Abstract
Culture systems that support development and maturation of oocytes in vitro with a high efficiency would have great impact not only on research addressed at underlying mechanisms of oocyte development but also on preservation of fertility. Recently, attention has turned to using culture systems that preserve follicle integrity, in contrast to existing systems that do not maintain follicle integrity, with the hope of improving oocyte development. We report that an alginate-based follicle culture system supports both follicular and oocyte growth in vitro, with little effect on the oocyte transcriptome. Nevertheless, oocytes obtained from these follicles exhibit an increased incidence of defects in spindle formation and chromosome alignment as well as pronounced abnormalities in cortical granule biogenesis. Developmental competence is also highly compromised, because few matured oocytes develop into 1-cell embryos with pronuclei. This situation contrasts with a high incidence of pronuclear formation following development using an existing in vitro culture system that does not preserve follicle integrity.
Keywords: assisted reproductive technology, gamete biology, gene expression, meiotic maturation, oocyte development
Although an alginate-based system that preserves follicle integrity supports oocyte development in vitro, developmental competence of the oocytes is compromised.
INTRODUCTION
An in vitro follicle culture system that supports acquisition of developmental competence of the enclosed oocytes would provide a valuable option for fertility preservation before gonadotoxic chemotherapy treatment. Currently, women wishing to preserve their fertility must undergo gonadotropin stimulation and oocyte or embryo cryopreservation, which often leads to a limited number of embryos available for transfer. The ability to isolate from a fragment of ovarian tissue hundreds of immature follicles that could develop in vitro to produce developmentally competent oocytes would provide a valuable alternative not only for these patients but also for prepubertal patients with cancer who are unable to undergo ovarian stimulation.
In a mouse model, multiple culture systems support growth of immature oocytes in vitro and have produced eggs capable of maturation, fertilization, and development into live offspring. These systems are classified as those that preserve or those that do not preserve three-dimensional follicle architecture and integrity. In the systems that do not preserve follicle integrity, granulosa cell-oocyte complexes are typically grown on a membrane for 10–12 days, until the oocytes are liberated from the complex to initiate maturation [1]. These systems, however, have been unsuccessful when translated to higher species [2, 3]. In a system that preserves follicle integrity, a fluid-filled antrum develops during the course of follicle culture [4]. The rationale for employing such a system is that it will better maintain cellular interactions between mural granulosa cells, cumulus cells, and oocytes and, thereby, improve the ability of the follicles and their enclosed oocytes to develop in vitro for long periods. Time becomes a critical factor for development of follicles from domestic animals and humans, in which folliculogenesis takes weeks to months [5–7] and mature follicles reach large diameters (e.g., 20 mm in humans).
Recently, a follicle culture system using an alginate matrix has been developed that is capable of producing live, healthy offspring in a mouse model. The cultured follicles, which reach diameters similar to those of follicles that develop in vivo, contain oocytes that achieve diameters indistinguishable from those of their in vivo brethren [8]. In the alginate system, granulosa cells proliferate and are hormonally active, secreting estradiol, progesterone, and androstenedione into the surrounding medium. Nevertheless, when early secondary follicles are cultured using the alginate system, the ability of the in vitro-grown oocytes to mature, be fertilized, and subsequently develop into blastocysts is quite limited [9].
To understand the molecular basis for why oocyte development is compromised following culture in this system, we examined the effect of culturing early secondary follicles in the alginate culture system on gene expression in the oocyte and oocyte maturation. We found that although the system supports robust oocyte growth and produces little perturbation of the transcriptome, the meiotic and developmental competence of the oocytes is highly compromised compared to oocytes that develop in a culture system that does not preserve follicle integrity.
MATERIALS AND METHODS
Follicle Collection and Culture
Twelve-day-old female CF1 mice were obtained from Harlan Sprague Dawley (Wilmington, MA) and housed in a temperature- and light-controlled environment at an animal facility at the University of Pennsylvania. Ovaries were isolated, and early secondary follicles (diameter, 100–130 μm) were manually isolated in L15 medium (Invitrogen) supplemented with bovine serum albumin (BSA; 1 mg/ml) using 27-gauge needles. Isolated follicles were maintained in a-minimal essential medium (aMEM; Invitrogen) supplemented with BSA (1 mg/ml) at 37°C in 5% CO2 in air. All experiments were performed with approval of and according to standards from the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Isolated follicles were encapsulated in 0.25% sodium alginate reconstituted in PBS; this concentration of alginate was previously shown to support the highest incidence of oocyte development [9]. Sodium alginate (55%–65% guluronic acid; a generous gift from Dr. Teresa Woodruff, Northwestern University, Feinberg School of Medicine, Chicago, IL) was purified as described previously [8]. Follicles were washed through a drop of alginate and then pipetted into an alginate droplet placed on a polypropylene mesh. The mesh was inverted and the droplet immersed into 50 mM calcium chloride for 2 min to allow cross-linking of the alginate. The alginate beads were then returned to maintenance medium and incubated at 37°C and 5% CO2 for 1 h. The alginate beads were then placed into 100-μl droplets of equilibrated growth medium (aMEM) supplemented with recombinant follicle-stimulating hormone [10 mIU/ml; EMD Serono], fetuin [1 mg/ml], insulin [5 μg/ml], transferrin [5 μg/ml], selenium [5 ng/ml], and BSA [3 mg/ml]) under washed mineral oil. Fetuin (provided by the Woodruff laboratory) was dialyzed against embryo-grade water and lyophilized before use. Follicles were cultured for 10 or 12 days at 37°C in an atmosphere containing 5% CO2 in air. Every other day, 50 μl of medium were removed and replaced with new equilibrated growth medium.
