Abstract
The success of postnatal uterine morphogenesis dictates, in part, the embryotrophic potential and functional capacity of the adult uterus. The definitive role of Wnt7a in postnatal uterine development and adult function requires a conditional knockout, because global deletion disrupts müllerian duct patterning, specification, and cell fate in the fetus. The Wnt7a-null uterus appears to be posteriorized because of developmental defects in the embryo, as evidenced by the stratified luminal epithelium that is normally found in the vagina and the presence of short and uncoiled oviducts. To understand the biological role of WNT7A after birth and allow tissue-selective deletion of Wnt7a, we generated loxP-flanked exon 2 mice and conditionally deleted Wnt7a after birth in the uterus by crossing them with PgrCre mice. Morphological examination revealed no obvious differences in the vagina, cervix, oviduct, or ovary. The uteri of Wnt7a mutant mice contained no endometrial glands, whereas all other uterine cell types appeared to be normal. Postnatal differentiation of endometrial glands was observed in control mice, but not in mutant mice, between Postnatal Days 3 and 12. Expression of morphoregulatory genes, particularly Foxa2, Hoxa10, Hoxa11, Msx1, and Wnt16, was disrupted in the Wnt7a mutant uteri. Conditional Wnt7a mutant mice were not fertile. Although embryos were present in uteri of mutant mice on Day 3.5 of pregnancy, blastocyst implantation was not observed on Day 5.5. Furthermore, expression of several genes (Foxa2, Lif, Msx1, and Wnt16) was reduced or absent in adult Wnt7a-deleted uteri on Day 3.5 postmating. These results indicate that WNT7A plays a critical role in postnatal uterine gland morphogenesis and function, which are important for blastocyst implantation and fertility in the adult uterus.
Keywords: developmental biology, female reproductive tract, pregnancy, transgenic/knockout model, uterus
Conditional deletion of Wnt7a in the neonatal mouse uterus compromises endometrial gland development and fertility.
INTRODUCTION
The histological organization of the adult uterine endometrium consists of a simple columnar luminal epithelium (LE) supported by stromal cells that contain coiled endometrial glands lined by simple columnar epithelial cells or glandular epithelium (GE) [1–3]. Circular and longitudinal layers of smooth muscle, termed the myometrium, surround the endometrium. Development of the uterus begins prenatally with formation, patterning, and then fusion of the müllerian ducts [4–6]. Although the organogenetic development and differentiation of most female reproductive tract organs from the müllerian ducts are complete at birth, the uterus is neither fully developed nor differentiated at birth. Establishment of tissue-specific uterine histoarchitecture is only completed postnatally in laboratory rodents, domestic animals, and humans [1, 7–9]. Postnatal radial patterning morphogenesis establishes the three classic histological elements of the uterine wall: the endometrium, the myometrium, and the perimetrium. Events common to postnatal morphogenesis of uteri include coordinated development of the endometrial glands from the LE, organization and stratification of endometrial stroma, and differentiation and growth of the myometrium [1, 6–8].
At birth, the neonatal mouse uterus lacks endometrial glands and consists of a simple LE supported by relatively undifferentiated mesenchyme [3]. Between birth (Postnatal Day [PND] 0) and PND 3, the three layers of mesenchyme are distinctly segregated into radially oriented endometrial stroma and inner circular and prospective outer longitudinal myometrial layers. By PND 6, epithelial invaginations appear that represent formation of GE buds [10]. By PND 12, endometrial glands extend from the LE into the surrounding endometrial stroma, and the outer longitudinal layer of the myometrium is fully organized into bundles [3]. The basic adult configuration of the uterus in mice is established by PND 15 [11]. Postnatal uterine morphogenesis is a critical period, because disruption of endometrial adenogenesis and mesenchymal specification and differentiation can cause permanent fertility problems in the adult [12–14]. Moreover, proper development of the endometrial stroma and myometrium is crucial for endometrial receptivity and decidualization as well as for expulsion of the fetus at term [15].
Postnatal uterine morphogenesis is complex and governed by intrinsic stromal-epithelial interactions that are precisely orchestrated by multifactorial gene networks as well as endocrine hormones after puberty [16]. Wnt genes encode secreted glycoproteins that are homologous to the Drosophila segment polarity gene wingless (wg) and regulate stem cell fate, cell differentiation, and tissue growth [17] via canonical and noncanonical signaling pathways [18]. The canonical WNT signaling pathway involves binding of frizzled (FZD) receptors and inhibition of catenin (cadherin-associated protein), beta 1 (CTNNB1) degradation, resulting in nuclear translocation and activation of target genes. Noncanonical WNT signaling encompasses a variety of signaling pathways that involve different WNT receptors (i.e., ROR2 and RYK) and mediators (i.e., calcium, MAPK8/9/10, and CAMK2) that regulate cell migration and movement. A subset of Wnts (Wnt4, Wnt5a, and Wnt7a) is involved in müllerian duct patterning and differentiation during development of the female reproductive tract in the embryo [14, 19, 20]. Postnatal uterine morphogenesis also involves a number of different Wnts and their signaling pathways. Total ablation of Wnt7a and Wnt5a or conditional ablation of Wnt4 and Ctnnb1 in the uterus after birth alters postnatal uterine development [21–23].
Adult Wnt7a-null mice are viable but infertile and exhibit malformations in the female reproductive tract, including shortened and uncoiled oviducts, hypoplastic uterine horns, and a vaginal septum tract [23, 24]. Wnt7a-null female mice display abnormal morphogenesis along the anteroposterior and radial axes of the uterine horn during postnatal development [20]. The most prominent feature is an absence of uterine glands, which are normally derived from the LE, and a reduction in the mesenchymally derived uterine stroma so that the inner circular and outer longitudinal layers of myometrium, which are present but reduced and disorganized, are much closer to the endometrial LE. Thus, in the absence of Wnt7a from conception, both epithelial and mesenchymal differentiation is disrupted in the uterus. Available evidence supports the idea that the abnormalities in epithelial growth and tubulogenesis in the uteri of Wnt7a-null mice is caused by müllerian duct dysgenesis in the embryo [25, 26]. The female reproductive tract of adult Wnt7a-null mice appears to be posteriorized, in which the posterior oviduct is more similar to the uterus and the uterus has characteristics of the vagina [23]. The Wnt7a-null uterus has a stratified LE (in contrast to simple columnar LE in the wild-type uterus) surrounded by a shallow stromal layer that does not contain glands. A stratified type of LE is characteristic of the vagina but not of normal uterus or oviduct. The myometrium appears to be hyperplastic and disorganized by 3 mo of postnatal development, and by 6 mo, the endometrial stroma is displaced by the myometrium [23, 27]. Although to our knowledge it has not been investigated in the uterus, WNT7A signals through the canonical WNT/catenin, beta 1 signaling pathway in other cells and organs [28–31]. Recent studies indicate that the canonical WNT-catenin signaling is required for mesenchymal differentiation into stroma and myometrium [32], endometrial gland development [21], estrogen-induced uterine growth [33], and implantation in adult mice [34].
