiogenic effect of nicotine), with observations that chronic smokers have an impairment of angiogenic processes (13,14).
Angiogenic effect of nicotine: role of the nAChR
We and others have previously shown that at clinically relevant doses, acute exposure to nicotine increases endothelial proliferation, migration, and capillary tube formation in vitro(7,9). In vivo, short term exposure to nicotine in animal models augments tumor vascularity, plaque neovascularization and retinal angiogenesis(10).
Other investigators have found that nAChR activation can enhance capillary formation in experimental myocardial infarction (11). The angiogenic effect of nicotine is mediated by endothelial nAChRs, the most prevalent being the α7-nAChR (7). The α7-nAChR selective partial agonist 3-(2,4-dimethoxybenzylidene) anabaseine (DXMB) mimics the effect of nicotine on endothelial tube formation, whereas antagonists of the α7-nAChR (such as α-bungarotoxin) inhibit endothelial tube formation in Matrigel. Other nAChR isoforms are present, and contribute to the angiogenic effect of nicotine, as nAChR-mediated angiogenesis is only partly inhibited in the α7-nAChR deficient mouse (9). Nevertheless, the endothelial α7-nAChR plays an important role in angiogenesis and is upregulated in hypoxic endothelial cells, and in ischemic tissue (9). Accordingly, we examined the effect of chronic nicotine exposure on expression of this key endothelial nACh receptor.
Downregulation of the nAChR
In the current study, we observed that after induction of hindlimb ischemia, the administration of nicotine to naïve animals augmented the angiogenic response to ischemia, as documented by a greater increase in capillary density. A short period (8weeks) of prior exposure to nicotine did not impair the cholinergic enhancement of angiogenesis. However, when animals were exposed to nicotine for 16 weeks prior to induction of hindlimb ischemia, the effect of nicotine to enhance angiogenesis was abolished. This effect was associated with a downregulation of the message for α7-nAChR. We were not able to reproducibly document the downregulation of the α7-nAChR using Western analysis, possibly because of cross-reactivity of our antibody with other nAChRs. In a separate series of experiments, we showed that animals exposed to nicotine for 52 weeks had impaired endothelial sprouting from vascular segments, an effect which was associated with a profound (>80%) downregulation of the α7-nAChR.
There is a substantial literature examining the effect of chronic nicotine exposure on receptor activity and expression in neuronal cells (19,20) With respect to the neuronal α7-nAChR, chronic nicotine exposure upregulates expression, but also induces a receptor desensitization. By contrast, little is known regarding the effect of nicotine exposure on the vascular nAChRs. In isolated rat aortic smooth muscle cells, exposure to nicotine for 48 hrs upregulates the expression of the α7-nAChR (21). In these cells the effect of nicotine to augment insulin-induced mitogenesis, is mediated by phosphorylation of p44/42 MAP kinase; is mimicked by the α7-nAChR agonist DXMB, and is blocked by the α7-nAChR antagonist methyllycaconitine. Our paper is the first to indicate that chronic exposure to nicotine downregulates message for the α7-nAChR.
Nicotine and VEGF
We observed that the effect of ischemia to elevate plasma VEGF levels was augmented by acute exposure to nicotine. This confirms previous reports by our group and others that the acute exposure to nicotine induces the release of VEGF from endothelial cells (9,22), and that nicotine administration augments VEGF levels in experimental models of ischemic or pathological angiogenesis(7,9). However, we now find that in animals exposed to nicotine for 16 weeks, the effect of nicotine to increase plasma VEGF levels is abolished.
This intriguing interaction between nAChR activation and VEGF release is consistent with our previous observations of interdependence between the pathways. In addition to stimulating the release of VEGF from endothelial cells in vitro, nicotine induces transactivation of the VEGF receptor, VEGFR-2 (9). This may explain the partial inhibition by VEGF-neutralizing antibodies of nicotine-induced endothelial capillary network formation in Matrigel (9). Conversely, antagonists of the nAChR inhibit VEGF-induced angiogenic processes such as endothelial cell migration and tube formation (9,23). As further evidence of interdependence between the pathways, there is a striking concordance in the transcriptional profile of nicotine or VEGF stimulated endothelial cells(23).
