Abstract
Congenital dysmorphic features are prevalent in schizophrenia and may reflect underlying neurodevelopmental abnormalities. A cluster analysis approach delineating patterns of dysmorphic features has been used in genetics to classify individuals into more etiologically homogeneous subgroups. In the present study, this approach was applied to schizophrenia, using a sample with a suspected genetic syndrome as a testable model. Subjects (n = 159) with schizophrenia or schizoaffective disorder were ascertained from chronic patient populations (random, n=123) or referred with possible 22q11 deletion syndrome (referred, n = 36). All subjects were evaluated for presence or absence of 70 reliably assessed dysmorphic features, which were used in a three-step cluster analysis. The analysis produced four major clusters with different patterns of dysmorphic features. Significant between-cluster differences were found for rates of 37 dysmorphic features (P < 0.05), median number of dysmorphic features (P = 0.0001), and validating features not used in the cluster analysis: mild mental retardation (P = 0.001) and congenital heart defects (P = 0.002). Two clusters (1 and 4) appeared to represent more developmental subgroups of schizophrenia with elevated rates of dysmorphic features and validating features. Cluster 1 (n = 27) comprised mostly referred subjects. Cluster 4 (n= 18) had a different pattern of dysmorphic features; one subject had a mosaic Turner syndrome variant. Two other clusters had lower rates and patterns of features consistent with those found in previous studies of schizophrenia. Delineating patterns of dysmorphic features may help identify subgroups that could represent neurodevelopmental forms of schizophrenia with more homogeneous origins.
Keywords: 22q11 deletion syndrome, cluster analysis, neurodevelopment, genetic subtype
INTRODUCTION
Schizophrenia is a complex disorder with substantial evidence for genetic causation [Jones and Murray, 1991; Waddington et al., 1999; Brzustowicz et al., 2000] and pathogenesis involving abnormal neurodevelopment [Weinberger, 1987; Murray et al., 1992]. There are undoubtedly multiple causes and mechanistic pathways to the clinical expression observable as schizophrenia. An approach based on genetic and neurodevelopmental strategies may be useful in identifying potential subgroups within schizophrenia. A similar approach has recently been advocated for the study of genetic disorders with significant behavioral components [Reiss et al., 2000]. We propose a subgrouping strategy based on the pattern of mild physical (dysmorphic) features in patients with schizophrenia to test this approach.
Dysmorphic features are defined as congenital physical features that, individually, are not associated with serious medical or cosmetic consequences [Jones, 1997]. Multiple dysmorphic features, however, often form recognizable patterns that may be associated with major organ malformations, such as congenital heart defects, and abnormal neurodevelopment manifest as learning disabilities, including mental retardation, and/or behavioral problems, including psychiatric disorders [Jones, 1997]. These patterns of physical and neurodevelopmental features may indicate the presence of specific genetic or other malformation syndromes [Jones, 1997]. There is consistent evidence for an increased prevalence of dysmorphic features (often referred to as minor physical anomalies) in schizophrenia [Gualtieri et al., 1982; Guy et al., 1983; Green et al., 1989, 1994a, 1994b; Lohr and Flynn, 1993; Murphy and Owen, 1996; Lane et al., 1997; Ismail et al., 2000]. Studies of dysmorphic features in schizophrenia have tended to focus on the number rather than the pattern of features, and these features have generally been attributed to prenatal insults to the fetus and/or birth or perinatal complications [Simonds et al., 1981; O’Callaghan et al., 1991; Cantor-Graae et al., 1994; Green et al., 1994a], rather than a possible association with underlying genetic complications. Also, despite the prevalent neurodevelopmental hypothesis of schizophrenia [Weinberger, 1987], studies of dysmorphic features in schizophrenia have generally not included subjects with learning disabilities who could help in understanding the genetics of neurodevelopment [Muir, 2000; Reiss et al., 2000].
22q11 deletion syndrome (22qDS) represents an identifiable genetic syndrome associated with specific patterns of dysmorphic features and increased rates of psychosis in adults [Murphy et al., 1999; Bassett et al., 2000]. 22qDS is characterized by a microdeletion on chromosome 22q11.2 [Scambler et al., 1992], a variable pattern of dysmorphic features (typically including long, narrow face, flat cheeks, narrow palpebral fissures, retruded jaw, and tapered fingers), hypernasal speech, sometimes congenital heart defects, and typically borderline to mild mental retardation [Cohen et al., 1999; Tobias et al., 1999]. About 25% of adults with 22qDS have schizophrenia [Pulver et al., 1994; Murphy et al., 1999], and chromosome 22q11.2 deletions are 40 to 80 times more common in schizophrenia than the general population [Bassett et al., 2000]. Increased rates of dysmorphic features, cognitive dysfunction, and structural brain abnormalities in both 22qDS [Shprintzen et al., 1978; Bingham et al., 1997; Murphy et al., 1998] and schizophrenia [Murray et al., 1992; Waddington et al., 1999] have led researchers to propose 22qDS as a model for a genetic neurodevelopmental subtype of schizophrenia (22qDS-SZ) [Bassett et al., 1998; Bassett and Chow, 1999; Murphy et al., 1999; Reiss et al., 2000]. Identification of 22qDS in populations of patients with schizophrenia [Karayiorgou et al., 1995; Bassett et al., 1998] suggests that determining patterns of dysmorphic features may reveal this and other potential etiologic subtypes of schizophrenia.
Cluster analysis is a statistical method that can be used to help classify patients into syndromes based on patterns of features. In the clinical genetics literature, the validity of this approach has been shown using dysmorphic features in subjects with known or suspected genetic syndromes [Preus, 1980, 1984; Preus and Ayme, 1983]. Similar to factor analysis, cluster analysis attempts to delineate patterns of associations and identify subgroups within a population of interest [Rapkin and Luke, 1993], therefore a normal control group is often not employed in this study design. Instead, a testable model to assess the overall validity of the clustering methods used can be included [Preus, 1980]. In the present study, we applied a cluster analysis approach using dysmorphic features to attempt to delineate subgroups within schizophrenia that may have increased homogeneity. We used a sample representative of patients with chronic schizophrenia at provincial (state) psychiatric hospitals. Following the methodology employed previously [Preus, 1980, 1984; Preus and Ayme, 1983], we included subjects with a clinical diagnosis of 22qDS-SZ as a testable model of a genetic subtype of schizophrenia. The specific hypotheses tested were that subgroups of schizophrenic subjects identified by cluster analysis would display differing patterns and rates of dysmorphic features; they would have differing rates of mild mental retardation and congenital heart defects as well as age at onset of psychosis; and one or more of these subgroups may represent more homogeneous genetic and/or neurodevelopmental subtypes of schizophrenia. As a testable model, most of the 22qDS patients should group together if the clustering methods used are valid.
MATERIALS AND METHODS
Subjects and Assessments
The 159 participating subjects had chronic schizophrenia (n = 143) or schizoaffective (n = 16) disorder. All subjects provided informed consent to participate in the study. Diagnoses were confirmed by research psychiatrists (A.S.B., E.W.C.C., W.G.H.) from extensive medical records using Structured Clinical Interviews for DSM-III-R (SCID-I) criteria [American Psychiatric Association, 1987]. Information was recorded from available medical records on age at onset of psychosis, defined as age at first hospitalization, and presence or absence of mild mental retardation and congenital heart defects requiring surgery. Subjects with mild mental retardation were deliberately included in the sample because community samples of schizophrenia demonstrate there is a significant prevalence (around 13.3%) of individuals with low IQ (estimated IQ < 74) in schizophrenia [David et al., 1997] and mild mental retardation is often present in genetic or other malformation syndromes [Winter, 1996; Jones, 1997]. In the majority (n = 20) of 30 subjects diagnosed with mild mental retardation (IQ < 70), the diagnosis was based on available standardized IQ testing results; chart diagnosis based on educational history and level of functioning was used for the remaining 10 subjects.
