Abstract
Since the recognition that plasminogen activator inhibitor-1 (PAI-1) is a powerful profibrotic molecule, there has been considerable interest in deciphering the extent to which this effect is mediated by its ability to inhibit serine proteases with downstream effects on fibrogenesis. This review will summarize current knowledge about the serine protease urokinase-type plasminogen activator and its high affinity receptor uPAR/CD87 as it pertains to chronic kidney disease (CKD) progression. An emerging theme is that the effects of PAI-1 and uPAR appear to be organ- and site-specific. Normal kidney tubules produce a large quantity of uPA that is secreted into the urinary space. Activity levels increase during CKD presumably due to new sources of production by macrophages and fibroblasts. By activating hepatocyte growth factor and degrading fibrinogen uPA may have anti-fibrotic effects. However CKD severity after experimental ureteral obstruction is not altered by endogenous uPA deficiency. Beneficial effects of exogenous uPA have been reported in experimental models of fibrosis in the lung and liver but CKD awaits exploration.
Absent in normal kidneys uPAR is expressed by both renal parenchymal cells and inflammatory cells in a variety of pathological states. Such expression appears beneficial based on studies performed in uPAR-deficient mice. The uPAR promotes bacterial clearance in infectious diseases. In CKD uPAR expression is associated with high uPA activity but its most important effect appears to be due to scavenging activities and effects on cell recruitment and migration. Although uPAR itself is a non-signaling receptor, it interacts with a variety of co-receptors to modify cellular behavior. Best known are interactions with the low-density lipoprotein receptor-related protein (LRP-1) that lead to PAI-1 endocytosis and degradation, and interactions with several integrins to regulate matrix-dependent cell migration. Contacts with the receptor for the complement C5a component and the interleukin −6 receptor gp130 are examples of other recently recognized interactions.
In addition to uPA, vitronectin and high molecular weight kininogen are alternate uPAR ligands that could be implicated in CKD progression. uPAR may also be shed from cell membranes. This soluble form (suPAR) has been detected in plasma and urine and is known to be a chemoattractant for leukocytes that express the formyl-peptide-receptor-like receptor 1/lipoxin A4 receptor. In addition to uPAR several other receptors, including some of the uPAR co-receptors, may also bind directly to uPA and activate cell signaling pathways. The roles of these newer uPAR ligands and uPA receptors are just beginning to be investigated. Since many of them are expressed in the kidney, their potential participation in CKD pathogenesis will be of interest.
Keywords: urokinase, urokinase receptor, serine protease, plasminogen activator inhibitor-1, low density lipoprotein receptor-related protein, fibrosis, vitronectin, integrin
2. INTRODUCTION
Accumulation of extracellular matrix proteins in the interstitium of the kidney begins as a dynamic process that is preceded by an increase in microvascular permeability, an influx of inflammatory cells and activation/transformation of resident kidney cells – fibroblasts and tubular epithelial cells in particular (1). Interstitial matrix proteins may be derived from several cellular sources but fibroblasts and myofibroblasts are thought to be the major contributors. If the inciting renal injury is sustained, over time the molecular composition of the interstitial “scar” also undergoes significant changes in both matrix composition and interactions between individual macromolecules. Studies performed almost 20 years ago suggested that decreased matrix turnover was a major feature of the renal fibrogenic response, yet precisely which endogenous matrix-degrading proteases normally regulate renal matrix remodeling remains unclear (2).
In principle ramping up the activity of the endogenous proteases or administration of proteases as therapeutics appears to be an attractive approach to chronic kidney disease. Of the four major protease families, the metalloproteinases (MMP) are considered the predominant matrix-degrading proteases. With few exceptions, the MMPs are synthesized as latent enzymes that require proteolytic cleavage for activation. Since plasmin can activate certain MMPs, at least in vitro, members of the serine protease family become candidates as endogenous regulators of renal fibrosis severity. Interest in the serine protease family strengthened when it was shown that plasminogen activator inhibitor-1 (PAI-1) is produced in large quantities by damaged kidneys, that fibrosis severity is significantly decreased in the absence or inhibition of PAI-1 and that fibrosis is significantly worse when PAI-1 is genetically over-expressed (3).
However, it now appears that the conceptual model of renal fibrosis as tightly regulated by a thermostat of matrix-degrading proteases is too simplistic because protease activity is not limited to the extracellular space (1). Furthermore, many of these proteases bind to cellular receptors, activate intracellular signaling pathways and have a profound effect on cellular behavior. These effects may influence not only the early “inflammatory” phase of renal fibrogenesis but also the important later phase of cellular destruction characterized by peritubular capillary rarefaction and tubular atrophy as the interstitial matrix expands and consolidates. This latter phase marks the point of no return in chronic kidney disease when functional nephron units are destroyed. Prior to this point, the reversal of renal scarring, as has been described in recent animal studies, is a desirable therapeutic goal and identifying the endogenous matrix-degrading pathways is an important area of ongoing investigation (4–6). This review will focus on what is currently known about the serine protease urokinase-type plasminogen activator (uPA) and its cellular receptors in chronic kidney disease. This appears to be another Pandora’s box that we have only recently opened and begun to understand.
3. UROKINASE-TYPE PLASMINOGEN ACTIVATOR (uPA)
3.1. Functional Overview
The uPA is produced in significant quantities by proximal and distal tubular epithelial cells and secreted across the apical membrane into the urinary space. Other sources include monocytes/macrophages, fibroblasts and myofibroblasts. Initially synthesized as a latent single chain proenzyme, uPA is proteolytically cleaved into a two chain active enzyme. Several enzymes can serve as uPA activators including plasmin, kallikrein, cathepsin B and the matrix metalloproteinase PUMP. Despite high uPA levels, its primary physiological function in the kidney is still unknown. One suggestion is that it may prevent renal stone formation (7). Plasminogen is the preferred uPA substrate (Figure 1) although it may also degrade fibronectin and fibrin and it activates latent hepatocyte growth factor [HGF] (8) and membrane-type MMP (9).
Figure 1.
Schematic summary of uPA extracellular and cell-dependent pathways.
3.2. uPA in chronic kidney disease
The role of fibrinolytic pathways in the pathogenesis of acute glomerular disease has been of interest for decades. Most evidence suggests that endogenous activity is due to tissue-type plasminogen activator (tPA) (10). Until recently it has been unclear if uPA is involved in the pathogenesis of glomerulosclerosis and/or interstitial fibrosis. In experimental models of chronic kidney disease uPA expression and activity increase, as has been shown in the model of unilateral ureteral ligation (UUO) (Figure 2) (11–13). Based on the known biological functions of uPA, it could be argued that it may have pro-fibrotic effects as a consequence of plasminogen (via enhanced TGF-β activation and PAR-1-dependent epithelial-to-mesenchymal transition) and FGF activation or anti-fibrotic effects by activating HGF, MT-MMP and by limited direct matrix protein (fibronectin) degradation. Although tPA is the primary physiological mediator of fibrin/fibrinogen degradation, uPA also has fibrinolytic activity and it has been used effectively in clinical settings for this purpose. Fibrin may serve as an early provisional matrix and in chronic lung disease; molecules with fibrinolytic activity usually attenuate pulmonary fibrosis. This includes studies that have administered uPA by gene therapy or recombinant protein to animals with bleomycin-induced pulmonary fibrosis (Table 1) (14–17). Despite the popular view of fibrin as a key early provisional matrix in the lung, when fibrosis severity was compared between bleomycin-treated wild-type and fibrinogen-deficient mice, no difference was found (18, 19). Similar studies have not been performed to determine if fibrinogen plays a role in chronic kidney disease.
Figure 2.
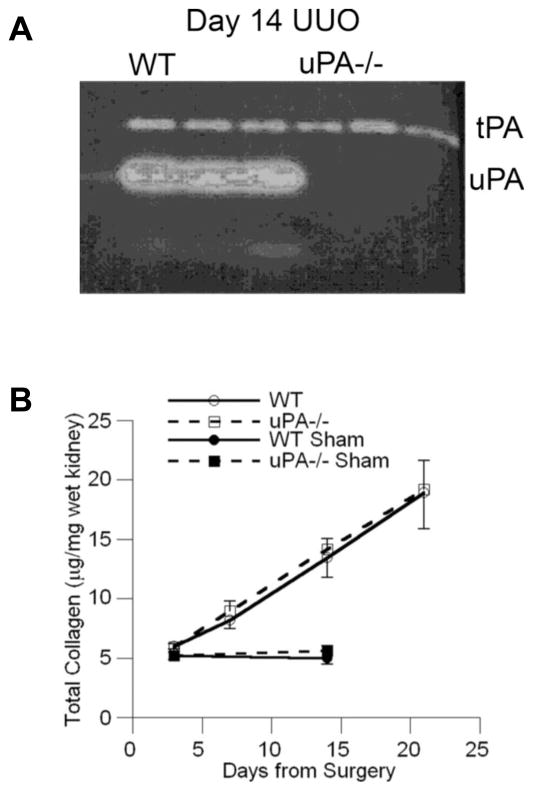
Genetic uPA deficiency does not alter renal fibrosis severity in response to unilateral ureteral obstruction (UUO). (A) A casein-plasminogen zymogram demonstrates high kidney uPA activity in wild-type (WT) mice 14 days after UUO while activity is absent in uPA−/− mice. Tissue plasminogen activator (tPA) activity was similar between the genotypes. (B) After 7, 14 and 21 days of UUO, the kidney collagen content measured as hydroxyproline levels was significantly increased compared to sham kidney levels (solid symbols) but were similar in both genotypes (open symbols). Data reproduced with permission from Yamaguchi et al (13).
TABLE 1.
