Abstract
Commentary on Oatley et al., “Inhibitor of DNA binding 4 is expressed selectively by single spermatogonia in the male germline and regulates the self-renewal of spermatogonial stem cells.”
Spermatogonial stem cells (SSCs) are undifferentiated male germ cells that have the potential to self-renew and differentiate into committed progenitors that maintain spermatogenesis throughout adult life. Rodent SSCs can be identified in whole-mount preparations of testicular seminiferous tubules (initially described by Clermont and Bustos-Obregon [1]) as isolated Asingle spermatogonia. These Asingle SSCs are present on the basement membrane of seminiferous tubules and can be distinguished from committed, transit-amplifying progenitor spermatogonia (some Apaired and Aaligned chains of 4–16 cells) because committed cells are clonally arranged and connected by intercellular cytoplasmic bridges. Here we define progenitors as undifferentiated spermatogonia that are committed to differentiate and can undergo a finite number of self-renewing divisions. SSCs can be definitively identified in a retrospective manner by observing their ability to produce and maintain spermatogenesis in a functional transplantation assay as initially described by Brinster et al. [2, 3]. It has been widely assumed that all Asingles (as the classically-defined SSCs) possess stem cell properties [4–7]. However, it has never been shown whether all or only some Asingles are bona fide SSCs, and cells with a particular morphological/clonal arrangement (e.g., Asingle) may or may not comprise the entire SSC pool [8].
Antibody-based studies using intact (whole-mount) seminiferous tubules have provided an avenue for defining proteins with expression patterns that are limited to classically described undifferentiated spermatogonia and, by extension, spermatagonial stem cells (SSCs). Such studies have revealed that CDH1, GFRA1, LIN28, NANOS2, NANOS3, NEUROG3, ZBTB16, and POU5F1 are expressed by undifferentiated stem and progenitor spermatogonia, including Asingle, Apaired, and Aaligned 4–16 [9–16]. However, a unique marker that distinguishes Asingle spermatogonia and perhaps spermatogonial stem cells has been elusive.
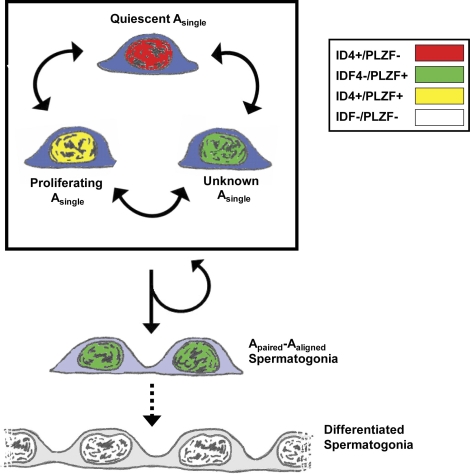
In the present issue, Oatley et al. identified ID4 as a marker restricted to Asingle spermatogonia in the testis and a protein essential for normal spermatogonial stem cell renewal both in vitro and in vivo [17]. The authors found that only a portion of ID4+ spermatogonia also stained for ZBTB16 (i.e., PLZF) in seminiferous tubule cross sections, indicating the presence of both ID4+/PLZF− and ID4+/PLZF+ Asingle spermatogonia (Fig. 1) and perhaps distinct subpopulations of SSCs. From the data provided, it is not known what proportion of total Asingle spermatogonia are marked by either ID4 or PLZF, so the possible existence of ID4−/PLZF+ or ID4−/PLZF− Asingles cannot be excluded (Fig. 1). These ID4 data support a growing theory in the field that the pool of rodent Asingle spermatogonia (and SSCs) is phenotypically heterogeneous, which may define unique subpopulations of these cells with potential functional differences. Heterogeneity among Asingles has been observed for CDH1, LIN28, NANOS2, and NANOS3 [13, 16, 18, 19]. Heterogeneity among transplantable SSCs has been revealed by studies on NEUROG3 and GFRA1 [10, 20]. While the significance of this heterogeneity is not well understood, it seems clear that a one-size-fits-all definition of Asingle spermatogonia and SSCs oversimplifies the stem cell system underlying spermatogenesis.
