Abstract
Frizzled receptors are components of the Wnt signalling pathway, but how they activate the canonical Wnt/β-catenin pathway is not clear. Here we use three distinct vertebrate frizzled receptors (Xfz3, Xfz4 and Xfz7) and describe whether and how their C-terminal cytoplasmic regions transduce the Wnt/β-catenin signal. We show that Xfz3 activates this pathway in the absence of exogenous ligands, while Xfz4 and Xfz7 interact with Xwnt5A to activate this pathway. Analysis using chimeric receptors reveals that their C-terminal cytoplasmic regions are functionally equivalent in Wnt/β-catenin signalling. Furthermore, a conserved motif (Lys-Thr-X-X-X-Trp) located two amino acids after the seventh transmembrane domain is required for activation of the Wnt/β-catenin pathway and for membrane relocalization and phosphorylation of Dishevelled. Frizzled receptors with point mutations affecting either of the three conserved residues are defective in Wnt/β-catenin signalling. These findings provide functional evidence supporting a role of this conserved motif in the modulation of Wnt signalling. They are consistent with the genetic features exhibited by Drosophila Dfz3 and Caenorhabditis elegans mom-5 in which the tryptophan is substituted by a tyrosine.
Keywords: Dishevelled/frizzled/signal transduction/Wnt/Xenopus
Introduction
Wnts are a family of secreted proteins involved in a wide range of developmental processes such as segmentation in Drosophila, control of asymmetric divisions in Caenorhabditis elegans, axis formation and patterning of the central nervous system in vertebrates (for reviews see Moon, 1993; Cadigan and Nusse, 1997; Wodarz and Nusse, 1998). The canonical Wnt/β-catenin signalling pathway that involves Dishevelled (Dsh), GSK3β/Shaggy and β-catenin/armadillo, is conserved from Drosophila to vertebrates. Genetic epistasis experiments suggest that Dsh lies upstream of and represses the activity of GSK3β. As a consequence, β-catenin is stabilized in the cytoplasm and forms a complex with members of the TCF/LEF family of DNA-binding molecules to activate transcription of target genes. The signal transduction pathway involving β-catenin stabilization represents a common mechanism underlying early development and carcinogenesis. In Xenopus, Wnt/β-catenin signalling plays a crucial role in dorso-ventral axis specification. Overexpression of certain Wnts, Dsh and β-catenin leads to axis duplication (for reviews see Moon and Kimelman, 1998; Wodarz and Nusse, 1998; Sokol, 1999). Conversely, overexpression of GSK3β and axin or depletion of oocytes of β-catenin mRNA inhibit the formation of dorsal axial structures (Heasman et al., 1994; He et al., 1995; Yost et al., 1996; Zeng et al., 1997). In human, inappropriate activation of the Wnt/β-catenin signalling pathway leads to carcinogenesis (reviewed by Gumbiner, 1997). Thus, mutations of adenomatous polyposis coli (APC) or β-catenin that stabilize β-catenin were found in the melanoma and colorectal cancers (Korinek et al., 1997; Morin et al., 1997; Rubinfeld et al., 1997).
Functional analysis in Xenopus embryos has led to the proposal that Wnts can be grouped into two distinct classes, which might stimulate distinct signalling pathways (reviewed by Moon et al., 1997; Miller et al., 1999). Axis-inducing Xenopus Wnts (Xwnts) fall into the first group, which includes Xwnt1, Xwnt3A, Xwnt8 and Xwnt8b; this group of Xwnts activates the Wnt/β-catenin signalling pathway and transcription of target genes Siamois and Xnr3 (Carnac et al., 1996; Brannon et al., 1997; McKendry et al., 1997; Fan et al., 1998). The second class of Xwnts represented by Xwnt4, Xwnt5A and Xwnt11 does not have axis-inducing activity and may be involved in the control of morphogenetic movements (Moon et al., 1993; Du et al., 1995; Ungar et al., 1995; Tada and Smith, 2000). Similarly, in the mesoderm of chick limb bud, Wnt3A and Wnt7A elicit, respectively, β-catenin-dependent and -independent responses (Kengaku et al., 1998).
Wnt proteins have been shown to bind to members of the frizzled family of serpentine receptors (Bhanot et al., 1996; Yang-Snyder et al., 1996). To date, there are at least nine members identified in mammals (Wang et al., 1996; reviewed by Wodarz and Nusse, 1998). All frizzled proteins have an extracellular cysteine-rich domain (CRD) followed by seven putative transmembrane (TM) segments; their C-terminal cytoplasmic regions differ significantly in length and in sequence similarity. In a similar way to Wnt proteins, vertebrate frizzled homologues have been shown to activate distinct signalling pathways. Some frizzled receptors induce the expression of Siamois when overexpressed in Xenopus ectodermal cells while others trigger intracellular calcium release in a G-protein-dependent manner (Yang-Snyder et al., 1996; Slusarski et al., 1997; Sheldahl et al., 1999). Nevertheless, the lack of activity of a frizzled receptor in Wnt/β-catenin signalling in a particular context may be due to the absence of an appropriate ligand (He et al., 1997; Medina et al., 2000; Sumanas et al., 2000). Similarly, genetic evidence suggests that the prototypic Drosophila frizzled (Dfz1) is required for the establishment of tissue polarity (Adler, 1992; Krasnow and Adler, 1994; reviewed by Mlodzik, 1999) through the c-Jun N-terminal kinase (JNK) pathway via Dsh and RhoA (Strutt et al., 1997; Axelrod et al., 1998; Boutros et al., 1998; reviewed by Boutros and Mlodzik, 1999). However, interference of the Dfz1 gene reveals that it is also required in neurogenesis, where it acts downstream of Wingless and upstream of GSK3β (Bhat, 1998; Kennerdell and Carthew, 1998; Bhanot et al., 1999). These studies raise the question of whether distinct frizzled receptors are functionally equivalent in Wnt/β-catenin signalling. Furthermore, given that the C-terminal cytoplasmic regions of various frizzled receptors do not share any significant similarity, it is important to determine the structural basis underlying the activation of the Wnt/β-catenin pathway.
Dsh acts downstream of frizzled receptors both in planar polarity signalling and in segmental polarity of the epidermis in Drosophila, which are β-catenin independent and dependent, respectively (reviewed by Wodarz and Nusse, 1998; Boutros and Mlodzik, 1999). Several lines of evidence suggest that Dsh activates distinct signalling pathways through distinct domains. For example, the C-terminal DEP domain is required for membrane translocation and activation of the JNK pathway, but is dispensable for the Wnt/β-catenin pathway (Axelrod et al., 1998; Boutros et al., 1998; Moriguchi et al., 1999; Rothbächer et al., 2000; reviewed by Boutros and Mlodzik, 1999). Dsh is phosphorylated both in vivo and in vitro in response to Wnt and frizzled receptors (Yanagawa et al., 1995; Willert et al., 1997; Rothbächer et al., 2000; Tada and Smith, 2000). The phosphorylation of Dsh requires a functional DEP domain and is closely associated with membrane relocalization, indicating that these properties are important for some aspects of Dsh function in signal transduction. Thus, analysis of how frizzled receptors mediate intracellular signalling and interact with Dsh should help to elucidate molecular mechanisms of different aspects of Wnt signalling.
In the present study, we analysed the signalling activity of three distinct Xenopus frizzled receptors, Xfz3, Xfz4 and Xfz7 (Shi et al., 1998; Djiane et al., 2000; Shi and Boucaut, 2000). We have taken the advantages of the Xenopus model in which Wnt/β-catenin signalling leads to transcriptional activation of target genes Siamois and Xnr3 in the animal caps of late blastula to show that the C-terminal cytoplasmic regions of these frizzled receptors are functionally equivalent in Wnt/β-catenin signalling, despite their difference in sequence similarity. Most importantly, we provide the first demonstration that a short conserved C-terminal cytoplasmic motif (Lys-Thr-X-X-X-Trp) is important for activation of Wnt/β-catenin signalling and may be involved in the modulation of Wnt signalling during development.