The definitive role of Wnt7a in postnatal uterine development and function requires a conditional knockout, because global deletion of this gene in the embryo disrupts fetal müllerian duct patterning, specification, and cell fate. To understand the biological role of WNT7A in the neonate and adult, we generated loxP-flanked exon 2 mice and conditionally deleted Wnt7a after birth by crossing them with PgrCre mice. The conditional ablation of Wnt7a after birth disrupted endometrial gland development and expression of morphoregulatory genes in the neonate, which resulted in a failure of blastocyst implantation in the adult. Thus, Wnt7a has an important biological role in postnatal uterine development.
MATERIALS AND METHODS
Animals and Tissue Collection
Mice were maintained in the designated animal care facility at Texas A&M University-College Station according to the institutional guidelines for the care and use of laboratory animals, and animal procedures were approved by the Institutional Animal Care and Use Committee. Postnatal samples were obtained after parturition, and the day that pups were observed was considered to be PND 1. Pregnancy samples were obtained by the mating of mice, and the day that a vaginal plug was observed was considered to be Gestational Day (GD) 0.5. At the time of necropsy, reproductive tract tissues were flash-frozen and stored at −80°C or fixed with 4% (vol/vol) paraformaldehyde and paraffin embedded.
Construction of the Targeting Vectors and Generation of Chimeric Mice
The Wnt7a gene contains four exons and three introns, and targeted deletion of exon 2 was used to create the original Wnt7a-null allele [24]. A targeting construct was made using a bacterial artificial chromosome (BAC) library clone (RPCI-23: Female (C57BL/6J) Mouse BAC Library) containing mouse Wnt7a from BACPAC Resources (Children's Hospital Oakland Research Institute). The targeting construct, containing Wnt7a fragment with exons 1 and 2, with 2 kb of the 5′ arm and 2 kb of the 3′ arm homology regions (Supplemental Fig. S1A, all Supplemental Data are available online at www.biolreprod.org), was transfected into 5–10 × 107 E14TG2a embryonic stem cells from 129Sv strain using a Bio-Rad gene pulser. Embryonic stem cell clones were selected positively with G418 (200 μg/ml; Mediatech, Inc.) for presence of the PGK-neo cassette and negatively with ganciclovir (2 μM; InvivoGen) for absence of the MC1TK cassette. Southern blot analysis was used to screen genomic DNA with XbaI digestion using 3′ external probes (Supplement Fig. S1B). Correctly targeted clones were microinjected into blastocysts derived from C57BL/6 mice. The F1 agouti male offspring mice were analyzed by PCR genotyping and Southern blot analysis to validate germ line transmission of the Wnt7a flox-neo (Wnt7aflneo) allele.
Generation of a Wnt7a Conditional Null Allele
B6.129-Pgrtm2(cre)Lyd (PgrCre) mice were provided by Drs. Franco DeMayo and John Lydon of Baylor College of Medicine [35]. B6.Cg-Tg(ACTFLPe)9205Dym (FLPe, Jax 005703) were obtained from the Jackson Laboratory. A PGK-neo cassette flanked by two Frt sites was removed from the Wnt7afloxneo/+ allele by crossing the mice to FLPe mice [36], which resulted in generating the Wnt7af/+ allele. Wnt7af/f mice were then obtained by intercrossing heterozygous Wnt7af/+ mice. Both the Wnt7afloxneo/+ and the Wnt7af/+ mice were viable and did not display any abnormalities.
The PCR analysis was used to genotype DNA extracts from tail biopsy specimens. Genomic DNA was extracted from mouse tail biopsy specimens via digestion with 300 μl of tail lysis buffer (5 mM ethylenediaminetetra-acetic acid, 200 mM NaCl, 100 mM Tris, and 0.2% SDS) with 0.25 mg of Proteinase K (Sigma Chemical Co., St. Louis, MO) overnight at 55°C. Following incubation, 1 ml of 100% ethanol was added to each tube, and samples were then vortexed and centrifuged at 16 000 × g for 30 min. Pelleted DNA was washed with 1 ml of 70% ethanol and resuspended in 150 μl of Tris-EDTA buffer (10 mM Tris, 50 mM EDTA).
Genomic DNA from each sample was reverse transcribed in a total reaction volume of 50 μl. Briefly, DNA (50 ng) was combined with appropriate primer mix (W1 and W2 or P1, P2, and P3; Integrated DNA Technologies), dNTP MIX (2.5 mM each; TaKaRa Bio, Inc.), 10× ExTaq Buffer (TaKaRa Bio, Inc.), and TaKaRa Ex Taq (TaKaRa Bio, Inc.). Primers P1, P2, and P3 (P1, 5′-ATG TTT AGC TGG CCC AAA TG-3′; P2, 5′-TAT ACC GAT CTC CCT GGA CG-3′; P3, 5′-CCC AAA GAG ACA CCA GGA AG-3′) were used to amplify the Pgr wild-type (285-bp) and Cre (590-bp) alleles (Supplementary Fig. S2A). To detect the Wnt7a flox allele, primers W1 and W2 (W1, 5′-CAC AGC CAC CCC TAG AGA GCT CAA TT-3′; W2, 5′-ATG CTT TGC CAG GGA ACA CCC-3′) were designed to amplify the fragments from the wild-type (135-bp) and floxed (180-bp) alleles (Supplemental Fig. S2B). All PCR reactions were carried out for 35 cycles of 95°C for 45 sec, 61°C for 1 min, and 72°C for 1 min.
RNA Isolation and Quantitative Real-Time RT-PCR Analysis
To investigate the effect of Wnt7a deletion on changes of gene expression in the uterus, quantitative real-time RT-PCR analysis was conducted on RNA extracted from uterine tissues using the Qiagen RNeasy Mini Kit. Total RNA from each sample was reverse transcribed in a total reaction volume of 20 μl. Briefly, total RNA (800 ng) was combined with primer mix containing oligo(dT) primer (0.2 μg/ml), random hexamer primer (300 μg/ml; Invitrogen), and dNTP MIX (10 mM each) and incubated at 65°C for 5 min. An RT mix containing 5× First-Strand Buffer, 0.1 M dithiothreitol, and SuperScript II Reverse Transcriptase (Invitrogen) was added to the reaction, and reverse transcription was performed under the following conditions: 25°C for 10 min, 42°C for 60 min, and 70°C for 5 min. Control reactions in the absence of reverse transcriptase were prepared for each sample to test for genomic DNA contamination. Real-time PCR analysis was performed using an ABI prism 7900HT system (Applied Biosystems). Expression levels of Fzd6, Fzd10, Hoxa10, Hoxa11, Ihh, Lif, Msx1, Msx2, Wnt4, Wnt5a, Wnt7a, Wnt11, Wnt16, and Vangl2 were measured with Power SYBR Green PCR Master Mix (Applied Biosystems) using specific oligonucleotide primers designed by the Oligo 5 program (Molecular Biology Insights, Inc.) (Supplemental Table S1). Mouse Rpl13a was used as a reference gene. Expression of Foxa2 was determined by real-time RT-PCR TaqMan analysis with TaqMan Gene Expression Master Mix (Applied Biosystems) and normalized against Rn18s. Real-time probes and primers for Foxa2 and Rn18s were purchased from Applied Biosystems. Each individual sample was run in triplicate using the following conditions: 50°C for 2 min, 95°C for 10 min, and then 40 cycles of 95°C for 15 sec and 60°C for 1 min. A dissociation curve was generated at the end of amplification to ensure that a single product was amplified. The threshold line was set in the linear region of the plots above the baseline noise, and threshold cycle (Ct) values were determined as the cycle number at which the threshold line crossed the amplification curve.