Tobacco and angiogenesis
Michaud and colleagues report that chronic exposure to cigarette smoke impairs the angiogenic response in a murine hindlimb ischemia model, associated with a reduction in HIF-1α transactivation of VEGF expression (24). However, their study differs from ours in that the stimulus is quite different (tobacco smoke) and that the period of prior exposure was only 2 weeks. We did not observe any impairment of angiogenesis at 8 weeks of chronic nicotine exposure. After 16 weeks of nicotine exposure, we observed that the nicotine-induced enhancement of the angiogenic response was abolished. Notably, capillary density of the ischemic limbs in chronically treated animals was not different from that of ischemic limbs in vehicle-treated animals (ie. the impairment of angiogenic response was restricted to abrogation of the nAChR-mediated component). The differences between our study and that of Michaud and colleagues indicate that the tobacco-mediated impairment of angiogenesis is not entirely mediated by nicotine. Indeed, Michaud and colleagues found that cigarette smoke extract acutely impaired endothelial cell migration and tube formation (24). By contrast, short-term exposure to nicotine increases endothelial cell proliferation, tube formation and migration(7,23,25). Indeed, it is known that carbon monoxide, another component of cigarette smoke, impairs angiogenesis (26). By increasing oxidative stress, smoking also reduces the bioavailability of NO (27–29), a critical modulator of angiogenesis(30). Finally, smokers are known to have an impairment in the number and function of circulating endothelial progenitor cells, which can be reversed by cessation of smoking (13,14). Notably, smokers who ceased smoking but continued to use a nicotine patch experienced an increase in the number of endothelial progenitor cells which was at least as great as those who ceased smoking without the use of supplemental nicotine(14). These studies highlight that the stimulus of nicotine is quite different from that of tobacco smoke, which includes 4000 additional compounds.
NO and angiogenesis
We and others have observed a prominent role of nitric oxide synthase (NOS) in ischemia. Endothelial cell survival, proliferation, migration and tube formation are enhanced by NO in vitro. In vivo, inhibition of NO synthase, as by asymmetric dimethylarginine (ADMA), suppresses angiogenesis (30). Transgenic animals with genetically reduced levels of ADMA synthesize increased amounts of NO, and manifest a more robust angiogenic response to ischemia (31). In the current study, we found that ischemia, as well as nicotine, modulated the expression of NOS. In the vehicle treated animals, the expression of eNOS and iNOS were significantly increased by ischemia (by comparison to the sham-operated extremity). The effect of ischemia to increase eNOS and iNOS expression was abolished or significantly attenuated by chronic exposure to nicotine. The effect of ischemia to increase eNOS (as well as iNOS) expression may reflect a counter-regulatory mechanism to enhance angiogenesis. That this counter-regulatory phenomenon is abrogated by chronic exposure to nicotine is consistent with our observation that chronic exposure to nicotine has an adverse impact on angiogenesis.
Limitations
Although we documented that the effect of nicotine to increase capillary density and serum VEGF levels were abolished by chronic nicotine exposure, we did not assess functional measures of limb perfusion. Nevertheless, we and others have previously found that changes in capillary density correlate well with changes in perfusion (31). We also did not examine the contribution of endothelial progenitor cells (EPCs) to our observations. Previously, we have shown that acute exposure to nicotine recruits EPCs to the ischemic limb (8). Certainly, chronic exposure to nicotine may have the opposite effect. That this might be the case is suggested by human studies where smokers have been found to have a reduction in circulating numbers and migratory activity of EPC (32).