The follicle culture system described by Eppig et al. [10] was also used. Briefly, early secondary follicles were obtained from 12-day-old B6SJLF1 mice by digestion in aMEM supplemented with collagenase (type 1; 3 mg/ml; Worthington). The follicles were then placed on collagen-impregnated membranes (Costar Corp.) in aMEM supplemented with fetuin (1 mg/ml), insulin (5 μg/ml), transferrin (5 μg/ml), selenium (5 ng/ml), and BSA (3 mg/ml). After 10 days in culture, the cumulus-oocyte complexes were matured in aMEM supplemented with BSA (3 mg/ml), epidermal growth factor (EGF; 10 ng/ml), and fetuin (1 mg/ml).
Oocyte Maturation and In Vitro Fertilization
After 12 days in culture, antral follicles were removed from the alginate using growth medium supplemented with alginate lyase (10 IU/ml) for 15 min. Follicles were then placed in maturation medium (aMEM supplemented with 10% fetal bovine serum, EGF [5 ng/ml], and human chorionic gonadotropin [hCG; 1.5 IU/ml]) at 37°C and 5% CO2 in air for 16 h. Oocytes/eggs were liberated from the follicles by gentle pipetting with a glass-drawn pipette and briefly placed in 0.3% hyaluronidase. Once free of the surrounding cumulus cells, the oocytes were examined for the presence of a germinal vesicle (GV) or a polar body (PB). Eggs with a PB were placed in a drop of equilibrated TYH medium (Specialty Media) under mineral oil. Control eggs were obtained from 20-day-old female mice superovulated with 5 IU of equine chorionic gonadotropin (eCG) followed by 5 IU of hCG 48 h later. Eggs were obtained from the oviduct 13–15 h after hCG injection, the surrounding cumulus cells removed with a brief incubation in hyaluronidase, and the eggs placed in a drop of TYH medium.
Sperm was collected from the caudae epididymides of B6SJLF1 male mice (age, 8–12 wk; Jackson Laboratories). The epididymides were opened and the sperm allowed to swim into TYH medium and capacitate for 90 min. Eggs were inseminated with 500 000 sperm and maintained at 37°C in 5% O2, 5% CO2, and 90% N2 for 3 h. The eggs were then washed free of unbound sperm and placed in KSOM+AA (Specialty Media). The incidence of fertilization was assessed by calculating the number of embryos with two visible pronuclei (PN) over the total number of eggs inseminated in each group. The embryos were incubated in KSOM+AA at 37°C in 5% O2, 5% CO2, and 90% N2. The incidence of development to the blastocyst stage was calculated as the percentage of fertilized embryos that developed to the blastocyst stage. To control for in vitro fertilization (IVF) conditions, only experiments with fertilization rates in the control eggs of greater than 75% were analyzed. IVF experiments were also conducted with 12-day-old hybrid F1 females (C57BL/6J × CBA/Ca) to replicate previously published data and to determine whether strain-specific differences existed in the extent of fertilization and development.
Transcription Assay
After 10 and 12 days, in vitro-cultured follicles from CF1 mice were removed from the alginate as described above, and oocytes were liberated by puncturing the follicle and then denuded of cumulus cells by successive pipetting through a narrow-bore pipette. Control oocytes were obtained from 22- and 24-day-old mice primed with eCG 48 h before collection as previously described [11]. Oocytes were cultured in the presence of 2.5 μM milrinone and 4 mM 5-ethynyl uridine (5EU) in CZB [12] for 1 h and then fixed in 3.7% paraformaldehyde in PBS for 1 h at room temperature. 5EU incorporation into RNA was detected using the Click-IT EdU assay (Invitrogen) per the manufacturer's protocol. Fluorescence was detected on a Leica TCS SP laser-scanning confocal microscope and processed using Adobe PhotoShop software.
Transcript Profiling Using Microarrays
After 10 days in culture, oocytes were removed from the alginate and stripped of the surrounding cells as described above. Oocytes were snap-frozen in liquid nitrogen in groups of 20, which is sufficient for isolation, amplification, and labeling of RNA for microarray analysis [13]. Five groups of 20 in vitro-cultured oocytes were obtained from two consecutive experiments. Control oocytes were obtained from 22-day-old CF1 mice primed with 5 IU of eCG. The surrounding cumulus cells were similarly removed and the oocytes snap-frozen in groups of 20. RNA extraction and isolation were performed using the Acturus PicoPure RNA Isolation Kit (Applied Biosystems) according to the manufacturer's instructions. RNA was eluted in 11 μl of elution buffer.