Immunohistochemistry
Uteri were fixed overnight in 4% (vol/vol) paraformaldehyde, followed by thorough washing in 70% ethanol, and tissues were processed, embedded in paraffin, and sectioned. Uterine sections from paraffin-embedded tissue were cut at a thickness of 5 μm and mounted on slides, deparaffinized, and rehydrated in a graded alcohol series. Sections were subjected to antigen retrieval using boiling citrate buffer, preincubated with 10% normal goat serum in PBS (pH 7.5), and then incubated with appropriate primary antibody. Sections were incubated with either rabbit anti-FOXA2 antibody (catalog no. WRAB-FOXA2; Seven Hills Bioreagents) diluted 1:2000 in 10% normal serum in PBS (pH 7.5) or rabbit anti-MKI67 antibody (catalog no. ab66155; Abcam) diluted 1:750 in 1% bovine serum albumin in PBS (pH 7.5). On the following day, sections were washed in PBS and incubated with biotinylated secondary antibody (5 μg/ml; Vector Laboratories) for 1 h at room temperature. Immunoreactive protein was detected using the Vectastain Elite ABC Kit (Vector Laboratories) and diaminobenzidine tetrahydrocholoride as the chromagen.
Statistical Analyses
All quantitative data were subjected to least-squares ANOVA using the general linear models procedures of the Statistical Analysis System (SAS Institute, Inc.). In all analyses, error terms used in tests of significance were identified according to the expectation of the mean squares for error. For analysis of real-time PCR data, the Ct values of the target mRNA were analyzed for effects of day, genotype (control or mutant), and their interaction with the Rpl13a values used as a covariate. Significance (P < 0.05) was determined by probability differences of least-square means (LSMs). The data are presented as the LSM and overall SEM.
RESULTS
Generation of Mice with Conditional Ablation of Wnt7a in the Uterus
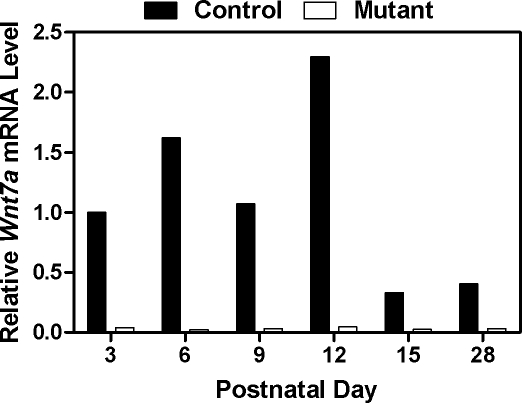
Ablation of Wnt7a leads to disruption of paramesonephric duct differentiation in the fetus [23]. To further investigate the biological role of Wnt7a in the uterus after birth, the Wnt7a gene was floxed and used to create Wnt7a floxed (Wnt7af/+) mice (see Supplemental Fig. S1A for the targeting strategy). Conditional ablation of Wnt7a in the uterus was conducted by crossing Wnt7af/f mice with PgrCre/+ mice [35]; PgrCre mice are an excellent model to conditionally ablate genes in the uterus after birth [37]. Cre excision activity in the PgrCre mouse model is restricted to cells that express the PGR after birth, including the uterus, ovary, oviduct, pituitary gland, and mammary gland [35]. In the neonate, PGR expression is initiated after birth in the uterine epithelia by PND 3 and in the stroma by PND 6 [37, 38]. As expected, real-time PCR analysis confirmed the deletion of Wnt7a expression in the developing postnatal uterus of Pgrcre/+Wnt7af/f mutant mice as compared to Pgr+/+Wnt7af/f control mice (Fig. 1).
FIG. 1.
Analysis of Wnt7a conditionally ablated in the uterus. The expression level of Wnt7a was measured in the uterus of Pgr+/+Wnt7af/f control and PgrCre/+Wnt7af/f mutant mice by real-time RT-PCR. The primers were specific for exon 2, which is floxed and deleted by Cre. Data are presented as the fold-change relative to the Wnt7a mRNA level on PND 3 in uteri from control mice.
Impact of Conditional Ablation of Wnt7a on Female Fertility
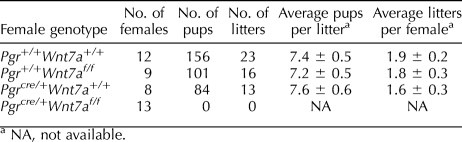
To determine the effect of conditional ablation of WNT7A on female fertility, female control mice (Pgr+/+Wnt7a+/+, Pgrcre/+Wnt7a+/+, and Pgr+/+Wnt7af/f) and female Pgrcre/+Wnt7af/f mutant mice were bred to Pgr+/+Wnt7a+/+ male mice. During the time of observation, control mice exhibited normal fecundity, whereas the Pgrcre/+Wnt7af/f mutant mice were completely infertile (Table 1). Thus, postnatal ablation of WNT7A after birth in the uterus is detrimental to female fertility.
TABLE 1.
Effect of conditional ablation of Wnt7a on female fertility.
Postnatal Ablation of Wnt7a Inhibits Endometrial Gland Development in the Uterus
Because the PgrCre mouse model recombines alleles in multiple reproductive tissues [35], we first assessed the impact of Wnt7a ablation on postnatal development of the female reproductive tract. On PND 28, no gross anatomical differences were observed in the female reproductive tract of control and mutant mice (Fig. 2), and weight of the uterus was not different (P > 0.10) (data not shown). As illustrated in Figure 2, no histoarchitectural differences were observed in the ovary and oviduct from control and mutant mice on PND 28. Although the uteri of control mice contained many glands in the endometrium, the uteri of mutant mice contained no endometrial glands. No other consistent histoarchitectural differences were noted in the endometrium or myometrium of the uterus from control and mutant mice. Furthermore, no obvious differences were observed in the ovary or oviducts between control and mutant mice (Supplemental Fig. S3).
FIG. 2.
