Abstract
Preserving the uterus in a state of relative quiescence is vital to the maintenance of a successful pregnancy. Elevated cytoplasmic levels of uterine caspase 3 during pregnancy have been proposed as a potential regulator of uterine quiescence through direct targeting and disabling of the uterine contractile architecture. However, despite highly elevated levels of uterine caspase 3 during pregnancy, there is minimal evidence of apoptosis. This current study defines the mechanism whereby the pregnant uterine myocyte may harness the tocolytic activity of active caspases while avoiding apoptotic cell death. Using the pregnant mouse model, we have analyzed the uterus for changes in pro- and antiapoptotic signaling patterns associated with the advancing stages of pregnancy. Briefly, we have found that members of the IAP family, such as SURVIVIN and XIAP, and the Bcl2 family members, such as MCL1, are elevated in the uterine myocyte during late gestation. The IAP family members are the only endogenous inhibitors of active caspase 3, and MCL1 limits activation of caspase 3 by suppressing proapoptotic signaling. Elevated XIAP levels partner with SURVIVIN, resulting in increased levels of the antiapoptotic MCL1 via NFKB activation; these together have the potential to limit both the activity and level of active caspase 3 in the pregnant uterus as term approaches. We propose that modification of these antiapoptotic signaling partners allows the pregnant uterus to escape the apoptotic action of elevated active caspase 3 levels but also functions to limit the levels of active uterine caspase 3 near term.
Keywords: apoptosis, caspase, female reproductive tract, labor, myometrium, NFKB, pregnancy, uterus
Uterine anti-apoptotic signaling prevents the apoptotic action of elevated levels of active caspase 3 in the uterine myocyte cytoplasmic and nuclear compartments during pregnancy.
INTRODUCTION
Apoptosis is an evolutionary conserved cell death signaling pathway essential in maintaining tissue homeostasis and development and removal of damaged cells. Deregulation of apoptosis contributes to human disease, including cancer and neurodegenerative disorders [1, 2]. We have identified the pregnant uterus as a site at which deregulation of and resistance to apoptosis occurs. We propose that the pregnant uterus harnesses caspase 3 activity to regulate uterine myocyte quiescence while using a gestationally regulated antiapoptotic signaling cascade to maintain resistance to the apoptotic effects of caspase 3.
In preparation for labor, the uterus undergoes a remarkable transition from a state of relative quiescence to that of an active contractile unit during the final trimester of pregnancy. The molecular mechanisms controlling the onset of uterine contractions at term include increased oxytocin receptors [3], gap junction formation [4], and prostaglandin production [5, 6]. A precipitous decline in circulating levels of P4 heralds the onset of labor in most mammalian species but not the human [7]. However, in both human and mouse uterus at term, a functional withdrawal of P4 action occurs at the PR level [8–12].
Recently we identified uterine caspase 3 as a progesterone-regulated gene and a potential regulator of uterine quiescence [13]. Although activation of caspase 3 is typically associated with the onset of apoptosis [14], many studies have identified caspase 3 as a negative regulator of myocyte contractility in skeletal [15], cardiac [16–19], and smooth muscle cells [16] that doesn't induce cell death [17]. Many components of the contractile apparatus have specific caspase recognition sites that mediate a caspase-regulated proteolysis, thus isolating the contractile architecture of the cell as a target of the protease action of caspase 3 [18]. However, nuclear damage caused by caspase 3-mediated, internucleosomal cleavage of DNA, the final step in the death of the cell, is reduced or often does not accompany caspase 3 proteolytic action. The lack of cell death despite elevated caspase 3 activation is a phenomenon that has been demonstrated previously in the pregnant uterus [19], fetal membranes [20], and the human heart [17].
The current study is focused on the mechanism by which the pregnant uterus resists apoptotic cell death despite elevated levels of uterine caspase 3 activity. It has been determined in other cell types that despite activation of the apoptotic pathway, there is often a lack of terminal morphological features of apoptosis [21]. Initially we tested the hypothesis that the pregnant uterus escaped apoptotic cell death as a result of active caspase 3 exclusion from the nuclear fraction. We examined the pregnant uterus from Embryonic Day 12 to Embryonic Day 19 (E12 to E19) for cleavage of the nuclear enzyme poly (ADP-ribose) polymerase (PARP), one of the first proteins identified as a substrate for active caspases [22]. PARP is essential for DNA repair and functions by catalyzing the synthesis of poly (ADP-ribose). The ability of PARP to repair DNA damage is prevented following cleavage of PARP by caspase 3. We also monitored uterine genomic DNA fragmentation across gestation as another indicator of uterine caspase 3-mediated apoptotic activity. We examined the profile of pro- and antiapoptotic signaling molecules expressed in the pregnant mouse uterus from E12 to E19. We hypothesized that modification of pro- and antiapoptotic signaling events in the pregnant uterus may function to protect the myometrium from the apoptotic action of caspase 3 in vivo.
MATERIALS AND METHODS
Mouse Studies
All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Timed-pregnant female ICR (an outbred Institute for Cancer Research strain) mice (8 wk old) were obtained from The Jackson Laboratory (Bar Harbor, ME). Uterine tissues (n = 3 for each gestational time point) were harvested at 1000 h from E12 to E19. The uterine horn was cleared of all embryonic material and maternal decidua. The remaining whole uterine tissue was washed in 1× PBS and flash frozen for subsequent protein and mRNA analysis.
