Abstract
The brain is considered to be an immune privileged site, because the blood-brain barrier limits entry of blood borne cells and proteins into the central nervous system (CNS). As a result, the detection and clearance of invading microorganisms and senescent cells as well as surplus neurotransmitters, aged and glycated proteins, in order to maintain a healthy environment for neuronal and glial cells, is largely confined to the innate immune system. In recent years it has become clear that many factors of innate immunity are expressed throughout the brain. Neuronal and glial cells express Toll like receptors as well as complement receptors, and virtually all complement components can be locally produced in the brain, often in response to injury or developmental cues. However, as inflammatory reactions could interfere with proper functioning of the brain, tight and fine tuned regulatory mechanisms are warranted. In age related diseases, such as Alzheimer’s disease (AD), accumulating amyloid proteins elicit complement activation and a local, chronic inflammatory response that leads to attraction and activation of glial cells that, under such activation conditions, can produce neurotoxic substances, including pro-inflammatory cytokines and oxygen radicals. This process may be exacerbated by a disturbed balance between complement activators and complement regulatory proteins such as occurs in AD, as the local synthesis of these proteins is differentially regulated by pro-inflammatory cytokines. Much knowledge about the role of complement in neurodegenerative diseases has been derived from animal studies with transgenic overexpressing or knockout mice for specific complement factors or receptors. These studies have provided insight into the potential therapeutic use of complement regulators and complement receptor antagonists in chronic neurodegenerative diseases as well as in acute conditions, such as stroke. Interestingly, recent animal studies have also indicated that complement activation products are involved in brain development and synapse formation. Not only are these findings important for the understanding of how brain development and neural network formation is organized, it may also give insights into the role of complement in processes of neurodegeneration and neuroprotection in the injured or aged and diseased adult central nervous system, and thus aid in identifying novel and specific targets for therapeutic intervention.
Keywords: Complement, brain, neurons, glia, neurodegeneration, neuroprotection
1. INTRODUCTION
1.1 Complement
Complement (C) is a major component of innate immunity, recognizing danger, as well as discriminating self from non-self (Ricklin et al., 2010). The C system is best known for its role in the recognition and killing of pathogenic microbes. Activation of the C system, which consists of over 30 soluble and cell-associated factors, can occur through three pathways, each triggered by different types of agents. All three pathways lead to the assembly of C3 convertases that, in turn, can cleave C3 resulting in formation of C3b and C3a activation products. The larger C3b fragment, a major effector molecule of the C system, acts as an opsonin and, in addition, together with other factors can assemble the C5 convertase, which enables further activation of the C cascade ultimately leading to generation of the chemotactic C5a fragment and the formation of the terminal complement complex C5b-9, also called membrane attack complex (Figure 1). Thus, when the cascade is fully activated, C leads to assembly of the membrane attack complex (C5b-9; MAC) and lysis of invading microorganisms. However, if C5b-9 production is excessive or targeted to host cells, C5b-9 can induce host cell death. On the other hand, some C activation products also facilitate the generation of adaptive immune responses (Carroll, 2004;Erdei et al., 2009), while other components contribute to the control of autoimmunity during the clearance of apoptotic cells (Sjoberg et al., 2009;Fraser et al., 2009;van Kooten et al., 2008). Interestingly, sublytic amounts of C5b-9 on host cells may cause an influx of extracellular calcium that leads to activation and/or proliferation of the cells and resistance to induction of apoptosis (Cole and Morgan, 2003), further illustrating the diverse functions of this ancient pathway. Binding of C1, a Ca2+-dependent complex of the recognition unit C1q and a tetramer of the proenzymes C1r and C1s, to an activator is the initial event in classical pathway (CP) activation of C. Activators can be immune complexes, certain microbes, apoptotic cells, and other specific protein motifs, such as amyloid in a fibrillar beta sheet structure found in the plaques in brain from Alzheimer’s disease (AD) patients.