Synthesis of cDNA, amplification, generation of sense-target cDNA, fragmentation, and labeling were performed using the WT-Ovation Pico RNA Amplification system, WT-Ovation Exon Module, and FL-Ovation cDNA Biotin Module V2 (all from NuGEN Technologies, Inc.) per the manufacturer's protocol. All samples were processed together, and 5 μg of each sample were submitted to the Penn Micro-Array Facility for Gene-Chip Hybridization. Samples of cRNA were hybridized to the Affymetrix GeneChip Mouse Exon 1.0 ST Array, then washed and stained on fluidics stations and scanned at a resolution of 3 μm according to the manufacturer's instructions (GeneChip Expression Analysis Technical Manual).
Statistical analysis of the microarray data was performed by the Penn Bioinformatics Core using Partek Genomics Suite to generate a gene list with statistical differences in gene expression. Hierarchical clustering analysis and principal components analysis were performed to assess the variability of the samples. A false-discovery rate of 5% was used to generate a list of genes with statistically significant differences in expression between the in vitro-cultured oocytes and the controls. The Database for Annotation, Visualization, and Integrated Discovery (DAVID) was used to assess whether the resulting gene list represented disruption of specific pathways or molecular interactions.
Live Imaging
Following in vitro culture, oocytes were stripped of the cumulus and placed in groups of 10 in 10 μl of equilibrated CZB medium under mineral oil. Control GV oocytes were isolated from mice primed with eCG and placed in 10-μl drops of medium. Using a Leica DMI4000B inverted microscope with computer-driven motorized X-Y-Z stage, groups of oocytes were imaged by differential interference contrast microscopy every 15 min for 17 h and recorded. The oocytes were maintained at 37°C and 5% CO2 in air throughout the imaging period using a heated stage and an environmental chamber. At each time point, the oocytes were analyzed for the number of oocytes having undergone GV breakdown (GVBD) and PB extrusion. After 17 h, the oocytes were fixed and stained as described below.
Immunocytochemistry
The GV oocytes and eggs from in vitro-cultured follicles and controls were obtained and matured as described above. Eggs were fixed in 3.7% paraformaldehyde in PBS for 1 h at room temperature, permeabilized in PBS with 0.3% BSA and 0.1% Triton X-100 for 15 min, and then blocked in PBS containing 0.3% BSA and 0.01% Tween 20. To visualize the spindle, eggs were incubated in anti-tubulin:fluorescein isothiocyanate (anti-tubulin:FITC; 1:150; Abcam) for 1 h, then washed and mounted in Vectashield (Vector Laboratories) and TO-PRO3 (1:250; Molecular Probes). To detect the actin cap, Alexa Fluor 633 phalloidin (1:100; Molecular Probes) was added during the incubation with anti-tubulin:FITC. Cortical granules (CGs) were detected with rhodamine-conjugated Lens culinaris agglutinin (1:200; Vector Laboratories) during incubation with anti-tubulin:FITC. Fluorescence was detected on a Leica TCS SP laser-scanning microscope and processed using Adobe PhotoShop software.
Statistical Analysis
Student t-test and Fischer exact tests were utilized to evaluate statistical significance between in vivo-grown and in vitro-cultured oocytes using Prism (GraphPad Software). A P-value of less than 0.05 was considered to be statistically significant.
RESULTS
Follicle Culture and IVF
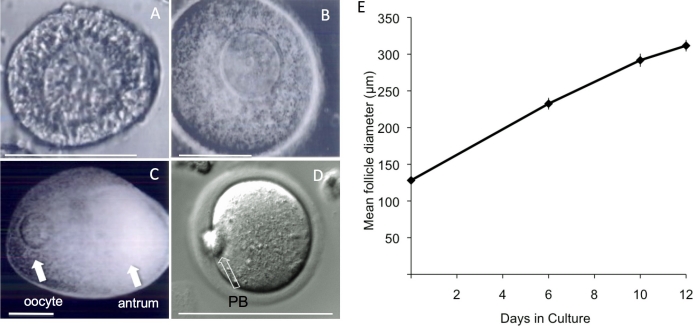
We first aimed to determine whether secondary oocytes from CF1 mice would develop in the alginate system. Not only was follicle growth supported by the alginate system, with formation of a clearly defined antrum (Fig. 1), the system also supported oocyte growth; no significant difference (P > 0.05) in oocyte diameter was found between oocytes that developed in vitro (70.7 ± 0.4 μm, mean ± SEM) and their in vivo-grown counterparts (71.8 ± 0.5 μm). Furthermore, no significant difference in oocyte size was found between oocytes grown in vitro for 10 days (70.7 ± 0.4 μm) versus those grown for 12 days (73.9 ± 0.7 μm).
FIG. 1.
Images of a representative follicle isolated from the ovaries of a 12-day-old CF1 mouse, in culture from day of encapsulation to Day 12 of culture. A) On Day 0, an early secondary follicle with an oocyte is surrounded by several layers of granulosa cells. B) By Day 6, the oocyte has moved eccentric within the follicle, and the granulosa cells continue to proliferate. C) By Day 12, a fluid-filled antrum has developed that separates mural granulosa cells from the cumulus cells around the oocyte. D) After removal from the alginate, the follicle is exposed to hCG. The majority of oocytes resume meiosis, as evident by the presence of a PB. E) Average growth over the 12-day culture period. The experiment was performed three times, and at least 80 follicles were analyzed for any stage. The data are expressed as mean ± SEM. Bar = 100 μm (A–D).