FIG. 1.
Phenotypic and functional heterogeneity among rodent SSCs. Within the pool of Asingle spermatogonia, some express only ID4 (red nuclei), while other Asingles also express PLZF (yellow nuclei), suggesting subpopulations of Asingles and SSCs. It is not known whether some Asingles express PLZF only (green nuclei) or neither marker (not shown). It is also not known whether all or some Asingle spermatogonia (or cells beyond Asingles) make up the SSC pool. Based on results of in vitro knockdown and in vivo knockout studies that suggest that ID4 participates in SSC self-renewal, Oatley et al. propose that ID4 delineates different functional subpopulations of SSCs (i.e., quiescent and proliferating).
Oatley et al. used two approaches to posit a functional role for ID4 in SSCs. First, ID4 knockdown in cultured SSCs resulted in stunted stem cell renewal without changes in germ cell amplification, suggesting a role for ID4 in maintenance of the SSC pool (i.e., self-renewal). Second, ID4 null mice showed progressive spermatogenic failure characteristic of a SSC self-renewal defect. Based on these data, Oatley's group suggested a model whereby the phenotypically distinct subpopulations of Asingle spermatogonia may represent functionally discrete populations. For example, ID4+/PLZF− Asingle spermatogonia may represent Asingles that are quiescent (Fig. 1, red nuclei) and/or that have self-renewing capacity and that these cells acquire PLZF expression on entering the cell cycle (green/yellow nuclei). Under this scenario, presence or persistence of ID4 at the subsequent cell division would favor a self-renewal fate decision, while absence or loss of ID4 might favor commitment to differentiation (e.g., production of Apaired and Aaligned spermatogonia).
The concept of a quiescent population of SSCs is similar to the A0/A1 model that was originally advanced for mouse and rat spermatogenesis [1, 21–23]. The A0/A1 model holds that normal spermatogenesis is maintained by an “active” pool of SSCs (A1) and that a quiescent “reserve” pool of A0 is mobilized following an insult to spermatogenesis. This model is also consistent with the Adark/Apale “reserve stem cell” model of primate SSCs [24]. Ultimately, the A0/A1 model was supplanted by the Asingle model [25, 26], in which a single population of stem cells (Asingle spermatogonia) divides regularly but infrequently and gives rise to the spermatogenic lineage. Recent pulse-chase experiments lend support for continual, steady-state renewal of the SSC pool in mice [27] but do not rule out the possibility of a phenotypically distinct, quiescent component. A model of rodent SSC contribution to normal spermatogenesis that incorporates a quiescent intermediate (whether truly “reserve” or simply “long cycling” [28]) would help unify our disparate understanding of spermatogenic lineage development in rodents and primates.
ID4 has the most restricted pattern of expression in undifferentiated spermatogonia observed to date and clearly delineates subpopulations of Asingle spermatogonia in the mouse testis. It is likely that molecular heterogeneity in the pool of Asingle spermatogonia have functional correlates that will be the focus of ongoing investigations. Whether the stem cell pool resides entirely in the population of Asingle spermatogonia or might be extended to include some Apaired or possibly larger chains, as suggested by Nakagawa et al. [8], is the subject of ongoing debate.
Footnotes
K.E.O. is supported by NIH grants R01 HD055475, R21 HD061289, and U54 HD08160 and the Magee-Womens Research Institute and Foundation. B.P.H. is supported by NIH grant K99 HD62687.
