Summary
This article reviews experimental and modeling methods for determining the critical roles played by the various factors that control nanocarrier drug delivery to vascular endothelial cells.
Keywords: Targeted drug delivery, nanocarriers, hemodynamics, binding, affinity
Introduction
The use of targeted nanocarriers for delivering therapeutic compounds to sites of pathology is gaining increased scientific attention and popularity [1]. The endothelial cells lining the vasculature are an obvious target for drug delivery, and design of nanocarriers having molecular specificity for capture on the cell surface is desirable. The ability of the nanocarriers to anchor to the vascular cells primarily depends on two factors: a) fluid mechanics controlling the flow and motion of the carriers and their propensity to approach the desired binding sites; and b) binding mechanism controlling attachment and motion of carriers at the sites. Ultimately the efficacy of the release of the cargo drugs depends on the nanocarrier and its anchoring mechanism. The combination of these steps will resultantly determine whether a nanocarrier loaded with a suitable drug for disease treatment is effective in delivering it to the chosen site.
Vascular hydrodynamics and blood as a colloidal fluid
The precursor to carrier anchoring on the vascular cells is their motion in the vasculature. The choice of the targeted vessel (e.g., capillaries, venules, arterioles) and the prevalent hemodynamics therein establish the leading criteria for the design and modeling of carriers and their subsequent anchoring. In this section, some of these factors are examined.
The first design parameter for selecting an appropriate carrier is its size. The choice of carrier size is directly related to the targeted vessel dimensions. Micron-size carriers have been found to have prolonged residency in prelysosomal compartments, whereas submicron carriers traffick to lysosomes more readily [2]. This broadly suggests that larger size particles are more suitable for vessels of larger diameter and smaller size particles are more suitable for the smaller vessels. Charoenphol et al. [3] suggest that spheres 25 μm in size are optimal for targeting the wall in medium to large vessels relevant in several cardiovascular diseases. However, if the larger carriers are designed to remain in circulation for a prolonged period, they must be designed to avoid entrapment in the capillaries (~5 μm diameter). For a spherical particle this means a radius in the submicron range. However, if the particle shape is not restricted to being a sphere, the carriers can be submicron in size in just one dimension. Thus, the shape of the particle is also an important design factor. Non-spherical particles laterally migrate, even in laminar and linear flows [4]. Particles like discs [2] and flexible filomicelles [5] have been shown to demonstrate superior circulation pro les, explained by their alignment with the flow guiding them to avoid excessive collisions with blood and vascular cells. Moghimi et al. [6] have reviewed some of the desirable characteristics of long-circulating drug carrier systems. Some of the filtering units in the spleen are described as slits through which spherical particles 200 nm in diameter cannot pass, but flexible RBCs routinely transit the spleen. Geng et al. [7] showed in rodent testing that flexible filomicelles persist in the circulation ten times longer than do spherical particles of comparable volume. Champion et al. [8] present an overview of some of the fabrication techniques of non-spherical carriers; e.g., ab initio synthesis of non-spherical particles, manipulation of previously fabricated spherical particles into non-spherical geometries.
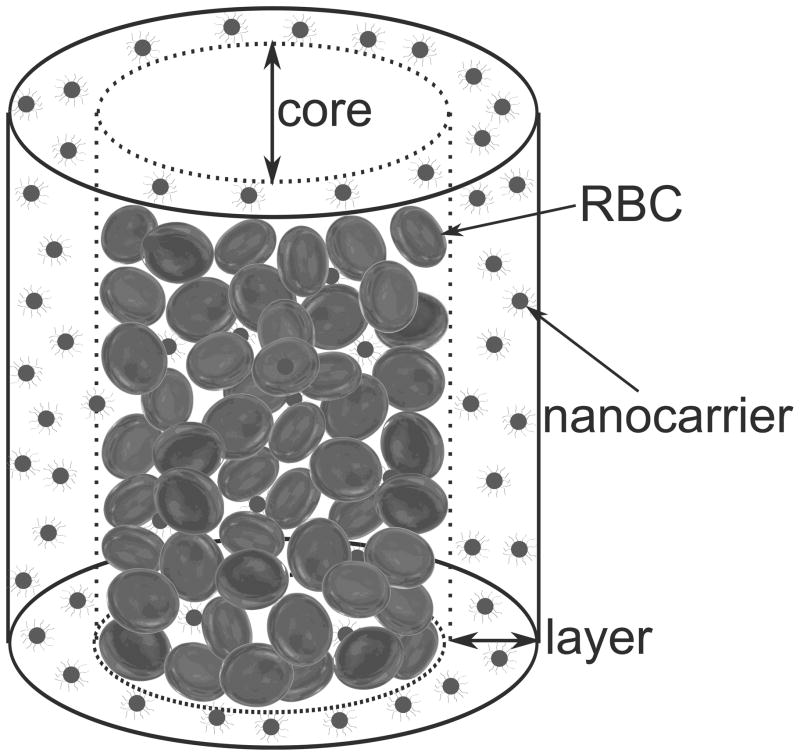
At the micron and submicron size, particle interaction with erythrocytes assumes great importance. RBCs are known to aggregate near the center in vessel sizes between 10 and 300 μm leading to changes in the discharge hematocrit and viscosity characterized by the Fåhraeus and Fåhraeus-Lindqvist effects [9]. Sharan and Popel [10] predict that the effective viscosity of the cell-free layer is different from that of blood plasma due to the occasional presence of RBCs near the wall. Small particles (like platelets) exhibit an inverse Fåhraeus effect and are expelled toward the plasma layer near the wall due to collision interaction with RBCs [11] resulting in a nearly seven fold increase in concentration. A schematic representation of this inverse Fåhraeus effect is shown in Figure 1, in which the smaller nanocarriers are expelled into the annular cell free plasma layer. Decuzzi et al. [12] based on their model, state that particles used for drug delivery should have a radius smaller than a critical value (in the range of 100 nm) to facilitate this margination and subsequent interaction with the endothelium. On the other hand, Gentile et al. report that in shear flow experiments, dense particles having a diameter > 200 nm have a greater propensity to marginate toward the vessel wall in gravitational fields [13]. Modeling and experimental studies [14] have also examined how the RBC deformation is a key factor in the near-wall excesses of platelet sized particles in flow.