Results
Exogenous ligand-dependent and -independent activation of the Wnt/β-catenin pathway by frizzled receptors
All frizzled receptors have an extracellular CRD with 10 invariant cysteines; however, the sequence similarity of this region differs significantly (ranging from 30 to 50% overall identity). The C-terminal cytoplasmic regions of frizzled receptors also differ in length and in sequence similarity (Wang et al., 1996). Therefore, it is unclear whether and how distinct frizzled receptors activate Wnt/β-catenin signalling. In the present study, we sought to determine the function of three distinct Xenopus frizzled receptors (Xfz3, Xfz4 and Xfz7) in the activation of the Wnt/β-catenin pathway during Xenopus early development. We used a homologous system in which Wnt/β-catenin signalling activates the transcription of target genes Siamois and Xnr3 (Carnac et al., 1996; Brannon et al., 1997; McKendry et al., 1997; Fan et al., 1998). Synthetic mRNAs were injected into the animal pole region at the 2-cell stage. Animal cap explants were dissected at mid-blastula stage and cultured to early gastrula stage for RT–PCR analysis. Uninjected animal cap explants did not express Siamois and Xnr3, while injection of Xwnt8 mRNA (5 pg) specifically activated the expression of both genes (Figure 1A). Injection of 500 pg Xfz3 mRNA, but not Xfz4 and Xfz7 mRNAs, induced the expression of Siamois and Xnr3 in animal caps (Figure 1A). Furthermore, the activity of Xfz3 was blocked by coinjection of 500 pg GSK3β mRNA, which encodes a negative regulator of the Wnt/β-catenin pathway. Therefore, we conclude that Xfz3, but not Xfz4 and Xfz7, is capable of activating the Wnt/β-catenin pathway when expressed in animal cap cells.
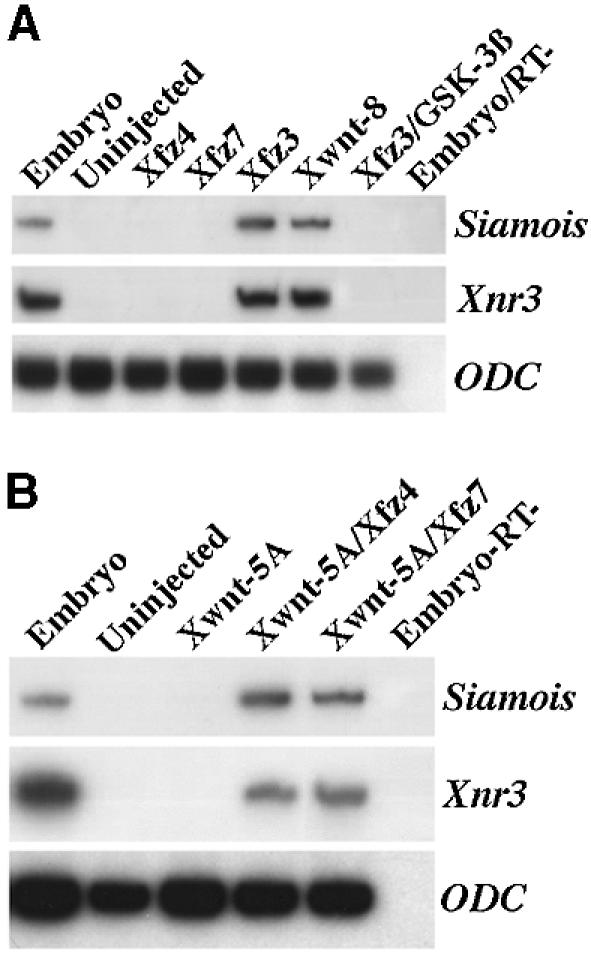
Fig. 1. Differential activation of Wnt/β-catenin target genes Siamois and Xnr3 by Xfz3, Xfz4 and Xfz7. Embryos at the 2-cell stage were injected at the animal pole region with the mRNAs indicated, whole embryos and animal caps were analysed by RT–PCR at stage 10.5 for the expression of Siamois and Xnr3. (A) Injection of Xfz3 and Xwnt8 mRNAs (500 pg and 5 pg, respectively), but not Xfz4 and Xfz7 mRNAs (500 pg), induces the expression of Siamois and Xnr3 in the animal caps. Coinjection of GSK3β mRNA (500 pg) blocks the effect of Xfz3. (B) Xwnt5A mRNA (5 pg) was either injected alone or coinjected with Xfz4 or Xfz7 mRNA (500 pg). Notice that Xwnt5A synergizes with Xfz4 and Xfz7 to induce Siamois and Xnr3 expression in the animal caps. ODC was used as a control for the level of input RNA. RT–, whole embryo control sample without reverse transcriptase.
The lack of effect following expression of Xfz4 and Xfz7 in animal caps may be simply because they cannot activate the Wnt/β-catenin pathway or may be attributable to the absence of a specific ligand for these receptors. To test whether they could activate the Wnt/β-catenin pathway if they are provided with a suitable ligand, we coinjected 500 pg Xfz4 or Xfz7 mRNA with 5 pg Xwnt5A mRNA. Overexpression of Xwnt5A alone did not induce the expression of Siamois and Xnr3 in animal cap explants (Figure 1B). However, when coinjected with Xfz4 or Xfz7 mRNA, expression of both genes was detected (Figure 1B). These results show that Xfz4 and Xfz7 can interact with Xwnt5A to activate the Wnt/β-catenin pathway.
Ectopic activation of Wnt/β-catenin signalling in the ventral region induces the formation of Spemann organizer. We injected Xfz3 mRNA alone (500 pg) or Xfz7 mRNA (500 pg) mixed with Xwnt5A mRNA (5 pg) into the ventral vegetal blastomeres at the 4-cell stage. The induction of an ectopic Spemann organizer was monitored by the expression of the Spemann organizer gene chordin (Sasai et al., 1994) by whole-mount in situ hybridization at the early gastrula stage. Ventral injection of Xwnt8 mRNA (5 pg) or Xfz3 mRNA (500 pg) led to ectopic chordin expression (Figure 2A–C), while injection of Xwnt5A mRNA (5 pg) or Xfz7 mRNA (500 pg) alone failed to induce ectopic chordin expression (Figure 2D and E). However, coinjection of Xwnt5A and Xfz7 led to ectopic chordin expression (Figure 2F), further arguing that Xfz7 can interact with Xwnt5A to activate the Wnt/β-catenin signalling pathway. These comparative analyses therefore indicate that distinct frizzled receptors could activate the Wnt/β-catenin signalling pathway either in an exogenous ligand-independent or -dependent fashion.
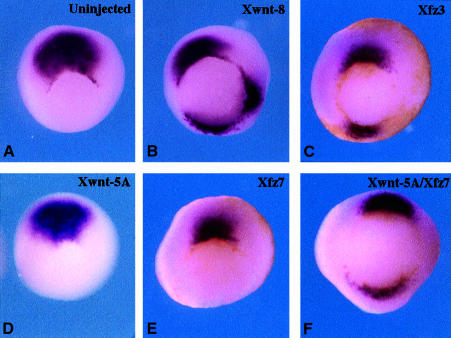
Fig. 2. Ectopic chordin expression induced by Xfz3 and Xwnt5A plus Xfz7 in stage 10.5 gastrulae. Four-cell stage embryos were injected at the ventral vegetal region with the mRNAs indicated and allowed to develop to stage 10.5 for in situ hybridization. (A) Expression of chordin in the Spemann organizer in an uninjected embryo. (B and C) Ventral injection of Xwnt8 or Xfz3 (5 pg and 500 pg mRNAs, respectively) induces ectopic chordin expression. (D and E) Ventral injection of Xwnt5A or Xfz7 alone (5 pg and 500 pg mRNAs, respectively) has no effect. (F) Coinjection of Xwnt5A and Xfz7 mRNAs induces ectopic chordin expression.
The C-terminal cytoplasmic regions from Xfz3, Xfz4 and Xfz7 are functionally equivalent in transducing the Wnt/β-catenin signal
Results from the above analyses argue that differential activation of Wnt/β-catenin signalling by frizzled receptors depends on the availability of a ligand. Furthermore, we took advantage of the fact that Xfz3 activates Wnt/β-catenin signalling in the absence of exogenous ligands and asked whether the C-terminal cytoplasmic regions of distinct frizzled receptors are functionally equivalent in Wnt/β-catenin signalling. To this purpose, we have analysed the activity of different chimeric receptors in the induction of Siamois expression. Xfz3/4 and Xfz3/7 are chimeric receptors made by replacing the C-terminal cytoplasmic region of Xfz3 with the corresponding region of Xfz4 or Xfz7. This approach circumvents the delicate overexpression of exogenous ligands and will allow us to examine directly whether the C-terminal cytoplasmic region of Xfz4 and Xfz7 is capable of transducing a Wnt/β-catenin signal. Conversely, Xfz7/3 is a receptor with the C-terminal cytoplasmic region of Xfz7 replaced by the corresponding region of Xfz3 (Figure 3A). The ability of these chimeric receptors to induce Siamois expression was examined using the animal cap assay after injection of the corresponding mRNA. As expected, overexpression of Xfz3/4 or Xfz3/7 alone was sufficient to induce Siamois expression like the wild-type Xfz3; however, Xfz7/3 induced Siamois expression only in the presence of Xwnt5A (Figure 3B). These results clearly demonstrate that the C-terminal cytoplasmic regions of all three receptors are functionally equivalent in Wnt/β-catenin signalling.