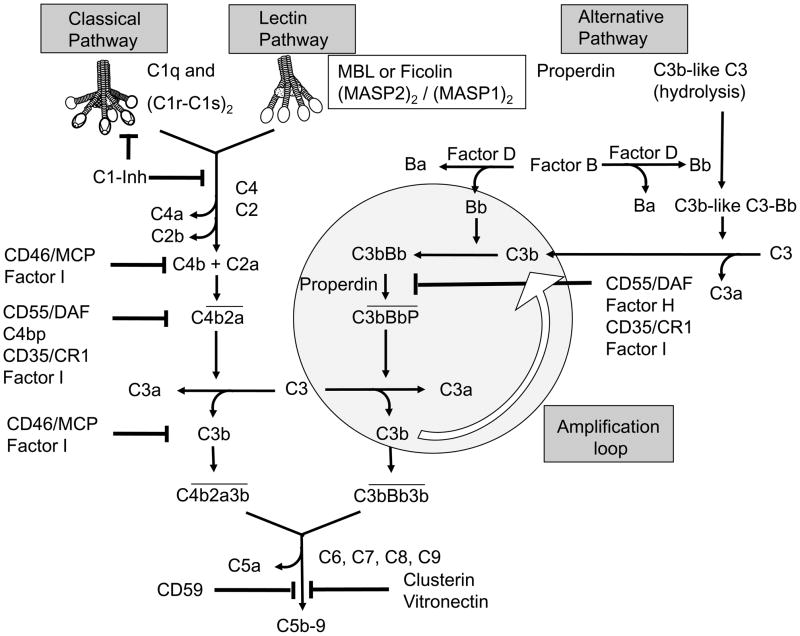
Figure 1. Complement activation and regulation.
Binding of the C1 macromolecule to the immune complexes, DNA, SAP and Aβ can initiate the CP, binding of mannose-binding lectin (MBL) or ficolins, complexed with a homodimer of MASP2, to carbohydrates (on bacterial cell walls) or attachment of spontaneously hydrolyzed C3 via active thio-ester to permissive surfaces or to properdin bound to an activating surface, generates a C3 convertase (C4b2a or C3bBb), and subsequently C5 convertases (C4b2a3b or C3bBb3b). Soluble and membrane-bound complement inhibitors regulate C activation. The soluble inhibitors C1-Inhibitor regulates activated C1, while factor I (fI) and C4b-binding protein (C4bp) control activation at the C4 and C3 level of the CP and LP, and fI together with factor H (fH) at the C3 and C5 convertase level of the AP. In addition, the membrane bound inhibitors CD35 and CD46 act as co-factors for fI, and CD55, decay accelerating factor (DAF) that accelerates the decay of C3 convertases. The fluid phase regulators vitronectin and clusterin and the membrane bound regulator CD59 can prevent formation of the C5b-9 complex on host cell membranes.
Mannose-binding lectin (MBL) and ficolins (Ficolin-1,-2 and -3) bind to mannan and other carbohydrate moieties or acetylated moieties on microorganisms or dying cells initiating C activation, through the lectin pathway (LP). MBL and ficolins share homology with C1q and, like C1q, are associated with proenzymes, MBL-associated serine proteases (MASPs).
The alternative pathway (AP) is initiated by spontaneous hydrolysis of the internal thioester within C3, resulting in C3b-like C3 (“tick-over”) or by recruitment of C3 by properdin bound to specific targets (Kemper and Hourcade, 2008). A range of microbial and also eukaryotic cell surfaces with a low sialic acid content allow AP C activation, whereas the inactivation of C3b by the C regulatory proteins (Creg) factor H (fH) and factor I (fI) is more efficient on surfaces rich in sialic acid (AUSTEN and Fearon, 1979). C3b generated by the CP or MP activation pathways can enlist the alternative pathway components, thereby amplifying the amount of downstream complement cascade events (Figure 1).
1.2 Complement in the brain
The brains of organisms with a well developed central nervous system are shielded by the blood-brain-barrier (BBB) with tight junction formations at three principal barrier sites i) the BBB formed by endothelial cells in the cerebral capillaries ii) the arachnoid barrier formed by the arachnoid multi-layered epithelium and iii) the blood-CSF barrier formed by the CSF-secreting choroid plexus epithelium. The integrity properties are further defined by BBB-associated cells including pericytes and astrocytes. Together these brain barriers efficiently prevent infiltration of circulating immune cells, such as B- and T lymphocytes, and minimize influx of plasma proteins as well as neuroexcitatory and neurotoxic substances from the blood (reviewed in (Abbott et al., 2010)). The functions of immune surveillance and differentiation between “self” and “nonself” in non-CNS tissue, provided by neutrophils, dendritic cells, macrophages and natural killer cells in the periphery, are in the CNS attributed to resident glial cells including astrocytes, microglia, oligodendrocytes, and NG2 chondroitin sulphate (NG2) and platelet-derived growth factor-α receptor (PDGFα) positive oligodendrocyte precursor cells (NG2+PDGFα+ OPCs) ((Butt et al., 2005) and reviewed in (Dong and Benveniste, 2001;Griffiths et al., 2009)). Much of our understanding of the occurrence and role of complement in the CNS derives from studies into the pathogenic mechanisms involved in various diseases affecting the brain. In a variety of CNS diseases, including bacterial meningitis, transmissible spongiform encephalopathies (TSE; prion disease), stroke and in more chronic conditions such as multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), Huntington’s (HD) and Parkinson’s disease (PD), AD, as well as age-related macular degeneration (AMD), C contributes to the inflammatory process (Woodruff et al., 2008;Bonifati and Kishore, 2007). In this review we discuss the findings that have led to insight on the role of C in acute and chronic brain disorders and, importantly, its role in the normal homeostasis and brain functioning, as more recent studies (Stevens et al., 2007) indicate that C is also involved in brain development and synapse pruning.