Conclusion
To our knowledge, this is the first report that chronic nicotine exposure impairs the angiogenic response to ischemia. Chronic exposure to nicotine profoundly reduced capillary sprouting from aortic segments ex vivo. Furthermore, in the hindlimb ischemia model, the acute effect of nicotine to increase capillary density was completely abrogated by chronic exposure to nicotine. This effect may be mediated in part by downregulation of the vascular α7-nAChR, an inhibition of NO synthase expression, as well as by a reduction in plasma VEGF levels.
References
- 1.Grando SA, Horton RM, Pereira EF, et al. A nicotinic acetylcholine receptor regulating cell adhesion and motility is expressed in human keratinocytes. J Invest Dermatol. 1995;105:774–81. doi: 10.1111/1523-1747.ep12325606. [DOI] [PubMed] [Google Scholar]
- 2.Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 1998;287:435–9. [PubMed] [Google Scholar]
- 3.Wessler I, Kirkpatrick CJ, Racke K. The cholinergic ‘pitfall’: acetylcholine, a universal cell molecule in biological systems, including humans. Clin Exp Pharmacol Physiol. 1999;26:198–205. doi: 10.1046/j.1440-1681.1999.03016.x. [DOI] [PubMed] [Google Scholar]
- 4.Conti-Fine BM, Navaneetham D, Lei S, Maus AD. Neuronal nicotinic receptors in non-neuronal cells: new mediators of tobacco toxicity? Eur J Pharmacol. 2000;393:279–94. doi: 10.1016/s0014-2999(00)00036-4. [DOI] [PubMed] [Google Scholar]
- 5.Parnavelas JG, Kelly W, Burnstock G. Ultrastructural localization of choline acetyltransferase in vascular endothelial cells in rat brain. Nature. 1985;316:724–5. doi: 10.1038/316724a0. [DOI] [PubMed] [Google Scholar]
- 6.Kirkpatrick CJ, Bittinger F, Unger RE, Kriegsmann J, Kilbinger H, Wessler I. The non-neuronal cholinergic system in the endothelium: evidence and possible pathobiological significance. Jpn J Pharmacol. 2001;85:24–8. doi: 10.1254/jjp.85.24. [DOI] [PubMed] [Google Scholar]
- 7.Heeschen C, Jang JJ, Weis M, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7:833–9. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 8.Heeschen C, Weis M, Cooke JP. Nicotine promotes arteriogenesis. J Am Coll Cardiol. 2003;41:489–96. doi: 10.1016/s0735-1097(02)02818-8. [DOI] [PubMed] [Google Scholar]
- 9.Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Invest. 2002;110:527–36. doi: 10.1172/JCI14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooke JP, Bitterman H. Nicotine and angiogenesis: a new paradigm for tobacco-related diseases. Ann Med. 2004;36:33–40. doi: 10.1080/07853890310017576. [DOI] [PubMed] [Google Scholar]
- 11.Li XW, Wang H. Non-neuronal nicotinic alpha 7 receptor, a new endothelial target for revascularization. Life Sci. 2006;78:1863–70. doi: 10.1016/j.lfs.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Olale F, Gerzanich V, Kuryatov A, Wang F, Lindstrom J. Chronic nicotine exposure differentially affects the function of human alpha3, alpha4, and alpha7 neuronal nicotinic receptor subtypes. J Pharmacol Exp Ther. 1997;283:675–83. [PubMed] [Google Scholar]
- 13.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 14.Kondo T, Hayashi M, Takeshita K, et al. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–7. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 15.Zhu BQ, Heeschen C, Sievers RE, et al. Second hand smoke stimulates tumor angiogenesis and growth. Cancer Cell. 2003;4:191–6. doi: 10.1016/s1535-6108(03)00219-8. [DOI] [PubMed] [Google Scholar]