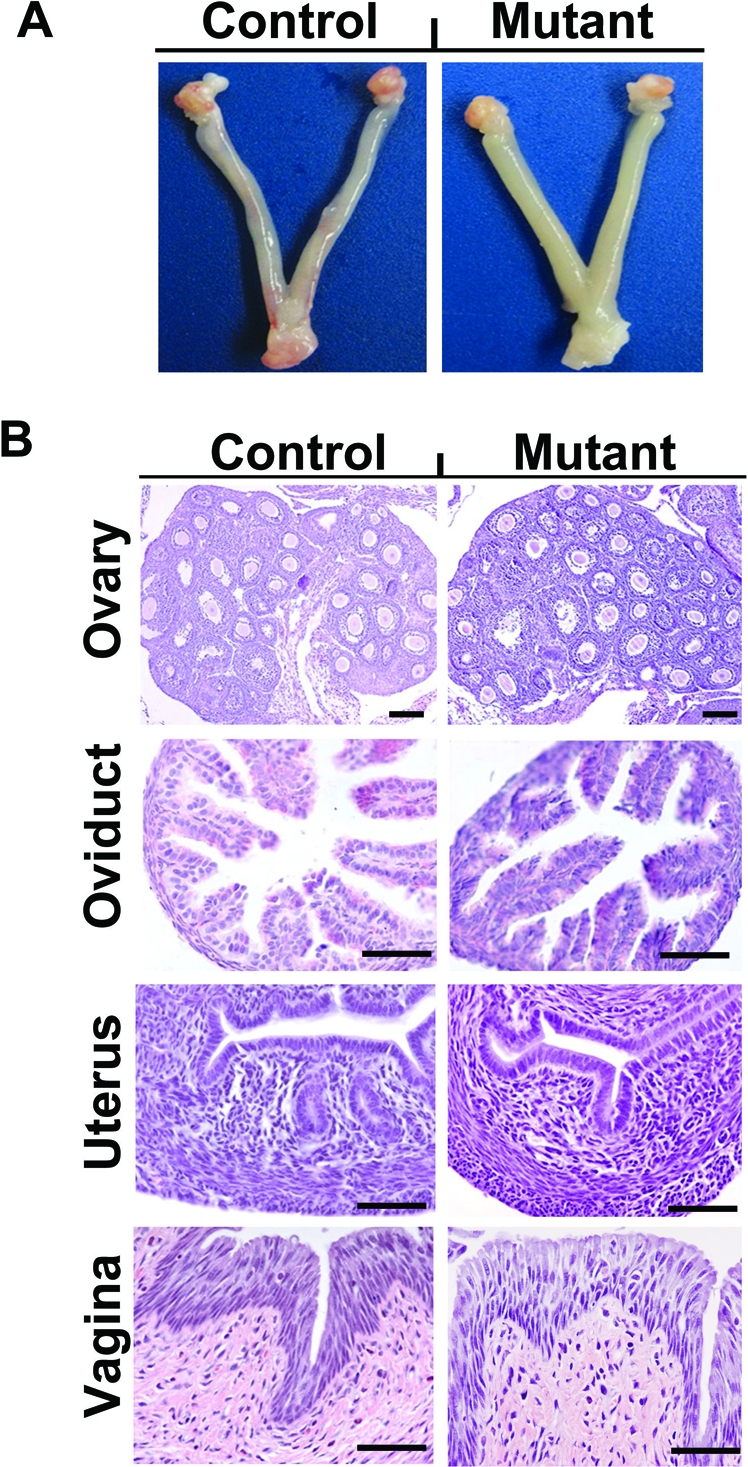
Postnatal ablation of Wnt7a disrupts uterine development. A) Gross anatomy of the female reproductive tract of Pgr+/+Wnt7a+/+ control and PgrCre/+Wnt7af/f mutant mice on PND 28. B) Histological analysis of the ovary, oviduct, uterus, and vagina of Pgr+/+Wnt7a+/+ control and PgrCre/+Wnt7af/f mutant mice on PND 28. Reproductive tract tissue sections were stained with hematoxylin and eosin. No differences in histoarchitecture of the ovary, oviduct, or vagina were observed between control and mutant mice. Note the absence of endometrial glands in the uteri of mutant as compared to control mice. Bar = 50 μm.
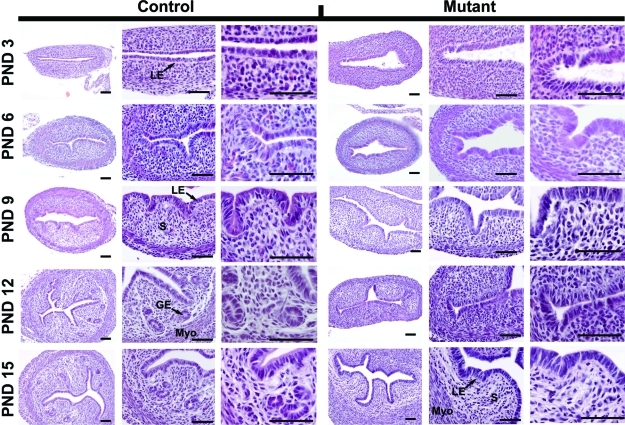
In the developing mouse uterus, endometrial glands begin to differentiate on PND 5 or 6 and are well developed by PND 15 [11, 16]. In control mice of the present study, endometrial glands were absent on PND 3 but were budding from the LE by PND 6 and present in the stroma by PND 12 (Fig. 3). In contrast, endometrial gland development was not observed in the uteri of mutant mice. As noted previously, no other histoarchitectural differences were observed in either the endometrium or myometrium. Therefore, conditional deletion of Wnt7a in the developing mouse uterus after birth disrupts endometrial gland morphogenesis, resulting in a glandless uterus in the adult.
FIG. 3.
Endometrial gland development is disrupted by postnatal ablation of Wnt7a. Histological analysis of uteri collected on PNDs 3, 6, 9, 12, and 15 from Pgr+/+Wnt7af/f control mice (left) and PgrCre/+ Wnt7af/f mutant mice (right). Myo, myometrium; S, stroma. Bar = 50 μm.
Postnatal Deletion of Wnt7a in the Uterus Does Not Affect Cell Proliferation But Alters Gene Expression
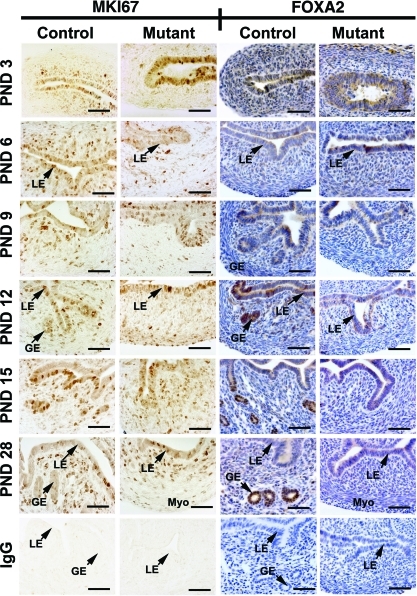
Null mutation of Wnt7a in the embryo alters cell proliferation and apoptosis in the postnatal reproductive tract [39]; therefore, we assessed effects of conditional deletion of Wnt7a on cell proliferation using immunohistochemistry for MKI67 (Ki67), a cellular marker of proliferation. In control mice, immunoreactive MKI67 protein was observed in the nuclei of both LE and stromal cells in the developing uterus (Fig. 4). Note the abundance of proliferating LE and GE cells in the PND 12 and PND 15 uteri from control mice as shown in Figure 4. Postnatal deletion of Wnt7a had no discernible effects on cell proliferation in any compartment of the uterus.