uPA and uPAR: Role in experimental fibrosis models
| Urokinase | |||||
|---|---|---|---|---|---|
| Organ | Model | Manipulation | Fibrosis | Inflammation | Reference |
| Kidney | Obstruction | uPA−/− mice | No change | No change | (13) |
| Crescentic GN | uPA−/− mice | No change | ↓Mφ | (22) | |
| Lung | Bleomycin | uPA−/− mice | ↑fibrosis | ↓early Mφ | (113) |
| Inducible uPA gene | ↓fibrosis | N.D. | (151) | ||
| uPA gene therapy | ↓fibrosis | N.D. | (16) | ||
| uPA protein | ↓fibrosis | N.D. | (14, 15, 17) | ||
| Silica particles | uPA−/− mice | ↑fibrosis | No change | (152) | |
| Liver | CCl4 | uPA−/− mice | Delayed clearance of necrotic hepatocytes | N.D. | (153) |
| uPA gene therapy | ↓fibrosis | N.D. | (119–121) | ||
| Heart | Pressure overload | uPA−/− mice | ↓fibrosis | ↓leukocytes | (118) |
| Myocarditis | uPA−/− mice | ↓fibrosis | ↓neutrophils | (117) | |
| Cardiac fibrosis | uPA-over-expressing macrophages | ↑fibrosis | ↑Mφ | (122) | |
| uPAR | |||||
| Kidney | Obstruction | uPAR−/− mice | ↑fibrosis | ↓Mφ | (50) |
| Crescentic GN | uPAR−/− mice | no change in proteinuria or fibrosis | No change Mφ | (22) | |
| Pyelonephritis | uPAR−/− mice | ↑inflammation | No change neutrophils | (27) | |
| Endotoxemia | uPAR−/− mice | ↑proteinuria | N.D. | (49) | |
| Lung | Bleomycin | uPAR−/− mice | No change in fibrosis; ↓ hemorrhage | ↓early Mφ | (113) |
| Liver | CCl4 | uPAR−/− mice | No change | N.D. | (153) |
| Heart | Cardiac fibrosis in uPA-over-expressing mice | uPAR−/− mice | No change | No change Mφ | (126) |
N.D. – not determined; Mφ - macrophages
Studies performed in uPA wild-type and knock-out mice have yielded surprising results (13). In the UUO model, the degree of fibrosis was identical in mice of both genotypes (Table 1, Figure 2). Compensatory changes in tPA activity and PAI-1 or urokinase receptor (uPAR) expression levels were not detected. While in the lung intratracheal administration of recombinant uPA increased levels of active HGF and reduced fibrosis in the bleomycin model (14), differences in active α-HGF and phosphorlyated HGF receptor levels were not detected between the kidneys of uPA+/+ and uPA−/− mice 7 days after UUO. These remarkable differences between the effect of uPA on lung and kidney fibrosis suggest that this serine protease may have significant tissue-specific effects. Alternatively the anti-fibrotic effects of HGF may be site-specific. It is known that renal tubule-derived uPA is secreted apically but whether endogenously generated uPA gains access to the interstitial space to modulate local HGF activity and interstitial fibrosis is unclear (20). Exogenous uPA as used in the lung fibrosis studies may have greater access to the sites of matrix protein deposition. We attempted to increase interstitial uPA activity by investigating the UUO model in mice genetically engineered to over-express uPA in macrophages (21). Differences in fibrosis severity compared to wild-type mice were not detected, but due to technical limitations we have not yet documented that interstitial uPA activity was significantly different between these genotypes (unpublished data). In a model of crescentic glomerulonephritis, acute disease severity was similar between uPA +/+ and uPA −/− mice while the severity of chronic glomerulosclerosis was not examined (22). It remains a possibility that the biological effects of uPA are redundant although tPA does not appear to serve that role. Definitive studies still need to be performed to determine if exogenous uPA or ectopically expressed uPA can modulate responses to injury in the kidney as has been demonstrated in other organs such as the liver, heart and lung (see section 6 below).
4. THE CLASSICAL HIGH AFFINITY UROKINASE RECEPTOR (uPAR/CD87)
4.1. Functional overview
Since its discovery on the surface of monocytes in 1985 as a receptor that binds to the amino terminal fragment (ATF) of uPA and concentrates protease activity (contained within the C-terminal fragment) on cell membranes, uPAR has become a very interesting molecule with diverse biological functions (23–25). The uPAR colocalizes with uPA at focal contacts in the leading edge of migrating cells (26). The uPAR has now been identified on a variety of other cell types, including inflammatory cells (monocytes, macrophages, neutrophils, activated T cells), vascular endothelial cells, epithelial cells (both glomerular and tubular), mesenchymal cells (fibroblasts, myofibroblasts, mesangial cells), and neurons (23, 27–32). The uPAR, a highly glycosylated 50-kD to 65-kD protein, is a transmembrane receptor with three extracellular domains (D1, D2 and D3) and a single membrane-inserted domain connected to a very short glycosyl-phosphatidylinositol (GPI)-anchored cytoplasmic tail. The crystal structure of uPAR in complex with a peptide antagonist was solved in 2005 (33). The subsequent identification of additional extracellular ligands and cell surface co-receptor suggests its possible roles independent of the enzymatic properties of its ligand urokinase (26, 34, 35). The currently known ligands are uPA ± PAI-1, vitronectin, and kininogen (23, 34, 36). The complex molecular interactions between uPAR and its ligands or co-receptors regulate key events during cell adhesion, migration, proliferation and survival. The most extensive studies performed to date pertain to its role in tumor invasion and metastasis (37–40).
The uPAR has pleomorphic functions (41–43). Studies in genetically engineered mice suggest that in addition to interactions with the amino terminal fragment (ATF) of uPA (both the single chain and the proteolytically active two chain enzyme), uPA and uPAR may also function independently. Three unique aspects of uPAR function underlie its great diversity. [1] In addition to uPA, vitronectin and high molecular weight kininogen are specific uPAR ligands. [2] uPAR can engage in lateral interactions with several other transmembrane cellular receptors. Together they elicit changes in cell motility and other signaling-dependent cellular functions. [3] uPAR domains D2 and D3 may be shed from the cell membrane as a soluble peptide that has significant chemotactic properties. While it is reasonable to suggest that some of these unique uPAR functions account for its ability to modulate the fibrogenic response of the kidney during chronic injury, the relative contribution of each specific function has yet to be determined.
4.2. uPAR in experimental kidney disease
Based on mRNA expression studies in mice, uPAR does not appear to be expressed in normal kidneys. However, de novo expression by glomerular and tubular epithelial cells and a variety of renal interstitial cells (fibroblasts, inflammatory cells and endothelial cells) has been confirmed in human renal diseases such as diabetic nephropathy, allograft rejection and pyelonephritis (29, 44, 45). There is a growing list of uPAR agonists that include interleukins-1, -2, -4, -6, -7, -10, -13; tumor necrosis factor-α and -β; TGF-β; interferon-α, -γ; thrombospondin-1 and monocyte chemoattractant proteins-1, -2, -3 (46–48).
Studies comparing renal disease between uPAR wild-type (+/+) and genetically deficient (−/−) mice have provided important insights into its diverse functions (Table 1). Mice with targeted disruption of the uPAR gene are phenotypically normal and fertile. The severity of acute crescentic glomerulonephritis was similar in uPAR+/+ and uPAR−/− mice (22). However in the absence of uPAR, mice were protected from endotoxin-induced proteinuria (49). The role of uPAR in the genesis of proteinuria is thought to involve 3 integrins and their collaborative effects on podocyte function. Acute pyelonephritis is more severe in uPAR −/− mice due to impaired bacterial phagocytosis (27). In a model of chronic kidney disease induced by UUO, induced uPAR expression in tubular and interstitial cells was associated with renoprotective effects including decreased numbers of myofibroblasts and reduced renal fibrosis compared to uPAR −/− mice (30, 50). It is of interest that the group A streptococcal surface dehydrogenase (GAPDH) also binds to uPAR (51).
4.3 Receptor-ligand interactions
4.3.1. uPAR stabilizes membrane-associated uPA activity
When the noncatalytic uPA A chain binds to the D1 domain of uPAR, it stabilizes and concentrates proteolytic activity in pericellular zones. While uPA activation may be achieved by a variety of proteases one interesting mechanism involves vascular endothelial growth factor receptor-2 that can activate uPAR-bound uPA via a process that involves MMP-2 (52). The receptor can also bind latent uPA, which retains its ability to be activated. In the study of the UUO model, uPA proteolytic activity (as assessed by plasminogen gel zymography) and levels of the anti-fibrotic hepatocyte growth factor (HGF) were significantly higher in the uPAR +/+ mice that had less fibrosis than the uPAR −/− mice (Figure 4) (50). HGF is known to have potent anti-fibrotic effects. Although these findings suggested that uPAR-dependent uPA catalytic activity might account for the receptor’s ability to attenuate renal scarring, our subsequent studies in uPA −/− mice did not reproduce the uPAR−/− phenotype after UUO (13). Bi-transgenic mice that over-express both uPAR and uPA in the skin develop extensive alopecia due to the enhanced urokinase catalytic activity while isolated deficiency of uPA or uPAR results in no cutaneous phenotype (53). This observation suggests a likely catalytic synergy between the receptor and its ligand.
Figure 4.
uPAR expression and function during experimental chronic kidney injury induced by UUO. (A) Northern blot analysis identifies uPAR mRNA in wild-type kidneys 7 days after UUO; no transcripts were detected in sham or uPAR−/− kidneys. (B) Kidney uPAR immunostaining identifies positive interstitial and tubular cells 7 days after UUO. (C) Measurement of interstitial area expressing mature sirius red+ collagen fibrils detected significantly more fibrosis in the uPAR-deficient mice (open bars) in comparison wth strain-identical uPAR wild-type mice (grey bars). Using immunohistochemical staining followed by computer-assisted image analysis, uPAR-deficient mice were found to have fewer F4/80+ macrophages (D), more alpha smooth muscle actin ( SMA)+ myofibroblasts (E) and more CD34+ interstitial vessels (endothelial and lymphatic) (F) compared to wild-type mice. *P < 0.05. Data reproduced with permission from Zhang et al (30).
Ligation of uPA to uPAR can initiate a variety of cellular responses that are discussed in greater detail in the subsequent sections. [1] Through transactivation of uPAR co-receptors including the epidermal growth factor receptor, several integrins, and gp130, extracellular signal regulated kinase (ERK) or signal transducer and activator of transcription (STAT) signaling pathways may be activated (28, 54–57). [2] The uPA-ligated cell surface uPAR may laterally interact with integrins, especially those that bind to the extracellular matrix proteins vitronectin and fibronectin (58, 59). [3] When uPAR-bound uPA interacts with PAI-1, this receptor complex can cluster with the scavenger receptor low density lipoprotein receptor-related protein (LRP) leading to the endocytic degradation of uPA/PAI-1 (60).
4.3.2. PAI-1/uPAR interactions
The renal anti-fibrotic effects of uPAR and uPA may also be indirect and regulated by interstitial levels of plasminogen activator inhibitor-1 (PAI-1) (3). Although PAI-1 has a very short half-life, it binds with high affinity to vitronectin. This interaction stabilizes PAI-1 and may account for the accumulation of PAI-1 within the renal interstitium during fibrosis. PAI-1 is one of the most potent pro-fibrotic molecules known to be expressed in the kidney. PAI-1 binds and inhibits uPA whether receptor-bound or free. The trimolecular complex of uPAR + uPA + PAI-1 initiates a shuttle that internalizes and degrades PAI-1 within lysosomes. Meanwhile uPAR is salvaged and recycled back to the cell membrane. LRP-1 is the critical uPAR partner in this shuttling process (Figure 5). It has also been reported that PAI-1 may interact directly with LRP-1 to promote cellular mobility (61). Whether the PAI-1 degrading cellular shuttle can function in the absence of uPA bound to uPAR is unclear. In the obstructed kidneys of uPAR −/− mice, PAI-1 protein levels were significantly higher than kidneys from uPAR +/+ with less fibrosis, suggesting a potential link between PAI-1 and uPAR during renal fibrosis (30). Ongoing and future studies need to address the role of uPAR in the context of its other known cellular and molecular functions.
Figure 5.
Schematic summary of uPAR and its co-receptors. (A) Extracellular uPAR (domain 1) interacts with uPA, both latent and active forms. (B) Recruitment of PAI-1 to receptor-bound uPA is an important event. Not only does it neutralize uPA activity but the trimolecular complex may co-associate with LRP leading to internalization of the entire complex, PAI-1 degradation and re-shuffling of uPAR back to the cell membrane. (C) uPAR itself a non-signaling receptor is predisposed to establishing lateral membrane interactions with several other receptors. Together they trigger proteolysis-independent intracellular signaling to modulate a variety of cellular behaviors. Many of these co-receptors also express uPA-binding motifs.
Interactions with PAI-1 may have additional effects on uPAR function. PAI-1 enhances uPAR-mediated, uPA-induced ERK signal activation (62). It has also been suggested that PAI-1 may directly bind to uPAR, activating ERK signaling and stimulating TGF-β expression (63). The uPAR-mediated cellular effects of PAI-1 are supported by the inhibitory effect of anti-uPAR antibody (64). By confocal microscopy it has been observed that the addition of PAI-1 alone induces significant cell surface LRP clustering in uPAR+/+ but not uPAR-null primary kidney fibroblast cultures, suggesting a possible direct interaction between PAI-1 and uPAR (65, 66). PAI-1 binds with high affinity to vitronectin that can bind directly to uPAR, and is also a ligand for certain integrin receptors known to function as uPAR co-receptors (see section 4.3.3.).