- 16.Jacobi J, Jang JJ, Sundram U, Dayoub H, Fajardo LF, Cooke JP. Nicotine accelerates angiogenesis and wound healing in genetically diabetic mice. Am J Pathol. 2002;161:97–104. doi: 10.1016/S0002-9440(10)64161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds LE, Wyder L, Lively JC, et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 18.Shimada T, Takeshita Y, Murohara T, et al. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation. 2004;110:1148–55. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]
- 19.Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr Drug Targets CNS Neurol Disord. 2002;1:359–85. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen HN, Rasmussen BA, Perry DC. Binding and functional activity of nicotinic cholinergic receptors in selected rat brain regions are increased following long-term but not short-term nicotine treatment. J Neurochem. 2004;90:40–9. doi: 10.1111/j.1471-4159.2004.02482.x. [DOI] [PubMed] [Google Scholar]
- 21.Wada T, Naito M, Kenmochi H, Tsuneki H, Sasaoka T. Chronic nicotine exposure enhances insulin-induced mitogenic signaling via up-regulation of alpha7 nicotinic receptors in isolated rat aortic smooth muscle cells. Endocrinology. 2007;148:790–9. doi: 10.1210/en.2006-0907. [DOI] [PubMed] [Google Scholar]
- 22.Conklin BS, Zhao W, Zhong DS, Chen C. Nicotine and cotinine up-regulate vascular endothelial growth factor expression in endothelial cells. Am J Pathol. 2002;160:413–8. doi: 10.1016/S0002-9440(10)64859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng MK, Wu J, Chang E, et al. A central role for nicotinic cholinergic regulation of growth factor-induced endothelial cell migration. Arterioscler Thromb Vasc Biol. 2007;27:106–12. doi: 10.1161/01.ATV.0000251517.98396.4a. [DOI] [PubMed] [Google Scholar]
- 24.Michaud SE, Menard C, Guy LG, Gennaro G, Rivard A. Inhibition of hypoxia-induced angiogenesis by cigarette smoke exposure: impairment of the HIF-1alpha/VEGF pathway. Faseb J. 2003;17:1150–2. doi: 10.1096/fj.02-0172fje. [DOI] [PubMed] [Google Scholar]
- 25.Villablanca AC. Nicotine stimulates DNA synthesis and proliferation in vascular endothelial cells in vitro. J Appl Physiol. 1998;84:2089–98. doi: 10.1152/jappl.1998.84.6.2089. [DOI] [PubMed] [Google Scholar]
- 26.Huang LE, Willmore WG, Gu J, Goldberg MA, Bunn HF. Inhibition of hypoxia-inducible factor 1 activation by carbon monoxide and nitric oxide. Implications for oxygen sensing and signaling. J Biol Chem. 1999;274:9038–44. doi: 10.1074/jbc.274.13.9038. [DOI] [PubMed] [Google Scholar]
- 27.Raij L, DeMaster EG, Jaimes EA. Cigarette smoke-induced endothelium dysfunction: role of superoxide anion. J Hypertens. 2001;19:891–7. doi: 10.1097/00004872-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation. 2002;105:1155–7. doi: 10.1161/hc1002.105935. [DOI] [PubMed] [Google Scholar]
- 29.Heitzer T, Yla-Herttuala S, Luoma J, et al. Cigarette smoking potentiates endothelial dysfunction of forearm resistance vessels in patients with hypercholesterolemia. Role of oxidized LDL. Circulation. 1996;93:1346–53. doi: 10.1161/01.cir.93.7.1346. [DOI] [PubMed] [Google Scholar]
- 30.Jang JJ, Ho HK, Kwan HH, Fajardo LF, Cooke JP. Angiogenesis is impaired by hypercholesterolemia: role of asymmetric dimethylarginine. Circulation. 2000;102:1414–9. doi: 10.1161/01.cir.102.12.1414. [DOI] [PubMed] [Google Scholar]
- 31.Dayoub H, Achan V, Adimoolam S, Jacobi J, Stuehlinger M, Wang B, Tsao PS, Kimoto M, Vallance P, Patterson AJ, Cooke JP. DDAH Regulates NO Synthesis: Genetic and physiological evidence. Circulation. 2003;108:1043–1048. doi: 10.1161/01.CIR.0000101924.04515.2E. [DOI] [PubMed] [Google Scholar]
- 32.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001 Jul 6;89(1):E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]