First-Strand cDNA Synthesis and Quantitative PCR
Frozen uterine tissue specimens (n = 3 for each gestational time point) were pulverized and homogenized in Trizol (Invitrogen, Carlsbad, CA). Total RNA was isolated and reverse transcribed to cDNA by a two-step method, followed by quantitative PCR (Q-PCR), as described previously in detail [23]. The ABI PRISM Sequence Detection System 7900HT (Perkin-Elmer; Applied Biosystems, Waltham, MA) was used to monitor the increase of reporter fluorescence following PCR. Reporter signal was normalized to the emission of a passive reference. PCR amplification was carried out using a Sybrgreen PCR Master Mix (Applied Biosystems). Primer Express software (Applied Biosystems) was used to design gene-specific mouse primers, with a preferable amplicon size of 50–150 bp for the proapoptotic members of the of B-cell lymphoma 2 (Bcl2) family, BH3 interacting domain death agonist (Bid), Bcl2 associated X protein (Bax), Bcl2 associated death promoter (Bad), and Bcl2 homologous antagonist killer 1 (Bak1). Gene-specific mouse primers were also designed for members of the antiapoptotic Bcl2 family (Bcl2)—myeloid cell leukemia sequence 1 (Mcl1), B cell lymphoma extra large (Bcl2l1), and Bcl2-like protein 2 (Bcl2l2)—and members of the antiapoptotic inhibitor of apoptosis (IAP) family—x-linked inhibitor of apoptosis protein (Xiap), cellular inhibitor of apoptosis 1 (Birc2/Ciap1), cellular inhibitor of apoptosis 2 (Birc3/Ciap2), and baculoviral inhibitor of apoptosis repeat-containing 5 (Survivin/Birc5). Primers were also designed for members of the IAP family antagonists: second mitochondria-derived activator of caspases (Diablo/Smac), XIAP-associated factor 1 (Xaf), and the house keeping gene, mouse acidic ribosomal phosphoprotein primers (Rplp0). We have found Rplp0 levels to be suitable for its use as a housekeeping gene for the pregnant mouse uterus, as its expression remains stable across gestation. Each uterine sample (n = 3 for each gestational time point) was assayed in triplicate. Amplified mRNAs identified by Q-PCR were normalized to Rplp0 [24]. Primer pairs can be found in Table 1.
TABLE 1.
Gene-specific primer pairs for pro- and anti-apoptotic factors examined in the pregnant mouse uterus across gestation.
Subcellular Fractionation
Isolation of cytoplasmic extracts from uterine tissue was done as described previously [23, 25]. Briefly, frozen myometrial tissues were pulverized in liquid nitrogen and homogenized in ice-cold NE1 buffer containing 10 mM Hepes (pH 7.5), 10 mM MgCl2, 5 mM KCl, and 0.1% Triton X-100. The homogenate was centrifuged at 3000 × g for 10 min at 4°C, and the supernatant was retained as cytoplasmic fraction. The pellet was washed in NE1 and then resuspended in ice-cold NE2 buffer containing 25% glycerol, 20 mM HEPES (pH 7.9), 500 mM NaCl, 1.5 mM MgCl2, and 0.2 mM ethylenediaminetetraacetic acid (EDTA; pH 8.0) and was incubated on ice for 30 min, vortexed every 5 min, and centrifuged at 10 600 × g for 10 min at 4°C. The supernatant was retained as the nuclear fraction. Subcellular protein concentrations were determined by bicinchoninic acid (BCA) protein assay (Promega, Madison, WI).
Immunoblotting
Equal amounts of cytoplasmic and nuclear proteins, quantified by colorimetric BCA protein assay, were separated in gradient polyacrylamide gels (Invitrogen) and transferred onto Hybond-P (Amersham Pharmacia, Piscataway, NJ). Blots were probed using primary antibodies against anti-GAPDH (1:250; #IMG 5019A-1; IMGENEX, San Diego, CA), anti-ATF6 (1:200; #IMG 273; IMGENEX), anti-caspase 3 cleaved form (1:100; #AB3623; Chemicon, Billerica, MA), PARP (1:500; #9542; Cell Signaling, Boston, MA), XIAP (1:100; #2042; Cell Signaling), Bid (1:200; #Pc645; Calbiochem, Darmstadt, Germany), MCL1 (1:200; #Sc-819; Santa Cruz Biotechnology, Santa Cruz, CA), and SURVIVIN (1:200; #Sc-10811; Santa Cruz). Primary antibodies were diluted in 5% milk + 1× Tris-buffered saline Tween 20 (TBS-T) buffer, followed by incubation with horseradish peroxidase-conjugated secondary antibodies diluted in 5% milk + 1× TBS-T buffer. Immunoreactive bands were visualized using an ECL detection system (Amersham Pharmacia). NIH Image quant (http://rsb.info.nih.gov/nih-image/) was used to identify immunoreactive protein band intensity. The relative optical density (ROD) was obtained by normalizing immunoreactive cytoplasmic protein band intensity to GAPDH, and nuclear band intensity was normalized to a 36-kDa cleavage product of the transcription factor ATF6, which we have found to be maintained at constant levels in the pregnant mouse uterine nuclear fraction across gestation.