Figure 1.
Schematic representation of nanoparticle segregation in smaller blood vessels.
Thus, there are primarily two geometric parameters (i.e., shape and size) that should be controlled in considering nanocarrier design. If the goal is to achieve maximal margination of the carriers, they should be spherical and less than 100 nm in size. Small non-spherical nanocarriers will marginate but will experience lateral motions based on the relative alignment with the flow and this will decrease their residence time near endothelial cells. On the other hand, large micron sized non-spherical particles with one dimension in the submicron range will not marginate, but will remain in circulation for longer durations and are therefore more suitable for drug release within the vasculature without necessitating carrier anchoring. The specific effects of particle size on binding and adhesion has been discussed in a subsequent section.
Specific individual nanocarrier motions can be predicted by modeling the colloidal interactions between carriers and RBCs. Such modeling gives useful information about the effects of nanocarrier concentration in the bulk medium, and what percentage of the carriers are likely to be captured near the desired vascular region. These interactions are inherently random in nature and so only the relevant “statistically averaged” quantities should be examined. The collisions between the RBCs and the nanocarriers in such a statistical model are typically represented as “fluctuations”. Munn et al. [15] present such mathematical models to give statistical measures of fluctuations. Temperature induced Brownian motion is not seen to influence platelet behavior near a wall [16]. An alternate method of measuring the averaged motion of the nanocarriers undergoing multiple collisions with RBCs is by an effective diffusion coefficient. Gentile et al. [17] have modeled the dispersion of nanocarriers this way. They capture this effect by an effective diffusion coefficient which quantifies the longitudinal mass transport in blood vessels.
Specific molecular targeting criteria
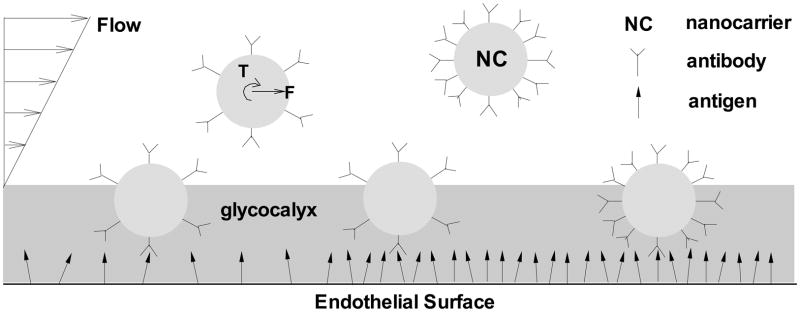
Figure 2 is a two dimensional depiction of various factors contributing to the capture of nanocarriers onto the endothelial cell surface in targeted vascular drug delivery. As shown, the local shear flow introduces both torque (T) and drag forces (F), which regulate the nanocarrier transportation inside blood vessel. The presence of the glycocalyx layer on the endothelial cell surface effectively reduces the nanocarrier binding by providing an energy barrier. Both the antibody density on the nanocarrier surface and the antigen density on the endothelial cell surface impact the nanocarrier binding. Under conditions in which both of these densities are sufficiently high, multivalent binding interactions yielding enough strength to capture carriers in flow are possible.
Figure 2.
Schematic illustration of factors influencing targeted nanocarrier capture by antigen expressing endothelial cell surfaces.
Effect of particle size and shape
Besides the physico-chemical properties of the particles, their geometric parameters (i.e., size and shape) have also been shown to play important roles in the vascular drug delivery. Particles need to be sufficiently small to be transported effectively inside the vasculature, yet the particles have to be large enough to carry some meaningful dosage of therapeutic cargo. Decuzzi et al. [18] developed a mathematical model to investigate systematically the important roles of particle size and shape on particle transport at the vascular level, as well as the strength of adhesion and internalization of particles at the cellular level. Their numerical model [18] allows prediction of the adhesive and endocytotic performances of particular systems based on geometrical, biophysical and biological properties. These investigators also generated a design map, which is used to relate the ratio of ligand-to-receptor surface density with the non-specific attractive force parameter for given particles. The design map is capable of predicting particle adherence to the targeted vasculature and if internalization occurs. Significant attention has also been devoted to developing new techniques for the fabrication of nanocarriers with various sizes and shapes.