REFERENCES
- Clermont Y, Bustos-Obregon E. Re-examination of spermatogonial renewal in the rat by means of seminiferous tubules mounted “in toto.” Am J Anat 1968; 122: 237 247. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A 1994; 91: 11303 11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A 1994; 91: 11298 11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrinski I, Ogawa T, Avarbock MR, Brinster RL. Computer assisted image analysis to assess colonization of recipient seminiferous tubules by spermatogonial stem cells from transgenic donor mice. Mol Reprod Dev 1999; 53: 142 148. [DOI] [PubMed] [Google Scholar]
- Nagano M, Avarbock MR, Brinster RL. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol Reprod 1999; 60: 1429 1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwig KE, Shinohara T, Avarbock MR, Brinster RL. Functional analysis of stem cells in the adult rat testis. Biol Reprod 2002; 66: 944 949. [DOI] [PubMed] [Google Scholar]
- Nagano MC. Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol Reprod 2003; 69: 701 707. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 2010; 328: 62 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, Yamamoto M, Suda T. Nabeshima Yi. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Developmental Biology 2004; 269: 447 458. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell 2007; 12: 195 206. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 2004; 36: 647 652. [DOI] [PubMed] [Google Scholar]
- Greenbaum MP, Yan W, Wu MH, Lin YN, Agno JE, Sharma M, Braun RE, Rajkovic A, Matzuk MM. TEX14 is essential for intercellular bridges and fertility in male mice. Proc Natl Acad Sci U S A 2006; 103: 4982 4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda M, Kadokawa Y, Kurahashi H, Marunouchi T. CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol Reprod 2007; 76: 130 141. [DOI] [PubMed] [Google Scholar]
- Schlesser HN, Simon L, Hofmann MC, Murphy KM, Murphy T, Hess RA, Cooke PS. Effects of ETV5 (ets variant gene 5) on testis and body growth, time course of spermatogonial stem cell loss, and fertility in mice. Biol Reprod 2008; 78: 483 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Sada A, Yoshida S, Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev Biol 2009; 336: 222 231. [DOI] [PubMed] [Google Scholar]
- Zheng K, Wu X, Kaestner KH, Wang PJ. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev Biol 2009; 9: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley MJ, Kaucher AV, Racicot KE, Oatley JM. Inhibitor of DNA binding 4 is expressed selectively by single spermatogonia in the male germline and regulates the self-renewal of spermatogonial stem cells in mice. Biol Reprod 2011; 85: 347– 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada A, Suzuki A, Suzuki H, Saga Y. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science 2009; 325: 1394 1398. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Nabeshima Y, Nakagawa T. Stem cell heterogeneity: actual and potential stem cell compartments in mouse spermatogenesis. Ann N Y Acad Sci 2007; 1120: 47 58. [DOI] [PubMed] [Google Scholar]
- Buageaw A, Sukhwani M, Ben-Yehudah A, Ehmcke J, Rawe VY, Pholpramool C, Orwig KE, Schlatt S. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod 2005; 73: 1011 1016. [DOI] [PubMed] [Google Scholar]
- Dym M, Clermont Y. Role of spermatogonia in the repair of the seminiferous epithelium following x-irradiation of the rat testis. Am J Anat 1970; 128: 265 282. [DOI] [PubMed] [Google Scholar]
- Clermont Y, Hermo L. Spermatogonial stem cells in the albino rat. Am J Anat 1975; 142: 159 175. [DOI] [PubMed] [Google Scholar]
- Bartmanska J, Clermont Y. Renewal of type A spermatogonia in adult rats. Cell Tissue Kinet 1983; 16: 135 143. [PubMed] [Google Scholar]
- Clermont Y. Two classes of spermatogonial stem cells in the monkey (Cercopithecus aethiops). Am J Anat 1969; 126: 57 71. [DOI] [PubMed] [Google Scholar]
- Huckins C. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat Rec 1971; 169: 533 557. [DOI] [PubMed] [Google Scholar]
- Oakberg EF. Spermatogonial stem-cell renewal in the mouse. Anat Rec 1971; 169: 515 531. [DOI] [PubMed] [Google Scholar]
- Klein AM, Nakagawa T, Ichikawa R, Yoshida S, Simons BD. Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell 2010; 7: 214 224. [DOI] [PubMed] [Google Scholar]
- Huckins C. The spermatogonial stem cell population in adult rats. 3. Evidence for a long-cycling population. Cell Tissue Kinet 1971; 4: 335 349. [DOI] [PubMed] [Google Scholar]