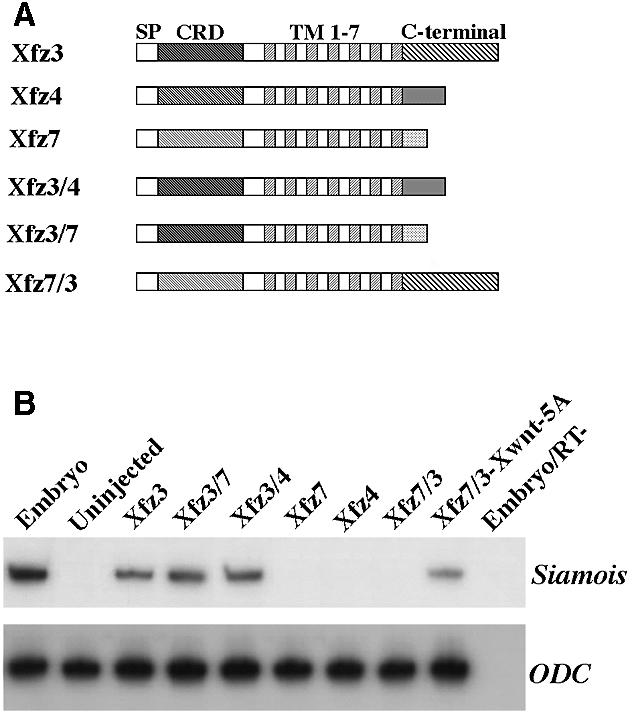
Fig. 3. Induction of the Wnt/β-catenin target gene Siamois by chimeric frizzled receptors. (A) Schematic representation of wild-type and chimeric frizzled constructs. Wild-type Xfz3, Xfz4 and Xfz7 contain an N-terminal signal peptide (SP) and an extracellular cysteine-rich domain (CRD), the seven transmembrane domains (TM 1–7) are followed by a C-terminal cytoplasmic region. Xfz3/4 and Xfz3/7 are chimeric receptors in which the C-terminal cytoplasmic region of Xfz3 is replaced by the corresponding region of Xfz4 or Xfz7; Xfz7/3 is the converse. (B) Xfz3, Xfz3/4 and Xfz3/7 induce the expression of Siamois in animal cap cells, while Xfz4 and Xfz7/3 have no effect. Note that Xfz7/3 synergizes with Xwnt5A to induce Siamois expression.
A Lys-Thr-X-X-X-Trp motif after the seventh TM is involved in the activation of Wnt/β-catenin signalling
We have thus established that the C-terminal cytoplasmic region of all three frizzled receptors is equivalent in transducing Wnt/β-catenin signal. Since this region from different frizzled receptors differs significantly in length and sequence similarity, we were interested to determine the structural basis that may confer this activity. A serial deletion of the 165 amino acids from the C-terminal cytoplasmic region of Xfz3 (Shi et al., 1998) was performed. We observed initially that deletion of the C-terminal-most 125 amino acids did not affect its ability to induce Siamois expression in animal caps (data not shown). Based on this result, we made further deletions of the Xfz3 receptor (Figure 4A). Xfz3-ΔC153 retains 12 amino acids after the seventh TM (Lys-Lys-Thr-Cys-Phe-Glu-Trp-Ala-Ser-Phe-Phe-His) while Xfz3-ΔC160 has five amino acids (Lys-Lys-Thr-Cys-Phe). Interestingly, we found that Xfz3-ΔC153 had the same activity as the wild-type Xfz3 in Siamois induction in animal caps, whereas Xfz3-ΔC160 had completely lost this activity (Figure 4B). Consistent with this in vitro analysis, we found that injection of Xfz3-ΔC153 mRNA in the ventral region of the embryo was able to induce ectopic chordin expression (data not shown). Therefore, these in vitro and in vivo analyses allowed us to define a short motif of the C-terminal cytoplasmic region that is required to activate the Wnt/β-catenin pathway.
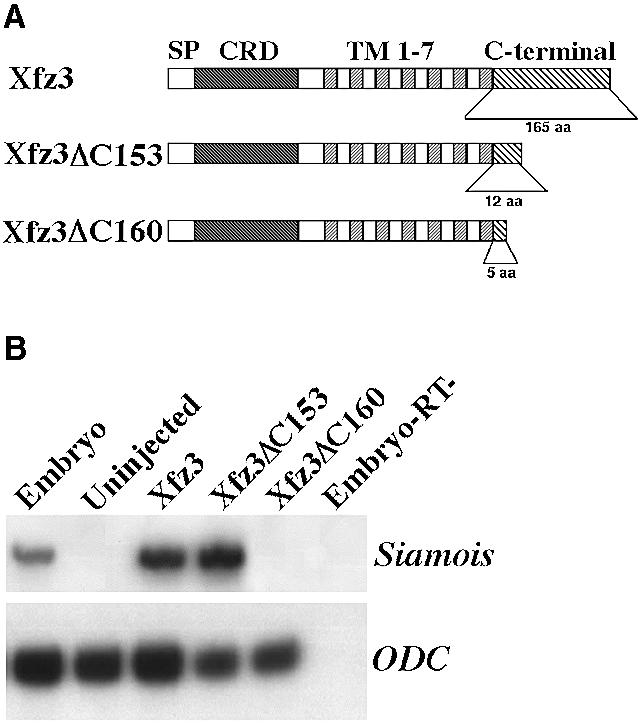
Fig. 4. Functional mapping of the C-terminal cytoplasmic portion of frizzled receptors. (A) Schematic representation of the Xfz3 deletion constructs. The C-terminal cytoplasmic region of Xfz3 protein has 165 amino acids. Xfz3-ΔC153 and Xfz3-ΔC160 proteins retain 12 and five amino acids after the seventh TM, respectively. (B) Embryos were injected with the mRNAs indicated and animal caps were analysed at stage 10.5 by RT–PCR for Siamois expression. Injection of 500 pg Xfz3-ΔC153 mRNA, but not the same amount of Xfz3-ΔC160 mRNA, induces Siamois expression.
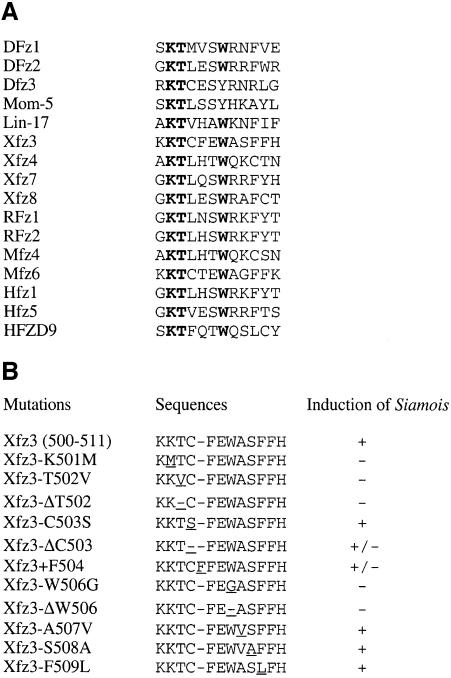
This finding prompted us to examine more closely the C-terminal cytoplasmic region among known frizzled receptors. Alignment of the 12 amino acids just after the seventh TM revealed that three residues were well conserved among different frizzled receptors; they are the consecutive lysine (Lys) and threonine (Thr), and a tryptophan (Trp) at the +6 position (Figure 5A). This motif of six amino acids (Lys-Thr-X-X-X-Trp) is located two amino acids after the seventh TM. We performed point mutations within this motif of Xfz3 to examine whether the conserved amino acids were involved in Wnt/β-catenin signalling using the convenient animal cap assay. We have substituted these conserved amino acids by those that display distinct chemical characters and functions. Our result indicated that substitution of the lysine at position 501 by a methionine (Xfz3-K501M), threonine at position 502 by a valine (Xfz3-T502V) or the tryptophan at position 506 by a glycine (Xfz3-W506G) completely abolished the activity of Xfz3 to induce Siamois expression in animal caps. Deletion of the threonine (Xfz3-ΔT502) or the tryptophan (Xfz3-ΔW506) residue similarly abolished the activity of Xfz3. In contrast, mutation of the non-conserved cysteine at position 503 to serine (Xfz3-C503S), alanine at position 507 to valine (Xfz3-A507V) or phenylalanine at position 509 to leucine (Xfz3-F509L) had no effect (Figure 5B). Therefore, the three conserved residues are required for the function of Xfz3 in transducing a Wnt/β-catenin signal. We further demonstrated that deletion or addition of a non-conserved amino acid within the Lys-Thr-X-X-X-Trp motif, or introducing a stop codon two amino acids after the tryptophan reduced but did not completely abolish the activity of Xfz3 in Siamois induction (Figure 5B). Therefore, it seems that the Lys-Thr-X-X-X-Trp motif should be in a particular context and followed by at least five amino acids for full function.