DNA Fragmentation Assay
Nuclear genomic DNA was extracted from uteri from E12 to E19 (n = 3 for each gestational time point). Briefly, frozen myometrial tissues were pulverized in liquid nitrogen, and the pulverized tissue was gently homogenized in 100 mM NaCl, 20 mM Tris-HCl (pH 8.0), 25 mM EDTA, 0.5% SDS, and 100 μg/ml of RNase A and incubated for 1 h at 37°C. Proteinase K was added to the sample and incubated at 55°C overnight. The DNA was extracted with equal parts phenol, chloroform, and isoamylalcohol and dissolved in Tris EDTA buffer. Equal amounts of DNA were separated on 1.5% agarose gel containing ethidium bromide and photographed under UV light. The positive control apoptotic DNA ladder, apoptotic U937 cell extract, was obtained from Roche, Mannheim, Germany.
Statistical Analysis
All data are representative of at least three individual experiments performed in triplicate. Individual data were expressed as mean ± SEM. Gestational profiles were subjected to a one-way ANOVA followed by pair-wise multiple comparison procedures (Student-Newman-Keuls method) to determine differences between groups. Statistical analysis was performed in StatPlus:mac for Mac OS version 2009 (AnalystSoft, Inc., Alexandria, VA). P < 0.05 was considered significant.
RESULTS
Elevated Levels of Active Caspase 3 Were Detected in the Nuclear Fraction of the Pregnant Mouse Uterus
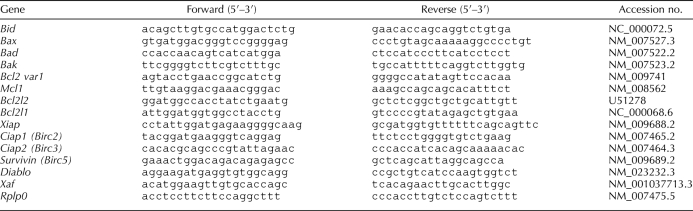
Nuclear proteins were freshly isolated from pregnant mouse uteri from E12 to E19 (n = 3 for each gestational time point). Western blots were performed using an antibody that only recognizes the cleaved form of active caspase 3 at 17 kDa. As shown in Figure 1, abundant active caspase 3 was found in the nuclear fraction of pregnant mouse uteri from E12 to E16, with maximal levels being found on E13. As term approached (E19), active nuclear caspase 3 decreased to barely detectable levels.
FIG. 1.
The effect of gestational age on the levels of active uterine caspase 3 in the nuclear faction of the mouse uterus. A) Uteri collected from three gestational series from E11 to E19 were examined individually by Western blot analysis. A representative Western blot is shown. Nuclear levels of active caspase 3 were normalized to the 36-kDa fragment of the transcription factor ATF6 found at constant levels in the nucleus across gestation. B) Data are presented as mean ± SEM from three experiments. ROD, relative optical density.
Minimal Nuclear Caspase 3-Mediated PARP Cleavage in the Pregnant Mouse Uterus
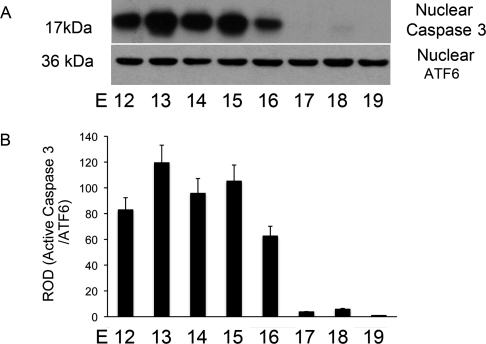
Nuclear proteins were freshly isolated from the pregnant mouse uteri from E12 to E19 (n = 3 for each gestational time point). Western blots were performed using an antibody that recognized both the full-length and caspase 3-cleaved forms of PARP (116 and 89 kDa, respectively). As shown in Figure 2A, abundant levels of the full-length, intact PARP were identified across gestation. In contrast, low levels of the caspase 3-cleaved form of PARP were observed and were isolated to E12 and E13. As defined by ROD (Fig. 2, B and C), the caspase 3-cleaved PARP fragments levels represent 16% and 10% of the full-length PARP on E12 and E13, respectively.
FIG. 2.
The effect of gestational age on the levels of intact and caspase 3-cleaved PARP in the nuclear fraction of the mouse uterus. Uteri collected from three gestational series from E11 to E19 were examined individually by Western blot analysis. A representative blot is shown (A). Nuclear levels of full length (B) and caspase 3-cleaved PARP (C) were normalized to the 36-kDa fragment of ATF6 found at constant levels in the nucleus across gestation. Data are represented as mean ± SEM from three experiments.
Genomic DNA Is Preserved in an Intact State in the Pregnant Mouse Uterus Across Gestation
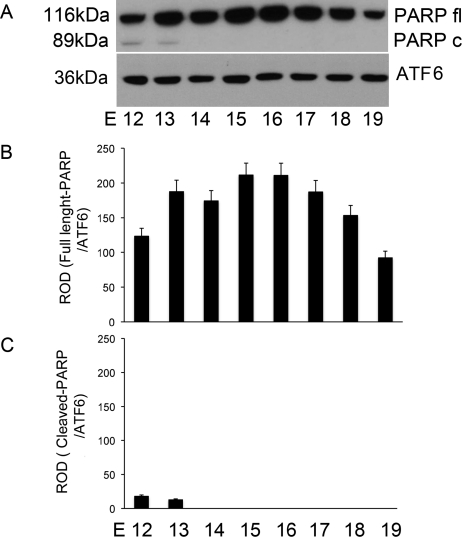
Genomic DNA was freshly isolated from pregnant mouse uteri from E12 to E19 (n = 3 for each gestational time point). Equal amounts of the uterine genomic DNA were electrophoresed on a 1% agarose gel using apoptotic U937 cells as a positive control (Fig. 3). Uterine genomic DNA is maintained in its intact state across gestation, which is indicated by a lack of DNA laddering, as was clearly identified in genomic DNA isolated from our positive control, the apoptotic U937 cell.