4.3.3. Alternative uPAR ligands: vitronectin and high molecular weight kininogen
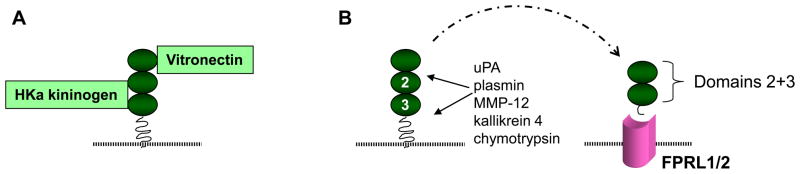
Nine years after the discovery of uPAR, it was further identified as a high affinity adhesion receptor for extracellular matrix vitronectin (VN) (Figure 6) (34). VN binds to both membrane GPI-anchored uPAR and soluble uncleaved uPAR at domain D1 and the D1-D2 linker region (65, 67, 68). The VN-uPAR or VN-suPAR binding is facilitated by concurrent receptor binding to urokinase. Indeed, VN can concentrate proteolytic activity by five-fold on cell surface and extracellular matrix via the formation/trapping of a ternary uPA/suPAR/VN complex (34). This complex can be disassembled when VN is cleaved by plasmin (that may be activated by uPA-mediated cleavage of the zymogen plasminogen) or when PAI-1 competes with uPAR for the VN somatomedin B domain (69). The ability of PAI-1 to competitively inhibit uPAR’s binding to the extracellular matrix VN, thereby regulating VN-uPAR and VN-integrin-uPAR interactions, plays a key role in uPAR-dependent cell adhesion and migration (70, 71). Vitronectin expression in the tubulointerstitium is known to be up-regulated in response to chronic damage (30).
Figure 6.
uPAR: alternative ligands and uPAR as a ligand. (A) The uPAR also expresses specific binding sites for vitronectin in domain 1 and the flanking interdomain linker region and for high molecular weight kininogen in domains 2 and 3, (B) A variety of proteases may release uPAR domains 2 and 3 which have been detected in plasma and urine in certain human diseases. This soluble peptide (suPAR) possesses chemotactic properties for cells that express receptors that are formyl-peptide-receptor-like (FPRL). FPRL1 is also the lipoxin A4 receptor.
In 1997 uPAR was also shown to be a cellular receptor for the cleaved high molecular weight kininogen (HKa) (36). The domain 5 of the HKa binds to uPAR at a site within domains 2 and 3 (36, 72). The uPAR-mediated cellular effect of HKa depends on the composition of the extracellular matrix. HKa selectively induces apoptosis of endothelial cells grown on VN, but not cells grown on fibronectin; under these conditions it also inhibits angiogenesis (73, 74). When HKa binds to uPAR on endothelial cells, it can physically disrupt the VN-uPAR signaling complex formed with co-receptors alpha(v)beta3 or alpha5 beta1 integrins, caveolin, and Src kinase resulting in anti-adhesive effect and apoptosis (73). The ligation of uPAR by HKa can stimulate cytokine and chemokine gene expression in mononuclear cells via mitogen-activated protein kinases (JNK and p38) and the nuclear factor kappa B (NFκB) signaling pathway (75). Careful studies still need to be performed to determine if VN and/or HKa participate in uPAR-dependent anti-fibrotic effects in the kidney.
4.3.4. uPAR lateral interactions with co-receptors
Integrins
Despite the fact that cellular uPAR lacks a signal transducing cytoplasmic tail, it is able to profoundly influence cellular behavior such as cell migration by interacting with several other cell surface molecules (Figure 6). First to be recognized was the association with the leukocyte integrin (CD11b/CD18) (76). Subsequently, it has been shown that uPAR can interact with several integrin family members including α3, α5, αv, β1, β2, β3 and β5. Such interactions initiate cytoskeletal reorganization and activate cell signaling pathways that regulate cell migration within extracellular matrices (77, 78). When uPA binds to uPAR it promotes cell adhesion by increasing the affinity of the receptor for both VN and integrins (58, 59). VN-uPAR interactions are critical for cell adhesion and migration (67). The most commonly identified effect is one of “deadhesion”, where cells can be released from one matrix footprint (often fibronectin) in order to move forward and attach to another matrix protein such as VN (24). PAI-1 and the locally concentrated active enzyme urokinase are regulators that promote cell detachment and migration by disrupting these VN-uPAR and VN-integrin interactions (70, 71, 79). In the obstructive uropathy model, uPAR deletion was associated with significantly fewer numbers of interstitial macrophages, perhaps due to the disruption of this pathway (30).
The uPAR appears necessary for adequate host defense during infections including acute pyelonephritis (27), peritonitis (80), pneumococcal pneumonia and meningitis (81). In these studies the mice lacking uPAR manifest a blunted leukocyte response to the infection and delayed pathogen clearance. In an experimental model of allergic encephalitis in which leukocyte recruitment requires β2 integrin function, the absence of uPAR reduced neutrophil recruitment (82).
Glomerular podocytes and renal tubules express various integrins during specific disease processes and they may also co-express uPAR (30, 49, 50). Given the importance of the integrin family for migration and crosstalk with extracellular matrix proteins, the specific role of uPAR in those processes deserve further exploration. In addition to effects on cell adhesion and migration, clustering between activated uPAR and one of its co-receptors may lead to trans-activation and initiation of intracellular signaling (83). Examples that may be relevant to renal disease include the JAK1/STAT1 described in the human kidney epithelial tumor cell line TCL-598 (84), the gp130/Tyk2/STAT3 reported in human mesangial cells (28), the ERK/MAPK activated in fibroblasts, endothelial cells and tumor cells (66, 85, 86), and the JNK/MAPK and p38/MAPK pathways in mononuclear cells (75).
Low density lipoprotein receptor related protein-1 (LRP-1)
The uPAR has been characterized as a scavenger receptor by virtue of its ability to work in collaboration with other scavenger receptors, especially LRP-1 to degrade ligands by endocytosis (60, 87, 88). This endocytic pathway is the primary route of elimination of extracellular PAI-1. PAI-1 and uPA are subsequently degraded within lysosomes while uPAR is recycled to the cell surface. The uPAR-associated protein (uPARAP/Endo180) is another internalization receptor connected with cell surface uPAR, that engages in matrix turnover during tissue remodeling (89, 90).
LRP-1 is itself a multi-ligand receptor with several important biological functions (91). These ligands include PAI-1, uPA and tPA. Recent studies have shown that LRP-1 activated by tPA triggers intracellular signaling to promote matrix metalloproteinase-9 expression, macrophage migration and fibroblast survival. Other known LRP-1 ligands include coagulation factors (VIIa, VIIIa, IXa, anti-thrombin III, tissue factor pathway inhibitor, heparin cofactor II), metalloproteinases (MMP-9, MMP-13), complement (C3, C1 inhibitor), thrombospondin −1 and −2, apolipoprotein E, α2 macroglobulin and LRP-receptor-associated protein (RAP). Whether uPAR influences the interaction of LRP with any of these ligands is unknown.
The growing list of uPAR co-receptors
Several uPAR co-receptors have themselves been implicated in fibrogenic reactions. Glycoprotein 130 (gp130) is a key member of the interleukin-6 superfamily. Homodimers serve as the signaling receptor for interleukin-6 and interleukin-11 while gp130 heterodimers constitute receptors for ciliary neurotrophic factor, cardiolipin-like cytokine, leukemia inhibitory factor, oncostatin M and neuropoietin (92).
The receptor for complement component C5a (C5aR) is expressed in response to acute and chronic kidney injury. Studies in C5aR deficient mice reported reduced interstitial disease severity in a chronic glomerulonephritis model while a specific C5a receptor antagonist has been shown to reduce renal disease in several experimental models (93–96). In a model of immune complex pulmonary inflammation, uPAR was necessary for C5a signaling (28). The insulin-like growth factor-II/mannose-6-phosphate (IGFII/M6P) receptor has been shown to be involved in directing uPAR to lysosomes (97). In an experimental model of partial hepatectomy the early regenerative phase was characterized by interactions between uPA, uPAR the HGF receptor c-Met (98). Recently uPAR has been shown to interact with platelet-derived growth factor B receptor and epidermal growth factor receptor (99–101).
4.3.5. Additional uPAR activities
In addition to regulating uPA catalytic activity, promoting PAI-1 degradation and modulating various cell-cell and cell-matrix interactions, uPAR may enhance cell survival and promote angiogenesis. The uPA-uPAR interaction stimulates proliferation in most cell lines (85, 101–103). PAI-1 promotes this effect by further enhancing intracellular ERK/MAPK signaling (62). uPAR interaction with uPA can stimulate proliferation; uPAR expression also confers resistance to caspase-mediated apoptosis and anoikis (104, 105).
Numerous studies have documented that strategies to block uPAR expression are associated with decreased angiogenesis and tumor growth and metastasis (85, 103, 106). In contrast, uPAR has been shown to promote angiogenesis in the eye (107). In the UUO model of chronic kidney disease uPAR +/+ mice have significantly fewer numbers of CD34+ interstitial cells, either as a direct uPAR effect or as an indirect consequence of enhanced PAI-1 clearance, as PAI-1 may have anti-angiogenic properties (Figure 4).
5. SOLUBLE uPAR (suPAR): A CHEMOTACTIC PEPTIDE
GPI-anchored uPAR can be cleaved by active urokinase at its D1-D2 linker region, leaving on the cell surface a truncated receptor (D2 and D3) where the p88-92 chemotactic sequence is exposed (72, 108). With the D1 domain removed, the truncated receptor gains chemotactic properties while its ability to interact with uPA, vitronectin and various co-receptors is lost. If the truncated D2+D3 receptor or even the intact receptor is shed from the cell surface, it is called the soluble form of uPAR (suPAR) (Figure 5) (45). SuPAR has been detected in human plasma and urine. Far from being an inactive degradation product, suPAR has important chemotactic properties due to its ability to bind to the G protein-coupled receptor formyl-peptide-receptor-like-1/lipoxin A4 receptor (FPRL1/LXA4R). Whether this truncated suPAR-induced signaling is mediated by the fMLP receptor homologue FPRL1 and FPRL2 is still unclear (55, 56). FPRL1/LXA4R is expressed by leukocytes and epithelial cells. Interaction between suPAR and its receptor appears to be biologically important for innate and adaptive immune responses. For example, plasma suPAR levels correlate with disease activity in AIDS and bacteremia (45, 109).
SuPAR also inactivates CXCR4, the chemokine receptor primarily responsible for hematopoietic stem cell retention in bone marrow (110). Following parenteral administration of suPAR to mice along with granulocyte-colony stimulating factor, leukocytosis and enhanced stem cells mobilization is observed (111, 112).
Plasma and urinary suPAR levels are increased in humans with acute pyelonephritis, an effect that could be reproduced experimentally when endotoxin was injected into a group of volunteers (45). Renal studies in uPAR genetic mice demonstrated significantly fewer interstitial macrophages in the uPAR−/− group (30). While this difference might be due to the absence of suPAR, lateral interactions of membrane-anchored uPAR with leukocyte intergrins, the anaphylatoxin C5a receptor and/or Endo180 (involved in directional sensing during chemotaxis) may also contribute to uPAR-dependent monocyte recruitment. In experimental pyelonephritis, although the absence of uPAR did not affect neutrophil recruitment, it was required for their bacterial phagocytic function (27).