Fig. 5. Induction of the Wnt/β-catenin target gene Siamois by Xfz3 point mutants in the C-terminal cytoplasmic region. (A) Alignment of 12 amino acids after the seventh TM among 16 frizzled homologues. Note that three amino acids (bold) are well conserved, except in Dfz3 and mom-5. (B) Xfz3 point mutants (see text for detail) and their ability to induce Siamois expression in animal caps. Mutations or deletions of either of the three conserved amino acids (Lys, Thr and Trp) abolish the function of Xfz3 to activate Siamois expression in animal caps, while mutations in other non-conserved amino acids have no effect. Note that deletion (Xfz3-ΔC503) or addition (Xfz3 + F504) of an amino acid between the threonine and tryptophan also affects Xfz3 activity.
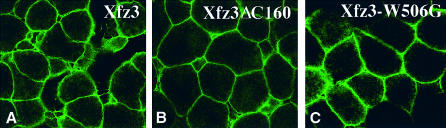
By western blotting and confocal microscopy we examined the expression and membrane localization of myc-tagged versions of the above constructs. We found that all myc-tagged Xfz3 have the same capacity to induce Siamois expression as non-tagged versions (data not shown). Western blotting first indicated that both wild-type and mutant receptors were correctly translated from injected mRNAs (not shown). Examination by confocal microscope showed that different forms of frizzled receptors were correctly expressed at the cell surface (Figure 6). Therefore, the lack of activity of different mutant frizzled receptors to transduce Wnt/β-catenin signal is probably due to the absence of a functional Lys-Thr-X-X-X-Trp motif.
Fig. 6. Expression of myc-tagged wild-type and mutant Xfz3 proteins at the cell surface. Synthetic mRNAs encoding myc-tagged Xfz3 (A), Xfz3-ΔC160 (B) and Xfz3-W506G (C) were injected at 2-cell stage near the animal pole region and animal caps were analysed at stage 9 by whole-mount immunostaining using 9E10 monoclonal antibody and confocal microscopy. All three proteins are correctly expressed at the cell surface.
Although Xfz3 receptors with point mutations within the Lys-Thr-X-X-X-Trp motif were not active in Wnt/β-catenin signalling when overexpressed alone, it is still unclear whether they respond to exogenous ligand. To test this possibility, we coinjected 500 pg Xfz7/3-W506G mRNA with 5 pg Xwnt5A mRNA. In contrast to the chimeric Xfz7/3 receptor, Xfz7/3-W506G did not synergize with Xwnt5A to induce Siamois expression, even at higher doses of injected mRNA (data not shown). This implies that frizzled receptors with point mutations within the Lys-Thr-X-X-X-Trp motif have lost the capacity to activate Wnt/β-catenin signalling.
Membrane relocalization and phosphorylation of Xdshmyc by wild-type and mutant frizzled receptors
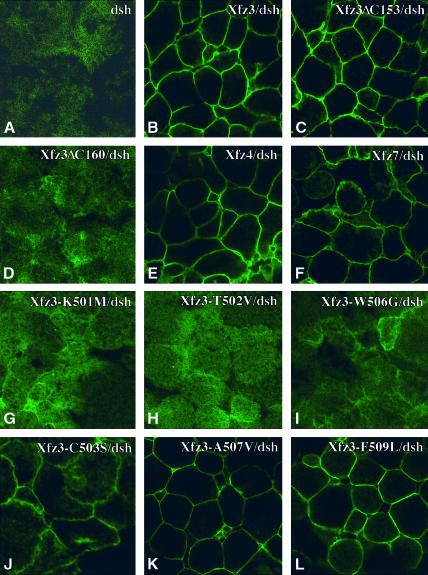
Dsh is at present the known component immediately downstream of frizzled receptors. We were interested to see whether different mutant frizzled receptors could interact with and phosphorylate Dsh protein. Synthetic mRNA (250 pg) encoding Xdshmyc (Sokol, 1996) was either injected alone or coinjected with mRNAs (500 pg) encoding wild-type or mutated frizzled receptors into the animal pole region at the 4-cell stage. Animal cap explants were cultured until stage 10 early gastrula to examine the localization of Xdshmyc by confocal microscopy and its phosphorylation by western blotting. When dsh mRNA was injected alone, diffuse fluorescent staining could be observed in the cytoplasm; there was no membrane association of Xdshmyc (Figure 7A). Wild-type Xfz3, Xfz4 and Xfz7 qualitatively affected the subcellular localization of Xdshmyc, which was relocalized to the plasma membrane and showed low levels of cytoplasmic distribution (Figure 7B, E and F). Xfz3-ΔC153 with 12 amino acids after the seventh TM recruited Xdshmyc at the plasma membrane similarly to the wild-type receptors (Figure 7C). In contrast, Xfz3-ΔC160, which retained only five amino acids after the seventh TM, did not relocalize Xdshmyc to the plasma membrane (Figure 7D). The distribution of Xdshmyc in this case was indistinguishable from that when Xdshmyc was expressed alone. We next examined the ability of different Xfz3 point mutants to interact with Dsh. Xfz3-K501M, Xfz3-T502V and Xfz3-W506G failed to recruit Xdshmyc to the plasma membrane (Figure 7G–I). Xfz3-ΔT502 and Xfz3-ΔW506 also failed to relocalize Xdshmyc (not shown). In contrast, Xfz3-C503S, Xfz3-A507V and Xfz3-F509L qualitatively relocalized Xdshmyc (Figure 7J–L) as the wild-type receptor.
Fig. 7. Recruitment of Xdshmyc to the plasma membrane by wild-type and mutant frizzled receptors. Embryos at 4-cell stage were either injected with 250 pg Xdshmyc mRNA alone or coinjected with mRNAs (500 pg) encoding the indicated wild-type and mutant frizzled receptors into the animal pole region of all four blastomeres. Animal caps were analysed at stage 9 by whole-mount immunostaining using 9E10 monoclonal antibody and confocal microscopy for the subcellular localization of Xdshmyc. (A) Expression of Xdshmyc alone results in diffuse fluorescent staining in the cytoplasm, no membrane localization is apparent. (B and C) Coexpression of Xdshmyc with Xfz3 (B) or Xfz3-ΔC153 (C) results in qualitative membrane localization of Xdshmyc. (D) Xfz3-ΔC160 does not recruit Xdshmyc to the plasma membrane. (E and F) Xfz4 and Xfz7 recruit Xdshmyc to the plasma membrane similarly to Xfz3. (G, H and I) Xdshmyc does not relocalize to the plasma membrane in response to expression of Xfz3-K501M (G), Xfz3-T502V (H) or Xfz3-W506G (I). (J) Xfz3-C503S significantly recruits Xdshmyc to the plasma membrane. (K and L) Xfz3-A507V (K) and Xfz3-F509L (L) recruit Xdshmyc at the plasma membrane similarly to the wild-type Xfz3.
We further analysed whether wild-type and mutant receptors were able to cause Dsh phosphorylation. Western blotting using 9E10 monoclonal antibody was performed on proteins extracted from embryos injected as above. It was shown previously that frizzled receptor causes a hyperphosphorylation of exogenous and endogenous Xenopus Dsh proteins, resulting in a slower-migrating form of Dsh when analysed by western blotting (Rothbächer et al., 2000). Consistent with this observation, we found that coexpression of Xdshmyc with Xfz3 or Xfz7 significantly increased the proportion of the slower-migrating form of Xdshmyc. In contrast, coinjection with mRNAs encoding Xfz3-ΔC160, Xfz3-K501M, Xfz3-T502V and Xfz3-W506G did not cause a hyperphosphorylation of Xdshmyc (data not shown), indicating that these mutant receptors have lost the capacity to induce the phosphorylation of Dsh proteins. Taken together, these results show that an intact Lys-Thr-X-X-X-Trp motif is required for Dsh relocalization and phosphorylation in response to frizzled receptors.
Discussion
In this report we have demonstrated that the C-terminal cytoplasmic regions from three distinct Xenopus frizzled receptors (Xfz3, Xfz4 and Xfz7) are functionally equivalent in the activation of Wnt/β-catenin signalling. The availability of a ligand determines whether a receptor is active in this pathway. In the case of Xfz3, we found that it could function in an exogenous ligand-independent manner. Most importantly, we provide the first demonstration that the conserved Lys-Thr-X-X-X-Trp motif located two amino acids after the seventh TM is required for activation of the Wnt/β-catenin signalling pathway, as well as for membrane relocalization and phosphorylation of Dsh.