The sample comprised two groups. Subjects in the first group (random, n = 123) were ascertained from three provincial psychiatric hospitals, randomly (n = 63 from one hospital and n = 30 from another) or from consecutive admissions (n = 30). Subjects in the second group (referred, n = 36) were referred by clinicians from the same chronic patient population as the random sample. Thirty-one subjects were referred for evaluation of possible 22qDS and met two or more clinical referral criteria suggestive of 22qDS [Bassett and Chow, 1999]: 30 (96.8%) had two or more characteristic facial features, 27 (87.1%) had a history of learning disabilities and/or mental retardation, 23 (74.2%) had palatal abnormalities, 11 (35.5%) had other major congenital dysmorphic features (e.g., talipes), 7 (22.6%) had a history of congenital heart defects, and 1 (3.2%) had a history of hypocalcemia. The remaining five subjects were referred with known 22q11.2 deletions.
Subjects were assessed for dysmorphic features using a Standardized Physical Examination for Dysmorphic Features (SPEDF) developed by our group (A.S.B. and R.W.) to aid in the comprehensive assessment of congenital dysmorphic features in adults. Most subjects were assessed by a single physician (e.g., A.S.B. or E.W.C.C.) blind to the specific degree of any learning disability. The SPEDF was created to capture dysmorphic features in the general population rather than features associated with specific syndromes. Therefore, some dysmorphic features associated with 22qDS (e.g., nose with bulbous tip) were not specified on the original form and were not included in analyses since they were not consistently rated for all subjects. Raters were blind to cytogenetic testing results, except for the five subjects referred with known 22qDS.
The SPEDF comprises 122 directly assessed features coded as present or absent. A feature had to be rated as both congenital and dysmorphic, and the age and ethnicity of each subject were considered in all ratings. Therefore, age-related variables (e.g., prematurely gray hair) were rated as unknown in older subjects, and variables associated with specific ethnic backgrounds (e.g., narrow palpebral fissures in Asian subjects) were rated as normal. Five additional features (short stature, microcephaly, macrocephaly, hypertelorism, hypotelorism) were determined post hoc to be present or absent, based on three continuous measurement variables (height, head circumference, and inner canthal distance) [Hall et al., 1989]. Height and maximum occipitofrontal head circumference (OFC) were measured to the nearest 0.1 cm as previously described [Bassett et al., 1996]. Height was used to define short stature (height < 10th percentile, < 164.0 cm for males and < 154.5 cm for females) [Hall et al., 1989]. OFC and height were used to determine microcephaly (<3rd percentile) and macrocephaly (> 97th percentile), based on standard curves for adults [Bushby et al., 1992]. Distance between inner canthi was measured to the nearest 0.1 cm using a transparent ruler to determine hypertelorism (inner canthal distance > 2 standard deviations [SD] from the published mean, or > 3.65 cm) and hypotelorism (inner canthal distance < 2 SD from the published mean, or 2.64 cm) [Hall et al., 1989].
Statistical Analyses
Demographics
Random and referred subjects were compared on basic demographic variables to evaluate possible clinical differences in the samples that could be important in the interpretation of clusters formed. All analyses were nonparametric due to nonnormal distribution of the samples. Group differences for binary-coded variables were evaluated using the chi-square statistic. Continuous variables were assessed using the Wilcoxon two-sample z-test or Kruskal-Wallis nonparametric analysis of variance (ANOVA) for comparison between three or more samples.
Interrater reliability
Reliability analyses were performed on a subset of subjects (n = 50) assessed by two or more raters. Of the SPEDF’s 127 binary variables, 49 were excluded from the cluster analysis for the following reasons: 30 had low (< 3%) frequency of occurrence and were dropped to minimize the number of variables needed for the cluster procedure [Anderberg, 1973; Rapkin and Luke, 1993], 10 were deemed likely to be acquired, and nine were urogenital abnormalities not consistently assessed in all subjects. Of the remaining 78 variables, Cohen’s kappa (κ) [Cohen, 1960] was used to assess the 73 variables directly rated as binary. Reliability of the three continuous variables used to determine the five other binary variables (short stature, microcephaly, macrocephaly, hypertelorism, and hypotelorism) was assessed using the intraclass correlation coefficient (ICC) [Shrout and Fleiss, 1979]. Variables with poor interobserver agreement (κ < 0.4) were excluded from further analyses [Mitchell, 1979]. Remaining variables were divided into craniofacial features and other features.
Cluster Analyses
Cluster analyses were performed with data from all 159 subjects. Dysmorphic features used in the cluster analysis were coded as 0 (absent) or 1 (present) to ensure equal weighting of variables [Anderberg, 1973; Sneath and Sokal, 1973]. A three-step procedure was followed in order to minimize the potential biases of correlated variables and a priori investigator expectations. In step 1, a principal components analysis (PCA) transformed dysmorphic feature variables into five clustering variables to control for the possible biasing effects of correlated variables [Borgen and Weiss, 1971; Lorr, 1983]. In step 2, the Ward minimum variance and average linkage hierarchical cluster methods were performed on the five PCA-derived clustering variables in order to determine the number of target clusters for step 3 [Rapkin and Luke, 1993]. In step 3, k means iterative cluster analysis was performed using the five PCA-derived clustering variables determined in step 1 and the number of target clusters determined in step 2.
The stability of clusters formed was assessed by repeating step 3 across the range of target clusters suggested by step 2. Demographic factors (sex, age at assessment, and ethnicity) were compared across clusters. Correlations between total number of dysmorphic features and age at onset of psychosis were examined and the number and distribution of dysmorphic features were examined in the entire sample and across clusters. Between-cluster validity was examined by comparing rates of individual dysmorphic features, mental retardation, and congenital heart defects as well as median age at onset of psychosis across clusters. These validating factors were chosen as they differ between clusters, are clinically relevant to the samples under study, and were not used in the cluster analysis procedure [Rapkin and Luke, 1993]. Within-cluster validity was examined by comparing rates of dysmorphic features between males and females (to assess possible sex differences) and between early (≤ 21 years) and later (> 21 years) age at onset of psychosis within each cluster (to assess differences that may be related to an early age at onset).
Differences in frequencies were assessed using chi-square and continuous variables were assessed using the Kruskal-Wallis one-way analysis of variance by ranks. All analyses were performed using the SAS system for Windows, version 6.12 (SAS, Cary, NC); SAS/STAT User’s Guide was consulted for analysis procedures [SAS Institute, 1990, 1997].
Posthoc Assessments
Specialized cytogenetic testing
The 31 previously undiagnosed referred group subjects who met clinical screening criteria for 22qDS [Bassett and Chow, 1999] were tested in a related study [Bassett and Chow, 1999] for a 22q11.2 deletion using standard fluorescence in situ hybridization (FISH) methods and a commercially available probe (either N25 or TUPLE 1) from the commonly deleted region [Demczuk and Aurias, 1995]. Subjects from the random group who were found to meet proposed clinical criteria for 22qDS [Bassett and Chow, 1999] or other possible genetic syndromes on review of features by a medical geneticist (R.W.) also had cytogenetic testing.