6. ROLE OF uPA AND uPAR DURING FIBROGENESIS: DISEASE AND ORGAN SPECIFICITY
The degree of overlapb in fibrogenic pathways in solid organs such as the kidney, liver, heart and lung is remarkable. However, when it comes to select members of the serine protease family and its associated receptors a divergence of effects is often observed based on studies performed in animal (Table 1). The reason for these differences is still not clear. In some situations worse fibrosis may be a secondary consequence of changes in inflammation severity. In the lung where fibrin is thought to form a critical early provisional matrix during fibrosis, uPA may be more effective as an anti-fibrotic protease. However, the fact that genetic fibrinogen deficiency has no effect on the severity of bleomycin-induced pulmonary fibrosis challenges this theory (18, 113). It is possible that there are important organ-specific differences in HGF activation mechanisms. In the lung and liver uPA plays an important role in the regulation of HGF activity while in the kidney an alternate pathway may serve this role - such a HGF activator and its specific inhibitors HAI-1 and HAI-2 (14, 114–116).
In the kidney where endogenous uPA is produced in large quantities, uPA deficiency had no effect on the severity of UUO-induced renal fibrosis (13). In contrast uPA deficiency worsened bleomycin-induced lung fibrosis and significantly reduced fibrosis in hearts that were damaged by viral myocarditis or left ventricular pressure overload (113, 117, 118). Delivery of exogenous uPA as a recombinant protein or via an uPA-expressing viral vector reduced fibrosis in the lung and liver while mice with macrophages engineered to over-express uPA spontaneously developed cardiac fibrosis (119–122). In chronic renal tubulointerstitial disease the induction of endogenous uPAR reduces fibrosis severity while in a model of crescentic glomerulonephritis, genetic deficiency of either uPA or uPAR did not worsen glomerulosclerosis although the degree of glomerular inflammation was reduced in the uPAR−/− mice (22, 50). In humans uPAR expression levels are higher on fibroblasts isolated form fibrotic lungs compared to normal lungs (123). In human keloid compared with normal scars, uPAR expression is enhanced (124). There is evidence that membrane-bound uPAR is necessary for myofibroblast differentiation (125). Following bleomycin administration, the degree of hemorrhage and inflammation is reduced in uPAR−/− mice but no difference in fibrosis was detected compared with uPAR+/+ mice (113). In the heart uPAR deficiency does not prevent the fibrogenic effects of uPA-over-expression (126). While a large majority of the cellular and molecular mediators of fibrosis elicit similar effects in all of these organs, the uPA-uPAR family is a clear exception which highlights the need to carefully elucidate mechanisms of fibrosis under a variety of pathological states.
In addition to matrix accumulation, another histological feature of chronic kidney disease is the presence of an interstitial infiltrate of mononuclear cells. Interstitial monocytes/macrophages in particular have been implicated in fibrogenic pathways making the mediators of monocyte recruitment and activation of interest as potential therapeutic targets (1). Several known uPA functions suggest that it may also promote inflammation. As a protease uPA may liberate fibrin and suPAR, both known monocytes chemoattractants. It may activate proinflammatory cytokines and through interactions with PAI-1 and LRP uPA may influence the movement of monocytes (61). The uPAR is known to facilitate cell migration via interactions with the 2 family of leucocyte integrins and with the fibronectin- or vitronectin-binding integrins, while suPAR is a leukocyte chemoattractant.
In animal models of acute inflammatory diseases such as bacterial pneumonia (81) or meningitis (127), endotoxin-induced peritonitis (80), immune complex-induced pneumonia (82, 128) and allergic encephalitis, inflammation (primarily neutrophils) is attenuated in uPAR-deficient mice. In a model of acute muscle injury accumulation of monocytes was shown to involve uPA but not its uPAR receptor (129). However, in chronic disease processes associated with fibrosis, the effects of uPA and uPAR deficiency on the associated inflammatory response are less consistent, as summarized in Table 1. The phenotypic heterogeneity of tissue macrophages has recently been appreciated (130–132). Classically activated M1 macrophages are typically associated with tissue injury, while M2 alternatively activated macrophages are usually associated with tissue repair and remodeling. Administration of M2 macrophages has recently been reported to reduce fibrosis in obstructive nephropathy and adriamycin nephrosis (133, 134). Although more studies are necessary, it is tempting to speculate that macrophage uPAR expression is associated with the reparative M2 phenotype as renal fibrosis is worse in uPAR−/− mice despite less interstitial inflammation compared to uPAR+/+ mice (30).
7. uPAR AS A THERAPEUTIC TARGET
uPAR has become a therapeutic target of interest for cancer and other pathologic conditions, including bone homeostasis (osteoporosis), neuron development, and atherosclerosis (32, 135–137). Pharmaceutical strategies of uPAR inactivation or blockade for in vivo evaluation have included RNA interference (85), adenovirus-mediated transfer of anti-sense uPAR (138), uPAR signaling blocked with anti-sense oligonucleotides or other small molecule inhibitors (139), adenovirus-mediated delivery of an uPA/uPAR antagonist (107), inhibitory monoclonal antibodies (140), and thalidomide (141). Soluble uPAR has also been investigated as a potential inhibitor of tumor growth (142). These intervention strategies have been shown to decrease tumor invasion/metastasis, angiogenesis and growth in breast cancer, ovarian cancer, brain tumor, gliomas, glioblastoma multiform, osteosarcoma, non-small cell lung cancer, prostate cancer, and melanoma (85, 138, 139, 141, 142); to reduce retinal neovascularization in retinopathy (107, 143); and to suppress macrophage infiltration into the vascular wall of ApoE-deficient mice (144).
8. OTHER uPA RECEPTORS
In addition to high-affinity binding of the growth factor-like uPA domain to uPAR, specific uPA binding to an alternative, unidentified low-affinity cellular receptor has been reported (145–147). We have found that urokinase signals renal fibroblast activation via the MAPK pathway (30). This regulation appears to be mediated at least in part by an alternative urokinase receptor as uPA can initiate mitogenesis in uPAR−/− fibroblasts. Using phage display technology Liang et al (148) identified a putative uPA-binding consensus sequence in 12 membrane proteins, further supporting the existence of additional urokinase receptors. Among these candidate receptors, several are already known as uPAR co-receptors: LRP, gp130, integrins, Endo180, and the IGF-II/M6P receptor. This suggests that different uPA sites might simultaneously bind to uPAR and one of its co-receptors (149). In other words, urokinase may serve as a bridge to cluster and coordinate the functions of uPAR and co-receptors.
We have recently identified the muscle type nicotinic receptor alpha1 (nAChR 1) as an alternative urokinase cellular signaling receptor for renal fibroblasts (150). It controls a complex of signaling proteins by tyrosine-phosphorylation, including calcium-binding proteins annexin II and AHNAK, cytoskeletal proteins actin, actinin and vimentin, and nucleoprotein histone 4. This new “outside-in” signaling pathway appears to initiate important profibrotic effects in the kidney (manuscript in preparation).
9. PERSPECTIVE
uPAR and suPAR are multifunctional proteins. The final outcome of the intricate interactions between uPAR and its ligands and co-receptors is a significant change in cellular phenotype that modulates cell adhesion, migration, proliferation, phagocytosis and survival. These uPAR-regulated cellular functions have been shown to be crucial in a variety of physical and pathologic processes in vivo including inflammation, wound healing and fibrogenesis, neoangiogenesis, tumor growth and metastasis, and tissue destruction and regeneration (28, 30, 85, 124). During fibrogenesis current evidence suggests that the specific role of uPA and uPAR are organ- and site-specific. In the kidney de novo uPAR expression during acute and chronic injury appears to represent a beneficial defense response. The recent recognition of several alternative uPA receptor candidates introduces a new level of complexity to uPA pathophysiology that has yet to be explored.
Figure 3.
uPAR deficiency reduces kidney uPA activity. (A) Casein-plasminogen zymogram illustrates uPA and tPA activity 7 days after UUO. (B) Analysis of the mean size of the lytic bands found significantly higher uPA activity in the uPAR-expressing kidneys (uPAR+/+, closed diamonds) 3, 7, and 14 days after UUO compared to uPAR-deficient kidneys (uPAR−/−, open circles). *P < 0.05. Data reproduced with permission from Zhang et al (50).
Acknowledgments
The authors acknowledge support from the National Institutes of Health Grants DK54500 and DK44757 (AAE), the American Heart Association (GZ) and Seattle Children’s Hospital Research Institute. We would also like to acknowledge members of the Pediatric Nephrology Research Program who performed some of our cited studies: Drs. Ikuyo Yamaguchi, Jesús López-Guisa, Takashi Oda, Heungsoo Kim, Shunya Matsuo, Daryl Okamura; and excellent technical assistance from Stella Cai, Sarah Collins and Megan Burger.