Next, we assessed meiotic competence of oocytes grown in vitro by examining the ability of the alginate-cultured oocytes to resume meiosis and expel the first PB. A high proportion (95%) of oocytes obtained from follicles from CF1 mice that developed in vitro for 12 days resumed meiosis and underwent GVBD, and 75% of these reached metaphase II (MII) as determined by the presence of a PB. We routinely observed that approximately 90% of oocytes matured in vitro reached and arrested at MII. Thus, acquisition of meiotic competence occurred during the course of follicle development in vitro.
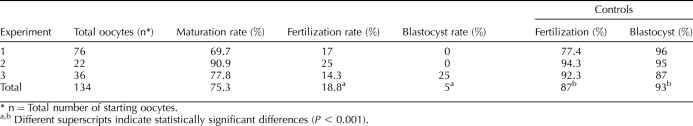
To assess developmental competence of the oocytes, we performed IVF using eggs derived from follicles that developed in vitro for 12 days and then were treated with EGF and hCG to induce oocyte maturation; oocytes that developed in vivo served as the control (Table 1). A total of 101 eggs obtained from follicles isolated from 12-day-old CF1 mice that developed in vitro were inseminated in three separate experiments, and fertilization was assessed by the presence of two PN. In addition, the embryos were cultured and later scored for blastocyst formation. Nineteen (19%) of the eggs were fertilized, and of these, one (5%) developed to the blastocyst stage. In contrast, of the 230 control eggs, 200 (87%) were fertilized, of which 186 (93%) developed to the blastocyst stage. Thus, acquisition of developmental competence was severely compromised after follicle development in vitro.
TABLE 1.
Percentage of oocytes following in vitro culture reaching MII, 2PN, and blastocyst stage after IVF compared to in vivo-grown control oocytes: CF1 mice.
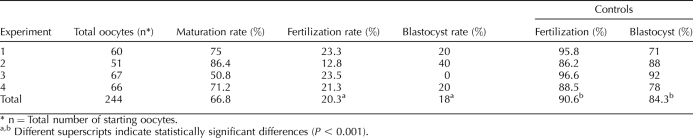
We repeated these experiments using the same mouse strain (F1 mice from a C57BL/6J × CBA/Ca cross) employed in a previous study [9] to replicate that study more closely. We found no difference (P > 0.05) in the percentage of oocytes able to expel a PB following maturation of oocytes obtained after follicle culture using CF1 mice (75%) or the F1 hybrid strain (67%). A total of 163 eggs from follicles grown in vitro from 12-day-old hybrid F1 females from a C57BL/6J × CBA/Ca were inseminated in four separate experiments, and fertilization was assessed by the presence of two PN and the embryos later scored for blastocyst formation (Table 2). Similar to what we observed with CF1 mice, developmental competence was highly compromised after follicle culture in vitro. The difference in the incidence of development to the blastocyst stage for the two strains was not significant (Fisher exact test, P > 0.05). Moreover, the low incidence of development to the blastocyst stage starting with MII eggs is similar to that previously reported (11%) [9].
TABLE 2.
Percentage of oocytes following in vitro culture reaching MII, 2PN, and blastocyst stage after IVF compared to in vivo-grown control oocytes: F1 from C57BL/6J x CBA/Ca.
Transcription and Gene Expression
To determine potential causes of poor fertilization and development of in vitro-grown oocytes, oocytes were examined for their ability to shut down transcription. A progressive decrease in transcription initiates around midgrowth, the time of antrum formation [14, 15], such that full-grown oocytes essentially are transcriptionally quiescent. Although it is not apparent why transcription ceases before completion of growth, it appears to be a universal hallmark, because it is observed in oocytes of all species examined to date (e.g., after eCG treatment, at least 80% of full-grown GV-stage oocytes become transcriptionally quiescent [16]). We examined at least 46 oocytes in two separate experiments and found that for CF1 mice, 89% of oocytes harvested from their follicles after 10 days in culture were transcriptionally silent; a similar incidence (97%; P > 0.05) was observed when oocytes were harvested after 12 days of follicle culture. In addition, no difference was found in the fraction of oocytes that were transcriptionally silent after follicle culture in vitro when compared to oocytes that developed in vivo (80.4%; P > 0.05).
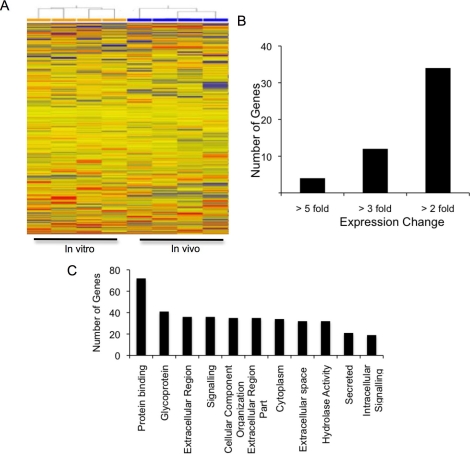
We performed microarray analysis to identify if differences in gene expression were responsible for the low fertilization and development of oocytes derived from follicles cultured in vitro. Hierarchical clustering analysis showed clustering of in vivo-grown oocytes and of in vitro-cultured oocytes (Fig. 2A). Using a false-discovery rate of 5% or less, only 135 genes were differentially expressed between the in vitro-cultured oocytes and in vivo-grown oocytes. Of these 135 differentially expressed genes, only 50 showed a greater than 2-fold difference in expression between the two groups (Fig. 2B and Supplemental Table S1, available online at www.biolreprod.org). DAVID annotation [17] is shown in Figure 2C.