FIG. 4.
Immunohistochemical localization of MKI67 and FOXA2 protein in the uteri of Pgr+/+Wnt7af/f control mice (left) and PgrCre/+ Wnt7af/f mutant mice (right). Cell proliferation was assessed by immunostaining uteri for MKI67. Sections were not counterstained with hematoxylin and eosin after MKI67 localization but were counterstained after FOXA2 localization. Myo myometrium; S, stroma. Bar = 50 μm.
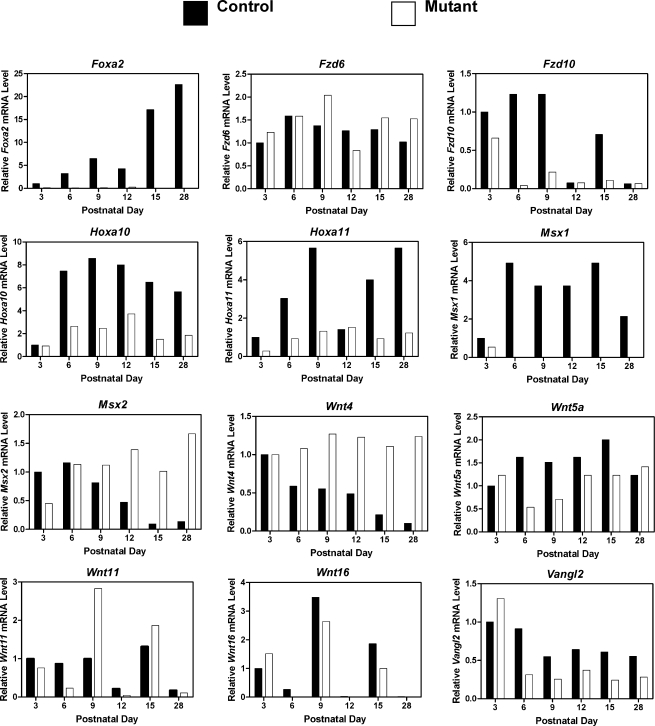
A carefully orchestrated interplay of Hox and Wnt genes regulate the prenatal morphogenesis of the female paramesonephric duct [4, 5, 40]. Several of those genes (Hoxa10, Hoxa11, Wnt4, Wnt5a, and Wnt7a), their signaling pathways, and target genes such as Msx1 are hypothesized or have been found to regulate endometrial gland development as well as myometrial differentiation in the postnatal uterus [41]. As illustrated in Figure 5, real-time RT-PCR analysis revealed that postnatal deletion of Wnt7a disrupted normal patterns of Fzd10, Hoxa10, Hoxa11, Msx1, Msx2, Wnt4, Wnt5a, Wnt11, and Vangl2 expression in the uterus (day × genotype, P < 0.01). Note that Msx1 expression is essentially lost after PND 3 in the uteri of mutant mice. In contrast, relative levels of Msx2 and Wnt4 mRNA were elevated in mutant uteri during postnatal development (day × genotype, P < 0.01). Furthermore, expression patterns of Fzd6 and Wnt16 were not different (P > 0.10) in uteri of control and mutant mice. CTNNB1 protein was present predominantly in the uterine epithelia and was not different between control and mutant uteri during postnatal development (data not shown).
FIG. 5.
Postnatal ablation of Wnt7a alters gene expression in the developing uterus. The relative mRNA levels of the indicated genes was measured in the uterus of Pgr+/+Wnt7af/f control and PgrCre/+Wnt7af/f mutant mice by real-time RT-PCR. Data are presented as the fold-change relative to the mRNA level on PND 3 in uteri from control mice.
Recently, Jeong et al. [12] found that conditional deletion of Foxa2 in the postnatal uterus using PgrCre resulted in a complete loss of endometrial glands and that FOXA2 protein was present solely in the endometrial glands of the adult uterus. As illustrated in Figure 5, Foxa2 mRNA levels increase substantially in the uteri of control mice after PND 3 in association with the development of endometrial glands. Note the presence of FOXA2 protein in the nuclei of endometrial glands in control mice after PND 9 as shown in Figure 4. Interestingly, FOXA2 protein is observed in the cytoplasm of LE cells on PNDs 3–15 and in some uterine mesenchymal and endometrial stromal cells. However, FOXA2 protein is restricted to the nuclei of endometrial glands in PND 28 control uteri. In contrast, no increase in Foxa2 expression was found (day × genotype, P < 0.001) in the uteri of mutant mice that lack endometrial glands (Fig. 5). Although cytoplasmic FOXA2 protein was observed in the LE of both control and mutant mice from PND 3 to PND 28, FOXA2 protein was only observed in the nuclei of endometrial glands after PND 9 in uteri of control mice (Fig. 4).
Postnatal Deletion of Wnt7a Impacts Adult Uterine Function and Compromises Blastocyst Implantation
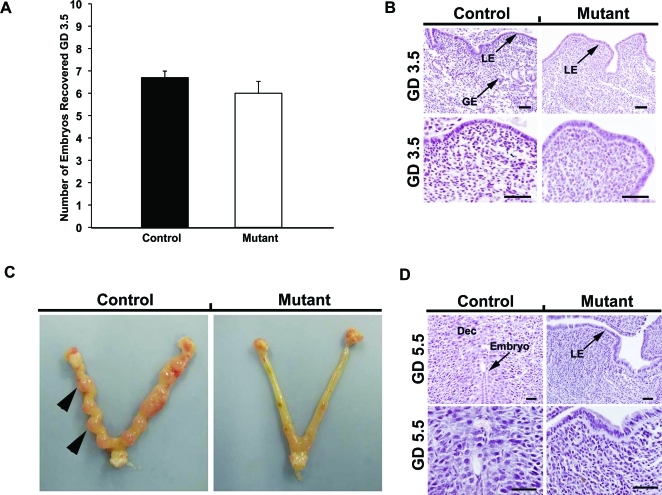
Conditional ablation of Wnt7a after birth resulted in complete infertility (Table 1). To determine the underlying cause of the infertility, control and mutant mice were bred and the uterus flushed on GD 3.5. As illustrated in Figure 6A, no difference (P > 0.10) was found in the number of embryos recovered from the uterus of control and mutant mice. The embryos recovered from both control and mutant mice were blastocysts of normal size and morphology. The uteri of both control and mutant mice were histoarchitecturally similar except that mutant uteri lacked endometrial glands (Fig. 6B). Implantation was observed in the uteri of control mice on GD 5.5 but not in the uteri of mutant mice (Fig. 6C). Note the presence of an implanted embryo and decidualized stroma in the uteri of control mice on GD 5.5 as shown in Figure 6D.
FIG. 6.