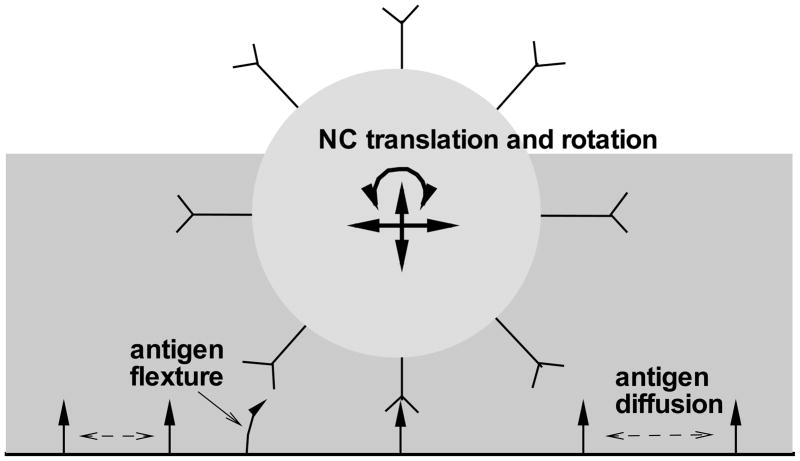
Liu et al. [19] developed a general computational protocol to calculate the binding affinities between functionalized nanocarriers and the endothelial cell surface. In their model, as illustrated in Figure 3, both the translational and rotational movements of the nanocarrier are considered. Antigens are allowed to diffuse freely on the cell surface, and antigen flexural movement is accounted for by allowing bending and rotation. These investigators have implemented the model to address the effect of particle size on binding, having shown that increasing the particle size only moderately enhances binding due to the entropy loss associated with bound receptors [20]. This conclusion is consistent with experimental measurements of binding dissociation constant (Kd) for 200 nm particles (77 pM, [21]) and 1 μm particles (1.6 pM, [22]).
Figure 3.
Cartoon depiction of flow-induced nanocarrier translational and rotational motion, antigen lateral diffusion within the cell membrane and antigen flexural movement.
Effect of antibody surface density
The antibody (Ab) surface coverage on nanocarriers (see Figure 2 for schematic illustration) is an experimentally tunable parameter and has been shown strongly to influence nanocarrier binding. The saturation limit (maximum number of molecules) for antibody surface coverage has been measured as ~7000 Ab/μm2 for anti-ICAMs [21]. Calderon et al. [23] studied the detachment of 1 μm diameter carriers with different antibody surface densities under shear flow. They found that no detachment occurs for carriers having 1100 and 4100 Ab/μm2 (16% and 59% of maximal coverage) under normal physiological flow conditions. However, carriers bearing 370 Ab/μm2 (5% of maximal coverage) detached significantly even at very low shear flows. Theoretically the binding of nanocarriers increases with the antibody surface coverage, but in practicality a sub-maximal antibody surface density may be preferred due to the fact that exceeding the optimal surface density of antibody or other affinity ligands on the nanocarrier surface may predispose patients to immune responses to the proteins. Calderon et al. [22] discussed the effects of antibody surface density on the performance of drug delivery using both in vivo and in vitro experiments. The in vivo experiments were performed using a radioisotope-tracing technique to measure the biodistribution of carriers targeted to ICAM1 in mice. Increasing the antibody density enhanced the carrier accumulation in the pulmonary vasculature compared to other tissues. The in vitro cell culture experiments, performed with fluorescence microscopy, showed the binding kinetics to be much faster for higher antibody density. Liu et al. [19] recently modeled the effect of antibody surface coverage on 100 nm nanocarriers and identified a threshold below which the binding affinity exponentially drops below that of free anti-ICAM to ICAM-1. This finding explains experimental observations by Calderon et al. [23]. Additionally, the computational model results agree remarkably well with experimental measurements of in vivo targeting of the anti-ICAM-1 coated nanocarriers to pulmonary endothelium in mice [19]. Inspection of the numerical results reveals a multivalency (number of bonds) change at the threshold value, clearly indicating that nanocarriers need not be maximally covered by antibodies to achieve binding efficiency. Rather, it is the minimum threshold number of surface molecules that must be specified to ensure that nanocarrier binding occurs.
Effect of glycocalyx
The gel-like endothelial glycocalyx layer usually extends a few hundred nanometers out from the lumenal surface of endothelial cells lining blood vessels in vivo. This brushy layer is an important structure affecting many cardiovascular diseases and biomedical procedures (see Figures 2 and 3 for schematic illustration). Recent studies of the mechanical and biochemical properties of the endothelial glycocalyx and its interactions with erythrocytes and leukocytes [24] have profited from the development of intravital microscopy. For example, Potter and Damiano [25] were directly able to probe the glycocalyx in vivo using fluorescent microparticle image velocimetry. They showed that the hydrodynamically relevant glycocalyx layer normally observed in vivo to be nearly absent under cell culture conditions in vitro. The endothelial glycocalyx plays a key role in mediating the binding of nanocarriers to the cell surface by effectively providing an energy barrier. Mulivor and Lipowsky [26] showed in vivo that binding of 100 nm diameter antibody coated microspheres increased up to 500 fold after chemical degradation of the glycocalyx. Adding to our understanding of glycocalyx mechanical function, Weinbaum et al. [27] examined the viscoelastic behavior of the endothelial surface layer and its response to fluid shear and cellular motion and were able to predict the bending rigidity of the glycocalyx core proteins to be in the range of 700 pN·nm2. Agrawal and Radhakrishnan [28] have now used a simplified model in which the glycocalyx effectively provides an energy barrier to reduce the binding between a nanocarrier and the endothelial cell surface. They obtained the glycocalyx stiffness by fitting the in vivo experimental data from [26]. However, the degree to which the glycocalyx thickness and stiffness are altered in vitro remains unclear, thus its true role in modulation of nanocarrier binding in vivo cannot yet be directly correlated to findings from cell culture experiments.