FIG. 3.
The effect of gestational age on the levels of uterine DNA fragmentation in the pregnant mouse uterus. Uteri collected from three gestational series from E11 to E19 were examined individually. A representative agarose gel is shown. Across gestation, uterine DNA retains an intact profile in comparison to the fragmented DNA observed as a ladder isolated from apoptotic U937 cells, which were used as a positive control (+Cnt).
Q-PCR Analysis of Pro- and Antiapoptotic Signaling Mediators Indicates Increased Uterine Antiapoptotic and Decreased Proapoptotic Signaling Events During Late Pregnancy
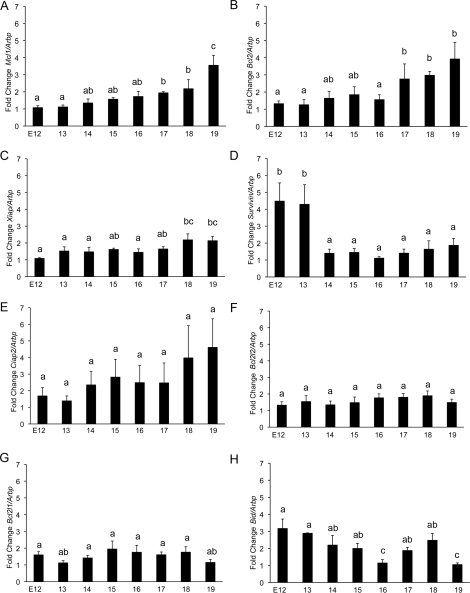
Messenger RNA isolated from uteri of three different gestational series of mice was analyzed for significant alterations in the levels of pro- and antiapoptotic mediators that would facilitate the preservation of the uterine nonapoptotic state despite elevated active caspase 3 levels present during pregnancy. Using oligonucleotides that primed with equal efficiency (Table 1), Q-PCR analysis demonstrated significant modification in Mcl1, Bcl2, Xiap, Survivin, and Bid mRNA levels from E12 to E19 (Fig. 4). Antiapoptotic factors Mcl1 (Fig. 4A), Bcl2 (Fig. 4B), and Xiap (Fig. 4C) increased significantly between E12 and E19. The antiapoptotic factor Survivin (Fig. 4D) was highly elevated on E12 and E13 but decreased dramatically at E14 and remained at low levels to term. The antiapoptotic factors Ciap2, Bcl2l2, and Bcl2l1 (Fig. 4, E–G) were detectable but remained unchanged across gestation. The proapoptotic factor Bid (Fig. 4H) decreased significantly from E12 to E19. Proapoptotic factors Bax, Bak, Bad, Diablo, Xaf, and Htra were also detectable but remained unchanged across gestation.
FIG. 4.
The gestational profiles of Mcl1, Bcl2, XIAP, Survivin, Ciap2 (Birc3), Bcl2l1, Bcl2l2, and Bid mRNA in the pregnant mouse uterus are shown. Uteri collected from three gestational series from E11 to E19 were examined individually in triplicate. Transcript levels were normalized to Arbp, an mRNA transcript that remains constant across gestation in the pregnant mouse uterus. Data labeled with different letters are significantly different from each other (P < 0.05). Data are represented as mean ± SEM from three experiments.
Protein Levels of MCL1, XIAP, and SURVIVIN Were Significantly Altered Across Gestation in Pregnant Mice
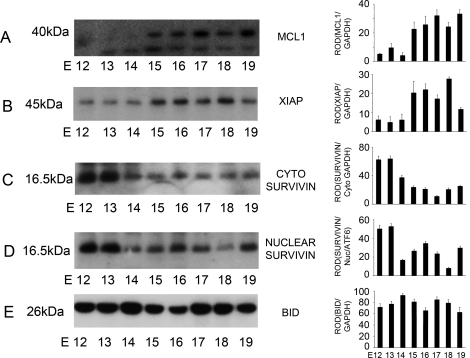
Cytoplasmic and nuclear extracts isolated from pregnant mouse uteri from E12 to E19 (n = 3 for each gestational time point) were examined by Western blot analysis using antibodies that specifically identified MCL1 at 40 kDa, XIAP at 45 kDa, SURVIVIN at 16.5 kDa, and BID at 26 kDa (Fig. 5). Increased levels of MCL1 (Fig. 5A) and XIAP (Fig. 5B) were identified in the pregnant mouse uterus from E15 to E19 in the cytoplasmic fraction. SURVIVIN was found to be elevated in both the cytoplasmic (Fig. 5C) and nuclear fraction (Fig. 5D) at E12 and E13, with a significant decrease in SURVIVIN levels in both cellular compartments from E14 to E19. The protein levels for MCL1, XIAP, and SURVIVIN paralleled the increased levels observed in the mRNA analysis seen in Figure 4. Cytoplasmic levels of BID (Fig. 5E) did not significantly change from E12 to E19.