Implantation defect in the PgrCre/+Wnt7af/f mutant mice. A) Embryos recovered from and histology of the control and mutant uterus on GD 3.5. The error bar denotes standard error of the mean number of embryos. B) Note the absence of endometrial glands (GE) in the uteri of mutant mice. C) Implantation sites in the control and mutant uterus on GD 5.5. The results represent results of five independent mice per genotype. Gross anatomy of the mutant uteri shows an absence of implantation sites (arrows) compared with controls. D) Implanted blastocysts are observed in the uteri of control mice on GD 5.5 but not in PgrCre/+Wnt7af/f mutant uteri. Dec, decidua; S, stroma. Bar = 50 μm.
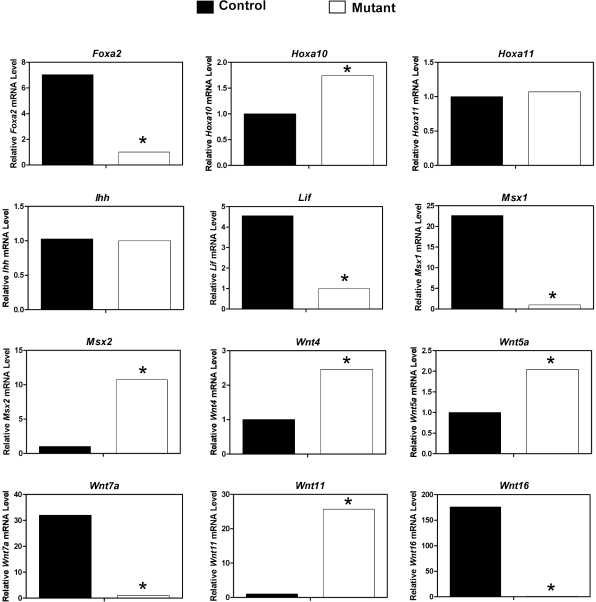
The expression of a number of genes that regulate endometrial receptivity and/or blastocyst implantation (Foxa2, Hoxa10, Hoxa11, Ihh, Lif, Msx1, and Wnt4) as well as Wnts and their receptors were measured in the uteri of control and mutant mice on GD 3.5 (Fig. 7). A striking reduction (P < 0.05) was found in expression of Foxa2, Lif, Msx1, Wnt7a, and Wnt16 in the uteri of mutant mice. In contrast, expression of Wnt11, Msx2, Wnt4, Wnt5a, and Hoxa10 was increased (P < 0.05) in the uteri of mutant as compared to control mice. No difference in expression of Ihh or Hoxa11 was detected (P > 0.10) in the uteri of control as compared to mutant mice.
FIG. 7.
Postnatal ablation of Wnt7a alters expression of genes in the uterus that are important for blastocyst implantation. The relative mRNA level for the indicated genes was measured in the uterus of Pgr+/+Wnt7af/f control and PgrCre/+Wnt7af/f mutant mice on GD 3.5 by real-time RT-PCR. Data are presented as the fold-change relative to the mRNA level on GD 3.5 in uteri from control mice. The asterisk denotes a significant difference (P < 0.05) in mRNA levels.
DISCUSSION
To understand the role of WNT7A in postnatal development of the uterus, we generated mice in which Wnt7a was ablated in the uterus using mice with floxed Wnt7a alleles and the PgrCre mouse model [35]. The resulting female mutant mice were infertile, with an inability of the uterus to support embryo implantation. We observed that loss of Wnt7a in the uterus after birth in the PgrCre/+Wnt7af/f neonatal mutant mice disrupted differentiation and growth of the endometrial glands without altering differentiation of the stroma or myometrium or inducing stratification of the LE. The uterine dysgenesis observed in the Wnt7a conditional mutant mice is less severe than that in global Wnt7a-null mice, likely because the uterine mesenchyme has already differentiated into endometrial stroma and myometrium by PND 3 and Wnt5a and Wnt7a expression becomes restricted to the uterine portion of the female reproductive tract after birth [20]. Indeed, the uteri of adult Wnt7a-null mice appear to be posteriorized, as evidenced by the stratified LE that is normally found in the vagina and the presence of short and uncoiled oviducts [23, 27]. Collectively, these results support the ideas that WNT7A is a secreted protein from the LE that acts on the mesenchyme in a paracrine manner during fetal life to regulate cell fate, differentiation, and survival in the müllerian duct and that global deletion of Wnt7a in the embryo disrupts epitheliomesenchymal interactions that elicit defects in all uterine cell types as well as other müllerian duct-derived structures, including the oviduct, cervix, and vagina, given that Wnt7a is expressed throughout müllerian duct epithelia [39].
Until recently, little was known about the cellular and molecular mechanisms governing uterine gland differentiation and development. However, a recent study found that conditional ablation of Foxa2 using the PgrCre mouse model results in an absence in uterine glands [12]. Foxa2 has an important role in epithelial budding and morphogenesis in many different epitheliomesenchymal organs, including the liver, lung, and prostate [42, 43]. FOXA2 likely has a biological role in gland specification via transcriptional regulation of differentiation genes [12]. One possible signaling pathway that either regulates FOXA2 or is regulated by FOXA2 is the WNT signaling pathway, because mouse models in which Wnt7a, Wnt5a, and Ctnnb1 are ablated all lack uterine glands [21–23, 39]. Indeed, FOXA2 has been shown to regulate the expression of multiple Wnts, including Wnt3a, Wnt8a, and Wnt7b [44–46], and a reciprocal interaction between FOXA2 and WNT signaling has been noted in that CTNNB1, a downstream effector of canonical WNT signaling, can promote Foxa2 [47]. WNT7A signals through the canonical WNT/catenin, beta 1 signaling pathway in other cells and organs [28–31]. In mice with conditional ablation of Foxa2, no differences in Wnt7a or Wnt5a expression were observed in the adult uterus [12]. The low levels of Foxa2 mRNA in the Wnt7a conditional mutant uterus is primarily due to the lack of endometrial glands that abundantly express Foxa2 in uteri of control mice. Whether or not WNT7A is upstream of FOXA2 in the regulatory cascade governing uterine adenogenesis remains to be determined.