Effect of flow dependent forces
The circulation of nanocarriers within the bloodstream is highly dependent on the local flow environment. As displayed in Figure 2, the fluid motion introduces a hydrodynamic drag force and a torque on the nanocarrier surface. Through these two mechanical effects, blood flow mediates the interaction between nanocarriers and cell surface. It has been observed in experiment that flow can actually enhance the adhesion between ligand bearing small particles (or leukocytes) and the receptor expressing vascular surface, as well as the ensuing cellular uptake of such particles. This is a counter-intuitive phenomenon, given that one would expect increases in flow to favor downstream displacement of particles. This observation has drawn tremendous attention due to its importance to many biological functions. The initial tethering and stable rolling of microparticles (or cells) requires a threshold flow shear rate. This appears to violate the well accepted and experimentally proven concept that the dissociation rate of the interacting pair increases exponentially with increasing applied force. This dissociation rate-force interaction is typically defined mathematically using the Bell spring model [29]:
| (1) |
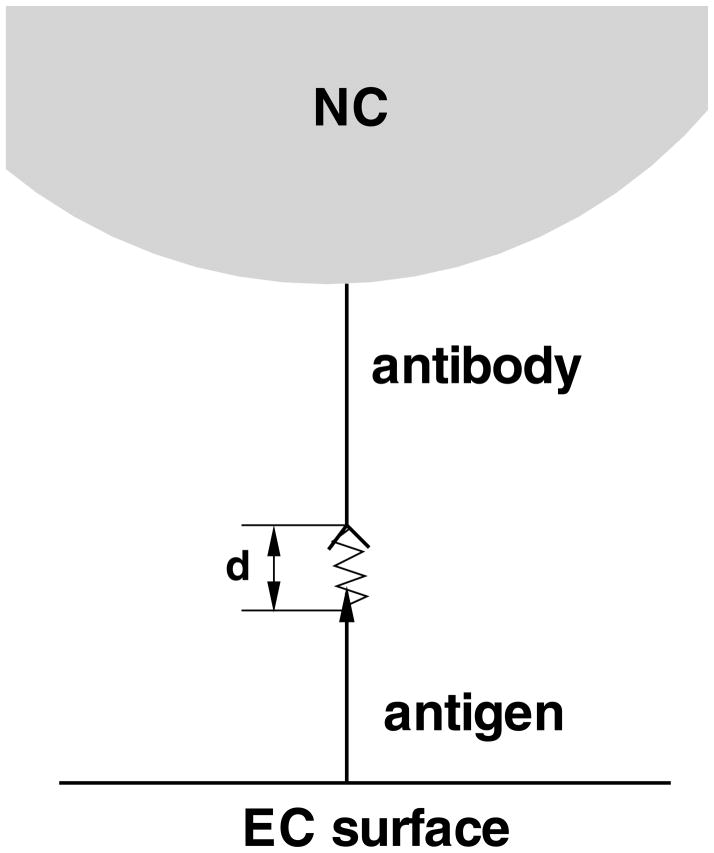
in which k(f) is the dissociation rate constant under applied force f, k0 is the dissociation rate constant with zero force, γ is the position of the transition state, kB is the Boltzmann constant and T is the temperature. As shown in Figure 4, the interaction pair between antibody and antigen is modeled as a simple mechanical spring. The binding free energy is computed as:
| (2) |
in which d is the distance between the two binding sites, ΔG0 is the equilibrium binding energy for d = 0, and k is the bond spring constant. Flow enhancement of adhesion can be divided into two stages: enhanced initial tethering and enhanced rolling after attachment. Flow enhanced tethering has been attributed to the convective transport in which the collision frequency between the antibody and antigen is enhanced by the convective flow [30]. To date, flow enhance rolling has been explained by “catch-slip” kinetics. The idea of “catch” bonds was proposed based on an observation that the lifetime of receptor-ligand bonds becomes prolonged by a tensile force acting on the cells [31]. It was also postulated that this was due to changes in molecular structure in response to the shear stress [32]. Based on the ideas of “catch-slip” bonds, several conceptual two-pathway models and mechanisms have been developed to account for the catch-slip transitions [33, 34], with two energy landscapes corresponding to “catch” and “slip” bonds respectively, having been postulated. These transitions are purportedly regulated by the external force. Despite the excellent agreement between model predictions and experiments, the fundamental physics of the “catch” bond is still far from conclusive due to the lack of experimental evidence. More recently, Beste and Hammer [35] attempted to characterize the phenomenological features of selectin-ligand bonds based on published data to connect the “catch-slip” kinetics to shear promoted adhesion. Two parameters have been identified: the critical force, fcr, and the kinetic efficiency, ∈. Each is assumed to play a critical role in the shear threshold behavior. Simulations of adhesion dynamics based on these two parameters demonstrate that precise knowledge of the mechanism is not required to capture the shear threshold rolling accurately. Nevertheless, recent fluorescence measurements of protein unfolding indicate no evidence of destabilization of folded proteins even at extremely high shear rates [36]. Therefore, the underlying physics and mechanisms of this flow promoted binding still remain unclear and under debate.