Abbreviations
- AHNAK
nucleoprotein also known as desmoyokin
- C5aR
complement component C5a receptor
- CKD
chronic kidney disease
- CXCR4
alpha-chemokine receptor specific for stromal-derived-factor-1
- ERK
extracellular signal-regulated kinase
- FGF
fibroblast growth factor
- FPRL1
formyl peptide receptor-like-1
- HGF
hepatocyte growth factor
- IGFII
insulin-like growth factor II
- JAK
Janus kinase
- JNK
c-jun N-terminal kinase
- LXA4R
lipoxin A4 receptor
- LRP
low density lipoprotein receptor-related protein
- MAPK
mitogen-activated protein kinase
- M6PR
mannose-6-phosphate
- MMP
matrix metalloproteinase
- MT-MMP
membrane-type matrix metalloproteinase
- PAI-1
plasminogen activator inhibitor-1
- RAP
receptor-associated protein
- STAT
signal transducer and activator of transcription
- suPAR
soluble uPAR
- tPA
tissue-type plasminogen activator
- Tyk2
tyrosine kinase 2
- uPA
urokinase-type plasminogen activator
- uPAR
high affinity urokinase receptor, CD87
- uPARAP
uPAR-associated protein also known as Endo180
- UUO
unilateral ureteral obstruction
- VN
vitronectin
Footnotes
Publisher's Disclaimer: This is an, un-copyedited, author manuscript that has been accepted for publication in the Frontiers in Bioscience". Cite this article as appearing in the Journal of Frontiers in Bioscience. Full citation can be found by searching the Frontiers in Bioscience (http://bioscience.org/search/authors/htm/search.htm) following publication and at PubMed (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=pubmed) following indexing. This article may not be duplicated or reproduced, other than for personal use or within the rule of "Fair Use of Copyrighted Materials" (section 107, Title 17, U.S. Code) without permission of the copyright holder, the Frontiers in Bioscience. From the time of acceptance following peer review, the full final copy edited article of this manuscript will be made available at http://www.bioscience.org/. The Frontiers in Bioscience disclaims any responsibility or liability for errors or omissions in this version of the un-copyedited manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.Eddy AA. Progression in chronic kidney disease. Adv Chronic Kidney Dis. 2005;12:353–65. doi: 10.1053/j.ackd.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 2.González-Avila G, Vadillo-Ortega F, Pérez-Tamayo R. Experimental diffuse interstitial renal fibrosis. A biochemical approach. Lab Invest. 1988;59:245–252. [PubMed] [Google Scholar]
- 3.Eddy AA, Fogo AB. PAI-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol. 2006;17:2999–3012. doi: 10.1681/ASN.2006050503. [DOI] [PubMed] [Google Scholar]
- 4.Cochrane AL, Kett MM, Samuel CS, Campanale NV, Anderson WP, Hume DA, Little MH, Bertram JF, Ricardo SD. Renal structural and functional repair in a mouse model of reversal of ureteral obstruction. J Am Soc Nephrol. 2005;16:3623–30. doi: 10.1681/ASN.2004090771. [DOI] [PubMed] [Google Scholar]
- 5.Ma LJ, Nakamura S, Aldigier JC, Rossini M, Yang H, Liang X, Nakamura I, Marcantoni C, Fogo AB. Regression of glomerulosclerosis with high-dose angiotensin inhibition is linked to decreased plasminogen activator inhibitor-1. J Am Soc Nephrol. 2005;16:966–76. doi: 10.1681/ASN.2004060492. [DOI] [PubMed] [Google Scholar]
- 6.Eddy A. Can renal fibrosis be reversed? Pediatr Nephrol. 2005;20:1369–1375. doi: 10.1007/s00467-005-1995-5. [DOI] [PubMed] [Google Scholar]
- 7.du Toit PJ, Van Aswegen CH, Steinmann CM, Klue L, Du Plessis DJ. Does urokinase play a role in renal stone formation? Med Hypotheses. 1997;49:57–9. doi: 10.1016/s0306-9877(97)90253-x. [DOI] [PubMed] [Google Scholar]
- 8.Naldini L, Vigna E, Bardelli A, Follenzi A, Galimi F, Comoglio PM. Biological activation of pro-HGF (hepatocyte growth factor) by urokinase is controlled by a stoichiometric reaction. J Biol Chem. 1995;270:603–11. doi: 10.1074/jbc.270.2.603. [DOI] [PubMed] [Google Scholar]
- 9.Murphy G, Stanton H, Cowell S, Butler G, Knauper V, Atkinson S, Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. Apmis. 1999;107:38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 10.Hertig A, Rondeau E. Role of the coagulation/fibrinolysis system in fibrin-associated glomerular injury. J Am Soc Nephrol. 2004;15:844–53. doi: 10.1097/01.asn.0000115400.52705.83. [DOI] [PubMed] [Google Scholar]
- 11.Oda T, Jung YO, Kim H, Cai x, Lopez-Guisa J, Ikeda Y, Eddy AA. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int. 2001;30:587–596. doi: 10.1046/j.1523-1755.2001.030002587.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo S, Lopez-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA. Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int. 2005;67:2221–38. doi: 10.1111/j.1523-1755.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi I, Lopez-Guisa JM, Cai X, Collins SJ, Okamura DM, Eddy AA. Endogenous urokinase lacks antifibrotic activity during progressive renal injury. Am J Physiol Renal Physiol. 2007;293:F12–9. doi: 10.1152/ajprenal.00380.2006. [DOI] [PubMed] [Google Scholar]
- 14.Hattori N, Mizuno S, Yoshida Y, Chin K, Mishima M, Sisson TH, Simon RH, Nakamura T, Miyake M. The plasminogen activation system reduces fibrosis in the lung by a hepatocyte growth factor-dependent mechanism. Am J Pathol. 2004;164:1091–8. doi: 10.1016/S0002-9440(10)63196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunther A, Lubke N, Ermert M, Schermuly RT, Weissmann N, Breithecker A, Markart P, Ruppert C, Quanz K, Ermert L, Grimminger F, Seeger W. Prevention of bleomycin-induced lung fibrosis by aerosolization of heparin or urokinase in rabbits. Am J Respir Crit Care Med. 2003;168:1358–65. doi: 10.1164/rccm.2201082. [DOI] [PubMed] [Google Scholar]
- 16.Sisson TH, Hattori N, Xu Y, Simon RH. Treatment of bleomycin-induced pulmonary fibrosis by transfer of urokinase-type plasminogen activator genes. Hum Gene Ther. 1999;10:2315–23. doi: 10.1089/10430349950016960. [DOI] [PubMed] [Google Scholar]
- 17.Hart DA, Whidden P, Green F, Henkin J, Woods DE. Partial reversal of established bleomycin-induced pulmonary fibrosis by rh-urokinase in a rat model. Clin Invest Med. 1994;17:69–76. [PubMed] [Google Scholar]
- 18.Ploplis VA, Wilberding J, McLennan L, Liang Z, Cornelissen I, DeFord ME, Rosen ED, Castellino FJ. A total fibrinogen deficiency is compatible with the development of pulmonary fibrosis in mice. Am J Pathol. 2000;157:703–8. doi: 10.1016/S0002-9440(10)64582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest. 2000;106:1341–50. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sappino AP, Huarle J, Vassalli JD, Belin D. Sites of synthesis of urokinase and tissue-type plasminogen activators in the murine kidney. J Clin Invest. 1991;87:962–970. doi: 10.1172/JCI115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moriwaki H, Cozen A, Deyoung M, Dichek D. Macrophage-specific overexpression of urokinase accelerates atherosclerosis and causes early mortality in Apo E null mice. Circulation. 2001;104:II–44 . [Google Scholar]
- 22.Kitching AR, Holdsworth SR, Ploplis VA, Plow EF, Collen D, Carmeliet P, Tipping PG. Plasminogen and plasminogen activators protect against renal injury in crescentic glomerulonephritis. J Exp Med. 1997;185:963–8. doi: 10.1084/jem.185.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vassalli JD, Baccino D, Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985;100:86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefansson S, Lawrence DA. Old dogs and new tricks: proteases, inhibitors, and cell migration. Sci STKE. 2003;2003:pe24 . doi: 10.1126/stke.2003.189.pe24. [DOI] [PubMed] [Google Scholar]
- 25.Mondino A, Blasi F. uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol. 2004;25:450–5. doi: 10.1016/j.it.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Margheri F, Manetti M, Serrati S, Nosi D, Pucci M, Matucci-Cerinic M, Kahaleh B, Bazzichi L, Fibbi G, Ibba-Manneschi L, Del Rosso M. Domain 1 of the urokinase-type plasminogen activator receptor is required for its morphologic and functional, beta2 integrin-mediated connection with actin cytoskeleton in human microvascular endothelial cells: failure of association in systemic sclerosis endothelial cells. Arthritis Rheum. 2006;54:3926–38. doi: 10.1002/art.22263. [DOI] [PubMed] [Google Scholar]
- 27.Roelofs JJ, Rouschop KM, Teske GJ, Claessen N, Weening JJ, van der Poll T, Florquin S. The urokinase plasminogen activator receptor is crucially involved in host defense during acute pyelonephritis. Kidney Int. 2006;70:1942–7. doi: 10.1038/sj.ki.5001947. [DOI] [PubMed] [Google Scholar]
- 28.Shushakova N, Tkachuk N, Dangers M, Tkachuk S, Park JK, Hashimoto K, Haller H, Dumler I. Urokinase-induced activation of the gp130/Tyk2/Stat3 pathway mediates a pro-inflammatory effect in human mesangial cells via expression of the anaphylatoxin C5a receptor. J Cell Sci. 2005;118:2743–53. doi: 10.1242/jcs.02409. [DOI] [PubMed] [Google Scholar]
- 29.Roelofs JJ, Rowshani AT, van den Berg JG, Claessen N, Aten J, ten Berge IJ, Weening JJ, Florquin S. Expression of urokinase plasminogen activator and its receptor during acute renal allograft rejection. Kidney Int. 2003;64:1845–53. doi: 10.1046/j.1523-1755.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang G, Kim H, Cai X, Lopez-Guisa J, Carmeliet P, Eddy A. Urokinase receptor modulates cellular and angiogenic responses in obstructive uropathy. J Am Soc Nephrol. 2003;14:1254–1271. doi: 10.1097/01.asn.0000064701.70231.3f. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Berrou J, Chen X, Fouqueray B, Callard P, Sraer JD, Rondeau E. Induction of urokinase receptor expression in nephrotoxic nephritis. Exp Nephrol. 2001;9:397–404. doi: 10.1159/000052638. [DOI] [PubMed] [Google Scholar]
- 32.Eagleson KL, Bonnin A, Levitt P. Region- and age-specific deficits in gamma-aminobutyric acidergic neuron development in the telencephalon of the uPAR(−/−) mouse. J Comp Neurol. 2005;489:449–66. doi: 10.1002/cne.20647. [DOI] [PubMed] [Google Scholar]
- 33.Llinas P, Le Du MH, Gardsvoll H, Dano K, Ploug M, Gilquin B, Stura EA, Menez A. Crystal structure of the human urokinase plasminogen activator receptor bound to an antagonist peptide. Embo J. 2005;24:1655–63. doi: 10.1038/sj.emboj.7600635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y, Waltz DA, Rao N, Drummond RJ, Rosenberg S, Chapman HA. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem. 1994;269:32380–32388. [PubMed] [Google Scholar]
- 35.Gargiulo L, Longanesi-Cattani I, Bifulco K, Franco P, Raiola R, Campiglia P, Grieco P, Peluso G, Stoppelli MP, Carriero MV. Cross-talk between fMLP and vitronectin receptors triggered by urokinase receptor-derived SRSRY peptide. J Biol Chem. 2005;280:25225–32. doi: 10.1074/jbc.M412605200. [DOI] [PubMed] [Google Scholar]