FIG. 2.
A) Hierarchical clustering analysis of oocytes derived from the ovaries of CF1 mice after follicle culture in vitro (GV oocytes obtained from the alginate culture system) and from oocytes that developed in vivo (GV oocytes from 22-day-old, eCG-primed mice). B) Number of genes showing 5-, 3-, or 2-fold differences in expression between oocytes grown in the alginate culture system and in vivo. C) DAVID annotations to identify enriched biological theme and functional-related gene groups among the genes showing differential expression.
Timing of Meiotic Resumption and Maturation
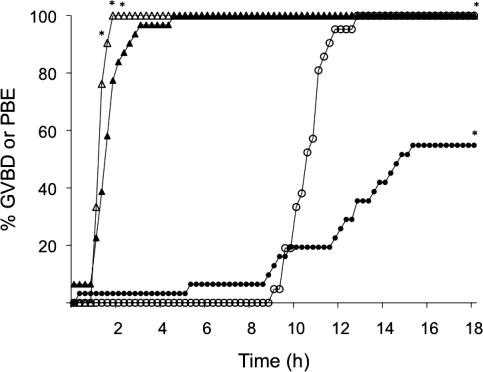
To ascertain whether oocyte maturation was compromised and could therefore account for the lower incidence of fertilization, we monitored the kinetics of maturation in vitro using live imaging (Fig. 3). Oocytes obtained from follicles harvested from ovaries of CF1 mice and developed in vitro resumed meiosis, as evidenced by GVBD, a little slower than control oocytes (e.g., >90% of in vivo-grown oocytes underwent GVBD within 90 min vs. 58% of in vitro-grown oocytes). Although no apparent difference was found in the time for onset of PB emission, significantly fewer of the in vitro-grown oocytes extruded a PB compared to the in vivo-grown group (55% vs. 100%, respectively) (Fig. 3).
FIG. 3.
Kinetics of maturation of oocytes derived from the ovaries of CF1 mice after follicle culture in vitro and from oocytes that develop in vivo. Triangles represent percentage of oocytes completing GVBD; circles represent percentage of oocytes expelling a PB (PBE). Solid symbols represent oocytes from the alginate culture system; open symbols represent oocytes from control mice. The experiment was performed two times, and at least 50 oocytes were examined for each time point. *P < 0.05.
Spindle and Chromosome Alignment
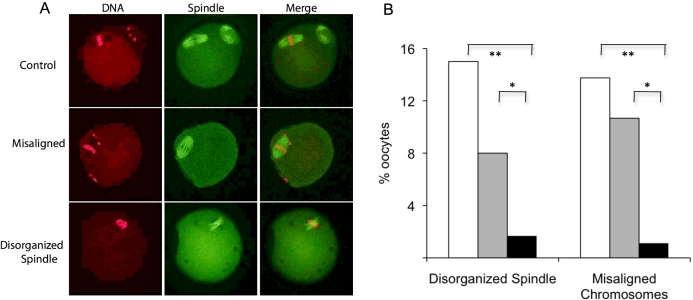
Problems in spindle formation and chromosome alignment on the spindle could contribute to the reduced developmental competence of oocytes obtained after follicle culture. Accordingly, we assessed the effect of culture on these parameters using denuded oocytes isolated from follicles and then matured in vitro. Control oocytes were full-grown oocytes that were developed in vivo, harvested from eCG-primed CF1 mice, and matured in vitro for 17 h. Oocytes were classified as either normal, having disorganized spindle configuration, or having misaligned chromosomes (Fig. 4A). If an oocyte had a disorganized spindle, chromosome alignment was not assessed; therefore, each oocyte received a single classification. Of the oocytes grown in vitro and matured free of the surrounding cumulus cells, 15% had a disorganized meiotic spindle, and 14% had at least one chromosome located outside of the metaphase plate, which was significantly higher than observed in the controls, in which only 2% had a disorganized spindle and 1% misaligned chromosomes.
FIG. 4.
Effect of follicle culture in vitro on oocyte maturation. A) MII eggs obtained from the ovaries of CF1 mice following culture in the alginate culture system and controls were fixed and stained for DNA (propidium iodide; red) and tubulin (green) and analyzed for chromosome alignment and spindle organization. If an egg had a disorganized spindle, it was not analyzed for chromosome alignment. B) Following in vitro follicle culture, oocytes were matured either outside of the cumulus cells (white bars) or within the follicle by exposure to hCG (gray bars). These oocytes were compared to oocytes that developed in vivo and were then matured in vitro (black bars). The experiment was performed three times, and at least 75 oocytes were analyzed for each group. *P ≤ 0.001, **P = 0.02.