Figure 4.
Schematic representation of the Bell spring model for the interaction pair between an antibody and an antigen.
Drug delivery
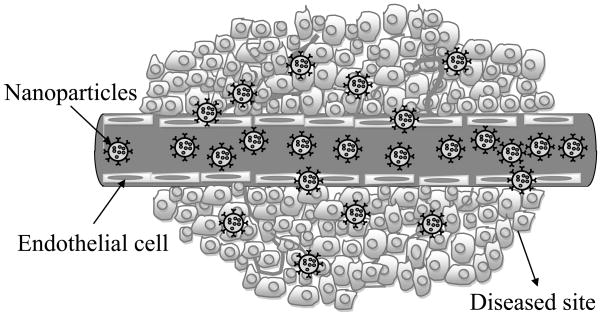
A major function of nanocarrier targeting is delivery of therapeutic cargo. The delivery of a cargo drug is effective only when it reaches the intracellular target which might be a) a nucleus, b) a signaling pathway, or c) enzymes within the cytoplasm. Different nanocarriers such as nanoparticles [37], polymeric micelles [38], liposomes [39], surface modified nanoparticles [40], protein nanoparticles [41] and solid lipid nanoparticles [42] are loaded with drugs to target the specific cell. Magnetic nanofilms or nanosheets (also called polymeric ultra thin films) produced using a spin coating process [43] are also used for delivering drug loaded patches on inner organ walls or on ulcers. Nanoparticle based drug delivery used in recent years holds promise in promoting the efficacy of various drugs [44]. Nanoparticles are made from a variety of biocompatible and biodegradable materials such as polymers (synthetic or natural) and proteins. The nanoparticle material depends on the physical and chemical properties of the drug. The technological advantages of nanoparticles as a drug delivery system are a) sustained drug release, b) relatively high carrier capacity for both hydrophobic and hydrophilic compounds, and c) variable routes of administration (e.g., oral, inhalation, parenteral). Figure 5 shows the passive tissue targeting in the blood vessels by nanoparticles. However, following the opening of endothelium tight intersections by hyperosmotic mannitol, nanoparticles may cross the blood brain barrier to treat central nervous system diseases such as brain tumors [45]. Targeting tissues passively with nanoparticles is fraught with hindrances; such as mucosal barriers, non-specific uptake of the nanoparticles and non-specific drug delivery in uncontrolled release. Controlled drug delivery involves the association of a drug with a carrier system and allows the modulation of the pharmacokinetic properties and biodistribution of the drug. Specific targeting of the diseased cells and the timed release of the drug are two very important aspects of drug delivery.
Figure 5.
Passive tissue targeting in the blood vessels by nanoparticles.
Targetability depends on the type of receptor or antigen expressed by endothelial cells which can specifically bind with ligand or antibodies on the surface of the nanoparticle. This receptor-ligand binding interaction captures nanoparticles onto the targeted cells through large adhesive forces. After initial binding, the nanoparticle may firmly adhere or may become unbound, depending on the bond strength and local flow forces. In a parallel plate flow chamber assays, Eniola et al. [46] have studied the effect of particle size with hemodynamics and the vessel size on the adhesion efficiency of targeting therapeutic material to the diseased site using biodegradable polymer microspheres. They found that the rolling velocity of sialyl Lewisx (sLex) coated microsphere is dependent on sLex site density, which gives the ability to adjust the contact time of carrier with the endothelial cells of the targeted site. Also they have shown that the average rolling velocity of microsphere depends linearly on wall shear stress within the parallel plate flow chamber. For the optimal drug targeting, it is required to understand the role of those key parameters that control the valency and affinity of carrier anchoring with the targeted site.
Haun and Hammer [47] characterized the kinetic rate constants of attachment and detachment of nanocarriers as a function of particle receptor density, substrate ligand density and shear rate. They have reported that the nanoparticle detachment is a time-dependent process with detachment probability decreases with elapsed attachment time. Binding affinity [21, 48] and multivalency, or the average number of receptor-ligand binding per bound nanocarrier [48, 49], are the important factors defining the efficacy of drug delivery to targeted cells. Muro et al. [21] have investigated the multivalent character of the anti-ICAM interaction with endothelial cell for 200 nm polymer nanocarrier possessing ~250 antibodies/carrier (~7000 antibodies/μm2) which facilitates anchoring with high affinity (70 pM) and binding capacity (Bmax ~300 particles/cell). As generally expected, the maximum binding sites available to carrier-coupled anti-ICAMs was lower than that for carrier-free anti-ICAMs (7.5×104 vs. 1.6×106 antibody per cell). In general, the targeting ligands are coupled directly to the carriers and compromise monoclonal antibodies, which confer specificity of binding. Dissociation constants in the nanomolar range or better are preferred. Multivalent binding can also be useful to enhance the avidity and reduce off rates so that binding persists long enough to permit imaging at convenient times after drug delivery. Hong et al. [48] have shown the quantitative and systematic evidence for multivalent interactions between nanocarrier and folate binding protein. Polyvalent binding is possible with more than one ligand type per carrier or with mixtures of ligand-carrier constructs directed at different targets.