FIG. 5.
The effect of gestational age on the levels of cytoplasmic (cyto) MCL1, XIAP, and cytoplasmic and nuclear (Nuc) SURVIVIN and BID in the mouse uterus from E12 to E19. Uteri collected from three gestational series from E11 to E19 were examined individually by Western blot analysis. Representative blots are shown. Cytoplasmic MCL1, XIAP, SURVIVIN, and BID (A, B, C, and E) were normalized to GAPDH, whereas nuclear levels of SURVIVIN (D) were normalized to the 36-kDa nuclear product of the transcription factor ATF6. Data are represented as mean ± SEM from three experiments.
Decreased NFKBIA (IκBα) and Elevated NFKB (NF-κB) Activation in the Pregnant Mouse Uterus as Term Approaches
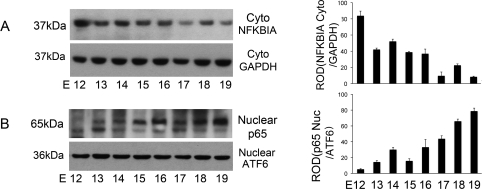
Cytoplasmic NFKBIA levels were examined as an indicator of activation of the NFKB pathway in the pregnant mouse uterus by Western blot analysis, which specifically identified NFKBIA at 37 kDa. Cytoplasmic NFKBIA levels decreased (Fig. 6A) in the pregnant mouse uterus as term approached, permitting the translocation of the NFKB subunit p65 into the nuclear fraction (Fig. 6B). Increasing levels of p65 were identified from E13 and reached maximal levels at term, E19, by Western blotting using an antibody that recognizes p65 at 65 kDa.
FIG. 6.
The effect of gestational age on the levels of NFKB activation on the pregnant mouse uterus from E12 to E19. Uteri collected from three gestational series from E11 to E19 were examined individually by Western blot analysis. Representative Western blots are shown. Cytoplasmic NFKBIA levels decreased as term approached (A), with a reciprocal increase in nuclear levels of p65 toward term, E19 (B). Cytoplasmic NFKBIA was normalized to GAPDH, whereas nuclear levels of p65 were normalized to the 36-kDa nuclear product of the transcription factor ATF6. Data are represented as mean ± SEM from three experiments.
DISCUSSION
This study defines a potential molecular mechanism whereby the pregnant uterus may maintain a nonapoptotic status despite elevated levels of active caspase 3 [13]. Our initial line of investigation was to determine if exclusion of active caspase 3 from the nucleus was used by the pregnant uterine myocyte to limit caspase 3's ability to perform its terminal apoptotic actions, which usually result in DNA damage and ultimately death of the cell. Active caspase 3 uses a nuclear localization sequence found at the cleavage site between the p17 and p12 subunits to actively translocate to the nucleus during the progression of an apoptotic event [26]. However, previous studies have found that nuclear damage caused by caspase 3-mediated internucleosomal cleavage of DNA, the final step in the death of the cell, is reduced or often does not accompany caspase 3's proteolytic action [17, 19, 20]. We first examined active caspase 3 levels in the nuclear fraction of the pregnant mouse uterus across gestation and found that similar to the cytoplasmic fraction [13] there were abundant levels of active caspase 3 from E12 to E16, which decreased to barely detectable levels at term (E19; Fig. 1). However, apoptotic indices of nuclear caspase 3 action such as PARP cleavage (Fig. 2) and nucleosomal DNA fragmentation (Fig. 3) were minor or absent, respectively. These data suggested that though active caspase 3 had translocated to the nucleus, its apoptotic activity was limited, resulting in inefficient initiation of apoptotic cell death in the pregnant mouse uterus.
Apoptosis is a genetically programmed process of controlled cell suicide that has a critical role in development and homeostasis of the organism. Dysregulation of apoptosis contributes to the pathogenesis of a host of diseases, including cancers [1], autoimmunity [27], and neurological disorders [2]. Our findings in the pregnant uterus suggest a similar dysregulation of apoptotic signaling. The pregnant uterine myocyte displays robust levels of active caspase 3 in the cytoplasm [13] and nucleus (Fig. 1), with little or no indication of an accompanying process of apoptotic cell death (Figs. 2 and 3). The IAP family of proteins are the only known endogenous inhibitors of active caspases in vivo and play a critical role in limiting active caspases by inhibiting the apoptotic process [28]. A Q-PCR screen of pro- and antiapoptotic factors expressed in the pregnant uterus (Fig. 4) revealed that there was an induction of an antiapoptotic response during late gestation. Two members of the IAP family, SURVIVIN and XIAP (Figs. 4 and 5), were found to be significantly altered at the mRNA and protein levels across gestation in the pregnant mouse uterus.