- 36.Colman RW, Pixley RA, Najamunnisa S, Yan W, Wang J, Mazar A, McCrae KR. Binding of high molecular weight kininogen to human endothelial cells is mediated via a site within domains 2 and 3 of the urokinase receptor. J Clin Invest. 1997;100:1481–7. doi: 10.1172/JCI119669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graf M, Reif S, Hecht K, Pelka-Fleischer R, Pfister K, Schmetzer H. High expression of urokinase plasminogen activator receptor (UPA-R) in acute myeloid leukemia (AML) is associated with worse prognosis. Am J Hematol. 2005;79:26–35. doi: 10.1002/ajh.20337. [DOI] [PubMed] [Google Scholar]
- 38.Saldanha RG, Molloy MP, Bdeir K, Cines DB, Song X, Uitto PM, Weinreb PH, Violette SM, Baker MS. Proteomic identification of lynchpin urokinase plasminogen activator receptor protein interactions associated with epithelial cancer malignancy. J Proteome Res. 2007;6:1016–28. doi: 10.1021/pr060518n. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed N, Riley C, Oliva K, Rice G, Quinn M. Ascites induces modulation of alpha6beta1 integrin and urokinase plasminogen activator receptor expression and associated functions in ovarian carcinoma. Br J Cancer. 2005;92:1475–85. doi: 10.1038/sj.bjc.6602495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lakka SS, Gondi CS, Dinh DH, Olivero WC, Gujrati M, Rao VH, Sioka C, Rao JS. Specific interference of urokinase-type plasminogen activator receptor and matrix metalloproteinase-9 gene expression induced by double-stranded RNA results in decreased invasion, tumor growth, and angiogenesis in gliomas. J Biol Chem. 2005;280:21882–92. doi: 10.1074/jbc.M408520200. [DOI] [PubMed] [Google Scholar]
- 41.Preissner KT, Kanse SM, May AE. Urokinase receptor: a molecular organizer in cellular communication. Curr Opin Cell Biol. 2000;12:621–8. doi: 10.1016/s0955-0674(00)00141-1. [DOI] [PubMed] [Google Scholar]
- 42.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–43. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 43.Ge Y, Elghetany MT. Urokinase plasminogen activator receptor (CD87): something old, something new. Lab Hematol. 2003;9:67–71. [PubMed] [Google Scholar]
- 44.Kenichi M, Masanobu M, Takehiko K, Shoko T, Akira F, Katsushige A, Takashi H, Yoshiyuki O, Shigeru K. Renal synthesis of urokinase type-plasminogen activator, its receptor, and plasminogen activator inhibitor-1 in diabetic nephropathy in rats: modulation by angiotensin-converting-enzyme inhibitor. J Lab Clin Med. 2004;144:69–77. doi: 10.1016/j.lab.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Florquin S, van den Berg JG, Olszyna DP, Claessen N, Opal SM, Weening JJ, van der Poll T. Release of urokinase plasminogen activator receptor during urosepsis and endotoxemia. Kidney Int. 2001;59:2054–61. doi: 10.1046/j.1523-1755.2001.00719.x. [DOI] [PubMed] [Google Scholar]
- 46.Degryse B. Is uPAR the center of a sensing system invloved in the regulation of inflammation? Curr Med Chem. 2003;2:237–259. [Google Scholar]
- 47.Albo D, Tuszynski GP. Thrombospondin-1 up-regulates tumor cell invasion through the urokinase plasminogen activator receptor in head and neck cancer cells. J Surg Res. 2004;120:21–6. doi: 10.1016/j.jss.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Nakayama M, Yoshida E, Sugiki M, Anai K, Maruyama M, Mihara H. Up-regulation of the urokinase-type plasminogen activator receptor by monocyte chemotactic proteins. Blood Coagul Fibrinolysis. 2002;13:383–91. doi: 10.1097/00001721-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Wei C, Moller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 50.Zhang G, Kim H, Cai X, Lopez-Guisa J, Alpers C, Liu Y, Carmeliet P, Eddy A. Urokinase receptor deficiency accelerates fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2003;14:1254–1271. doi: 10.1097/01.asn.0000064292.37793.fb. [DOI] [PubMed] [Google Scholar]
- 51.Jin H, Song YP, Boel G, Kochar J, Pancholi V. Group A streptococcal surface GAPDH, SDH, recognizes uPAR/CD87 as its receptor on the human pharyngeal cell and mediates bacterial adherence to host cells. J Mol Biol. 2005;350:27–41. doi: 10.1016/j.jmb.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 52.Prager GW, Breuss JM, Steurer S, Olcaydu D, Mihaly J, Brunner PM, Stockinger H, Binder BR. Vascular endothelial growth factor receptor-2-induced initial endothelial cell migration depends on the presence of the urokinase receptor. Circ Res. 2004;94:1562–70. doi: 10.1161/01.RES.0000131498.36194.6b. [DOI] [PubMed] [Google Scholar]
- 53.Zhou HM, Nichols A, Meda P, Vassalli JD. Urokinase-type plasminogen activator and its receptor synergize to promote pathogenic proteolysis. Embo J. 2000;19:4817–26. doi: 10.1093/emboj/19.17.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazzieri R, D’Alessio S, Kenmoe RK, Ossowski L, Blasi F. An uncleavable uPAR mutant allows dissection of signaling pathways in uPA-dependent cell migration. Mol Biol Cell. 2006;17:367–78. doi: 10.1091/mbc.E05-07-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, Romano M, Blasi F. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci U S A. 2002;99:1359–64. doi: 10.1073/pnas.022652999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Paulis A, Montuori N, Prevete N, Fiorentino I, Rossi FW, Visconte V, Rossi G, Marone G, Ragno P. Urokinase induces basophil chemotaxis through a urokinase receptor epitope that is an endogenous ligand for formyl peptide receptor-like 1 and -like 2. J Immunol. 2004;173:5739–48. doi: 10.4049/jimmunol.173.9.5739. [DOI] [PubMed] [Google Scholar]
- 57.Shetty S, Rao GN, Cines DB, Bdeir K. Urokinase induces activation of STAT3 in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L772–80. doi: 10.1152/ajplung.00476.2005. [DOI] [PubMed] [Google Scholar]
- 58.Wei Y, Czekay RP, Robillard L, Kugler MC, Zhang F, Kim KK, Xiong JP, Humphries MJ, Chapman HA. Regulation of alpha5beta1 integrin conformation and function by urokinase receptor binding. J Cell Biol. 2005;168:501–11. doi: 10.1083/jcb.200404112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei Y, Eble JA, Wang Z, Kreidberg JA, Chapman HA. Urokinase receptors promote beta1 integrin function through interactions with integrin alpha3beta1. Mol Biol Cell. 2001;12:2975–86. doi: 10.1091/mbc.12.10.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, Knisely JM, Lu W, McCormick LM, Wang J, Henkin J, Schwartz AL, Bu G. Low density lipoprotein (LDL) receptor-related protein 1B impairs urokinase receptor regeneration on the cell surface and inhibits cell migration. J Biol Chem. 2002;277:42366–71. doi: 10.1074/jbc.M207705200. [DOI] [PubMed] [Google Scholar]
- 61.Degryse B, Neels JG, Czekay RP, Aertgeerts K, Kamikubo Y, Loskutoff DJ. The low density lipoprotein receptor-related protein is a motogenic receptor for plasminogen activator inhibitor-1. J Biol Chem. 2004;279:22595–604. doi: 10.1074/jbc.M313004200. [DOI] [PubMed] [Google Scholar]
- 62.Webb DJ, Thomas KS, Gonias SL. Plasminogen activator inhibitor 1 functions as a urokinase response modifier at the level of cell signaling and thereby promotes MCF-7 cell growth. J Cell Biol. 2001;152:741–52. doi: 10.1083/jcb.152.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nicholas SB, Aguiniga E, Ren Y, Kim J, Wong J, Govindarajan N, Noda M, Wang W, Kawano Y, Collins A, Hsueh WA. Plasminogen activator inhibitor-1 deficiency retards diabetic nephropathy. Kidney Int. 2005;67:1297–307. doi: 10.1111/j.1523-1755.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- 64.Brodsky SV, Malinowski K, Golightly M, Jesty J, Goligorsky MS. Plasminogen activator inhibitor-1 promotes formation of endothelial microparticles with procoagulant potential. Circulation. 2002;106:2372–8. doi: 10.1161/01.cir.0000033972.90653.af. [DOI] [PubMed] [Google Scholar]
- 65.Gardsvoll H, Ploug M. Mapping of the vitronectin-binding site on the urokinase receptor: involvement of a coherent receptor interface consisting of residues from both domain I and the flanking interdomain linker region. J Biol Chem. 2007;282:13561–72. doi: 10.1074/jbc.M610184200. [DOI] [PubMed] [Google Scholar]
- 66.Zhang G, Cai X, Lopez-Guisa JM, Collins SJ, Eddy AA. Mitogenic signaling of urokinase receptor-deficient kidney fibroblasts: actions of an alternative urokinase receptor and LDL receptor-related protein. J Am Soc Nephrol. 2004;15:2090–102. doi: 10.1097/01.ASN.0000135057.41526.2C. [DOI] [PubMed] [Google Scholar]
- 67.Madsen CD, Ferraris GM, Andolfo A, Cunningham O, Sidenius N. uPAR-induced cell adhesion and migration: vitronectin provides the key. J Cell Biol. 2007;177:927–39. doi: 10.1083/jcb.200612058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoyer-Hansen G, Behrendt N, Ploug M, Dano K, Preissner KT. The intact urokinase receptor is required for efficient vitronectin binding: receptor cleavage prevents ligand interaction. FEBS Lett. 1997;420:79–85. doi: 10.1016/s0014-5793(97)01491-9. [DOI] [PubMed] [Google Scholar]
- 69.Kjaergaard M, Gardsvoll H, Hirschberg D, Nielbo S, Mayasundari A, Peterson CB, Jansson A, Jorgensen TJ, Poulsen FM, Ploug M. Solution structure of recombinant somatomedin B domain from vitronectin produced in Pichia pastoris. Protein Sci. 2007;16:1934–45. doi: 10.1110/ps.072949607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dellas C, Loskutoff DJ. Historical analysis of PAI-1 from its discovery to its potential role in cell motility and disease. Thromb Haemost. 2005;93:631–40. doi: 10.1160/TH05-01-0033. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi T, Suzuki K, Ihara H, Mogami H, Kazui T, Urano T. Plasminogen activator inhibitor type 1 promotes fibrosarcoma cell migration by modifying cellular attachment to vitronectin via alpha(v)beta(5) integrin. Semin Thromb Hemost. 2005;31:356–63. doi: 10.1055/s-2005-872444. [DOI] [PubMed] [Google Scholar]
- 72.Montuori N, Carriero MV, Salzano S, Rossi G, Ragno P. The cleavage of the urokinase receptor regulates its multiple functions. J Biol Chem. 2002;277:46932–9. doi: 10.1074/jbc.M207494200. [DOI] [PubMed] [Google Scholar]
- 73.Cao DJ, Guo YL, Colman RW. Urokinase-type plasminogen activator receptor is involved in mediating the apoptotic effect of cleaved high molecular weight kininogen in human endothelial cells. Circ Res. 2004;94:1227–34. doi: 10.1161/01.RES.0000126567.75232.46. [DOI] [PubMed] [Google Scholar]
- 74.Colman RW, Jameson BA, Lin Y, Johnson D, Mousa SA. Domain 5 of high molecular weight kininogen (kininostatin) down-regulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood. 2000;95:543–50. [PubMed] [Google Scholar]