Functional equivalence of the C-terminal cytoplasmic region of frizzled receptors in Wnt/β-catenin signalling
The C-terminal cytoplasmic regions of all known frizzled receptors differ in length and in sequence similarity (Wang et al., 1996). This raises the question of whether they are functionally equivalent in activating the canonical Wnt/β-catenin pathway. Although the activity of frizzled receptors in Wnt/β-catenin signalling in Xenopus has been analysed previously, the results were not fully consistent. These studies have allowed the conclusion that some members of the frizzled family transduce the Wnt/β-catenin signal while others do not have this property, at least when they are overexpressed alone in animal cap cells (Yang-Snyder et al., 1996; Slusarski et al., 1997; Deardorff et al., 1998; Itoh et al., 1998; Sheldahl et al., 1999). However, the lack of activity of a frizzled receptor in Wnt/β-catenin signalling in the animal cap assay may be due to the absence of an appropriate ligand (He et al., 1997; Medina et al., 2000; Sumanas et al., 2000). Our results from the present analyses using three distinct frizzled receptors (Xfz3, Xfz4 and Xfz7) both confirm and extend these previous observations. They suggest that the availability of a Wnt protein determines whether some frizzled receptors could activate the Wnt/β-catenin pathway. Therefore, in the context of Siamois induction using animal cap assay, distinct frizzled receptors may be functionally equivalent.
This conclusion is further supported by analyses using chimeric receptors that bypass the overexpression of exogenous ligands. We showed that Xfz3, but not Xfz4 and Xfz7, activates the expression of Siamois in an exogenous ligand-independent manner. Replacement of the C-terminal cytoplasmic portion of Xfz3 by the corresponding region of Xfz4 or Xfz7 did not affect its Siamois-inducing activity. These observations strongly suggest that the C-terminal cytoplasmic regions from distinct frizzled receptors are functionally equivalent in the induction of Wnt/β-catenin target genes in early Xenopus embryogenesis. Using a similar approach, it was reported that Wnt/β-catenin signalling could be activated by a chimeric receptor composed of the extracellular region of Dfz1 and the C-terminal cytoplasmic region of Dfz2. This led to the suggestion that the C-terminal cytoplasmic region of Dfz2 confers Wnt/β-catenin signalling specificity (Boutros et al., 2000). Although we cannot explain fully the apparent discrepancy between this result and ours, in this experiment, the wild-type Dfz1 alone was capable of inducing Siamois expression in a Xenopus animal cap assay, whereas in our experiments, neither Xfz7 nor Xfz7/3 alone induces Siamois expression, at least at the dose of mRNA injected. Thus, the difference in results might be due to the frizzled receptors used in different experiments. Since both Xfz7 and Xfz7/3 activate Wnt/β-catenin signalling in the presence of Xwnt5A, and Xfz3/4 and Xfz3/7 function as wild-type Xfz3, we conclude that the C-terminal cytoplasmic regions of Xfz3, Xfz4 and Xfz7 are interchangeable in Wnt/β-catenin signalling.
Our results are consistent with genetic analyses in Drosophila showing that Dfz1 also functions in neurogenesis where it acts downstream of Wingless and upstream of GSK3β (Bhat, 1998; Kennerdell and Carthew, 1998; Bhanot et al., 1999). Thus, whether a frizzled receptor activates the Wnt/β-catenin pathway will depend on the developmental context. The differential activation of Wnt/β-catenin signalling by distinct frizzled receptors in a particular context implies that they may have different affinity for endogenously expressed Wnt proteins. It is likely that frizzled receptors that activate Wnt/β-catenin signalling in the absence of exogenous ligands, such as Xfz3, interact with an endogenous ligand with high affinity. In contrast, those that interact with exogenous ligands to activate this pathway would display a low binding affinity with endogenous ligands, which is probably not sufficient to trigger receptor activation. It was reported previously that vertebrate frizzled receptors differentially induce the expression of Siamois when expressed alone in the animal caps; those that do not induce Siamois expression stimulate protein kinase C (PKC) in a G-protein-dependent manner (Sheldahl et al., 1999). Our observation that Xfz4 and Xfz7 interact with Xwnt5A to trigger Wnt/β-catenin signalling does not exclude the possibility that they can also stimulate PKC. It was shown that activation of PKC serves to augment the effects of GSK3β inhibition in Wnt/β-catenin signalling (Cook et al., 1996; Chen et al., 2000). Therefore, we may postulate that overexpression of Xwnt5A with Xfz4 or Xfz7 would trigger both a direct inhibition of GSK3β and activation of PKC. These two interactive components contribute to stabilization of β-catenin and expression of target genes.
Structural basis underlying Wnt/β-catenin signal transduction by the C-terminal cytoplasmic region of frizzled receptors
Our studies and those from other groups (Sawa et al., 1996; Itoh et al., 1998) clearly indicate that only a small portion of the C-terminal cytoplasmic region is required for Wnt/β-catenin signalling. Furthermore, these studies allow one to conclude definitively that the C-terminal Ser/Thr-X-Val motif is dispensable for Wnt/β-catenin signalling and interaction with Dsh. More importantly, we showed that the conserved Lys-Thr-X-X-X-Trp motif located two amino acids after the seventh TM of frizzled receptors is required for biological function. Xfz3, retaining only five amino acids after the seventh TM (Lys-Lys-Thr-Cys-Phe), did not possess the Siamois-inducing activity although it is correctly expressed at the plasma membrane. Mutation or deletion of either of the three conserved residues also abolished this activity, while mutation of other non-conserved residues did not. Furthermore, Xfz3 and Xfz7 receptors with point mutations affecting conserved residues of this motif were not able to interact with a ligand to activate Wnt/β-catenin signalling, they are therefore not active and probably function as antagonists in Wnt/β-catenin signalling (data not shown). These observations indicate that the conserved motif is important for signal transduction.
Our findings may help to explain some unexpected features of some invertebrate frizzled receptors in Wnt signalling. It is interesting to notice that the Lys-Thr-X-X-X-Trp motif is conserved among all frizzled receptors, except the Drosophila Dfz3 and C.elegans mom-5 in which the tryptophan is substituted by a tyrosine. Dfz3 stabilizes β-catenin/armadillo much less efficiently than Dfz2 in transfected Drosophila cells; it also reduces the activity of Wingless (Sato et al., 1999). In C.elegans, Wnt signalling is involved in endoderm formation. Mutation in mom-5, which encodes a frizzled, has a low penetrance of gutless phenotype, whereas mutation in mom-2, which encodes a Wnt, has a much higher penetrance. Double mutant mom-5/mom-2 shows similar penetrance of gutless phenotype to the mom-5 mutant. Therefore, mom-5 might not be activated by mom-2, but exerts a negative influence on endoderm formation (Rocheleau et al., 1997; Thorpe et al., 1997). Based on our functional analyses, it is possible that Dfz3 and mom-5 may represent two naturally occurring ‘defective’ frizzled receptors in Wnt signalling. They may regulate the activity of Wnt proteins in vivo as members of the family of secreted frizzled-related proteins identified in vertebrates (reviewed in Wodarz and Nusse, 1998). Our findings therefore provide a molecular framework underlying these unexpected genetic features in Drosophila and C.elegans. Taken together, these analyses suggest that the conserved Lys-Thr-X-X-X-Trp motif may be involved in the modulation of Wnt signalling during development.
Role of the Lys-Thr-X-X-X-Trp motif in protein–protein interaction
We showed that an intact Lys-Thr-X-X-X-Trp motif is also required for membrane relocalization and phosphorylation of Dsh. However, we did not observe any direct interaction between the C-terminal cytoplasmic region and Dsh by directional two-hybrid assay (data not shown); this suggests that recruitment of Dsh requires a synergic interaction involving the Lys-Thr-X-X-X-Trp motif and transmembrane helices. The exact function of the Lys-Thr-X-X-X-Trp motif in Wnt/β-catenin signalling is not clear at present. We can speculate that it may be involved in the proper activation of frizzled receptors, or in the interaction with a putative cytoplasmic component that would be upstream of Dsh. In both cases, mutations that generate a non-functional motif would disrupt both Wnt/β-catenin signalling and recruitment of Dsh. It is striking that frizzled receptors bearing mutations in either of the three conserved residues were not able to induce Siamois expression but also failed to phosphorylate Dsh and to relocalize it to the plasma membrane. Nevertheless, it should be pointed out that the ability of a frizzled receptor to relocalize Xdshmyc to the plasma membrane is not correlated with its ability to activate Siamois expression in our animal cap assay. We showed that overexpression of Xfz4 and Xfz7 alone did not induce Siamois expression although they efficiently relocalized Xdshmyc at the plasma membrane. Therefore, Xfz4 and Xfz7 may recruit Xdshmyc due to an overexpression of these receptors, which provides sufficient binding sites. This is consistent with several lines of evidence showing that activation of the Wnt/β-catenin pathway by Dsh is independent of its membrane relocalization and phosphorylation (Yang-Snyder et al., 1996; Axelrod et al., 1998; Moriguchi et al., 1999; Rothbächer et al., 2000; reviewed by Boutros and Mlodzik, 1999).