SURVIVIN, the smallest member of the IAP family at 16.5 kDa, was found at high levels in both the cytoplasmic and nuclear fraction of the pregnant mouse uterus on Gestation Days 12 and 13, declined on Gestation Day 14, and remained low to term (Fig. 5, C and D). Recombinant SURVIVIN has previously been identified in other systems to have the capacity to bind directly to and inhibit caspase 3 activity [28, 29]. We propose that the presence of cytoplasmic SURVIVIN in the pregnant mouse uterus at E12 and E13 likely limits cytoplasmic uterine caspase 3 apoptotic activity. Nuclear SURVIVIN was also identified in the pregnant uterus at E12 and E13 (Fig. 5D). Recent data suggest that SURVIVIN's role in the nucleus is to promote the cell's DNA repair capacity, rendering the cells less sensitive to apoptotic stimuli, providing a potential mechanism of action for SURVIVIN in the dysregulation of the uterine nuclear caspase 3-mediated DNA damage response [30]. After E13, SURVIVIN levels decrease; however, the pregnant uterus continues to retain its nonapoptotic status. We speculate that this is a consequence of elevated prosurvival antiapoptotic signaling by XIAP and MCL1 (Fig. 5, A and B).
Recent studies have described how SURVIVIN inhibits apoptosis via cooperative interactions with other partners [31]. An example of these interactions is an IAP-IAP complex between SURVIVIN and XIAP, which further increases the activity of XIAP-mediated caspase 3 inhibition by silencing its enzymatic activity [32]. We have indentified the presence of XIAP in the pregnant uterus from E12, and it increases from E14 to E19 (Fig. 5B). We propose that XIAP and SURVIVIN may have the capacity to work together to limit the apoptotic potential of active caspases in the pregnant uterus from E12 to E19. It has been described that XIAP not only inhibits active caspase 3 proteolytic potential, it also functions in an antiapoptotic manner by removing the active caspase 3 enzyme from the cell by using the ubiquitin-targeted proteasome degradation machinery [33, 34]. Therefore, we propose that the increased levels of XIAP in the pregnant uterus near term may play a critical role not only in inhibiting the action of active caspase 3 but also in acting in an antiapoptotic manner by clearing active caspases from the uterine myocyte as term approaches by targeting them for proteasomal degradation (Fig. 1).
Very recent data has demonstrated that the hallmark of a functioning SURVIVIN-XIAP complex is the activation of the NFKB pathway [35]. XIAP was identified as having the capacity to enhance NFKBIA phosphorylation and degradation, resulting in robust NFKB activation. It has previously been determined that in late gestation NFKB activation from E16 to term plays a critical role in regulating the timing of the onset of labor [36–38]. However, in this current study we have identified the onset of NFKBIA degradation (Fig. 6A) and the consequent NFKB activation at E13 (Fig. 6B), which reaches maximal levels at term. The increased uterine NFKB activation initiated at E13 may be a result of the interaction between both SURVIVIN and XIAP in the pregnant uterine myocyte as early as E12 (Fig. 5).
The role of NFKB activation in the pregnant uterus has been attributed to activation of prostaglandin production [39] and inhibition of uterine progesterone receptor function [40]. However, NFKB itself has also been promoted as both a pro- and antiapoptotic mediator. Among the NFKB-dependent antiapoptotic genes is Mcl1 [41–43], a prosurvival member of the Bcl2 family that maintains cell viability through inhibition of apoptosis. Mcl1 has been described as positively regulated at the transcriptional levels by NFKB activation [42, 44]. MCL1 functions in a prosurvival, nonapoptotic manner by inhibiting further activation of caspase 3 through sequestering proapoptotic signaling mediators [45, 46]. In the pregnant uterus we propose that NFKB may act as a potent antiapoptotic signaling molecule by promoting the upregulation of the antiapoptotic factors such as MCL1 (Fig. 5A) to limit further activation of uterine caspase 3 as term approaches.
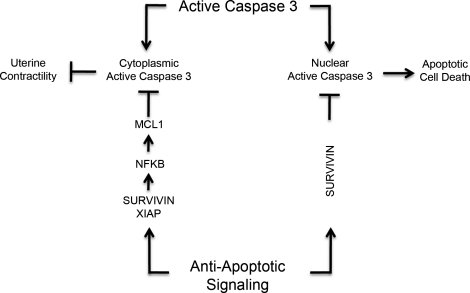
In conclusion, we propose that the pregnant mouse uterus may use an intact caspase 3 dysregulation mechanism to limit the apoptotic actions of active caspase 3 found in the myometrial cytoplasmic and nuclear compartments during pregnancy (Fig. 1). We have previously described how caspase 3 may play a role in maintaining uterine quiescence, and in this current study we now describe how the uterine myocyte avoids apoptotic cell death despite elevated levels of caspase 3, as is summarized in Figure 7. We propose that elevated levels of SURVIVIN limit the consequences of caspase 3 action in the both the cytoplasmic and nuclear fraction, when caspase 3 levels are at their highest (Fig. 5, C and D). We hypothesize that SURVIVIN partners with increasing levels of XIAP (Fig. 5) to further limit caspase 3 activity levels after E13 and to clear active caspase 3 from the cell through activation of the proteasomal degradation pathway. The partnership of XIAP and SURVIVIN may also lead to an upregulation of the antiapoptotic factor MCL1 (Fig. 5) through the degradation of NFKBIA and the consequent activation of an NFKB response initiating at E13 (Fig. 6).
FIG. 7.
The potential role of active caspase 3 and antiapoptotic signaling during pregnancy in the regulation of uterine quiescence.