RESULTS
Demographics
There were no significant differences between random (83 males, 40 females) and referred (19 males, 17 females) groups with respect to sex (chi-square = 2.62, df = 1, P = 0.11) or median age at onset of psychosis (21 years, range 13–51 years, and 19 years, range 11–36 years, respectively; z = −1.75, P = 0.08). The referred group had a younger median age at assessment (28.5 years, range 16–63 years, vs. 38 years, range 16–86 years; z = −4.27, P = 0.0001) and proportionately more Caucasian subjects (91.7% vs. 76.4%, df = 1, chi-square = 4.03, P = 0.05) than the random group. As expected, the proportion of subjects with mild mental retardation was significantly higher in the referred group (n = 18, 33.3%) than the random group (n = 12, 14.6%; chi-square = 6.36, df = 1, P = 0.012).
Dysmorphic Features and Interrater Reliability
Eight (11.0%) of 73 binary variables (asymmetrical ears, prominent nasal bridge, frontal bossing, mouth with downturning corners, large tongue, short neck, pectus excavatum, gap between first and second toes) with poor interobserver agreement were excluded from further analyses (κ < 0.4) [Mitchell, 1979]. The kappa statistic could not be computed for 13 (17.8%) variables (indicated in Table I) that were rated absent by all raters. For the remaining 52 (71.2%) variables, mean interobserver agreement as assessed by the Cohen kappa was 0.63 (SD = 0.22, range 0.4–1.00). In contrast to the ICC, mean kappa values greater than 0.6 are considered good to excellent [Mitchell, 1979; Keller et al., 1995]. Reliability was also high for height (ICC = 0.997), head circumference (ICC = 0.985), and inner canthal distance (ICC = 0.926). The 70 dysmorphic features (44 craniofacial and 26 other) used for the cluster analyses are listed in Table I. The median number of dysmorphic features for the entire sample was 9 (range, 1–30). Referred subjects had more dysmorphic features (median = 16, range 5–30) than random subjects (median = 7, range 1–20; z = 7.21, P = 0.0001).
TABLE I.
Validity of Cluster Solutions Using Dysmorphic Features
| Occurrencea (%)
|
Chi-square (df = 3) | P value | ||||
|---|---|---|---|---|---|---|
| Cluster 1 (n = 27) | Cluster 3 (n = 67) | Cluster 4 (n = 18) | Cluster 5 (n = 41) | |||
| Craniofacial dysmorphic features (n = 44) | ||||||
| Hypernasal voice | 92.6 | 4.5 | 0 | 4.9 | 111.0 | 0.001 |
| Flat cheeks | 92.6 | 26.9 | 44.4 | 53.7 | 34.13 | 0.001 |
| Narrow/slanted palpebral fissures | 85.2 | 9.0 | 55.6 | 12.2 | 66.58 | 0.001 |
| High palate | 70.4 | 20.9 | 0 | 12.2 | 40.0 | 0.001 |
| Small jaw | 66.7 | 11.9 | 11.1 | 2.4 | 50.16 | 0.001 |
| Large nose | 66.7 | 16.4 | 27.8 | 12.2 | 30.87 | 0.001 |
| Small eyes | 63.0 | 10.5 | 38.9 | 7.3 | 39.47 | 0.001 |
| Small mouth | 55.6 | 14.9 | 11.1 | 7.3 | 27.8 | 0.001 |
| Broad nasal bridge | 44.4 | 6.0 | 27.8 | 17.1 | 20.28 | 0.001 |
| Low-set ears | 40.7 | 10.5 | 11.1 | 9.8 | 15.58 | 0.001 |
| Posteriorly rotated ears | 37.0 | 6.0 | 5.6 | 12.2 | 17.54 | 0.001 |
| Narrow palate | 37.0 | 3.0 | 5.6 | 4.9 | 27.7 | 0.001 |
| Lip abnormalities | 37.0 | 13.4 | 22.2 | 2.4 | 15.59 | 0.001 |
| Flat occiput | 33.3 | 11.9 | 50.0 | 41.5 | 16.92 | 0.001 |
| Flat supraorbital ridges | 33.3 | 3.0 | 33.3 | 7.3 | 23.45 | 0.001 |
| Cleft palate | 14.8 | 0 | 0 | 0 | 19.17 | 0.001 |
| Hair whorl abnormalityb | 7.4 | 11.9 | 50.0 | 17.1 | 16.87 | 0.001 |
| Low hair line | 3.7 | 4.5 | 33.3 | 12.2 | 14.82 | 0.002 |
| Inner epicanthal folds | 3.7 | 1.5 | 27.8 | 12.2 | 15.35 | 0.002 |
| Small ears | 40.7 | 10.5 | 5.6 | 19.5 | 14.21 | 0.003 |
| Malformed ears | 33.3 | 7.5 | 33.3 | 14.6 | 12.99 | 0.005 |
| Small nose | 0 | 7.5 | 22.2 | 2.4 | 10.36 | 0.02 |
| Macrocephaly | 7.4 | 20.9 | 5.6 | 34.2 | 10.08 | 0.02 |
| Low nasal bridgeb | 11.1 | 0 | 0 | 4.9 | 8.46 | 0.04 |
| Widow’s peak | 22.2 | 9.0 | 33.3 | 24.4 | 7.93 | 0.05 |
| Hypertelorism | 3.7 | 9.0 | 27.8 | 9.8 | 7.27 | 0.06 |
| Asymmetrical face | 29.6 | 19.4 | 33.3 | 9.8 | 6.20 | 0.10 |
| Iris abnormalitiesb | 0 | 4.5 | 11.1 | 0 | 6.11 | 0.11 |
| Prominent supraorbital ridges | 0 | 7.46 | 16.7 | 9.8 | 4.46 | 0.22 |
| Hypotelorism | 11.1 | 1.5 | 5.6 | 4.9 | 4.15 | 0.25 |
| Long philtrum | 7.4 | 7.5 | 16.7 | 2.4 | 3.82 | 0.28 |
| Asymmetrical eyes | 25.9 | 14.9 | 22.2 | 9.8 | 3.67 | 0.30 |
| Short philtrum | 7.4 | 9.0 | 0 | 14.6 | 3.40 | 0.33 |
| Prominent occiputb | 7.4 | 1.5 | 0 | 4.9 | 3.08 | 0.38 |
| Large ears | 14.8 | 13.4 | 5.6 | 4.9 | 3.01 | 0.39 |
| Missing lingual frenulum | 14.8 | 6.0 | 5.6 | 12.2 | 2.59 | 0.46 |
| Eye pathology | 3.7 | 4.5 | 11.1 | 2.4 | 2.24 | 0.52 |
| Ears protuberant | 14.8 | 19.4 | 16.7 | 26.8 | 1.77 | 0.62 |
| Broad face | 7.4 | 6.0 | 11.1 | 12.2 | 1.47 | 0.69 |
| Flat face | 11.1 | 7.5 | 16.7 | 9.8 | 1.43 | 0.70 |
| Prematurely grey | 7.4 | 6.0 | 0 | 4.9 | 1.34 | 0.72 |
| Microcephaly | 3.7 | 1.5 | 5.6 | 2.4 | 1.07 | 0.78 |