Maturation of denuded oocytes can perturb spindle formation and chromosome spacing [18, 19]. It was formally possible that spindle formation and chromosome alignment was more susceptible to errors for oocytes that developed after follicle culture in vitro and then matured in the absence of their associated cumulus cells. Therefore, we matured oocytes that developed within follicles cultured in vitro by adding EGF and hCG to the medium. Seventeen hours later, the oocytes were harvested and analyzed for spindle formation and chromosome alignment. Oocytes cultured in vitro but initially matured within the surrounding follicle also displayed a higher incidence of spindle disorganization with misaligned chromosomes (11%) when compared to in vivo-grown controls (Fig. 4B). In addition, a trend toward a higher incidence of spindle abnormalities was found when oocytes were obtained from follicles cultured in vitro and then matured in the absence of their cumulus cells (15%) than when matured within the follicle (8%). This finding is consistent with previous studies demonstrating that maturation in the absence of surrounding cumulus cells predisposes the oocyte to errors of spindle assembly [19]. These findings suggest that in vitro follicle culture increases the susceptibility of oocytes to errors in spindle assembly during maturation.
CG Formation
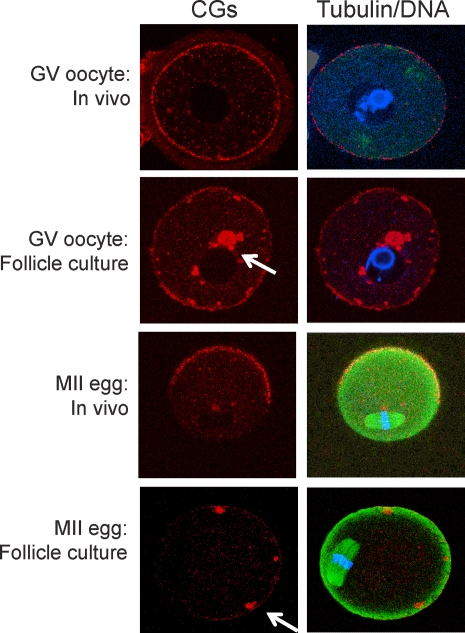
Cortical granules are secretory granules found in oocytes, and CG exocytosis plays a role in inducing the zona pellucida block to polyspermy [20, 21]. CGs display a uniform cortical distribution in the oocyte and in MII eggs. CGs are absent in the region of the spindle, the so-called CG-free domain (CGFD) [22, 23] (Fig. 5). We noted an absence of a CGFD in MII eggs that were derived from oocytes obtained from follicles from CF1 mice that developed in vitro (Fig. 5). The likely explanation for this finding is that CGs present in oocytes derived from follicles that developed in vitro were clumped and did not display a uniform cortical distribution. Thus, CG biogenesis/localization appeared to be impaired in these oocytes.
FIG. 5.
Effect of follicle culture in vitro on CG distribution. Following the isolation of secondary follicles from the ovaries of CF1 mice, follicles were cultured in vitro for 12 days. GV oocytes were then removed from the follicle or allowed to mature within the follicle. GV and MII eggs were fixed and stained for DNA (propidium iodide; blue), tubulin (green), or CGs (rhodamine-conjugated Lens culinaris agglutinin; red). All oocytes from the alginate culture system showed abnormal CG distribution in both the GV oocytes and the MII eggs, with clumping of the CGs (arrows). The experiment was performed two times, and at least 25 oocytes or eggs were analyzed in each group.
Oocyte Development in a Culture System That Does Not Preserve Follicle Integrity
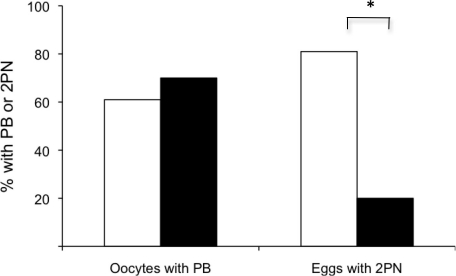
Because mouse strain did not appear to affect developmental competence, it was formally possible that the low incidence of developmental competence we observed after follicle development in vitro using the alginate culture system could be attributed to the experimental technique. Accordingly, we obtained oocytes using a culture system developed by Eppig et al. [24] that does not maintain follicle integrity and performed IVF on the resulting eggs and on in vivo-grown controls. Of the 334 oocytes obtained from 12-day-old B6SJLF1 mice following development in vitro with this system, 64% resumed meiosis and expelled the first PB, an incidence similar to that observed after follicle culture using the alginate system and the F1 mice from a C57BL/6J × CBA/Ca cross. Nevertheless, 81% of the eggs were fertilized, which equaled the incidence of fertilization of the in vivo-grown controls, an incidence far greater than that observed for the alginate system (Fig. 6). Thus, it is unlikely that poor experimental technique was responsible for the low incidence of developmental competence acquired after follicle development in vitro using the alginate system.
FIG. 6.
Acquisition of meiotic and developmental competence following culture in a two-dimensional (2D) system. Oocytes from a 2D culture system (white bars; n = 218) have significantly greater incidence of fertilization than oocytes from the three-dimensional alginate system (black bars; n = 163), as documented by the presence of two PN (2PN) after IVF* (P < 0.001). No significant difference was found in the percentage of oocytes able to reach the MII stage (P > 0.05).