Caspase activation has also been shown to be engaged in myometrial cell differentiation during pregnancy. The transition of the uterine myocyte between myocyte hyperplasia during the first half of gestation and cellular hypertrophy during the second part of gestation has been associated with the activation of the caspase cascade in the pregnant rat uterus [19]. Whether activation of the antiapoptotic signaling cascade contributes to or is as a result of uterine myocyte hypertrophy during late pregnancy has yet to be determined; however, a recent study demonstrated that mice overexpressing XIAP display mild skeletal muscle hypertrophy [47]. Similarly, in rats undergoing aortic ligation for the induction of cardiomyocyte hypertrophy, activation of NFKB and the antiapoptotic signaling cascade were induced significantly [48]. Active caspase 3 levels are also robustly induced postpartum in rat uteri [49]. It has yet to be determined if activation of caspase 3 postpartum in the rodent model is associated with apoptotic cell death. However, in the human, extensive apoptosis has also been associated with an early stage of uterine involution that follows the onset of labor [50]. Future studies will determine whether the switch to caspase 3-mediated apoptotic cell death postpartum is regulated by decreased antiapoptotic signaling.
We speculate that during pregnancy, modification of the tightly regulated antiapoptotic signaling cascade may result in the inappropriate activation of apoptotic actions of caspase 3 in the pregnant uterus. Future screening of preterm and term uterine myometrial tissues may identify activation of the anti-caspase 3 pathway as a marker for aberrant and premature priming of the pregnant uterus, resulting in the onset of preterm labor.
Footnotes
Supported by the March of Dimes (21-FY2008-555) and the NICHD (1R01HD065011-01A1).
REFERENCES
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995; 267: 1456 1462. [DOI] [PubMed] [Google Scholar]
- Roth KA. Caspases, apoptosis, and Alzheimer disease: causation, correlation, and confusion. J Neuropathol Exp Neurol 2001; 60: 829 838. [DOI] [PubMed] [Google Scholar]
- Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol 1984; 150: 734 741. [DOI] [PubMed] [Google Scholar]
- Garfield RE, Sims SM, Kannan MS, Daniel EE. Possible role of gap junctions in activation of myometrium during parturition. Am J Physiol 1978; 235: C168 C179. [DOI] [PubMed] [Google Scholar]
- Zuo J, Lei ZM, Rao CV, Pietrantoni M, Cook VD. Differential cyclooxygenase-1 and -2 gene expression in human myometria from preterm and term deliveries. J Clin Endocrinol Metab 1994; 79: 894 899. [DOI] [PubMed] [Google Scholar]
- Tuross N, Mahtani M, Marshall JM. Comparison of effects of oxytocin and prostaglandin F2-alpha on circular and longitudinal myometrium from the pregnant rat. Biol Reprod 1987; 37: 348 355. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 2000; 21: 514 550. [DOI] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci U S A 2003; 100: 9518 9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 2002; 87: 2924 2930. [DOI] [PubMed] [Google Scholar]
- Madsen G, Zakar T, Ku CY, Sanborn BM, Smith R, Mesiano S. Prostaglandins differentially modulate progesterone receptor-A and -B expression in human myometrial cells: evidence for prostaglandin-induced functional progesterone withdrawal. J Clin Endocrinol Metab 2004; 89: 1010 1013. [DOI] [PubMed] [Google Scholar]
- Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab 2007; 92: 1927 1933. [DOI] [PubMed] [Google Scholar]
- Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod 2001; 7: 875 879. [DOI] [PubMed] [Google Scholar]
- Jeyasuria P, Wetzel J, Bradley M, Subedi K, Condon JC. Progesterone-regulated caspase 3 action in the mouse may play a role in uterine quiescence during pregnancy through fragmentation of uterine myocyte contractile proteins. Biol Reprod 2009; 80: 928 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem 1994; 269: 30761 30764. [PubMed] [Google Scholar]
- Supinski GS, Callahan LA. Caspase activation contributes to endotoxin-induced diaphragm weakness. J Appl Physiol 2006; 100: 1770 1777. [DOI] [PubMed] [Google Scholar]
- Hong SK, Son H, Kim SW, Oh SJ, Choi H. Effect of glycine on recovery of bladder smooth muscle contractility after acute urinary retention in rats. BJU Int 2005; 96: 1403 1408. [DOI] [PubMed] [Google Scholar]
- Narula J, Haider N, Arbustini E, Chandrashekhar Y. Mechanisms of disease: apoptosis in heart failure—seeing hope in death. Nat Clin Pract Cardiovasc Med 2006; 3: 681 688. [DOI] [PubMed] [Google Scholar]
- Communal C, Sumandea M, de Tombe P, Narula J, Solaro RJ, Hajjar RJ. Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci U S A 2002; 99: 6252 6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shynlova O, Oldenhof A, Dorogin A, Xu Q, Mu J, Nashman N, Lye SJ. Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprod 2006; 74: 839 849. [DOI] [PubMed] [Google Scholar]
- Runic R, Lockwood CJ, LaChapelle L, Dipasquale B, Demopoulos RI, Kumar A, Guller S. Apoptosis and Fas expression in human fetal membranes. J Clin Endocrinol Metab 1998; 83: 660 666. [DOI] [PubMed] [Google Scholar]