In the developing neonatal mouse uterus, Hoxa10, Hoxa11, Wnt4, Wnt5a, and Wnt16 are expressed in the endometrial stroma, whereas Msx1, Wnt7a, Wnt11, Fzd6, Fzd10, and Vangl2 are expressed in the uterine epithelia of neonatal mice [40, 41, 48]. In the present study, analyses of gene expression revealed that several genes were reduced (Fzd10, Hoxa10, Hoxa11, Msx1, Wnt5a, Wnt11, Wnt16, and Vangl2) or increased (Msx2, Wnt4, and Wnt11) by Wnt7a ablation and, thus, may be implicated in endometrial gland dysgenesis observed in the conditional mutant mice. The complete absence of Msx1 and Wnt16 in the neonatal and adult uteri of conditional mutant mice indicates that they, along with Foxa2 governed by WNT7A in the postnatal uterus, are primary genes. The phenotype of Wnt16 ablated mice has not been reported, but Wnt16 is expressed in the stroma of the developing and adult mouse uterus [41, 49]. In other cells, Msx1 and several Fzds are regulated by canonical WNT signaling [50], and conditional deletion of Ctnnb1 using the PgrCre mouse model ablates uterine gland differentiation and induces stratification and squamous cell metaplasia of the LE [21]. Null mutation of the Msx1 gene results in perinatal lethality [51]. To our knowledge, conditional mutation of Msx1 has not been reported, although it has been implicated as a regulator of implantation in the mouse [26]. Alterations in other genes that are implicated in uterine development in the fetus and neonate, such as Hoxa10, Hoxa11, and Vangl2 [48, 52–54], were also reduced only in the uteri of neonatal Wnt7a mutant mice in the present study. In contrast, Hoxa10 and Hoxa11 were reduced in the uterine stroma of Wnt7a-null adult mice [23]. Interestingly, the conditional loss of Wnt7a in the present study increased expression of Msx2, Wnt4, and Wnt11 in both the neonate and adult, suggesting that Wnt7a negatively regulates their expression. Conditional ablation of Wnt4 using the PgrCre mouse model results in reduced numbers of uterine glands as well as stratification of the LE and defects in decidualization and embryo implantation. In contrast, conditional ablation of Wnt11 using the PgrCre mouse model had no effects on uterine gland development or fertility [41]. However, the effect of overexpression of Msx2, Wnt4, and Wnt11 on uterine development and function has not been reported. Available studies support the idea that WNT7A from the LE in the developing postnatal uterus acts on the stroma and, perhaps, the LE itself and signals via the canonical catenin, beta 1 pathway to orchestrate changes in gene expression governing endometrial gland differentiation and development in the neonate. The conditional Wnt7a mouse should be useful to determine genes and regulatory cascades important for tissue development and growth.
The major histological defect in the uterus was the absence of endometrial glands. Because the PgrCre mouse model recombines alleles in all compartments of the uterus as well as the ovary, oviduct, vagina, and pituitary [35], we determined if infertility was caused by the uterine defect or, perhaps, pituitary or ovarian defects. No histological or functional defects were observed in the ovary, oviduct, vagina, or pituitary, because unimplanted and morphologically normal embryos could be recovered on Day 3.5 postmating. Therefore, the lack of uterine glands is likely the key defect that underlies the infertility and lack of embryo implantation in the mutant mice. Indeed, the lack of endometrial glands in Foxa2 conditional mutant mice [12] as well as uterine gland knockout ewes [13] results in severe subfertility or infertility, respectively, that manifests in a lack of embryo implantation. Whether or not delayed embryo attachment is observed in mice lacking Wnt7a or Foxa2 remains to be determined. Endometrial glands and their secretions are critical regulators of peri-implantation survival of the conceptus and implantation as well as establishment of uterine receptivity and decidualization [13, 55–57]. Several signaling pathways necessary for implantation have been identified [37, 58]. Foxa2 and Lif mutant mice exhibit defects in both implantation and decidualization [12, 59]. Leukemia inhibitory factor (LIF) is secreted by the uterine glands in response to nidatory estrogen on GD 3.5 [60, 61] and is also expressed in the subluminal stroma at the implantation site [62]. In the present study, Foxa2 and Lif were much reduced in the GD 3.5 uterus of Wnt7a mutant mice, which is likely caused by the absence of endometrial glands. Indeed, uterine gland production of LIF is critical for embryo implantation [61]. Thus, the absence of uterine glands and LIF is probably the cause of embryo implantation failure in the Wnt7a conditional mutant. Future studies will determine the impact of Wnt7a conditional ablation on decidualization, which is likely given that decidualization defects occur in the Foxa2 conditional mutant [12]. Thus, products of the endometrial glands are definitively required for embryo implantation as well as subsequent decidualization that regulates placental growth and development.
In summary, the present results concerning conditional ablation of Wnt7a in the uterus support the hypothesis that WNT7A is a critical regulator of postnatal uterine morphogenesis and, in particular, plays a pivotal role in the specification, differentiation, and development of the GE within the endometrium. The lack of endometrial glands resulted in an inability of the uterus to support embryo implantation. Future studies are needed to determine the regulatory cascades promulgated by WNT7A that regulate the critical process of endometrial morphogenesis after birth and potential roles of WNT7A in adult uterine function. The Wnt7a conditional mouse will be a particularly valuable model to undertake those studies as well as determine the role of WNT7A in other organs.
ACKNOWLEDGMENTS
The authors appreciate the assistance and advice of Dr. Allison Stewart of the University of Texas M.D. Cancer Center and Dr. Heather Franco of Baylor College of Medicine.
Footnotes
Supported by National Institutes of Health grants 1 R21 HD054679 (to T.E.S.) and 1 R01 HD30284 (to R.R.B.). Portions of this work will be presented at the 44th Annual Meeting of the Society for the Study of Reproduction, July 31–August 4, 2011, Portland, Oregon.