DISCUSSION
We report that full-grown oocytes with a normal morphological appearance readily form after follicle culture in the alginate system and mature to MII at a high incidence in response to gonadotropin stimulation. Moreover, the developing oocytes undergo the transition to transcription quiescence that normally occurs, and the transcriptome of these oocytes is very similar (∼99.5%) to that of oocytes that develop in vivo. Nevertheless, consistent with results obtained from previous studies using the alginate system [9, 25], the developmental competence of these oocytes is compromised, as evidenced by the very low incidence of fertilization following maturation and insemination in vitro. A likely contributing factor to their reduced developmental competence is the increased incidence of abnormal spindle formation and chromosome alignment as well as abnormal CG biogenesis and failure to emit the first PB.
In spite of these aforementioned differences, it is striking that the transcriptome of oocytes that develop either in vitro or in vivo is virtually identical, with the relative abundance of only 135 transcripts differing between the two, and yet the developmental competence of the oocytes that develop in vitro is markedly reduced compared to their in vivo counterparts. It should be noted that because pools of oocytes were analyzed and these pools contained oocytes of both full and impaired developmental competence, the observed differences following culture may be underestimated. Examination of the affected transcripts affords no clear insight as to why oocytes that develop within follicles in the alginate culture system exhibit problems in CG biogenesis, spindle formation, and chromosome alignment.
The transcriptome of oocytes that develop in vitro using a culture system that does not maintain follicle integrity is also very similar to the transcriptome of oocytes that develop in vivo [13]. Nevertheless, only four of the affected transcripts are common—namely, Prkcb, Rai14, Cd200, and Optn. The relative abundance of each transcript is decreased to a similar extent following culture relative to that in oocytes that develop in vivo. That so few affected transcripts are shared between the two groups is perhaps not surprising, because the phenotypes differ: Oocytes that develop using the alginate system, but not the Eppig system, exhibit numerous problems in maturing properly to MII. What is surprising is the minimal impact of culture on the transcriptome that nevertheless leads to reduced developmental competence, albeit to different degrees, in both systems. A likely explanation is that posttranscriptional and posttranslational regulation is perturbed following culture in vitro. Transcript profiling, however, would not detect perturbations in such regulation. The molecular basis for the markedly reduced meiotic and developmental competence of oocytes that develop following culture using the alginate system remains unknown, and unfortunately, transcript profiling affords no insights regarding how to improve the culture conditions.
A goal of developing systems that support oocyte development in vitro has been to generate an experimentally tractable system that not only will yield insights regarding the molecular and cellular basis for acquisition of meiotic and developmental competence but also generate “high-quality” oocytes with all of the apparent clinical applications. An outcome of decades of research is that what is essential for oocyte development is preservation of follicle cell-oocyte interactions, which are often mediated by gap junction communication but also are medicated via paracrine effects. For example, oocytes apparently circumvent the problem of having a minimal ratio of surface area to volume by deriving metabolites (e.g., nucleotides and amino acids) via gap junction mediated-communication with their companion follicle cells [26]. In certain instances, transport systems and metabolic pathways are outsourced to the surrounding follicles cells [27]. Paracrine systems also operate in which oocyte-derived factors, such as bone morphogenetic protein 15 and growth differentiation factor 9, act on the follicle cells to generate a signal (e.g., kit ligand), which then acts on the oocyte [26], or control cholesterol synthesis in cumulus cells [28].
Given that maintaining proper oocyte-somatic cell interactions is paramount to supporting oocyte development, it may be premature to abandon efforts to improve systems that do not preserve follicle integrity, which in principle should not be constrained by follicle size. For example, one problem with these systems is their ability to maintain meiotic arrest for extended periods of time [29]. Cyclic GMP derived from follicle cells plays a central role in maintaining meiotic arrest by inhibiting phosphodiesterase 3A [30, 31]. Recent work has shown that natriuretic peptide type C (NPPC) derived from mural granulosa cells activates a guanylyl cyclase, natriuretic peptide receptor 2, present on the oocyte's companion somatic cells and, thereby, helps maintain meiotic arrest [32]. Thus, in principle, addition of NPPC to the culture medium could maintain meiotic arrest for longer periods of time and, thereby, enhance the acquisition of developmental competence that occurs at the end oocyte development [33].
Based on the present results and other published observations to date, it appears that the originally described alginate-based system does not efficiently generate meiotically and developmentally competent oocytes [34]. A recently published two-step strategy employing a fibrin-alginate matrix shows improvement over the initially described system [35], and further improvement may be possible by altering the time of culture or the culture medium. Unfortunately, the present lack of understanding as to the cause of poor oocyte development using the alginate culture system makes it challenging to design a systematic approach for improvement. In addition, additional and significant barriers likely will be encountered in species for which follicle diameter is much greater than that in mice (e.g., oxygen diffusion). Alginate-based systems, however, may potentially be very useful to study theca and mural granulosa cell development and function [9].
ACKNOWLEDGMENTS
The authors thank Christos Coutifaris, Marisa S. Bartolomei, and Karen Schindler for critically reading the manuscript. M.A.M. thanks Teresa Woodruff for supplying the alginate and fetuin as well as members of her laboratory for demonstrating the alginate system. M.A.M. also thanks John Eppig and Karen Wigglesworth for supplying culture media and instruction with their culture system and Paula Stein for teaching her many of the methods used in this study.