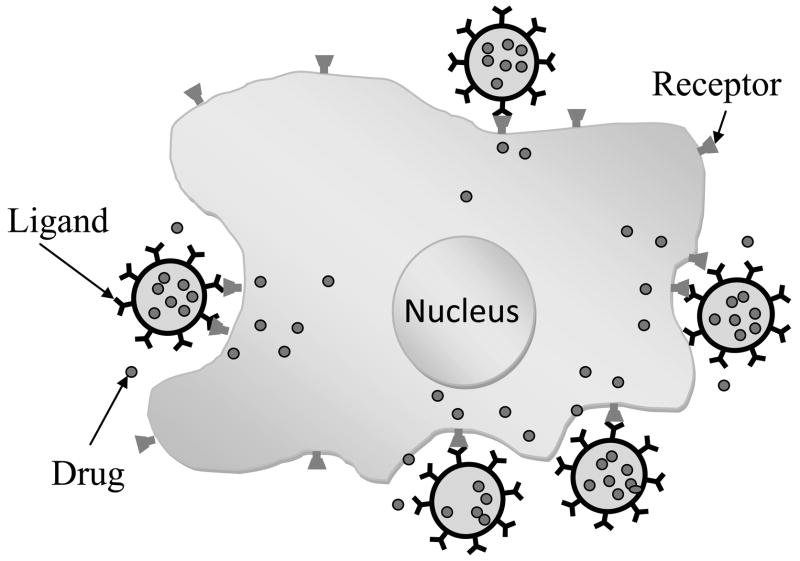
The kinetics of the release of drug from the nanoparticles to the targeted cell (see Figure 6) depends on a) the strength of the interactions between the polymer and the drug as well as the permeability of the polymer, when the drug is non-covalently encapsulated inside nanoparticles and b) the rate of hydrolysis of the covalent bond between the drug and the polymer, when the drug is covalently conjugated to the polymer [50]. Also the rate of drug release depends on the ratio of surface area to volume. With smaller particles, a large fraction of the drug is at or near the particle surface, which leads to faster release of drug but has higher risk of accumulation during dispersion, transport and storage. Larger particles might allow more drug to be encapsulated in the particle, but this can lead to slower release of the drug [51]. Drug-laden nanoparticles can be endocytosed as a whole, and this is followed by the leaking of the drug inside the cell [52]. If instead the carrier is positioned in the proximity of the diseased tissue, then passive diffusion must transport the drug from inside the particle to the cell interior. Concentration pro les for the drug released from nanoparticles depend upon the nature of the delivery system. For example, in the case of nanospheres, the drug is uniformly distributed or dissolved in the matrix and then release occurs by diffusion or erosion of the matrix. The method of incorporation of a drug into a polymer has an effect on its release pro le. The initial rapid release of a drug is mainly ascribed to the amount of drug adsorbed by the surface of nanoparticles [53]. If the nanoparticle is coated with a polymer, the release of the drug from the particle is controlled by the diffusion of the drug through the polymeric membrane. The membrane coating acts as a barrier to the drug release. Therefore, in the drug release, the solubility and diffusion of the drug in or across the polymeric membrane has to be determined. The interaction between the drug and the auxiliary ingredients also affects the rate of drug release. A variety of experimental techniques have been used to quantify drug release in vitro, including: a) side-by-side diffusion cells with artificial or biological membranes, b) equilibrium dialysis technique, c) reverse dialysis sac technique, d) ultracentrifugation, e) ultrafiltration and f) centrifugal ultrafiltration technique [54, 55]. However, these techniques are difficult to scale-up for industrial use.
Figure 6.
Schematic representation of the drug release from the nanoparticles to the targeted site.
Conclusions
The two important factors determining targeted nanocarrier anchoring on vascular endothelial cells are the geometry and the binding mechanisms between the cell surface antibodies and the nanocarrier surface antigens. The size and shape of the particle contributes greatly to its motion with in the vasculature, its margination propensity, circulation time and residence time near endothelial cells. The binding interactions control the motion of the carrier near the endothelial cell luminal surface, its attachment and detachment, its drug release characteristics and other interactions with the cell such as internalization. Together, the two parameters of carrier size and shape can be used to predict the suitability of a drug-laden nanocarrier to deliver its therapeutic cargo to a specific vascular target.
Acknowledgments
This work has been supported by NIH grant R01 EB006818.