The fact that membrane relocalization and phosphorylation of Dsh are not necessary for activation of the Wnt/β-catenin pathway raises the question of their biological significance. In Drosophila, Dsh is also involved in planar polarity signalling in which it activates JNK and requires a functional DEP domain. Mutation in the DEP domain of Dsh impairs its membrane translocation in response to frizzled and disrupts its function in planar polarity signalling but not in Wnt/β-catenin signalling (Axelrod et al., 1998; reviewed by Boutros and Mlodzik, 1999). Thus, the recruitment of Dsh by frizzled receptors is required for activation of the JNK pathway (Boutros et al., 1998; Moriguchi et al., 1999). In Xenopus, Wnt/frizzled signalling is also implicated in the control of morphogenetic movements through a pathway via Dsh, but not β-catenin (Djiane et al., 2000; Tada and Smith, 2000; Wallingford et al., 2000). It is worth noting that a specific dominant-negative mutant for Xwnt-11 inhibits morphogenetic movements and Dsh phosphorylation (Tada and Smith, 2000). Thus, it is of interest to examine the effects of our mutant receptors in morphogenetic movements.
In conclusion, Wnt signalling through frizzled receptors plays a crucial role in the control of cell fates and morphogenetic movements. Our functional analyses have allowed the identification of a conserved Lys-Thr-X-X-X-Trp motif, which is required for signal transduction. Mutation in either of the three conserved residues impairs the activity of a frizzled receptor in Wnt/β-catenin signalling. Therefore, our approaches should help to understand the molecular mechanism underlying certain genetic features in Drosophila and C.elegans; they suggest that the Lys-Thr-X-X-X-Trp motif may modulate Wnt activity during development.
Materials and methods
Plasmid constructs
The Xfz3 coding sequence in pSP64T vector (pSP64T-Xfz3) has been described previously (Shi et al., 1998). Xfz3-ΔC153 and Xfz3-ΔC160, which retain 12 and five amino acids after the seventh TM, respectively, were generated by digesting the pSP64T-Xfz3 plasmid using convenient restriction sites. All Xfz3 point mutants were generated by PCR-based mutagenesis using primers with either a mutated or a deleted codon:
Xfz3-K501M, 5′-CCCATTCGAAACAGGTCATCTTGCTTCC-3′;
Xfz3-T502V, 5′-CCCTTCGAAACAGACCTTCTTGCTTC-3′;
Xfz3-ΔT502, 5′-CCATTCGAAACACTTCTTGCTTCC-3′;
Xfz3-C503S, 5′-CCATTCGAAAGAGGTCTTCTTGC-3′;
Xfz3-ΔC503, 5′-CCATTCGAAGGTCTTCTTGC-3′;
Xfz3+F505, 5′-CCATTCGAAGAAACAGGTCT-3′;
Xfz3-W506G, 5′-CTGTTTCGAAGGGGCCAGCTT-3′;
Xfz3-ΔW506, 5′-TGTTTCGAAGCCAGCTTTTTCC-3′;
Xfz3-A507V, 5′-TGTTTCGAATGGGTCAGCTTTTTC-3′;
Xfz3-S508A, 5′-CATTCGAAACAGGTCTTCTTGGCTCCGACC-3′;
Xfz3-F509L, 5′-TGTTTCGAATGGGCCAGCCTTTTCCATG-3′.
Myc-tagged versions of all Xfz3 constructs described above were also cloned in the pCS2 vector containing six myc epitopes (Rupp et al., 1994; Turner and Weintraub, 1994) to ensure that they were expressed at the cell surface by confocal microscopy. The six myc epitopes were placed between the signal peptide and the CRD.
Xfz4 (Shi and Boucaut, 2000) and Xfz7 (Djiane et al., 2000) constructs were obtained by subcloning the corresponding coding sequence into pCS2+ vector. The chimeric receptor Xfz3/7 was obtained by replacing the C-terminal cytoplasmic region of Xfz3 by the corresponding region of Xfz7 amplified by PCR, with an upstream primer containing an added BstBI site (underlined), 5′-CGGTTCGAATGGCGCAGGTTCTAC-3′ and the T7 primer. The cDNA was cloned in-frame with the seventh TM of Xfz3 into an internal BstBI site. Xfz3/4, which has the C-terminal cytoplasmic region of Xfz3 replaced by the corresponding region of Xfz4, was generated by sequential PCR steps using the following overlapping primers: 5′-GGTCGGAAGCGCTAAAACCCTGC-3′ and 5′-GGGTTT TAGCGCTTCCGACCCAG-3′. Xfz7/3, which has the C-terminal cytoplasmic region of Xfz7 replaced by the corresponding region of Xfz3, was generated in a similar way using the following overlapping primers: 5′-TGGATCTGGTCGAAGAAGACCTGT-3′ and 5′-ACA GGTCTTCTTCGACCAGATCCA-3′. Xfz7/3-W506G was obtained by replacing the C-terminal cytoplasmic region of Xfz7/3 with the corresponding region of Xfz3-W506G. All constructs were sequenced and were found to be identical to the original cDNA sequence.
Xwnt8myc and Xwnt5Amyc plasmids (Yang-Snyder et al., 1996) were from Dr R.T.Moon. The Xdshmyc plasmid (Sokol, 1996) was provided by Dr S.Sokol and the pSP-GSK3β plasmid (He et al., 1995) was from Dr X.He.
Xenopus embryos and mRNA microinjections
Xenopus eggs were obtained from females injected with 500 IU of human chorionic gonadotrophin (Sigma), and artificially fertilized. Eggs were dejellied with 2% cysteine hydrochloride pH 7.8 and embryos were staged according to Nieuwkoop and Faber (1967).
Capped mRNAs were synthesized from linearized plasmids using SP6 RNA polymerase (Boehringer Mannheim) in the presence of 500 µM 5′-mGpppG-3′ cap analogue, 500 µM each of rUTP, rATP, rCTP and 50 µM rGTP. The synthetic mRNAs were purified using a Sephadex G-50 column (Pharmacia) and recovered by ethanol precipitation. The amounts of mRNAs were determined both by ethidium bromide staining in comparison with standard RNA and by incorporation of [3H]UTP in the reaction mixture. Aliquots of RNA in diethyl pyrocarbonate-treated water were stored at –80°C. Microinjection of embryos was performed in 0.1× MBS (modified Barth’s solution) containing 3% Ficoll 400 (Sigma) using a PLI-100 reproducible pico-injector (Medical Systems Corp.). A volume of 10 nl was injected into each blastomere at the animal pole region of 2-cell stage embryos or into the two ventral vegetal blastomeres of 4-cell stage embryos. After injection, embryos were maintained in this medium for 2 h, they were then cultured in 0.1 × MBS supplemented with 50 µg/ml gentamicin for an appropriate period.
RT–PCR and in situ hybridization
Animal cap explants from control and injected embryos were dissected at mid-blastula stage (stage 8) and cultured to early gastrula stage (stage 10.5) in 1 × MBS. Extraction of RNA was as previously described (Shi et al., 1998). For RT–PCR, RNA samples were treated with RNase-free DNase I (Boehringer Mannheim) to remove further genomic DNA. Approximately 5 µg of total RNA were reverse-transcribed in the presence of 200 U SuperScript RNase H– reverse transcriptase (Life Technologies). PCR primers are the following: Siamois (Lemaire et al., 1995; 5′-AAGGAACCCCACCAGGATAA-3′ and 5′-TACTGGTGGCT GGAGAAATA-3′); Xnr3 (Smith et al., 1995; 5′-TCCACTTGTGCA GTTCCACAG-3′ and 5′-ATCTCTTCATGGTGCCTCAGG-3′) and ornithine decarboxylase (ODC; Bassez et al., 1990), which was used as a control for the level of input RNA (5′-GTCAATGATGGAGTG TATGGATC-3′ and 5′-TCCATTCCGCTCTCCTGAGCAC-3′). In all experiments, 5 µg of total RNA from whole embryos treated the same way but minus the reverse transcriptase served as a control for the specificity of PCR. One-twentieth of the reverse-transcribed cDNA was used for PCR amplification in a 25 µl reaction mixture consisting of 1× PCR buffer (Perkin Elmer Cetus), dNTP at 0.2 mM each, 1 µCi of [α-32P]dCTP (Amersham), 25 pmol of sense and antisense primers and 2.5 U of Taq DNA polymerase (Perkin Elmer Cetus). We used 20 amplification cycles for ODC and 26 amplification cycles for Siamois and Xnr3. In all cases, amplification was found to be in the linear range (not shown). PCR products were resolved on a 5% non-denaturing polyacrylamide gel and visualized by autoradiography.
Whole-mount in situ hybridization was performed according to standard protocol (Harland, 1991) except that chromogenic reaction was carried out using BM purple as substrate (Boehringer Mannheim).