- Haider N, Narula N, Narula J. Apoptosis in heart failure represents programmed cell survival, not death, of cardiomyocytes and likelihood of reverse remodeling. J Card Fail 2002; 8: S512 S517. [DOI] [PubMed] [Google Scholar]
- Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 1994; 371: 346 347. [DOI] [PubMed] [Google Scholar]
- Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol 2006; 20: 764 775. [DOI] [PubMed] [Google Scholar]
- van Wijngaarden P, Brereton HM, Coster DJ, Williams KA. Stability of housekeeping gene expression in the rat retina during exposure to cyclic hyperoxia. Mol Vis 2007; 13: 1508 1515. [PubMed] [Google Scholar]
- Blough E, Dineen B, Esser K. Extraction of nuclear proteins from striated muscle tissue. Biotechniques 1999; 26: 202 204, 206. [DOI] [PubMed] [Google Scholar]
- Kamada S, Kikkawa U, Tsujimoto Y, Hunter T. Nuclear translocation of caspase-3 is dependent on its proteolytic activation and recognition of a substrate-like protein(s). J Biol Chem 2005; 280: 857 860. [DOI] [PubMed] [Google Scholar]
- Zhou T, Song L, Yang P, Wang Z, Lui D, Jope RS. Bisindolylmaleimide VIII facilitates Fas-mediated apoptosis and inhibits T cell-mediated autoimmune diseases. Nat Med 1999; 5: 42 48. [DOI] [PubMed] [Google Scholar]
- Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein SURVIVIN inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res 1998; 58: 5315 5320. [PubMed] [Google Scholar]
- Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry (Mosc) 2001; 40: 1117 1123. [DOI] [PubMed] [Google Scholar]
- Capalbo G, Dittmann K, Weiss C, Reichert S, Hausmann E, Rodel C, Rodel F. Radiation-induced survivin nuclear accumulation is linked to DNA damage repair. Int J Radiat Oncol Biol Phys 2010; 77: 226 234. [DOI] [PubMed] [Google Scholar]
- Srinivasula SM, Ashwell JD. IAPs: what's in a name? Mol Cell 2008; 30: 123 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep 2006; 7: 988 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi T, Okada K, Xia F, Wilford CE, Samuel T, Welsh K, Marusawa H, Zou H, Armstrong R, Matsuzawa S, Salvesen GS, Reed JC, Altieri DC. An IAP-IAP complex inhibits apoptosis. J Biol Chem 2004; 279: 34087 34090. [DOI] [PubMed] [Google Scholar]
- Dohi T, Xia F, Altieri DC. Compartmentalized phosphorylation of IAP by protein kinase A regulates cytoprotection. Mol Cell 2007; 27: 17 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra S, Languino LR, Raskett CM, Mercurio AM, Dohi T, Altieri DC. IAP regulation of metastasis. Cancer Cell 2010; 17: 53 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. Human labour is associated with nuclear factor-kappaB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod 2001; 7: 581 586. [DOI] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A 2004; 101: 4978 4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman NR, Europe-Finner GN, Robson SC. Expression and deoxyribonucleic acid-binding activity of the nuclear factor kappaB family in the human myometrium during pregnancy and labor. J Clin Endocrinol Metab 2004; 89: 5683 5693. [DOI] [PubMed] [Google Scholar]
- Belt AR, Baldassare JJ, Molnar M, Romero R, Hertelendy F. The nuclear transcription factor NF-kappaB mediates interleukin-1beta-induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol 1999; 181: 359 366. [DOI] [PubMed] [Google Scholar]
- Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem 1996; 271: 6217 6224. [DOI] [PubMed] [Google Scholar]
- Reynolds JE, Li J, Craig RW, Eastman A. BCL-2 and MCL-1 expression in Chinese hamster ovary cells inhibits intracellular acidification and apoptosis induced by staurosporine. Exp Cell Res 1996; 225: 430 436. [DOI] [PubMed] [Google Scholar]
- Buggins AG, Pepper C, Patten PE, Hewamana S, Gohil S, Moorhead J, Folarin N, Yallop D, Thomas NS, Mufti GJ, Fegan C, Devereux S. Interaction with vascular endothelium enhances survival in primary chronic lymphocytic leukemia cells via NF-kappaB activation and de novo gene transcription. Cancer Res 2010; 70: 7523 7533. [DOI] [PubMed] [Google Scholar]
- Kupfner JG, Arcaroli JJ, Yum HK, Nadler SG, Yang KY, Abraham E. Role of NF-kappaB in endotoxemia-induced alterations of lung neutrophil apoptosis. J Immunol 2001; 167: 7044 7051. [DOI] [PubMed] [Google Scholar]
- Akgul C, Turner PC, White MR, Edwards SW. Functional analysis of the human MCL-1 gene. Cell Mol Life Sci 2000; 57: 684 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuconati A, Mukherjee C, Perez D, White E. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev 2003; 17: 2922 2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 2003; 426: 671 676. [DOI] [PubMed] [Google Scholar]
- Hu J, Du J, Zhang L, Price SR, Klein JD, Wang XH. XIAP reduces muscle proteolysis induced by CKD. J Am Soc Nephrol 2010; 21: 1174 1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Mir SA, Dutta D, Mitra A, Pathak K, Sarkar S. Analysis of p53 and NF-kappaB signaling in modulating the cardiomyocyte fate during hypertrophy. J Cell Physiol 2010; (in press). Published online ahead of print 13 December 2010; DOI 10.1002/jcp.22599. [DOI] [PubMed]
- Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol 2009; 144 (suppl 1): S2 S10. [DOI] [PubMed] [Google Scholar]
- Leong AS, Norman JE, Smith R. Vascular and myometrial changes in the human uterus at term. Reprod Sci 2008; 15: 59 65. [DOI] [PubMed] [Google Scholar]