References
- 1.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1 targeted carriers. Mol Ther. 2008;16:1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charoenphol P, Huang RB, Eniola-Adefeso O. Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials. 2010;31:1392–1402. doi: 10.1016/j.biomaterials.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Swaminathan TN, Mukundakrishnan K, Hu HH. Sedimentation of an ellipsoid inside an infinitely long tube at low and intermediate Reynolds numbers. J Fluid Mech. 2006;551:357–385. [Google Scholar]
- 5.Cai S, Vijayan K, Cheng D, Lima E, Discher D. Micelles of different morphologies - advantages of worm-like filomicelles of peo-pcl in paclitaxel delivery. Pharm Res. 2007;24:2099–2109. doi: 10.1007/s11095-007-9335-z. [DOI] [PubMed] [Google Scholar]
- 6.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target specific nanoparticles: Theory to practice. Pharm Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 7.Geng Y, Dalhaimer P, Cai S, Tsal R, Tewari M, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nano. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champion JA, Katare YK, Mitragotri S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J Control Release. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayyaswamy PS. In: Fluid Mechanics. Kundu PK, Cohen IM, editors. Academic Press; Maryland Heights, Missouri: 2008. pp. 765–840. [Google Scholar]
- 10.Sharan M, Popel AS. A two-phase model for blood flow in narrow tubes with increased viscosity near the wall. Biorheology. 2001;38:415–428. [PubMed] [Google Scholar]
- 11.Uijttewaal WS, Nijhof EJ, Bronkhorst PJ, Den Hartog E, Heethaar RM. Near-wall excess of platelets induced by lateral migration of erythrocytes in flowing blood. Am J Physiol Heart Circ Physiol. 1993;264:H1239–1244. doi: 10.1152/ajpheart.1993.264.4.H1239. [DOI] [PubMed] [Google Scholar]
- 12.Decuzzi P, Lee S, Bhushan B, Ferrari M. A theoretical model for the margination of particles within blood vessels. Ann Biomed Eng. 2005;33:179–90. doi: 10.1007/s10439-005-8976-5. [DOI] [PubMed] [Google Scholar]
- 13.Gentile F, Curcio A, Indolfi C, Ferrari M, Decuzzi P. The margination propensity of spherical particles for vascular targeting in the microcirculation. J Nanobiotechnology. 2008;6:9. doi: 10.1186/1477-3155-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckstein EC, Tilles AW, Millero FJ. Conditions for the occurrence of large near-wall excesses of small particles during blood flow. Microvasc Res. 1988;36:31–39. doi: 10.1016/0026-2862(88)90036-2. [DOI] [PubMed] [Google Scholar]
- 15.Munn LL, Melder RJ, Jain RK. Role of erythrocytes in leukocyteendothelial interactions: mathematical model and experimental validation. Biophys J. 1996;71:466–478. doi: 10.1016/S0006-3495(96)79248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mody NA, King MR. Influence of brownian motion on blood platelet flow behavior and adhesive dynamics near a planar wall. Langmuir. 2007;23:6321–6328. doi: 10.1021/la0701475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentile F, Decuzzi P. Time dependent dispersion of nanoparticles in blood vessels. J Biomedical Science and Engineering. 2010;3:517–524. [Google Scholar]
- 18.Decuzzi P, Ferrari M. Design maps for nanoparticles targeting the diseased microvasculature. Biomaterials. 2008;29:377–384. doi: 10.1016/j.biomaterials.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Weller GER, Zern B, Ayyaswamy PS, Eckmann DM, et al. Computational model for nanocarrier binding to endothelium validated using in vivo, in vitro, atomic force microscopy experiments. Proc Natl Acad Sci USA. 2010;107:16530–16535. doi: 10.1073/pnas.1006611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Agrawal NJ, Calderon AJ, Ayyaswamy PS, Eckmann DM, et al. Multivalent nature of nanocarrier binding to endothelial cells can induce flow enhanced binding behavior in shear flow. 2010. Submitted. [Google Scholar]
- 21.Muro S, Dziubla T, Qiu W, Leferovich J, Cui X, et al. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule. J Pharmacol Exp Ther. 2006;317:1161–1169. doi: 10.1124/jpet.105.098970. [DOI] [PubMed] [Google Scholar]
- 22.Calderon AJ, Bhowmich T, Leferovich J, Burman B, Pichette B, et al. Optimizing endothelial targeting by modulating the antibody density and particle concentration of anti-ICAM coated carriers. J Control Release. 2011;150:37–44. doi: 10.1016/j.jconrel.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calderon AJ, Muzykantov VR, Muro S, Eckmann DM. Flow dynamics, binding and detachment of spherical carriers targeted to ICAM-1 on endothelial cells. Biorheology. 2009;46:323–341. doi: 10.3233/BIR-2009-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 25.Potter DR, Damiano ER. The hydrodynamically relavant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res. 2008;102:770–776. doi: 10.1161/CIRCRESAHA.107.160226. [DOI] [PubMed] [Google Scholar]