| Triangular face | 7.4 | 7.5 | 11.1 | 9.8 | 0.38 | 0.95 |
| Abnormal hair patternb | 3.7 | 4.5 | 5.6 | 4.9 | 0.097 | 0.99 |
| Other dysmorphic features (n = 26) | ||||||
| Scoliosis | 37.4 | 11.9 | 0 | 90.2 | 79.38 | 0.001 |
| Clinodactyly of fingers | 33.3 | 10.5 | 0 | 36.6 | 18.19 | 0.001 |
| Small hands | 25.9 | 6.0 | 44.4 | 4.9 | 23.85 | 0.001 |
| Cubitus valgus | 25.9 | 1.5 | 16.7 | 2.4 | 19.55 | 0.001 |
| Sacral dimpleb | 3.7 | 3.0 | 38.9 | 2.4 | 30.76 | 0.001 |
| Sparse body hair | 18.5 | 1.5 | 55.6 | 22.0 | 32.09 | 0.001 |
| Loose skinb | 0 | 3.0 | 33.3 | 4.9 | 24.62 | 0.001 |
| Loose skin on neckb | 0 | 1.5 | 27.8 | 2.4 | 25.38 | 0.001 |
| Kyphosis | 55.6 | 23.9 | 16.7 | 17.1 | 14.59 | 0.002 |
| Short toes | 33.3 | 31.3 | 0 | 14.6 | 11.06 | 0.01 |
| Skin pigmentation abnormalities | 37.0 | 17.9 | 5.6 | 12.2 | 9.32 | 0.025 |
| Long toes | 37.0 | 16.4 | 5.6 | 17.1 | 8.23 | 0.04 |
| Simian crease | 3.7 | 5.97 | 0 | 17.07 | 7.33 | 0.062 |
| Inguinal hernia | 14.8 | 3.0 | 5.6 | 2.4 | 6.34 | 0.10 |
| Webbed toes | 29.6 | 35.8 | 33.3 | 14.6 | 5.82 | 0.12 |
| Pectus carinatumb | 0 | 4.5 | 0 | 9.8 | 4.68 | 0.20 |
| Short fingers | 18.5 | 9.0 | 0 | 12.2 | 4.27 | 0.23 |
| Small feet | 14.8 | 6.0 | 5.6 | 2.4 | 4.22 | 0.24 |
| Hyperflexible joints | 25.9 | 10.5 | 11.1 | 14.6 | 3.92 | 0.27 |
| Short stature | 14.8 | 14.9 | 33.3 | 14.6 | 3.86 | 0.28 |
| Long fingersb | 14.8 | 4.9 | 5.6 | 7.3 | 3.16 | 0.37 |
| Asymmetrical feetb | 3.7 | 3.0 | 11.1 | 2.4 | 2.87 | 0.41 |
| Umbilical hernia | 3.7 | 1.5 | 5.6 | 7.3 | 2.44 | 0.49 |
| Longneckb | 0 | 4.5 | 5.6 | 4.9 | 1.38 | 0.71 |
| Winged scapulab | 3.7 | 3.0 | 0 | 4.9 | 0.98 | 0.81 |
| Hirsute body hair | 7.4 | 6.0 | 5.6 | 2.4 | 0.98 | 0.81 |
Frequencies in boldface are those dysmorphic features that were significantly different across clusters and occurred in at least 20% of subjects within an individual cluster.
Dysmorphic features (n = 13) with no available kappa statistic.
Cluster Analyses
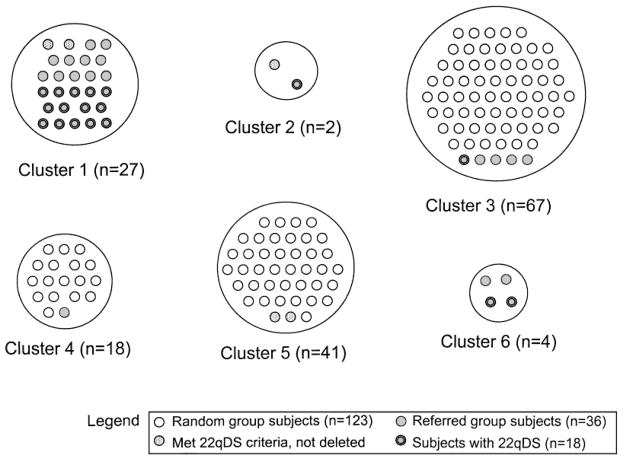
The Ward minimum variance and average linkage cluster analyses suggested seven-cluster and four-cluster solutions, respectively. Results were essentially unchanged when the five subjects who had already been diagnosed prior to their physical examination were omitted (results not shown), therefore they were included for all analyses. The k means cluster analysis technique was performed with four, five, six, and seven target clusters to assess stability of the clusters. The most logical solution as determined by the investigators was the six-cluster solution, based on the assignment of most referred subjects to cluster 1 (Fig. 1). Clustering of subjects together was relatively consistent across four-, five-, six-, and seven-target-cluster solutions, demonstrating stability of clusters. As expected, 14 of the 18 22qDS subjects (78%) clustered together in cluster 1; the remaining subjects were allocated to clusters 2 (n = 1), 3 (n = 1), and 6 (n = 2; Fig. 1). Clusters 2 and 6 were considered outliers as they contained less than 5% of the total sample size [Edelbrock, 1979] and were dropped from subsequent analyses, leaving four major clusters (1, 3, 4, and 5).
Fig. 1.
The six-cluster solution grouping 159 subjects with schizophrenia, as produced by the k means cluster analysis. The four major clusters (1, 3, 4, and 5) comprise 153 subjects. Clusters 2 and 6 each contained less than 5% of the sample size, therefore they were considered outliers and dropped from analyses.
Between-Cluster Validity
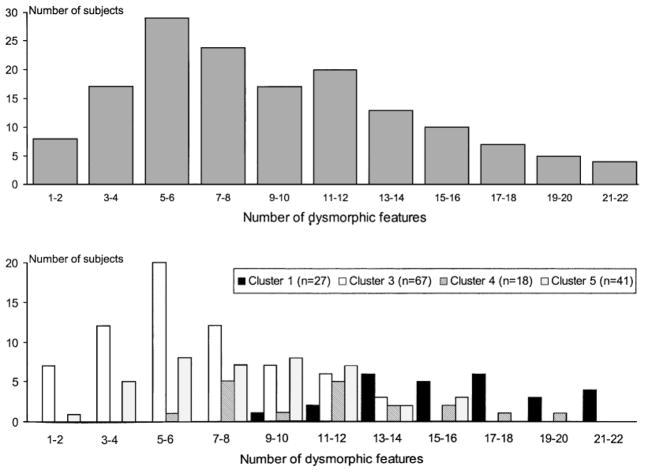
The four major clusters differed qualitatively (Table I) and quantitatively (Fig. 2) with respect to occurrence of dysmorphic features. Thirty-seven (52.9%) of the 70 dysmorphic features listed in Table I differed significantly (P = 0.05 to P = 0.001) in frequency across the four major clusters. The median number of dysmorphic features was significantly different across the clusters (medians were 16, 11.5, 8, and 6 for clusters 1, 4, 5, and 3, respectively; F = 60.26, P < 0.0001; Fig. 2). Clusters 1 and 4 had higher rates of craniofacial dysmorphic features than clusters 3 and 5 (medians: 11, 7.5, 5, and 4 for clusters 1, 4, 5, and 3, respectively; F = 74.8, P < 0.0001). Rates of other dysmorphic features were lowest for cluster 3 subjects (medians: 4, 4.5, 4, and 3 for clusters 1, 4, 5, and 3, respectively; F = 11.5, P < 0.0001). The distributions of rates of dysmorphic features for the total sample and each of the clusters appeared relatively normal and unimodal, with the exception of cluster 4, which appeared to be somewhat bimodal (Fig. 2).
Fig. 2.
The top graph shows the distribution of number of dysmorphic features for the sample of 153 subjects from the four major clusters (1, 3, 4, and 5). The bottom graph reveals the distributions of dysmorphic features for clusters 1 (n = 27), 3 (n = 67), 4 (n = 18), and 5 (n = 41) separately.