Footnotes
Supported by a grant from the Reproductive Scientists Development Program/NIH K12 HD000849-22 and T90/TL1-CA-133837 to M.A.M.
REFERENCES
- Eppig JJ. Mouse oocyte development in vitro with various culture systems. Dev Biol 1977; 60: 371 388. [DOI] [PubMed] [Google Scholar]
- Abir R, Franks S, Mobberley MA, Moore PA, Margara RA, Winston RM. Mechanical isolation and in vitro growth of preantral and small antral human follicles. Fertil Steril 1997; 68: 682 688. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Wright C, Krausz T, Hardy K, Winston RM. Human primordial, primary and secondary ovarian follicles in long-term culture: effect of partial isolation. Hum Reprod 1999; 14: 2519 2524. [DOI] [PubMed] [Google Scholar]
- Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials 2006; 27: 714 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod 1986; 1: 81 87. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Some aspects of the dynamics of ovarian follicular growth in the human. Acta Eur Fertil 1989; 20: 185 192. [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 1996; 17: 121 155. [DOI] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng 2006; 12: 2739 2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod 2006; 75: 916 923. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle-stimulating hormone and insulin. Biol Reprod 1998; 59: 1445 1453. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol 1983; 97: 264 273. [DOI] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil 1989; 86: 679 688. [DOI] [PubMed] [Google Scholar]
- Pan H, O'Brien M J, Wigglesworth K, Eppig JJ, Schultz RM. Transcript profiling during mouse oocyte development and the effect of gonadotropin priming and development in vitro. Dev Biol 2005; 286: 493 506. [DOI] [PubMed] [Google Scholar]
- Moore GPM. The RNA polymerase activity of the preimplantation mouse embryo. J Embryol Exp Morphol 1975; 34: 291 298. [PubMed] [Google Scholar]
- Moore GPM, Lintern-Moore S. Transcription of the mouse oocyte genome. Biol Reprod 1978; 17: 865 870. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, Eppig JJ. Transcriptional activity of the mouse oocyte genome: companion granulosa cells modulate transcription and chromatin remodeling. Dev Biol 2001; 229: 224 236. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4: 44 57. [DOI] [PubMed] [Google Scholar]
- Barrett SL, Albertini DF. Cumulus cell contact during oocyte maturation in mice regulates meiotic spindle positioning and enhances developmental competence. J Assist Reprod Genet 2010; 27: 29 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfins A, Lee GY, Plancha CE, Overstrom EW, Albertini DF. Distinctions in meiotic spindle structure and assembly during in vitro and in vivo maturation of mouse oocytes. Biol Reprod 2003; 69: 2059 2067. [DOI] [PubMed] [Google Scholar]
- Wolf DE, Edidin M, Handyside AH. Changes in the organization of the mouse egg plasma membrane upon fertilization and first cleavage: indications from the lateral diffusion rates of fluorescent lipid analogs. Dev Biol 1981; 85: 195 198. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Kurasawa S, Duffy P, Kopf GS, Schultz RM. Regulation of the polyspermy block in the mouse egg: maturation-dependent differences in cortical granule exocytosis and zona pellucida modifications induced by inositol 1,4,5-trisphosphate and an activator of protein kinase C. Biol Reprod 1993; 48: 1251 1257. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Rangarajan S, Anderson E. The development of mouse oocyte cortical reaction competence is accompanied by major changes in cortical vesicles and not cortical granule depth. Dev Biol 1988; 130: 789 792. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Duffy P, Reindollar R, Su B. Changes in the distribution of mouse oocyte cortical granules and ability to undergo the cortical reaction during gonadotropin-stimulated meiotic maturation and aging in vivo. Biol Reprod 1990; 43: 870 876. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Daniel SA, Schroeder AC. Growth and development of mouse oocytes in vitro. Ann N Y Acad Sci 1988; 541: 205 210. [DOI] [PubMed] [Google Scholar]
- Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod 2005; 73: 942 950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K, Eppig JJ. Society for Reproductive Biology Founders' Lecture 2005. Control of metabolic cooperativity between oocytes and their companion granulosa cells by mouse oocytes. Reprod Fertil Dev 2005; 17: 667 674. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med 2009; 27: 32 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Wigglesworth K, O'Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 2008; 135: 111 121. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K. Factors affecting the developmental competence of mouse oocytes grown in vitro: oxygen concentration. Mol Reprod Dev 1995; 42: 447 456. [DOI] [PubMed] [Google Scholar]
- Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 2009; 136: 1869 1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari S, Weeks JL, II, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod 2009; 81: 595 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 2010; 330: 366 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod 1989; 41: 268 276. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Hosoe M, O'Brien MJ, Pendola FM, Requena A, Watanabe S. Conditions that affect acquisition of developmental competence by mouse oocytes in vitro: FSH, insulin, glucose and ascorbic acid. Mol Cell Endocrinol 2000; 163: 109 116. [DOI] [PubMed] [Google Scholar]
- Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early stage ovarian follicles in the mouse. Fertil Steril 2010; 93: 2633 2639. [DOI] [PMC free article] [PubMed] [Google Scholar]