2. Local Synthesis of Complement and Complement Regulators in the Brain
The liver is the main source of C proteins, but although the BBB is not absolute and some macromolecules can by-pass the barriers by use of extracellular routes (Broadwell and Sofroniew, 1993) most C proteins are unlikely to penetrate the brain parenchyma unless the BBB integrity is disrupted. Therefore, local synthesis of C components by resident cells in the brain is crucial to appropriate functions of the local defense system. Local synthesis of C proteins in the brain was suggested after identification of C in human brain tissue. In situ hybridization studies (Lampert-Etchells et al., 1993;Rozovsky et al., 1994;Veerhuis et al., 1998) confirmed that C factors are all locally produced and that the presence of C is not merely due to leakage of plasma proteins because of BBB damage.
2.1 Glial cells
Astrocytes are the most numerous cell type in the human brain and are dedicated to various functions such as regulation of synaptogenesis, metabolic support and control of the homeostatic system including regulation of extracellular ion concentrations and neurotransmitters, especially glutamate, and regulation of brain water homeostasis (Verkhratsky and Parpura, 2010). Microglia, the macrophages of the brain, are scattered throughout the brain tissue at a density of about 6×106 cells per mm3 and, when in a “resting” state are highly dynamic and estimated to completely scan the brain parenchyma once every few hours (Nimmerjahn et al., 2005). Microglia as well as astrocytes are considered CNS immune effector cells and are able to produce cytokines and chemokines as well as to phagocytose up targets upon stimulation (Dong and Benveniste, 2001;Nielsen et al., 2010;Familian et al., 2007;Nielsen et al., 2009;Fraser et al., 2010;Bohlson et al., 2007;Yang et al., 2010;Watabe et al., 1989). Another group of macroglial cells are the oligodendrocytes, which are responsible for myelinating axons in the CNS.
More than two decades ago Levi-Strauss and Mallat reported that primary cultures of murine astrocytes were capable of producing C components of the AP (Levi-Strauss and Mallat, 1987). Extensive research during the 1990’s, initially performed in several human astrocyte-derived tumor cell lines (118MG, T193, T98G), demonstrated the expression of components of the CP and also of terminal C components (Gasque et al., 1995;Gasque et al., 1993) by human glial cells. In further studies with cell lines and also primary cells isolated from mouse and adult human brain (Walker and McGeer, 1992;Veerhuis et al., 1999;Walker et al., 1995;Veerhuis et al., 1998) convincing evidence was obtained for local synthesis of most C components of both the classical and alternative pathway, including Cregs, by human astrocytes and microglia (Table 1). What cell type that is responsible for local production of fluid-phase C inhibitor C4b binding protein (C4bp) has yet to be determined in the brain as detectable levels of secreted C4bp appeared to be absent in cultures of primary human astrocytes as well as several cell lines (Trouw et al., 2008). Further, results from in vitro studies on primary human microglia and astrocytes suggest that synthesis of several C components, C1 subcomponents C1s and C1r, C3, C4 and C1-inh can be modulated by various factors like pro-inflammatory cytokines but as well as by the AD-related amyloid-β peptide (Aβ) perhaps via TLR stimulation (Veerhuis et al., 1999). The same study, in support of earlier investigations, also suggested that microglia, but not astrocytes, are a significant source of locally secreted C1q in the brain (Lampert-Etchells et al., 1993;Veerhuis et al., 1999).
Table 1.
Expression of C proteins by human astrocytes and microglia1
| Complement Components
|
||||||
|---|---|---|---|---|---|---|
| Cell Type | Classical | Alternative | Terminal | C-receptors | C-regulators | |
| Soluble | Membrane-bound | |||||
| Astrocytes | C1q,C1r, C1s C2, C3, C4 | C3, fB, fD | C5, C6, C7, C8, | C1qR, CR2, C3aR, C5aR | C1-inh, fH, fI, clusterin | CD59, DAF, MCP, CR1 |
| Microglia | C1q, C1r, C1s,C2, C3, C4 | C3 | C1qR, CR3, C3aR, CR4, C5aR, | C1-inh | CD59, CR1 | |
C components in the brain are inducible, most notably in re sponse to injury. Recently reviewed in (Woodruff et al., 2010).