Membrane recruitment of myc-tagged Dsh (Xdshmyc)
This was performed as described (Yang-Snyder et al., 1996; Axelrod et al., 1998). Briefly, 250 pg Xdshmyc mRNA were either injected alone or coinjected with 500 pg mRNA encoding different frizzled receptors into the animal pole region of 4-cell stage embryos. Animal caps were dissected at stage 9 and fixed in MEMFA (0.1 M MOPS pH 7.4, 2 mM EGTA, 1 mM MgSO4 and 3.7% formaldehyde) for 1 h at room temperature. After washing in Tris-buffered saline containing 2 mg/ml bovine serum albumin and 0.1% Triton X-100, whole-mount immunostaining was carried out with 9E10 anti-c-myc monoclonal antibody (Santa Cruz Biotechnology), followed by FITC-conjugated secondary antibody (Jackson ImmunoResearch Laboratories). The localization of Xdshmyc was observed using a Leica laser scanning confocal microscope.
Western blotting
Five whole early gastrulae previously injected as above were extracted in 200 µl of extraction buffer (100 mM NaCl, 5 mM EDTA, 0.5% NP-40 and 10 mM Tris–HCl pH 7.5) containing 2 mM PMSF, 25 µM leupeptin and 0.2 U/ml aprotinin (all from Sigma) in the presence of the serine and threonine phosphatase inhibitor NaF at 5 mM. Proteins extracted from an equivalent of one embryo were analysed by 7.5% SDS–PAGE. Following electrophoresis, proteins were electrophorestically transferred to nitrocellulose sheets (Amersham). The membranes were incubated with 9E10 monoclonal antibody followed by horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories). Bound secondary antibodies were visualized by diaminobenzidine substrate.
Acknowledgments
Acknowledgements
The authors would like to thank Drs R.T.Moon, S.Y.Sokol and Y.He for providing the cDNA constructs, A.Pascal and A.Bourdelas for excellent technical assistance, R.Schwartzmann for confocal microscopy and P.Nguyen for illustrations. This work was supported by grants from the Centre National de la Recherche Scientifique (CNRS), the Association Française contre les Myopathies (AFM), the Association pour la Recherche contre le Cancer (ARC) and the Ministère de l’Education Nationale et de la Recherche et Technologies (MENRT).
References
- Adler P.N. (1992) The genetic control of tissue polarity in Drosophila. BioEssays, 14, 735–741. [DOI] [PubMed] [Google Scholar]
- Axelrod J.D., Miller,J.R., Shulman,J.M., Moon,R.T. and Perrimon,N. (1998) Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev., 12, 2610–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassez T., Paris,J., Omilli,F., Dorel,C. and Osborne,H.B. (1990) Post-transcriptional regulation of ornithine decarboxylase in Xenopus laevis. Development, 110, 955–962. [DOI] [PubMed] [Google Scholar]
- Bhanot P., Brink,M., Samos,C.H., Hsieh,J.C., Wang,Y.S., Macke,J.P., Andrew,D., Nathans,J. and Nusse,R. (1996) A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature, 382, 225–230. [DOI] [PubMed] [Google Scholar]
- Bhanot P., Fish,M., Jemison,J.A., Nusse,R., Nathans,J. and Cadigan,K.M. (1999) Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development, 126, 4175–4186. [DOI] [PubMed] [Google Scholar]
- Bhat K.M. (1998) frizzled and frizzled 2 play a partially redundant role in Wingless signaling and have similar requirements to Wingless in neurogenesis. Cell, 95, 1027–1036. [DOI] [PubMed] [Google Scholar]
- Boutros M. and Mlodzik,M. (1999) Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech. Dev., 83, 27–37. [DOI] [PubMed] [Google Scholar]
- Boutros M., Paricio,N., Strutt,D.I. and Mlodzik,M. (1998) Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell, 94, 109–118. [DOI] [PubMed] [Google Scholar]
- Boutros M., Mihaly,J., Bouwmeester,T. and Mlodzik,M. (2000) Signaling specificity by frizzled receptors in Drosophila. Science, 288, 1825–1828. [DOI] [PubMed] [Google Scholar]
- Brannon M., Gomperts,M., Sumoy,L., Moon,R.T. and Kimelman,D. (1997) A β-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev., 11, 2359–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan K.M. and Nusse,R. (1997) Wnt signaling: a common theme in animal development. Genes Dev., 11, 3286–3305. [DOI] [PubMed] [Google Scholar]
- Carnac G., Kodjabachian,L., Gurdon,J.B. and Lemaire,P. (1996) The homeobox gene Siamois is a target of the wnt dorsalization pathway and triggers organizer activity in the absence of mesoderm. Development, 122, 3055–3065. [DOI] [PubMed] [Google Scholar]
- Chen R.H., Ding,W.V. and McCormick,F. (2000) Wnt signaling to β-catenin involves two interactive components: GSK-3β inhibition and activation of PKC. J. Biol. Chem., 275, 17894–17899. [DOI] [PubMed] [Google Scholar]
- Cook D., Fry,M.J., Hughes,K., Sumathipala,R., Woodgett,J.R. and Dale,T.C. (1996) Wingless inactivates glycogen synthase kinase-3 via an intracellular signaling pathway which involves a protein kinase C. EMBO J., 15, 4526–4536. [PMC free article] [PubMed] [Google Scholar]
- Deardorff M.A., Tan,C.G., Conrad,L.J. and Klein,P.S. (1998) Frizzled-8 is expressed in the Spemann organizer and plays a role in early morphogenesis. Development, 125, 2687–2700. [DOI] [PubMed] [Google Scholar]
- Djiane A., Riou,J.F., Umbhauer,M., Boucaut,J.C. and Shi,D.L. (2000) Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development, 127, 3091–3400. [DOI] [PubMed] [Google Scholar]
- Du S.J., Purcell,S.M., Christian,J.L., McGrew,L.L. and Moon,R.T. (1995) Identification of distinct classes and functional domains of wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol. Cell. Biol., 15, 2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M.J., Grüning,W., Walz,G. and Sokol,S.Y. (1998) Wnt signaling and transcriptional control of Siamois in Xenopus embryos. Proc. Natl Acad. Sci. USA, 95, 5626–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B.M. (1997) Carcinogenesis: a balance between β-catenin and APC. Curr. Biol., 7, 443–446. [DOI] [PubMed] [Google Scholar]
- Harland R.M. (1991) In situ hybridization: an improved whole mount method for Xenopus embryos. Methods Cell Biol., 36, 685–695. [DOI] [PubMed] [Google Scholar]
- He X., Saint-Jeannet,J.P., Woodgett,J.R., Varmus,H.E. and Dawid,I.B. (1995) Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature, 374, 617–622. [DOI] [PubMed] [Google Scholar]
- He X., Saint-Jeannet,J.P.,Wang,Y., Nathans,J., Dawid,I.B. and Varmus,H.E. (1997) A member of frizzled protein family mediating axis induction by Wnt-5A. Science, 275, 1652–1654. [DOI] [PubMed] [Google Scholar]
- Heasman J., Crawford,A., Goldstone,K., Garner-Hamrick,P., Gumbiner,B., McCrea,P., Kintner,C., Noro,C.Y. and Wylie,C. (1994) Overexpression of cadherins and underexpression of β-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell, 79, 791–803. [DOI] [PubMed] [Google Scholar]
- Itoh K., Jacob,J. and Sokol,S.Y. (1998) A role for Frizzled 8 in dorsal development. Mech. Dev., 74, 145–157. [DOI] [PubMed] [Google Scholar]
- Kengaku M., Capdevila,J., Rodriguez-Esteban,C., De La Pena,J., Johnson,R.L., Belmonte,J.C.I. and Tabin,C.J. (1998) Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science, 280, 1274–1277. [DOI] [PubMed] [Google Scholar]
- Kennerdell J.R. and Carthew,R.W. (1998) Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the Wingless pathway. Cell, 95, 1017–1026. [DOI] [PubMed] [Google Scholar]
- Korinek V., Barker,N., Morin,P.J., van Wichen,D., de Weger,R., Kinzler,K.W., Vogelstein,B. and Clevers,H. (1997) Constitutive transcriptional activation by a β-catenin–Tcf complex in APC–/– colon carcinoma. Science, 275, 1784–1787. [DOI] [PubMed] [Google Scholar]
- Krasnow R.E. and Adler,P.N. (1994) A single frizzled protein has a dual function in tissue polarity. Development, 120, 1883–1893. [DOI] [PubMed] [Google Scholar]
- Lemaire P., Garrett,N. and Gurdon,J.B. (1995) Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastula and able to induce a complete secondary axis. Cell, 81, 85–94. [DOI] [PubMed] [Google Scholar]