- 75.Khan MM, Bradford HN, Isordia-Salas I, Liu Y, Wu Y, Espinola RG, Ghebrehiwet B, Colman RW. High-molecular-weight kininogen fragments stimulate the secretion of cytokines and chemokines through uPAR, Mac-1, and gC1qR in monocytes. Arterioscler Thromb Vasc Biol. 2006;26:2260–6. doi: 10.1161/01.ATV.0000240290.70852.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xue W, Kindzelskii AL, Todd RF, 3rd, Petty HR. Physical association of complement receptor type 3 and urokinase-type plasminogen activator receptor in neutrophil membranes. J Immunol. 1994;152:4630–40. [PubMed] [Google Scholar]
- 77.Kjoller L. The urokinase plasminogen activator receptor in the regulation of the actin cytoskeleton and cell motility. Biol Chem. 2002;383:5–19. doi: 10.1515/BC.2002.002. [DOI] [PubMed] [Google Scholar]
- 78.Ossowski L, Aguirre-Ghiso JA. Urokinase receptor and integrin partnership: coordination of signaling for cell adhesion, migration and growth. Curr Opin Cell Biol. 2000;12:613–20. doi: 10.1016/s0955-0674(00)00140-x. [DOI] [PubMed] [Google Scholar]
- 79.Degryse B, Orlando S, Resnati M, Rabbani SA, Blasi F. Urokinase/urokinase receptor and vitronectin/alpha(v)beta(3) integrin induce chemotaxis and cytoskeleton reorganization through different signaling pathways. Oncogene. 2001;20:2032–43. doi: 10.1038/sj.onc.1204261. [DOI] [PubMed] [Google Scholar]
- 80.Renckens R, Roelofs JJ, Florquin S, van der Poll T. Urokinase-type plasminogen activator receptor plays a role in neutrophil migration during lipopolysaccharide-induced peritoneal inflammation but not during Escherichia coli-induced peritonitis. J Infect Dis. 2006;193:522–30. doi: 10.1086/499601. [DOI] [PubMed] [Google Scholar]
- 81.Rijneveld AW, Levi M, Florquin S, Speelman P, Carmeliet P, van Der Poll T. Urokinase receptor is necessary for adequate host defense against pneumococcal pneumonia. J Immunol. 2002;168:3507–11. doi: 10.4049/jimmunol.168.7.3507. [DOI] [PubMed] [Google Scholar]
- 82.East E, Baker D, Pryce G, Lijnen HR, Cuzner ML, Gveric D. A role for the plasminogen activator system in inflammation and neurodegeneration in the central nervous system during experimental allergic encephalomyelitis. Am J Pathol. 2005;167:545–54. doi: 10.1016/S0002-9440(10)62996-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chapman HA, Wei Y. Protease crosstalk with integrins: the urokinase receptor paradigm. Thromb Haemost. 2001;86:124–9. [PubMed] [Google Scholar]
- 84.Koshelnick Y, Ehart M, Hufnagl P, Heinrich PC, Binder BR. Urokinase receptor is associated with the components of the JAK1/STAT1 signaling pathway and leads to activation of this pathway upon receptor clustering in the human kidney epithelial tumor cell line TCL-598. J Biol Chem. 1997;272:28563–28567. doi: 10.1074/jbc.272.45.28563. [DOI] [PubMed] [Google Scholar]
- 85.Kunigal S, Lakka SS, Gondi CS, Estes N, Rao JS. RNAi-mediated downregulation of urokinase plasminogen activator receptor and matrix metalloprotease-9 in human breast cancer cells results in decreased tumor invasion, angiogenesis and growth. Int J Cancer. 2007;121:2307–16. doi: 10.1002/ijc.22962. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Wei Y, Tang CH, Kim Y, Robillard L, Zhang F, Kugler MC, Chapman HA. Urokinase receptors are required for alpha 5 beta 1 integrin-mediated signaling in tumor cells. J Biol Chem. 2007;282:3929–39. doi: 10.1074/jbc.M607989200. [DOI] [PubMed] [Google Scholar]
- 87.Chazaud B, Bonavaud S, Plonquet A, Pouchelet M, Gherardi RK, Barlovatz-Meimon G. Involvement of the [uPAR:uPA:PAI-1:LRP] complex in human myogenic cell motility. Exp Cell Res. 2000;258:237–44. doi: 10.1006/excr.2000.4934. [DOI] [PubMed] [Google Scholar]
- 88.Croucher D, Saunders DN, Ranson M. The urokinase/PAI-2 complex: a new high affinity ligand for the endocytosis receptor low density lipoprotein receptor-related protein. J Biol Chem. 2006;281:10206–13. doi: 10.1074/jbc.M513645200. [DOI] [PubMed] [Google Scholar]
- 89.Engelholm LH, Nielsen BS, Dano K, Behrendt N. The urokinase receptor associated protein (uPARAP/endo180): a novel internalization receptor connected to the plasminogen activation system. Trends Cardiovasc Med. 2001;11:7–13. doi: 10.1016/s1050-1738(01)00076-7. [DOI] [PubMed] [Google Scholar]
- 90.Behrendt N. The urokinase receptor (uPAR) and the uPAR-associated protein (uPARAP/Endo180): membrane proteins engaged in matrix turnover during tissue remodeling. Biol Chem. 2004;385:103–36. doi: 10.1515/BC.2004.031. [DOI] [PubMed] [Google Scholar]
- 91.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schroers A, Hecht O, Kallen KJ, Pachta M, Rose-John S, Grotzinger J. Dynamics of the gp130 cytokine complex: a model for assembly on the cellular membrane. Protein Sci. 2005;14:783–90. doi: 10.1110/ps.041117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Welch TR, Frenzke M, Witte D, Davis AE. C5a is important in the tubulointerstitial component of experimental immune complex glomerulonephritis. Clin Exp Immunol. 2002;130:43–8. doi: 10.1046/j.1365-2249.2002.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Vries B, Kohl J, Leclercq WK, Wolfs TG, van Bijnen AA, Heeringa P, Buurman WA. Complement factor C5a mediates renal ischemia-reperfusion injury independent from neutrophils. J Immunol. 2003;170:3883–9. doi: 10.4049/jimmunol.170.7.3883. [DOI] [PubMed] [Google Scholar]
- 95.Bao L, Osawe I, Puri T, Lambris JD, Haas M, Quigg RJ. C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur J Immunol. 2005;35:2496–506. doi: 10.1002/eji.200526327. [DOI] [PubMed] [Google Scholar]
- 96.Boor P, Konieczny A, Villa L, Schult AL, Bucher E, Rong S, Kunter U, van Roeyen CR, Polakowski T, Hawlisch H, Hillebrandt S, Lammert F, Eitner F, Floege J, Ostendorf T. Complement C5 mediates experimental tubulointerstitial fibrosis. J Am Soc Nephrol. 2007;18:1508–15. doi: 10.1681/ASN.2006121343. [DOI] [PubMed] [Google Scholar]
- 97.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220–6. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 98.Stolz DB, Mars WM, Petersen BE, Kim TH, Michalopoulos GK. Growth factor signal transduction immediately after two-thirds partial hepatectomy in the rat. Cancer Res. 1999;59:3954–60. [PubMed] [Google Scholar]
- 99.Kiyan J, Kiyan R, Haller H, Dumler I. Urokinase-induced signaling in human vascular smooth muscle cells is mediated by PDGFR-beta. Embo J. 2005;24:1787–97. doi: 10.1038/sj.emboj.7600669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guerrero J, Santibanez JF, Gonzalez A, Martinez J. EGF receptor transactivation by urokinase receptor stimulus through a mechanism involving Src and matrix metalloproteinases. Exp Cell Res. 2004;292:201–8. doi: 10.1016/j.yexcr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 101.Jo M, Thomas KS, Takimoto S, Gaultier A, Hsieh EH, Lester RD, Gonias SL. Urokinase receptor primes cells to proliferate in response to epidermal growth factor. Oncogene. 2007;26:2585–94. doi: 10.1038/sj.onc.1210066. [DOI] [PubMed] [Google Scholar]
- 102.Mazzieri R, Blasi F. The urokinase receptor and the regulation of cell proliferation. Thromb Haemost. 2005;93:641–6. doi: 10.1160/TH05-01-0021. [DOI] [PubMed] [Google Scholar]
- 103.Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. Intraperitoneal injection of a hairpin RNA-expressing plasmid targeting urokinase-type plasminogen activator (uPA) receptor and uPA retards angiogenesis and inhibits intracranial tumor growth in nude mice. Clin Cancer Res. 2007;13:4051–60. doi: 10.1158/1078-0432.CCR-06-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gondi CS, Kandhukuri N, Dinh DH, Gujrati M, Rao JS. Down-regulation of uPAR and uPA activates caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. Int J Oncol. 2007;31:19–27. [PMC free article] [PubMed] [Google Scholar]
- 105.Hasanuzzaman M, Kutner R, Agha-Mohammadi S, Reiser J, Sehgal I. A doxycycline-inducible urokinase receptor (uPAR) upregulates uPAR activities including resistance to anoikis in human prostate cancer cell lines. Mol Cancer. 2007;6:34. doi: 10.1186/1476-4598-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Binder BR, Mihaly J, Prager GW. uPAR-uPA-PAI-1 interactions and signaling: a vascular biologist’s view. Thromb Haemost. 2007;97:336–42. [PubMed] [Google Scholar]
- 107.Le Gat L, Gogat K, Bouquet C, Saint-Geniez M, Darland D, Van Den Berghe L, Marchant D, Provost A, Perricaudet M, Menasche M, Abitbol M. In vivo adenovirus-mediated delivery of a uPA/uPAR antagonist reduces retinal neovascularization in a mouse model of retinopathy. Gene Ther. 2003;10:2098–103. doi: 10.1038/sj.gt.3302122. [DOI] [PubMed] [Google Scholar]
- 108.Ragno P. The urokinase receptor: a ligand or a receptor? Story of a sociable molecule. Cell Mol Life Sci. 2006;63:1028–37. doi: 10.1007/s00018-005-5428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lawn SD, Myer L, Bangani N, Vogt M, Wood R. Plasma levels of soluble urokinase-type plasminogen activator receptor (suPAR) and early mortality risk among patients enrolling for antiretroviral treatment in South Africa. BMC Infect Dis. 2007;7:41. doi: 10.1186/1471-2334-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Selleri C, Montuori N, Ricci P, Visconte V, Baiano A, Carriero MV, Rotoli B, Rossi G, Ragno P. In vivo activity of the cleaved form of soluble urokinase receptor: a new hematopoietic stem/progenitor cell mobilizer. Cancer Res. 2006;66:10885–90. doi: 10.1158/0008-5472.CAN-06-1311. [DOI] [PubMed] [Google Scholar]
- 111.Selleri C, Montuori N, Ricci P, Visconte V, Carriero MV, Sidenius N, Serio B, Blasi F, Rotoli B, Rossi G, Ragno P. Involvement of the urokinase-type plasminogen activator receptor in hematopoietic stem cell mobilization. Blood. 2005;105:2198–205. doi: 10.1182/blood-2004-06-2424. [DOI] [PubMed] [Google Scholar]
- 112.Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, Mills M, Wanzeck J, Janowska-Wieczorek A, Ratajczak MZ. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40–8. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 113.Swaisgood CM, French EL, Noga C, Simon RH, Ploplis VA. The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system. Am J Pathol. 2000;157:177–87. doi: 10.1016/S0002-9440(10)64529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Matsuoka H, Sisson TH, Nishiuma T, Simon RH. Plasminogen-mediated activation and release of hepatocyte growth factor from extracellular matrix. Am J Respir Cell Mol Biol. 2006;35:705–13. doi: 10.1165/rcmb.2006-0006OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shimizu M, Hara A, Okuno M, Matsuno H, Okada K, Ueshima S, Matsuo O, Niwa M, Akita K, Yamada Y, Yoshimi N, Uematsu T, Kojima S, Friedman SL, Moriwaki H, Mori H. Mechanism of retarded liver regeneration in plasminogen activator-deficient mice: impaired activation of hepatocyte growth factor after Fas-mediated massive hepatic apoptosis. Hepatology. 2001;33:569–76. doi: 10.1053/jhep.2001.22650. [DOI] [PubMed] [Google Scholar]