REFERENCES
- Cunha GR. Epithelial-stromal interactions in development of the urogenital tract. Int Rev Cytol 1976; 47: 137 194. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Bigsby RM, Cooke PS, Sugimura Y. Stromal-epithelial interactions in adult organs. Cell Differ 1985; 17: 137 148. [DOI] [PubMed] [Google Scholar]
- Brody JR, Cunha GR. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: I. Normal development. Am J Anat 1989; 186: 1 20. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet 2003; 4: 969 980. [DOI] [PubMed] [Google Scholar]
- Yin Y, Ma L. Development of the mammalian female reproductive tract. J Biochem (Tokyo) 2005; 137: 677 683. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Hayashi K, Hu J, Carpenter KD. Comparative developmental biology of the mammalian uterus. : Schatten G. (ed.), Current Topics in Developmental Biology. Burlington, MA: Elsevier; 2005: 85 122. [DOI] [PubMed] [Google Scholar]
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod 2001; 65: 1311 1323. [DOI] [PubMed] [Google Scholar]
- Bartol FF, Wiley AA, Floyd JG, Ott TL, Bazer FW, Gray CA, Spencer TE. Uterine differentiation as a foundation for subsequent fertility. J Reprod Fertil Suppl 1999; 54: 287 302. [PubMed] [Google Scholar]
- Bartol FF, Wiley AA, Spencer TE, Vallet JL, Christenson RK. Early uterine development in pigs. J Reprod Fertil Suppl 1993; 48: 99 116. [PubMed] [Google Scholar]
- Branham WS, Sheehan DM, Zehr DR, Ridlon E, Nelson CJ. The postnatal ontogeny of rat uterine glands and age-related effects of 17beta-estradiol. Endocrinology 1985; 117: 2229 2237. [DOI] [PubMed] [Google Scholar]
- Hu J, Gray CA, Spencer TE. Gene expression profiling of neonatal mouse uterine development. Biol Reprod 2004; 70: 1870 1876. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Kwak I, Lee KY, Kim TH, Large MJ, Stewart CL, Kaestner KH, Lydon JP, DeMayo FJ. Foxa2 is essential for mouse endometrial gland development and fertility. Biol Reprod 2010; 83: 396 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 2002; 124: 289 300. [PubMed] [Google Scholar]
- Sassoon D. Wnt genes and endocrine disruption of the female reproductive tract: a genetic approach. Mol Cell Endocrinol 1999; 158: 1 5. [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev 2004; 25: 341 373. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Carpenter KD, Hayashi K, Hu J. Uterine glands. : Davies JA. (ed.), Branching Morphogenesis. Georgetown: Landes Biosciences; 2005. [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev 2000; 14: 1837 1851. [PubMed] [Google Scholar]
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 2009; 10: 468 477. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signaling. Nature 1999; 397: 405 409. [DOI] [PubMed] [Google Scholar]
- Miller C, Pavlova A, Sassoon DA. Differential expression patterns of Wnt genes in the murine female reproductive tract during development and the estrous cycle. Mech Dev 1998; 76: 91 99. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, Lydon JP, DeMayo FJ. Beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 2009; 28: 31 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development 2004; 131: 2061 2072. [DOI] [PubMed] [Google Scholar]
- Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 1998; 125: 3201 3211. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 1998; 395: 707 710. [DOI] [PubMed] [Google Scholar]
- Pavlova A, Boutin E, Cunha G, Sassoon D. Msx1 (Hox-7.1) in the adult mouse uterus: cellular interactions underlying regulation of expression. Development 1994; 120: 335 345. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Song H, Guo Y, Riesewijk A, Mosselman S, Das SK, Dey SK. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol Endocrinol 2004; 18: 1238 1250. [DOI] [PubMed] [Google Scholar]
- Miller C, Degenhardt K, Sassoon DA. Fetal exposure to DES results in deregulation of Wnt7a during uterine morphogenesis. Nat Genet 1998; 20: 228 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldahl LC, Park M, Malbon CC, Moon RT. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol 1999; 9: 695 698. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet 2000; 16: 279 283. [DOI] [PubMed] [Google Scholar]
- Caricasole A, Ferraro T, Iacovelli L, Barletta E, Caruso A, Melchiorri D, Terstappen GC, Nicoletti F. Functional characterization of WNT7A signaling in PC12 cells: interaction with a FZD5:LRP6 receptor complex and modulation by Dickkopf proteins. J Biol Chem 2003; 278: 37024 37031. [DOI] [PubMed] [Google Scholar]
- Katoh M, Kirikoshi H, Terasaki H, Shiokawa K. WNT2B2 mRNA, up-regulated in primary gastric cancer, is a positive regulator of the WNT-beta-catenin-TCF signaling pathway. Biochem Biophys Res Commun 2001; 289: 1093 1098. [DOI] [PubMed] [Google Scholar]
- Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol 2005; 288: 276 283. [DOI] [PubMed] [Google Scholar]
- Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol 2004; 18: 3035 3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/beta-catenin signaling is required for implantation. Proc Natl Acad Sci U S A 2005; 102: 8579 8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005; 41: 58 66. [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet 2000; 25: 139 140. [DOI] [PubMed] [Google Scholar]
- Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol 2008; 19: 178 186. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (mullerian) epithelial differentiation. Dev Biol 2001; 240: 194 211. [DOI] [PubMed] [Google Scholar]
- Carta L, Sassoon D. Wnt7a is a suppressor of cell death in the female reproductive tract and is required for postnatal and estrogen-mediated growth. Biol Reprod 2004; 71: 444 454. [DOI] [PubMed] [Google Scholar]
- Masse J, Watrin T, Laurent A, Deschamps S, Guerrier D, Pellerin I. The developing female genital tract: from genetics to epigenetics. Int J Dev Biol 2009; 53: 411 424. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Yoshioka S, Reardon SN, Rucker EB, III, Spencer TE, Demayo FJ, Lydon JP, Maclean JA., II WNTs in the neonatal mouse uterus: potential regulation of endometrial gland development. Biol Reprod 2011; 84: 308 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns 2004; 5: 193 208. [DOI] [PubMed] [Google Scholar]
- Kaestner KH. The FoxA factors in organogenesis and differentiation. Curr Opin Genet Dev 2010; 20: 527 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J Biol Chem 2005; 280: 13809 13816. [DOI] [PubMed] [Google Scholar]
- Tamplin OJ, Kinzel D, Cox BJ, Bell CE, Rossant J, Lickert H. Microarray analysis of Foxa2 mutant mouse embryos reveals novel gene expression and inductive roles for the gastrula organizer and its derivatives. BMC Genomics 2008; 9: e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. Beta-catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 2003; 278: 40231 40238. [DOI] [PubMed] [Google Scholar]
- Yu X, Wang Y, Jiang M, Bierie B, Roy-Burman P, Shen MM, Taketo MM, Wills M, Matusik RJ. Activation of beta-catenin in mouse prostate causes HGPIN and continuous prostate growth after castration. Prostate 2009; 69: 249 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg AL, Sassoon DA. Noncanonical Wnt signaling regulates cell polarity in female reproductive tract development via van gogh-like 2. Development 2009; 136: 1559 1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Erikson DW, Tilford SA, Bany BM, Maclean JA, III, Rucker EB, III, Johnson GA, Spencer TE. Wnt genes in the mouse uterus: potential regulation of implantation. Biol Reprod 2009; 80: 989 1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert J, Epping M, Pollack JR, Brown PO, Nusse R. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol 2002; 2: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satokata I, Maas R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet 1994; 6: 348 356. [DOI] [PubMed] [Google Scholar]
- Wong KH, Wintch HD, Capecchi MR. Hoxa11 regulates stromal cell death and proliferation during neonatal uterine development. Mol Endocrinol 2004; 18: 184 193. [DOI] [PubMed] [Google Scholar]
- Gendron RL, Paradis H, Hsieh-Li HM, Lee DW, Potter SS, Markoff E. Abnormal uterine stromal and glandular function associated with maternal reproductive defects in Hoxa-11 null mice. Biol Reprod 1997; 56: 1097 1105. [DOI] [PubMed] [Google Scholar]
- Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development 1996; 122: 2687 2696. [DOI] [PubMed] [Google Scholar]
- Bagchi IC, Cheon YP, Li Q, Bagchi MK. Progesterone receptor-regulated gene networks in implantation. Front Biosci 2003; 8: S852 S861. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab 2002; 87: 2954 2959. [DOI] [PubMed] [Google Scholar]
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol 2000; 223: 217 237. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. Mouse models of implantation. Trends Endocrinol Metab 2007; 18: 234 239. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukemia inhibitory factor. Nature 1992; 359: 76 79. [DOI] [PubMed] [Google Scholar]
- Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci U S A 1991; 88: 11408 11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, Stewart CL. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology 2000; 141: 4365 4372. [DOI] [PubMed] [Google Scholar]
- Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol 2000; 14: 1147 1161. [DOI] [PubMed] [Google Scholar]