- McKendry R., Hsu,S.C., Harland,R.M. and Grosschedl,R. (1997) LEF1/TCF proteins mediate wnt-inducible transcription from the Xenopus nodal-related 3 promoter. Dev. Biol., 192, 420–431. [DOI] [PubMed] [Google Scholar]
- Medina A., Reintsch,W. and Steinbeisser,H. (2000) Xenopus frizzled 7 can act in canonical and non-canonical Wnt signaling pathways: implications in early patterning and morphogenesis. Mech. Dev., 92, 227–237. [DOI] [PubMed] [Google Scholar]
- Miller J.R., Hocking,A.M., Brown,J.D. and Moon,R.T. (1999) Mechanism and function of signal transduction by the Wnt/β-catenin and Wnt/Ca2+ pathways. Oncogene, 18, 7860–7872. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. (1999) Planar polarity in the Drosophila eye: a multifaceted view of signaling specificity and cross-talk. EMBO J., 18, 6873–6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R.T. (1993) In pursuit of the functions of the Wnt family of developmental regulators: insights from Xenopus laevis.BioEssays, 15, 91–97. [DOI] [PubMed] [Google Scholar]
- Moon R.T. and Kimelman,D. (1998) From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus.BioEssays, 20, 536–545. [DOI] [PubMed] [Google Scholar]
- Moon R.T., Campbell,R.M., Christian,J.L., McGrew,L.L., Shih,J. and Fraser,S. (1993) Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis.Development, 119, 97–111. [DOI] [PubMed] [Google Scholar]
- Moon R.T., Brown,J.D. and Torres,M. (1997) WNTs modulate cell fate and behavior during vertebrate development. Trends Genet., 13, 157–162. [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Kawachi,K., Kamakura,S., Masuyama,N., Yamanaka,H., Matsumoto,K., Kikachi,A. and Nishida,E. (1999) Distinct domains of mouse dishevelled are responsible for the c-Jun N-terminal kinase/stress-activated protein kinase activation and the axis formation in vertebrates. J. Biol. Chem., 274, 30957–30962. [DOI] [PubMed] [Google Scholar]
- Morin P.J., Sparks,A.B., Korinek,V., Barker,N., Clevers,H., Vogelstein,B. and Kinzler,K.W. (1997) Activation of β-catenin–Tcf signaling in colon cancer by mutations in β-catenin or APC. Science, 275, 1787–1790. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P.D. and Faber,J. (1967) Normal Table of Xenopus laevis (Daudin). North Holland, Amsterdam. [Google Scholar]
- Rocheleau C.E., Downs,W.D., Lin,R.Y., Wittmann,C., Bei,Y.X., Cha,Y.H., Ali,M., Priess,J.R. and Mello,C.C. (1997) Wnt signaling and an APC-related gene specify endoderm in early C.elegans embryos. Cell, 90, 707–716. [DOI] [PubMed] [Google Scholar]
- Rothbächer U., Laurent,M.N., Deardorff,M.A., Klein,P.S., Cho,K.W. and Fraser,S.E. (2000) Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J., 19, 1010–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B., Robbins,P., El-Gamil,M., Albert,I., Porfiri,E. and Polakis,P. (1997) Stabilization of β-catenin by genetic defects in melanoma cell lines. Science, 275, 1790–1792. [DOI] [PubMed] [Google Scholar]
- Rupp R.A.W., Snider,L. and Weintraub,H. (1994) Xenopus embryos regulate the nuclear localization of XMyoD. Genes Dev., 8, 1311–1323. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu,B., Steinbeisser,H., Geissert,D., Gont,L.K. and De Robertis,E.M. (1994) Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell, 79, 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Kojima,T., Ui-Tei,K., Miyata,Y. and Saigo,K. (1999) Dfrizzled-3, a new Drosophila Wnt receptor, acting as an attenuator of Wingless signaling in wingless hypomorphic mutants. Development, 126, 4421–4430. [DOI] [PubMed] [Google Scholar]
- Sawa H., Lobel,L. and Horvitz,H.R. (1996) The Caenorhabditis elegans gene lin-17, which is required for certain asymmetric cell divisions, encodes a putative seven-transmembrane protein similar to the Drosophila Frizzled protein. Genes Dev., 10, 2189–2197. [DOI] [PubMed] [Google Scholar]
- Sheldahl L.C., Park,M., Malbon,C.C., and Moon,R.T. (1999) Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr. Biol., 9, 695–698. [DOI] [PubMed] [Google Scholar]
- Shi D.L. and Boucaut,J.C. (2000) Xenopus frizzled 4 is a maternal mRNA and its zygotic expression is localized to the neuroectoderm and trunk lateral plate mesoderm. Mech. Dev., 94, 243–245. [DOI] [PubMed] [Google Scholar]
- Shi D.L., Goisset,C. and Boucaut,J.C (1998). Expression of Xfz3, a Xenopus frizzled family member, is restricted to the early nervous system. Mech. Dev., 70, 35–47. [DOI] [PubMed] [Google Scholar]
- Slusarski D.C., Corces,V.G. and Moon,R.T. (1997) Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phos phatidylinositol signalling. Nature, 390, 410–413. [DOI] [PubMed] [Google Scholar]
- Smith W.C., McKendry,R., Ribisi,S.,Jr and Harland,R.M. (1995) A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell, 82, 37–46. [DOI] [PubMed] [Google Scholar]
- Sokol S.Y. (1996) Analysis of Dishevelled signalling pathways during Xenopus development. Curr. Biol., 6, 1456–1467. [DOI] [PubMed] [Google Scholar]
- Sokol S.Y. (1999) Wnt signalling and dorso-ventral specification in vertebrates. Curr. Opin. Genet. Dev., 9, 405–410. [DOI] [PubMed] [Google Scholar]
- Strutt D.I., Weber,U. and Mlodzik,M. (1997) The role of RhoA in tissue polarity and Frizzled signalling. Nature, 387, 292–295. [DOI] [PubMed] [Google Scholar]
- Sumanas S., Strege,P., Heasman,J. and Ekker,S.C. (2000) The putative Wnt receptor Xenopus frizzled-7 functions upstream of β-catenin in vertebrate dorsoventral mesoderm patterning. Development, 127, 1981–1990. [DOI] [PubMed] [Google Scholar]
- Tada M. and Smith,J.C. (2000) Xwnt-11, a target of Xenopus Brachyury, regulates gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development, 127, 2227–2238. [DOI] [PubMed] [Google Scholar]
- Thorpe C.J., Schlesinger,A., Clayton,J. and Bowerman,B. (1997) Wnt signaling polarizes an early C.elegans blastomere to distinguish endoderm from mesoderm. Cell, 90, 695–705. [DOI] [PubMed] [Google Scholar]
- Turner D.L. and Weintraub,H. (1994) Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to neural fate. Genes Dev., 8, 1434–1447. [DOI] [PubMed] [Google Scholar]
- Ungar A.R., Kelly,G.M. and Moon,R.T. (1995) Wnt4 affects morphogenesis when misexpressed in the zebrafish embryo. Mech. Dev., 52, 153–164. [DOI] [PubMed] [Google Scholar]
- Wallingford J.B., Rowning,B.A., Vogeli,K.M., Rothbächer,U., Fraser,S.E. and Harland,R.M. (2000) Dishevelled controls cell polarity during Xenopus gastrulation. Nature, 405, 81–85. [DOI] [PubMed] [Google Scholar]
- Wang Y.S., Macke,J.P., Abella,B.S., Andreasson,K., Worley,P., Gilbert,D.J., Copeland,N.G., Jenkins,N.A. and Nathans,J. (1996) A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J. Biol. Chem., 271, 4468–4476. [DOI] [PubMed] [Google Scholar]
- Willert K., Brink,M., Wodarz,A., Varmus,H. and Nusse,R. (1997) Casein kinase 2 associates with and phosphorylates Dishevelled. EMBO J., 16, 3089–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A. and Nusse,R. (1998) Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol., 14, 59–88. [DOI] [PubMed] [Google Scholar]
- Yanagawa S., van Leeuwen,F., Wodarz,A., Klingensmith,J. and Nusse,R. (1995) The Dishevelled protein is modified by Wingless signaling in Drosophila. Genes Dev., 9, 1087–1097. [DOI] [PubMed] [Google Scholar]
- Yang-Snyder J., Miller,J.R., Brown,J.D., Lai,C.J. and Moon,R.T. (1996) A frizzled homolog functions in a vertebrate wnt signaling pathway. Curr. Biol., 6, 1302–1306. [DOI] [PubMed] [Google Scholar]
- Yost C., Torres,M., Miller,J.R., Huang,E., Kimelman,D. and Moon,R.T. (1996) The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev., 15, 1443–1454. [DOI] [PubMed] [Google Scholar]
- Zeng L. et al. (1997) The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell, 90, 181–192. [DOI] [PubMed] [Google Scholar]