The clusters also differed on variables not used in the cluster analysis (Table II). Sex distributions differed significantly across clusters, with an equal sex distribution in clusters 1 and more women in cluster 4 compared with male excess in clusters 3 and 5. Clusters did not differ significantly on ethnicity. Rates of mental retardation and congenital heart defects were significantly different across clusters (Table II). These features were more prevalent in cluster 1, as expected, and in cluster 4. Median age at onset of psychosis (20 years, range 11–34 years; 19 years, range 14–46 years; 22 years, range 14–45 years; and 21 years, range 13–43 years, for clusters 1, 3, 4, and 5, respectively) was not significantly different between clusters (F = 1.69, P = 0.17) and was not correlated with the total number of dysmorphic features (r = −0.06 for all 153 subjects, and r = 0.13, −0.07, −0.19, and −0.05 for clusters 1, 3, 4, and 5, respectively).
TABLE II.
Demographic and Validating Variables Not Used in Cluster Analysis
| Variables not used in cluster analysis | Cluster 1 (n = 27) | Cluster 3 (n = 67) | Cluster 4 (n = 18) | Cluster 5 (n = 41) | Chi-square (df = 3) | P value |
|---|---|---|---|---|---|---|
| Sex (% male) | 51.9 | 62.7 | 44.4 | 85.4 | 12.97 | 0.005 |
| Ethnicity (% Caucasian) | 96.3 | 76.1 | 88.9 | 73.2 | 7.29 | 0.06 |
| Early age at onset (% ≤ 21 years) | 70.4 | 64.2 | 44.4 | 53.7 | 4.20 | 0.24 |
| Congenital heart defect | 22.2 | 1.82 | 7.69 | 0 | 14.39 | 0.002 |
| Mild mental retardation | 33.3 | 6.7 | 42.9 | 6.1 | 20.2 | 0.001 |
Within-Cluster Validity
There were no significant differences after Bonferroni correction was applied in rates of dysmorphic features between males and females or between subjects with early and later age at onset within any of the four major clusters.
(Post hoc) Results
Reviews by medical geneticist and specialized cytogenetic testing
Thirteen (41.9%) of the 31 referred subjects assessed using FISH were found to have a chromosome 22q11.2 deletion. Including the five subjects (all in cluster 1) referred with known deletions, the referred group comprised 18 22qDS subjects and 18 nondeleted subjects. Five random subjects, two in cluster 1, one in cluster 2, and two in cluster 5, were also found to meet proposed clinical screening criteria for 22qDS [Bassett and Chow, 1999]. None of these subjects was found to have a 22q11.2 deletion or other chromosomal anomaly. Three other random subjects had clinical features suggestive of other possible genetic syndromes and were assessed by a medical geneticist. A female subject from cluster 4 with mild mental retardation, short stature, minor ear anomalies, small hands and feet, cubitus valgus, and kyphosis met clinical criteria for possible Turner syndrome. Subsequent cytogenetic testing revealed a mosaic Turner syndrome variant (45,X in 10 cells; 46,XX in 19 cells; 47,XXX in one cell). A cluster 3 subject had features (tall stature, myopia, arm span greater than height, mild hypotonia, and a history of spontaneous pneumothoraces) suggestive of an unspecified connective tissue disorder. Another cluster 3 subject with multiple café-au-lait spots was assessed for possible neurofibromatosis, but did not meet criteria for a clinical diagnosis. Neither of the latter two subjects had a detectable chromosomal abnormality.
DISCUSSION
This study found four major subgroups in a large sample of subjects with chronic schizophrenia, with differing patterns and rates of dysmorphic features and other validating factors that did not appear to be related to ethnicity or age. The results indicate the potential importance of the pattern, in addition to the number, of dysmorphic features in helping to identify possible subgroups of schizophrenia that may have relevance to a genetic neurodevelopmental pathogenesis. This study presents a complementary strategy to research involving familial (transmitted) forms of schizophrenia and risk research attempting to differentiate schizophrenia from control samples. Many genetic syndromes occur as spontaneous (not inherited) mutations that may involve structural abnormalities of chromosomes [Muir, 2000], but variability of features in syndromes may be related to random (stochastic) effects [Kurnit et al., 1987]. Thus, the overall pattern of dysmorphic features may be more indicative of common etiology than an individual feature.
Clusters Suggestive of Genetic and/or Malformation Syndromes
Although the clusters identified in this study undoubtedly contain heterogeneous groups of subjects with schizophrenia, there is some evidence that two of the clusters may represent more homogeneous subgroups perhaps related to specific neurodevelopmentally related etiologies. Cluster 1 comprises mainly subjects with a known genetic subtype of schizophrenia, 22qDS, and/or clinical features suggestive of the syndrome. These subjects served as a testable model that validated the cluster analysis solutions. Cluster 1 had high rates of palatal anomalies, including narrow palate and high palate that were not part of the ascertainment criteria for the referred group. This is consistent with increased rates of palatal anomalies reported in 22qDS [Cohen et al., 1999] and schizophrenia [Green et al., 1987, 1989; McGrath et al., 1995; Waddington et al., 1995; Lane et al., 1997]. Cluster 4 is the other cluster that may be more likely to contain subjects with genetic or malformation syndromes and had the second highest median number of dysmorphic features. This cluster had elevated rates of mild mental retardation and congenital heart defects, but a different pattern of dysmorphic features from cluster 1, including abnormal hair whorls, inner epicanthal folds, and sacral dimple. These features were not suggestive of any single specific genetic syndrome, and the somewhat bimodal distribution of the number of dysmorphic features in this cluster suggests that more than one neurodevelopmental syndrome is likely to be present. Interestingly, one subject in this cluster was diagnosed with mosaic Turner syndrome. Mosaic Turner syndrome occurs in women and is characterized by a normal karyotype in some cells (i.e., 46,XX) and a missing X chromosome in other cells (i.e., 45,X). Dysmorphic features in this syndrome include short stature and webbed neck, sometimes congenital heart defects, specific learning disabilities (e.g., visual-spatial deficits) [Jones, 1997], and an association with schizophrenia [Bassett et al., 2000; Prior et al., 2000].
Clusters Consistent With Literature
The two other major clusters (clusters 3 and 5) had lower overall rates of dysmorphic features, but elevated rates of individual features found in previous studies to be relatively prevalent in schizophrenia: clinodactyly (cluster 5) [Green et al., 1989; McGrath et al., 1995], protuberant ears (cluster 5) [Lane et al., 1997], macrocephaly (clusters 3 and 5) [Green et al., 1989], and high palate (cluster 3) [Green et al., 1987; Ismail et al., 1998]. Individual dysmorphic features are usually of no significance [Jones, 1997]. However, the number and patterns of features in these clusters are consistent with generalized developmental disturbances in schizophrenia [Weinberger, 1995]. The lower rates of mental retardation and greater preponderance of males in these two clusters suggest these subjects may be more comparable to schizophrenia samples usually reported in the literature.
Comparison of Results to Literature
Results from the present study are consistent with reports suggesting that higher rates of dysmorphic features in schizophrenia may be driven by one or more subgroups of subjects with high rates [Green et al., 1994b; McGrath et al., 1995; Griffiths et al., 1998]. Although a unimodal and relatively normal distribution of dysmorphic features has been cited as evidence for the lack of a subgroup effect in schizophrenia [Lane et al., 1997; Ismail et al., 2000], results from the present study suggest that the overall frequency distribution may conceal varying rates (Fig. 2), as well as varying patterns (Table I), of dysmorphic features in subgroups. The overlap of referred and random subjects in three of the four major clusters is consistent with previous reports of overlap among individuals with known genetic syndromes [Preus and Ayme, 1983; Preus, 1984]. This is due to both the nonspecific nature of many dysmorphic features and the considerable variability among individuals with the same genetic syndrome [Jones, 1997]. Age at onset of psychosis did not differentiate clusters, and consistent with most [O’Callaghan et al., 1991; Lohr and Flynn, 1993; Alexander et al., 1994; McGrath et al., 1995] but not all [Green et al., 1987] studies, age at onset was not found to be correlated with the overall number of dysmorphic features. This is likely because most of the subjects had severe, chronic schizophrenia characterized by relatively early age at onset. Therefore, the present sample may represent multiple neurodevelopmental forms of schizophrenia where age at onset may not be a discriminating factor. These converging results, despite differing methodologies, suggest that dysmorphic features may be more useful than age at onset of psychosis for the identification of putative subgroups among neurodevelopmental forms of schizophrenia.