- 26.Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002;283:H1282–H1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- 27.Weinbaum S, Zhang X, Han Y, Vink H, Cowin C. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci USA. 2003;100:7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agrawal NJ, Radhakrishnan R. The role of glycocalyx in nanocarrier-cell adhesion investigated using a thermodynamics model and monte carlo simulations. J Phys Chem C. 2007;111:15848–15856. doi: 10.1021/jp074514x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bell GI, Dembo M, Bongrand P. Cell adhesion. competition between nonspecific repulsion and specific bonding. Biophys J. 1984;45:1051–1064. doi: 10.1016/S0006-3495(84)84252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yago T, Zarnitsyna VI, Klopocki AG, McEver RP, Zhu C. Transport governs flow-enhanced cell tethering through l-selectin at threshold shear. Biophys J. 2007;92:330–342. doi: 10.1529/biophysj.106.090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall BT, Long M, Piper JW, Yago T, McEver RP, et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423:190–193. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- 32.Lou J, Yago T, Klopocki AG, Mehta P, Chen W, et al. Flow-enhanced adhesion regulated by a selectin interdomain hinge. J Cell Biol. 2006;174:1107–1117. doi: 10.1083/jcb.200606056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barsegov V, Thirumalai D. Dynamics of unbinding of cell adhesion molecules: Transition from catch to slip bonds. Proc Natl Acad Sci USA. 2005;102:1835–1839. doi: 10.1073/pnas.0406938102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereverzev YV, Prezhdo OV, Forero M, Sokurenko EV, Thomas WE. The two-pathway model for the catch-slip transition in biological adhesion. Biophys J. 2005;89:1446–1454. doi: 10.1529/biophysj.105.062158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beste MT, Hammer DA. Selectin catch-slip kinetics encode shear threshold adhesive behavior of rolling leukocytes. Proc Natl Acad Sci USA. 2008;105:20716–20721. doi: 10.1073/pnas.0808213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaspe J, Hagen SJ. Do protein molecules unfold in a simple shear flow? Biophys J. 2006;91:3415–3424. doi: 10.1529/biophysj.106.089367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leroux JC, Cozens R, Roesel JL, Galli B, Kubel F, et al. Pharmacokinetics of a novel HIV-1 protease inhibitor incorporated into biodegradable or enteric nanoparticles following intravenous and oral administration to mice. J Pharm Sci. 1995;84:1387–1391. doi: 10.1002/jps.2600841202. [DOI] [PubMed] [Google Scholar]
- 38.Kataoka K, Kwon GS, Yokoyama M, Okano T, Sakurai Y. Block copolymer micelles as vehicles for drug delivery. J Control Release. 1993;24:119–132. [Google Scholar]
- 39.Bochot A, Fattal E, Boutet V, Deverre J, Jeanny J, et al. Intravitreal delivery of oligonucleotides by sterically stabilized liposomes. Invest Ophthalmol Vis Sci. 2002;43:253–259. [PubMed] [Google Scholar]
- 40.Araujo L, Löbenberg R, Kreuter J. Influence of the surfactant concentration on the body distribution of nanoparticles. J Drug Target. 1999;6:373–385. doi: 10.3109/10611869908996844. [DOI] [PubMed] [Google Scholar]
- 41.Jahanshahi M, Babaei Z. Protein nanoparticle: A unique system as drug delivery vehicles. African Journal of Biotechnology. 2008;7:4926–4934. [Google Scholar]
- 42.Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 43.Uma B, Usha R. A thin conducting liquid film on a spinning disk in the presence of a magnetic field: Dynamics and stability. J Applied Mech. 2009;76:041002. [Google Scholar]
- 44.Vijayanathan V, Thomas T, Thomas TJ. DNA nanoparticles and development of DNA delivery vehicles for gene therapy. Biochemistry. 2002;41:14085–14094. doi: 10.1021/bi0203987. [DOI] [PubMed] [Google Scholar]
- 45.Kroll RA, Pagel MA, Muldoon LL, Roman-Goldstein S, Fiamengo SA, et al. Improving drug delivery to intracerebral tumor and surrounding brain in a rodent model: A comparison of osmotic versus bradykinin modification of the blood-brain and/or blood-tumor barriers. Neurosurgery. 1998;43:879–886. doi: 10.1097/00006123-199810000-00090. [DOI] [PubMed] [Google Scholar]
- 46.Eniola AO, Hammer DA. Artificial polymeric cells for targeted drug delivery. J Control Release. 2003;87:15–22. doi: 10.1016/s0168-3659(02)00346-2. [DOI] [PubMed] [Google Scholar]
- 47.Haun JB, Hammer DA. Quantifying nanoparticle adhesion mediated by specific molecular interactions. Langmuir. 2008;24:8821–8832. doi: 10.1021/la8005844. [DOI] [PubMed] [Google Scholar]
- 48.Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR., Jr The binding avidity of a nanoparticle-based multivalent targeted drug delivery platform. Chemistry and Biology. 2007;14:107–115. doi: 10.1016/j.chembiol.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Kaittanis C, Santra S, Perez JM. Emerging nanotechnology-based strategies for the identification of microbial pathogenesis. Adv Drug Deliv Rev. 2010;62:408–423. doi: 10.1016/j.addr.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 51.Redhead HM, Davis SS, Illum L. Drug delivery in poly(lactide-coglycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: in vitro characterisation and in vivo evaluation. J Control Release. 2001;70:353–363. doi: 10.1016/s0168-3659(00)00367-9. [DOI] [PubMed] [Google Scholar]
- 52.Lobler M, Rohm HW, Schmitz KP, Johnston AH, Newman TA, et al. Drug delivery by nanoparticles facing the obstacles. IFMBE Proceedings. 2009;22:2335–2338. [Google Scholar]
- 53.Magenheim B, Levy MY, Benita S. A new in vitro technique for the evaluation of drug release profile from colloidal carriers - ultrafiltration technique at low pressure. Int J Pharm. 1993;94:115–123. [Google Scholar]
- 54.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 55.Singh R, Lillard JW., Jr Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]