- 116.Marchand-Adam S, Fabre A, Mailleux AA, Marchal J, Quesnel C, Kataoka H, Aubier M, Dehoux M, Soler P, Crestani B. Defect of pro-hepatocyte growth factor activation by fibroblasts in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:58–66. doi: 10.1164/rccm.200507-1074OC. [DOI] [PubMed] [Google Scholar]
- 117.Heymans S, Pauschinger M, De Palma A, Kallwellis-Opara A, Rutschow S, Swinnen M, Vanhoutte D, Gao F, Torpai R, Baker AH, Padalko E, Neyts J, Schultheiss HP, Van de Werf F, Carmeliet P, Pinto YM. Inhibition of urokinase-type plasminogen activator or matrix metalloproteinases prevents cardiac injury and dysfunction during viral myocarditis. Circulation. 2006;114:565–73. doi: 10.1161/CIRCULATIONAHA.105.591032. [DOI] [PubMed] [Google Scholar]
- 118.Heymans S, Lupu F, Terclavers S, Vanwetswinkel B, Herbert JM, Baker A, Collen D, Carmeliet P, Moons L. Loss or inhibition of uPA or MMP-9 attenuates LV remodeling and dysfunction after acute pressure overload in mice. Am J Pathol. 2005;166:15–25. doi: 10.1016/S0002-9440(10)62228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bueno M, Salgado S, Beas-Zarate C, Armendariz-Borunda J. Urokinase-type plasminogen activator gene therapy in liver cirrhosis is mediated by collagens gene expression down-regulation and up-regulation of MMPs, HGF and VEGF. J Gene Med. 2006;8:1291–9. doi: 10.1002/jgm.961. [DOI] [PubMed] [Google Scholar]
- 120.Lin Y, Xie WF, Chen YX, Zhang X, Zeng X, Qiang H, Chen WZ, Yang XJ, Han ZG, Zhang ZB. Treatment of experimental hepatic fibrosis by combinational delivery of urokinase-type plasminogen activator and hepatocyte growth factor genes. Liver Int. 2005;25:796–807. doi: 10.1111/j.1478-3231.2005.01098.x. [DOI] [PubMed] [Google Scholar]
- 121.Salgado S, Garcia J, Vera J, Siller F, Bueno M, Miranda A, Segura A, Grijalva G, Segura J, Orozco H, Hernandez-Pando R, Fafutis M, Aguilar LK, Aguilar-Cordova E, Armendariz-Borunda J. Liver cirrhosis is reverted by urokinase-type plasminogen activator gene therapy. Mol Ther. 2000;2:545–51. doi: 10.1006/mthe.2000.0210. [DOI] [PubMed] [Google Scholar]
- 122.Moriwaki H, Stempien-Otero A, Kremen M, Cozen AE, Dichek DA. Overexpression of urokinase by macrophages or deficiency of plasminogen activator inhibitor type 1 causes cardiac fibrosis in mice. Circ Res. 2004;95:637–44. doi: 10.1161/01.RES.0000141427.61023.f4. [DOI] [PubMed] [Google Scholar]
- 123.Shetty S, Kumar A, Johnson AR, Pueblitz S, Holiday D, Raghu G, Idell S. Differential expression of the urokinase receptor in fibroblasts from normal and fibrotic human lungs. Am J Respir Cell Mol Biol. 1996;15:78–87. doi: 10.1165/ajrcmb.15.1.8679225. [DOI] [PubMed] [Google Scholar]
- 124.Leake D, Doerr TD, Scott G. Expression of urokinase-type plasminogen activator and its receptor in keloids. Arch Otolaryngol Head Neck Surg. 2003;129:1334–8. doi: 10.1001/archotol.129.12.1334. [DOI] [PubMed] [Google Scholar]
- 125.Bernstein AM, Twining SS, Warejcka DJ, Tall E, Masur SK. Urokinase receptor cleavage: a crucial step in fibroblast-to-myofibroblast differentiation. Mol Biol Cell. 2007;18:2716–27. doi: 10.1091/mbc.E06-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stempien-Otero A, Plawman A, Meznarich J, Dyamenahalli T, Otsuka G, Dichek DA. Mechanisms of cardiac fibrosis induced by urokinase plasminogen activator. J Biol Chem. 2006;281:15345–51. doi: 10.1074/jbc.M512818200. [DOI] [PubMed] [Google Scholar]
- 127.Paul R, Winkler F, Bayerlein I, Popp B, Pfister HW, Koedel U. Urokinase-type plasminogen activator receptor regulates leukocyte recruitment during experimental pneumococcal meningitis. J Infect Dis. 2005;191:776–82. doi: 10.1086/427829. [DOI] [PubMed] [Google Scholar]
- 128.Shushakova N, Eden G, Dangers M, Menne J, Gueler F, Luft FC, Haller H, Dumler I. The urokinase/urokinase receptor system mediates the IgG immune complex-induced inflammation in lung. J Immunol. 2005;175:4060–8. doi: 10.4049/jimmunol.175.6.4060. [DOI] [PubMed] [Google Scholar]
- 129.Bryer SC, Fantuzzi G, Van Rooijen N, Koh TJ. Urokinase-type plasminogen activator plays essential roles in macrophage chemotaxis and skeletal muscle regeneration. J Immunol. 2008;180:1179–88. doi: 10.4049/jimmunol.180.2.1179. [DOI] [PubMed] [Google Scholar]
- 130.Gordon S. Macrophage heterogeneity and tissue lipids. J Clin Invest. 2007;117:89–93. doi: 10.1172/JCI30992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 132.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nishida M, Okumura Y, Fujimoto S, Shiraishi I, Itoi T, Hamaoka K. Adoptive transfer of macrophages ameliorates renal fibrosis in mice. Biochem Biophys Res Commun. 2005;332:11–6. doi: 10.1016/j.bbrc.2005.04.083. [DOI] [PubMed] [Google Scholar]
- 134.Wang Y, Wang YP, Zheng G, Lee VW, Ouyang L, Chang DH, Mahajan D, Coombs J, Wang YM, Alexander SI, Harris DC. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72:290–9. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 135.Pillay V, Dass CR, Choong PF. The urokinase plasminogen activator receptor as a gene therapy target for cancer. Trends Biotechnol. 2007;25:33–9. doi: 10.1016/j.tibtech.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 136.Furlan F, Galbiati C, Jorgensen NR, Jensen JE, Mrak E, Rubinacci A, Talotta F, Verde P, Blasi F. Urokinase plasminogen activator receptor affects bone homeostasis by regulating osteoblast and osteoclast function. J Bone Miner Res. 2007;22:1387–96. doi: 10.1359/jbmr.070516. [DOI] [PubMed] [Google Scholar]
- 137.Steins MB, Padro T, Schwaenen C, Ruiz S, Mesters RM, Berdel WE, Kienast J. Overexpression of urokinase receptor and cell surface urokinase-type plasminogen activator in the human vessel wall with different types of atherosclerotic lesions. Blood Coagul Fibrinolysis. 2004;15:383–91. doi: 10.1097/01.mbc.0000114441.59147.56. [DOI] [PubMed] [Google Scholar]
- 138.Rao JS, Gondi C, Chetty C, Chittivelu S, Joseph PA, Lakka SS. Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells. Mol Cancer Ther. 2005;4:1399–408. doi: 10.1158/1535-7163.MCT-05-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Margheri F, D’Alessio S, Serrati S, Pucci M, Annunziato F, Cosmi L, Liotta F, Angeli R, Angelucci A, Gravina GL, Rucci N, Bologna M, Teti A, Monia B, Fibbi G, Del Rosso M. Effects of blocking urokinase receptor signaling by antisense oligonucleotides in a mouse model of experimental prostate cancer bone metastases. Gene Ther. 2005;12:702–14. doi: 10.1038/sj.gt.3302456. [DOI] [PubMed] [Google Scholar]
- 140.Pass J, Jogi A, Lund IK, Rono B, Rasch MG, Gardsvoll H, Lund LR, Ploug M, Romer J, Dano K, Hoyer-Hansen G. Murine monoclonal antibodies against murine uPA receptor produced in gene-deficient mice: inhibitory effects on receptor-mediated uPA activity in vitro and in vivo. Thromb Haemost. 2007;97:1013–22. [PubMed] [Google Scholar]
- 141.Kobayashi H, Yagyu T, Kondo T, Kurita N, Inagaki K, Haruta S, Kawaguchi R, Kitanaka T, Sakamoto Y, Yamada Y, Kanayama N, Terao T. Suppression of urokinase receptor expression by thalidomide is associated with inhibition of nuclear factor kappaB activation and subsequently suppressed ovarian cancer dissemination. Cancer Res. 2005;65:10464–71. doi: 10.1158/0008-5472.CAN-04-3789. [DOI] [PubMed] [Google Scholar]
- 142.Jo M, Thomas KS, Wu L, Gonias SL. Soluble urokinase-type plasminogen activator receptor inhibits cancer cell growth and invasion by direct urokinase-independent effects on cell signaling. J Biol Chem. 2003;278:46692–8. doi: 10.1074/jbc.M308808200. [DOI] [PubMed] [Google Scholar]
- 143.McGuire PG, Jones TR, Talarico N, Warren E, Das A. The urokinase/urokinase receptor system in retinal neovascularization: inhibition by A6 suggests a new therapeutic target. Invest Ophthalmol Vis Sci. 2003;44:2736–42. doi: 10.1167/iovs.02-1160. [DOI] [PubMed] [Google Scholar]
- 144.Gu JM, Johns A, Morser J, Dole WP, Greaves DR, Deng GG. Urokinase plasminogen activator receptor promotes macrophage infiltration into the vascular wall of ApoE deficient mice. J Cell Physiol. 2005;204:73–82. doi: 10.1002/jcp.20262. [DOI] [PubMed] [Google Scholar]
- 145.Koopman JL, Slomp J, de Bart AC, Quax PH, Verheijen JH. Mitogenic effects of urokinase on melanoma cells are independent of high affinity binding to the urokinase receptor. J Biol Chem. 1998;273:33267–72. doi: 10.1074/jbc.273.50.33267. [DOI] [PubMed] [Google Scholar]
- 146.Mukhina S, Stepanova V, Traktouev D, Poliakov A, Beabealashvilly R, Gursky Y, Minashkin M, Shevelev A, Tkachuk V. The chemotactic action of urokinase on smooth muscle cells is dependent on its kringle domain. Characterization of interactions and contribution to chemotaxis. J Biol Chem. 2000;275:16450–8. doi: 10.1074/jbc.M909080199. [DOI] [PubMed] [Google Scholar]
- 147.Poliakov AA, Mukhina SA, Traktouev DO, Bibilashvily RS, Gursky YG, Minashkin MM, Stepanova VV, Tkachuk VA. Chemotactic effect of urokinase plasminogen activator: a major role for mechanisms independent of its proteolytic or growth factor domains. J Recept Signal Transduct Res. 1999;19:939–51. doi: 10.3109/10799899909038433. [DOI] [PubMed] [Google Scholar]
- 148.Liang OD, Chavakis T, Linder M, Bdeir K, Kuo A, Preissner KT. Binding of urokinase plasminogen activator to gp130 via a putative urokinase-binding consensus sequence. Biol Chem. 2003;384:229–36. doi: 10.1515/BC.2003.025. [DOI] [PubMed] [Google Scholar]
- 149.Franco P, Vocca I, Carriero MV, Alfano D, Cito L, Longanesi-Cattani I, Grieco P, Ossowski L, Stoppelli MP. Activation of urokinase receptor by a novel interaction between the connecting peptide region of urokinase and alpha v beta 5 integrin. J Cell Sci. 2006;119:3424–34. doi: 10.1242/jcs.03067. [DOI] [PubMed] [Google Scholar]
- 150.Zhang G, Kernan KA, Collins SJ, Lopez-Guisa J, Eddy AA. The novel ligand-receptor relationship identified between urokinase and the nicotinic receptor (nAChR♋1): its signaling and function in cell growth and chronic renal injury. J Am Soc Nephrol, Abs. 2007 [Google Scholar]
- 151.Sisson TH, Hanson KE, Subbotina N, Patwardhan A, Hattori N, Simon RH. Inducible lung-specific urokinase expression reduces fibrosis and mortality after lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1023–32. doi: 10.1152/ajplung.00049.2002. [DOI] [PubMed] [Google Scholar]
- 152.Lardot CG, Huaux FA, Broeckaert FR, Declerck PJ, Delos M, Fubini B, Lison DF. Role of urokinase in the fibrogenic response of the lung to mineral particles. Am J Respir Crit Care Med. 1998;157:617–28. doi: 10.1164/ajrccm.157.2.9707052. [DOI] [PubMed] [Google Scholar]
- 153.Shanmukhappa K, Sabla GE, Degen JL, Bezerra JA. Urokinase-type plasminogen activator supports liver repair independent of its cellular receptor. BMC Gastroenterol. 2006;6:40. doi: 10.1186/1471-230X-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]