Advantages
The current study applied a genetic approach to identifying possible subgroups in schizophrenia using dysmorphic features as markers of both abnormal development and possible genetic syndromes. The samples used ensured that a sufficient number of subjects with features of a genetic syndrome (22qDS) with known etiology would be present to act as a testable model of a genetic subtype of schizophrenia and therefore to validate the cluster analysis methods. Further, the present study included subjects with IQ < 70, an understudied group despite their clinical similarities to and prevalence in chronic populations of schizophrenia [Doody et al., 1998; Sanderson et al., 1999]. Mild mental retardation is a feature of many genetic syndromes involving abnormal neurodevelopment [Winter, 1996; Jones, 1997]. Therefore, including these subjects could have increased the likelihood of identifying genetically relevant subgroups.
The assessment tool used, the SPEDF, reliably assessed 70 dysmorphic features that may be associated with genetic syndromes in adults. Most previous studies of dysmorphic features in schizophrenia have employed varying modifications of the Waldrop scale [Waldrop et al., 1968]. Several researchers have pointed out the limitations of this scale [Alexander et al., 1994; O’Callaghan et al., 1995; Murphy and Owen, 1996], which is comprised of only 18 features and was developed to assess dysmorphic features, primarily of Down syndrome, in pediatric samples [Waldrop et al., 1968]. One study used an anthropometric approach [Lane et al., 1997], a comprehensive but labor-intensive method requiring special instruments. In contrast, the current study employed a 30-min clinical assessment of dysmorphic features.
Study Limitations
The present study has several limitations. First, referred group subjects were ascertained partly from patterns of dysmorphic features and developmental features such as mental retardation and congenital heart defects [Bassett and Chow, 1999]. Therefore, results for cluster 1 were anticipated from the study design. However, all subjects met clinical criteria [Bassett and Chow, 1999] for a suspected genetic syndrome with known etiology; therefore, the fact that they mostly clustered together provided an initial validator of the overall cluster solutions. Second, cluster analysis techniques are somewhat arbitrary, and it is up to the researcher ultimately to decide the number of clusters that makes the most sense [Lorr, 1983]. To minimize the possible effects of a priori expectations, hierarchical and iterative methods were combined to produce discrete clusters [Milligan and Sokol, 1980]. Third, most of the features were clinically determined as present or absent. However, the current study used only reliably assessed features, and a subjective approach to assessing dysmorphic features is commonly used for genetic and other malformation syndromes [Preus and Ayme, 1983; Jones, 1997]. This approach also predominates in the Waldrop scale, used in most other studies of dysmorphic features in schizophrenia [Gualtieri et al., 1982; Guy et al., 1983; Green et al., 1987, 1989, 1994b; O’Callaghan et al., 1991, 1995; Lohr and Flynn, 1993; Alexander et al., 1994; Cantor-Graae et al., 1994; McGrath et al., 1995; Waddington et al., 1995; Griffiths et al., 1998; Weinstein et al., 1999; Ismail et al., 2000]. Fourth, assessment of some features may have been confounded by age or ethnicity effects. For example, cluster 4 had an older median age of assessment suggesting that some dysmorphic features in this subgroup may have been acquired. However, age-related features are unlikely to be the primary reason for the clustering results, since the initial cluster analysis step should have minimized effects of correlated variables (e.g., age-related) and the common features in this subgroup (e.g., narrow/slanted palpebral fissures, flat occiput, hair whorl abnormalities) are unlikely to be age-related. Fifth, mild mental retardation was determined from chart diagnosis rather than available IQ testing results in one-third of cases. It is possible that some of these subjects could have had borderline mental retardation. Finally, subjects had a severe, chronic form of schizophrenia and a relatively high rate of mild mental retardation that is similar to rates in other unselected samples [David et al., 1997] but may be less generalizable to samples common in the research literature, which may have higher levels of education or less severe forms of the illness.
Summary and Future Research
The value of assessing dysmorphic features in schizophrenia may lie in the pattern, as well as the number, of features. The manner in which dysmorphic features group together may sometimes offer a clue to the identification of potential underlying genetic or teratogenic abnormalities. Future research in this area should include systematic specialized cytogenetic and/or molecular testing of individuals with schizophrenia with certain patterns and elevated rates of dysmorphic and/or other features, who may be at increased risk for specific genetic syndromes [Bassett et al., 2000]. Although the phenotype of two identifiable genetic syndromes (22qDS and mosaic Turner syndrome) associated with schizophrenia is variable and may be subtle, an increased index of suspicion for these syndromes and their pattern of features may enhance their identification [Bassett et al., 2000]. Investigation of brain structure and functioning and neurocognitive deficits could help identify specific neurodevelopmental abnormalities associated with subgroups identified by patterns of dysmorphic features and/or cytogenetic or molecular abnormalities. This behavioral neurogenetics approach has recently been advocated for the multilevel investigation of genetic syndromes with significant cognitive and neuropsychiatric components, such as 22qDS [Reiss et al., 2000]. Our group has documented qualitative [Chow et al., 1999] and quantitative [Chow et al., in press] structural brain abnormalities in adults with 22qDS and schizophrenia. Further delineation of patterns of dysmorphic features and cognitive functioning and structural brain abnormalities may lead to the identification of subgroups with other, as yet unrecognized genetic or developmental etiologies for schizophrenia. Studying such etiological subtypes may improve our understanding of the pathogenesis of neurodevelopmental forms of schizophrenia that could be relevant to the illness in general.
Acknowledgments
Grant sponsor: The Ontario Mental Health Foundation, the Scottish Rite Schizophrenia Research Program, and National Alliance for Research on Schizophrenia and Depression.
We thank Dr. B. MacGillvray and Dr. C. Haylock (University of British Columbia), Dr. R. Levitan (University of Toronto), J. Hogan, K. Dufour, and R. Roy for help with data collection. We also thank the many clinicians who helped to ascertain referred group subjects. Grateful thanks go to all of the patients.
Footnotes
First presented as a poster at the Winter Workshop in Schizophrenia in Davos, Switzerland, February 1998, and at the International Congress on Schizophrenia Research in Santa Fe, New Mexico, April 1999.
References
- Alexander RC, Mukherjee S, Richter J, Kaufmann CA. Minor physical anomalies in schizophrenia. J Nerv Ment Dis. 1994;182:639–644. doi: 10.1097/00005053-199411000-00007. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. DSM-III-R: diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- Anderberg MR. Cluster analysis for applications. New York: Academic Press; 1973. [Google Scholar]
- Bassett AS, Chow EWC. 22q11 deletion syndrome: a genetic subtype of schizophrenia. Biol Psychiatry. 1999;46:882–891. doi: 10.1016/s0006-3223(99)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC, Bury A, Ali F, Haylock CAH, Smith GN, Lapointe JS, Honer WG. Increased head circumference in schizophrenia. Biol Psychiatry. 1996;40:1173–1175. doi: 10.1016/S0006-3223(96)00288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Hodgkinson K, Chow EWC, Correia S, Scutt LE, Weksberg R. 22q11 deletion syndrome in adults with schizophrenia. Am J Med Genet (Neuropsychiatr Genet) 1998;81:328–337. [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC, Weksberg R. Chromosomal abnormalities and schizophrenia. Am J Med Genet. 2000;97:45–51. doi: 10.1002/(sici)1096-8628(200021)97:1<45::aid-ajmg6>3.0.co;2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham PM, Zimmerman RA, McDonald-McGinn D, Driscoll D, Emanuel BS, Zackai E. Enlarged sylvian fissures in infants with interstitial deletion of chromosome 22q11. Am J Med Genet (Neuropsychiatr Genet) 1997;74:538–543. [PubMed] [Google Scholar]
- Borgen FH, Weiss DJ. Research methodology: cluster analysis in counseling research. J Counsel Psychol. 1971;18:583–591. [Google Scholar]
- Brzustowicz LM, Hodgkinson KA, Chow EWC, Honer WG, Bassett AS. Location of a major susceptibility locus for familial schizophrenia on chromosome Iq21–q22. Science. 2000;288:678–682. doi: 10.1126/science.288.5466.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushby KMD, Cole T, Matthews JNS, Goodship JA. Centiles for adult head circumference. Arch Dis Child. 1992;67:1286–1287. doi: 10.1136/adc.67.10.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor-Graae E, McNeil TF, Torrey EF, Quinn P, Bowler A, Sjostrom K, Rawlings R. Link between pregnancy complications and minor physical anomalies in monozygotic twins discordant for schizophrenia. Am J Psychiatry. 1994;151:1188–1193. doi: 10.1176/ajp.151.8.1188. [DOI] [PubMed] [Google Scholar]
- Chow EWC, Zipursky RB, Mikulis DJ, Scutt LE, Weksberg R, Bassett AS. Qualitative MRI findings in adults with 22q11 deletion syndrome and schizophrenia. Biol Psychiatry. 1999;46:1436–1442. doi: 10.1016/s0006-3223(99)00150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EWC, Zipursky RB, Mikulis DJ, Bassett AS. Structural brain abnormalities in patients with schizophrenia and 22q11 Deletion Syndrome. Bio Psychiatry. doi: 10.1016/s0006-3223(01)01246-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- Cohen E, Chow EWC, Weksberg R, Bassett AS. The phenotype of adults with the 22q11 deletion syndrome: a review. Am J Med Genet. 1999;86:359–365. doi: 10.1002/(sici)1096-8628(19991008)86:4<359::aid-ajmg10>3.0.co;2-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David AS, Malmberg A, Brandt L, Allebeck P, Lewis G. IQ and risk for schizophrenia: a population-based cohort study. Psychol Med. 1997;27:1311–1323. doi: 10.1017/s0033291797005680. [DOI] [PubMed] [Google Scholar]
- Demczuk S, Aurias A. DiGeorge syndrome and related syndromes associated with 22q11.2 deletions: a review. Ann Genet. 1995;38:59–76. [PubMed] [Google Scholar]
- Doody GA, Johnstone EC, Sanderson TL, Cunningham Owens DG, Muir WJ. “Pfropfschizophrenie” revisited: schizophrenia in people with mild learning disability. Br J Psychiatry. 1998;173:145–153. doi: 10.1192/bjp.173.2.145. [DOI] [PubMed] [Google Scholar]
- Edelbrock C. Mixture model tests of hierarchical clustering algorithms: the problem of classifying everybody. Multivar Behav Res. 1979;14:367–384. doi: 10.1207/s15327906mbr1403_6. [DOI] [PubMed] [Google Scholar]
- Green MF, Satz P, Soper HV, Kharabi F. Relationship between physical anomalies and age at onset of schizophrenia. Am J Psychiatry. 1987;144:666–667. doi: 10.1176/ajp.144.5.666. [DOI] [PubMed] [Google Scholar]
- Green MF, Satz P, Galer DJ, Ganzell S, Kharabi F. Minor physical anomalies in schizophrenia. Schizophr Bull. 1989;15:91–99. doi: 10.1093/schbul/15.1.91. [DOI] [PubMed] [Google Scholar]
- Green MF, Bracha HS, Satz P, Christenson CD. Preliminary evidence for an association between minor physical anomalies and second trimester neurodevelopment in schizophrenia. Psychiatry Res. 1994a;53:119–127. doi: 10.1016/0165-1781(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Green MF, Satz P, Christenson C. Minor physical anomalies in schizophrenia patients, bipolar patients, and their siblings. Schizophr Bull. 1994b;20:433–440. doi: 10.1093/schbul/20.3.433. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Sigmundsson T, Takei N, Frangou S, Birkett PB, Sharma T, Reveley AM, Murray RM. Minor physical anomalies in familial and sporadic schizophrenia: the Maudsley family study. J Neurosurg Psychiatry. 1998;64:56–60. doi: 10.1136/jnnp.64.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri CT, Adams A, Shen CD, Loiselle D. Minor physical anomalies in alcoholic and schizophrenic adults and hyperactive and autistic children. Am J Psychiatry. 1982;139:640–643. doi: 10.1176/ajp.139.5.640. [DOI] [PubMed] [Google Scholar]
- Guy JD, Majorski LV, Wallace CJ, Guy MP. The incidence of minor physical anomalies in adult male schizophrenics. Schizophr Bull. 1983;9:571–582. doi: 10.1093/schbul/9.4.571. [DOI] [PubMed] [Google Scholar]
- Hall JG, Froster-Iskenius UG, Allanson JE. Handbook of normal physical measurements. Oxford: Oxford University Press; 1989. [Google Scholar]
- Ismail B, Cantor-Graae E, McNeil TF. Minor physical anomalies in schizophrenic patients and their siblings. Am J Psychiatry. 1998;155:1695–1702. doi: 10.1176/ajp.155.12.1695. [DOI] [PubMed] [Google Scholar]
- Ismail B, Cantor-Graae E, McNeil TF. Minor physical anomalies in schizophrenia: cognitive, neurological and other correlates. J Psychiatr Res. 2000;34:45–56. doi: 10.1016/s0022-3956(99)00034-5. [DOI] [PubMed] [Google Scholar]
- Jones KL. Smith’s recognizable patterns of human malformation. 5. Philadelphia: W.B. Saunders; 1997. [Google Scholar]
- Jones P, Murray RM. The genetics of schizophrenia is the genetics of neurodevelopment. Br J Psychiatry. 1991;158:615–623. doi: 10.1192/bjp.158.5.615. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec PS, Lasseter VK, Eisen H, Childs B, Kazazian HH, Kucherlapati R, Antonarakis SE, Pulver AE, Housman DE. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MB, Klein DN, Hirschfeld RMA, Kocsis JH, McCullough JP, Miller I, First MB, Holzer CP, III, Keitner GI, Marin D, Shea T. Results of the DSM-IV mood disorders field trial. Am J Psychiatry. 1995;152:843–849. doi: 10.1176/ajp.152.6.843. [DOI] [PubMed] [Google Scholar]
- Kurnit DM, Layton WM, Matthysse S. Genetics, chance, and morphogenesis. Am J Hum Genet. 1987;41:979–995. [PMC free article] [PubMed] [Google Scholar]
- Lane A, Kinsella A, Murphy P, Byrne M, Keenan J, Colgan K, Cassidy B, Sheppard N, Horgan R, Waddington JL, Larkin C, O’Callaghan E. The anthropometric assessment of dysmorphic features in schizophrenia as an index of its developmental origins. Psychol Med. 1997;27:1155–1164. doi: 10.1017/s0033291797005503. [DOI] [PubMed] [Google Scholar]
- Lohr JB, Flynn K. Minor physical anomalies in schizophrenia and mood disorders. Schizophr Bull. 1993;19:551–556. doi: 10.1093/schbul/19.3.551. [DOI] [PubMed] [Google Scholar]
- Lorr M. Cluster analysis for social scientists. Vol. 1983. San Francisco: Jossey-Bass; [Google Scholar]
- McGrath JJ, Van Os J, Hoyos C, Jones PB, Harvey I, Murray RM. Minor physical anomalies in psychoses: associations with clinical and putative aetiological variables. Schizophr Res. 1995;18:9–20. doi: 10.1016/0920-9964(95)00016-x. [DOI] [PubMed] [Google Scholar]
- Milligan GW, Sokol LM. A two-stage clustering algorithm with robust recovery characteristics. Educ Psychol Meas. 1980;40:755–759. [Google Scholar]
- Mitchell SK. Interobserver agreement, reliability, and generalizability of data collected in observational studies. Psychol Bull. 1979;86:376–390. [Google Scholar]
- Muir WJ. Genetics advances and learning disability. Br J Psychiatry. 2000;176:12–19. doi: 10.1192/bjp.176.1.12. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Owen MJ. Minor physical anomalies and their relationship to the aetiology of schizophrenia. Br J Psychiatry. 1996;168:139–142. doi: 10.1192/bjp.168.2.139. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones RG, Griffiths E, Thompson PW, Owen MJ. Chromosome 22q11 deletions: an under-recognised cause of idiopathic learning disability. Br J Psychiatry. 1998;172:180–183. doi: 10.1192/bjp.172.2.180. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones AL, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiat. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Murray RM, O’Callaghan E, Castle DJ, Lewis SW. A neurodevelopmental approach to the classification of schizophrenia. Schizophr Bull. 1992;18:319–332. doi: 10.1093/schbul/18.2.319. [DOI] [PubMed] [Google Scholar]
- O’Callaghan E, Larkin C, Kinsella A, Waddington JL. Familial, obstetric, and other clinical correlates of minor physical anomalies in schizophrenia. Am J Psychiatry. 1991;148:479–483. doi: 10.1176/ajp.148.4.479. [DOI] [PubMed] [Google Scholar]
- O’Callaghan E, Buckley P, Madigan C, Redmond O, Stack JP, Kinsella A, Larkin C, Ennis JT, Waddington JL. The relationship of minor physical anomalies and other putative indices of developmental disturbance in schizophrenia to abnormalities of cerebral structure on magnetic resonance imaging. Biol Psychiatry. 1995;38:516–524. doi: 10.1016/0006-3223(94)00381-C. [DOI] [PubMed] [Google Scholar]
- Preus M. The numerical versus intuitive approach to syndrome nosology. Birth Defects. 1980;16:93–104. [PubMed] [Google Scholar]
- Preus M. The Williams syndrome: objective definition and diagnosis. Clin Genet. 1984;25:422–428. doi: 10.1111/j.1399-0004.1984.tb02011.x. [DOI] [PubMed] [Google Scholar]
- Preus M, Ayme S. Formal analysis of dysmorphism: objective methods of syndrome definition. Clin Genet. 1983;23:1–16. doi: 10.1111/j.1399-0004.1983.tb00430.x. [DOI] [PubMed] [Google Scholar]
- Prior TI, Chue PS, Tibbo P. Investigation of Turner syndrome in schizophrenia. Am J Med Genet (Neuropsychiatr Genet) 2000;96:373–378. doi: 10.1002/1096-8628(20000612)96:3<373::aid-ajmg26>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS, Morrow B, Karayiorgou M, Antonarakis SE, Housman D, Kucherlapati R. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis. 1994;182:476–478. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- Rapkin BD, Luke DA. Cluster analysis in community research: epistemology and practice. Am J Coram Psychol. 1993;27:247–277. [Google Scholar]
- Reiss AL, Eliez S, Schmitt JE, Patwardhan A, Haberecht M. Brain imaging in neurogenetic conditions: realizing the potential of behavioral neurogenetics research. Ment Retard Dev Disabil Res. 2000;6:186–197. doi: 10.1002/1098-2779(2000)6:3<186::AID-MRDD6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sanderson TL, Best JJ, Doody GA, Owens DG, Johnstone EC. Neuroanatomy of comorbid schizophrenia and learning disability: a controlled study. Lancet. 1999;354:1867–1871. doi: 10.1016/s0140-6736(99)01049-1. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT user’s guide, version 6. 4. Cary, NC: SAS Institute; 1990. [Google Scholar]
- SAS Institute. SAS/STAT software: changes and enhancements through release 6.12. Cary, NC: SAS Institute; 1997. [Google Scholar]
- Scambler PJ, Kelly D, Lindsay E, Williamson R, Goldberg R, Shprintzen R, Wilson DI, Goodship JA, Cross IE, Burn J. Velo-cardio-facial syndrome associated with chromosome 22 deletions encompassing the DiGeorge locus. Lancet. 1992;339:1138–1139. doi: 10.1016/0140-6736(92)90734-k. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg RB, Lewin ML, Sidoti EJ, Berkman MD, Argamaso RV, Young D. A new syndrome involving cleft palate, cardiac anomalies, typical fades, and learning disabilities: velo-cardio-facial syndrome. Cleft Palate J. 1978;15:56–62. [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Simonds JF, Aston L. Relationship between minor physical anomalies, perinatal complications, and psychiatric diagnoses in children. Psychiatry Res. 1981;4:181–188. doi: 10.1016/0165-1781(81)90021-4. [DOI] [PubMed] [Google Scholar]
- Sneath PHA, Sokal RR. Numerical taxonomy: the principles and practice of numerical classification. New York: W.H. Freeman; 1973. [Google Scholar]
- Tobias ES, Morrison N, Whiteford ML, Tolmie JL. Towards earlier diagnosis of 22q11 deletions. Arch Dis Child. 1999;81:513–514. doi: 10.1136/adc.81.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington JL, O’Callaghan E, Buckley P, Madigan C, Redmond O, Stack JP, Kinsella A, Larkin C, Ennis JT. Tardive dyskinesia in schizophrenia: relationship to minor physical anomalies, frontal lobe dysfunction and cerebral structure on magnetic resonance imaging. Br J Psychiatry. 1995;167:41–44. doi: 10.1192/bjp.167.1.41. [DOI] [PubMed] [Google Scholar]
- Waddington JL, Lane A, Larkin C, O’Callaghan E. The neurodevelopmental basis of schizophrenia: clinical clues from cerebro-craniofacial dysmorphogenesis, and the roots of a lifetime trajectory of disease. Biol Psychiatry. 1999;46:31–39. doi: 10.1016/s0006-3223(99)00055-4. [DOI] [PubMed] [Google Scholar]
- Waldrop MF, Pedersen FA, Bell RQ. Minor physical anomalies and behavior in preschool children. Child Dev. 1968;39:391–400. [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiat. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. From neuropathology to neurodevelopment. Lancet. 1995;346:552–557. doi: 10.1016/s0140-6736(95)91386-6. [DOI] [PubMed] [Google Scholar]
- Weinstein DD, Diforio D, Schiffman J, Walker E, Bonsall R. Minor physical anomalies, dermatoglyphic asymmetries, and Cortisol levels in adolescents with schizotypal personality disorder. Am J Psychiatry. 1999;156:617–623. doi: 10.1176/ajp.156.4.617. [DOI] [PubMed] [Google Scholar]
- Winter RM. What’s in a face? Nat Genet. 1996;12:124–129. doi: 10.1038/ng0296-124. [DOI] [PubMed] [Google Scholar]