Results from initial studies on rodent oligodendrocytes suggested that these cells are vulnerable to C lysis due to a deficiency of C inhibitor expression (Wren and Noble, 1989;Piddlesden et al., 1994). Indeed, oligodendrocytes are susceptible to C attack which is particularly evident in multiple sclerosis (MS) (Schwab and McGeer, 2002). In one study, adult primary human oligodendrocyte cultures were found to produce only a limited Creg repertoire, which suggests that a relative deficiency in Creg expression may render oligodendrocytes sensitive to C damage in MS (Scolding et al., 1998). Interestingly, oligodendrocytes seem to not only be susceptible to C attack but also to themselves be a source of a large number of C proteins including C1q, C1s, C4, C2, C3, C5, C6, C7, C8 and C9 (Hosokawa et al., 2003). Little is known about C expression in various other cells in the CNS. However, initial studies suggest that primary human pericytes in vitro produce C1q (Verbeek et al., 1999) and that cultured endothelial cells from human brain microvessels produce soluble regulators fH and C1-inh and components of both the classical (C4), and the alternative (fB) complement pathway (Vastag et al., 1998). Ependymal cells, ciliated epithelial cells that line the lumen of the brain ventricular system, express Cregs CD59 and at a low level CD55, but no CD46 or CD35, however in inflammatory conditions (meningitis) CD46 and CD35 are highly expressed on epithelial cells of the ependymal lining, as well as in the choroid plexus (Canova et al., 2006). To what extent these cell types contribute to the levels of C factors in the brain parenchyma and of the CSF is unknown. Thus, future studies extending the knowledge on C expression in various cells of the CNS are warranted.
2.2 Neurons
Neuronal cells have long been considered innocent victims of C activation in neurodegenerative conditions, as a result of activation of C factors that had passed the BBB or that had been synthesized by activated glial cells. Robust activated complement system with C5b-9 insertion can lead to lysis and death of a targeted cell, a process which can be prevented by appropriate expression of complement regulators. However, neuronal cells were also found to be capable of de novo synthesis of complement factors both in vivo and in vitro. Neuronal mRNA expression of C1q, C2, C3, C4, C5, C6, C7, C8 and C9 was minimally detected using in situ hybridization in the temporal cortex and hippocampus in post mortem control brain tissue, with increased expression in AD tissue. The strongest signals were recorded over pyramidal neurons (Shen et al., 1997). Neuronal expression of C1-Inh was detectable in brain tissue from postmortem AD and control subjects as well as in the neuroblastoma cell line SK-N-SH (Veerhuis et al., 1998). In vitro expression of C1-Inh could only be upregulated by treatment with interferon γ (Veerhuis et al., 1998;Veerhuis et al., 1999), whereas expression of the fluid-phase regulators, MCP and CD59 in human neuroblastoma cell lines could be modulated by treatment with pro-inflammatory cytokines (Gasque et al., 1996). Extending those in vitro studies, Fontaine and colleagues showed that the above mentioned neuroblastoma cell lines and the human neuroblastoma cell lines SH-SY5Y and KELLY were able to express a complete set of C proteins and further suggested that the rate of synthesis was cell differentiation-dependent (Thomas et al., 2000). Interestingly, primary fetal human neurons in vitro were shown to spontaneously and independent of antibody activate the CP, possibly by expressing a molecule with affinity for C1q, leading to assembly of the cytolytic C5b-9 on their membranes. Limited neuronal expression of Cregs MCP and CD59, and lack of DAF and CR1 expression was suggested to underlie this vulnerability to complement damage (Singhrao et al., 2000). CD59 has been shown crucial to protection of for example NT2-N neurons (human NT2 cell line differentiated into post-mitotic neurons) against C attacks (Pedersen et al., 2007) and current strategies aiming at increasing neuronal protection against C include attempts of upregulation of Cregs like CD59. For example, the CD59 expression-regulating neural-restrictive silencer factor (REST) protected neurons from C-mediated lysis by a five-fold upregulation of CD59 expression in neuronal cultures (Kolev et al., 2010). Whether modulation of neuronal production of Cregs is a successful neuroprotective strategy remains to be elucidated as recent in vivo studies suggest that C activation products, including the anaphylatoxins C3a and C5a and sublytic levels of the MAC, may in fact have several neuroprotective functions ((Osaka et al., 1999;Tocco et al., 1997;O'Barr et al., 2001;Van Beek et al., 2003) and reviewed in (Woodruff et al., 2010).
3. The role of complement in normal CNS
Similar to other proteins that are part of the immune system, such as proinflammatory cytokines (e.g., TNFα, IL-6) and proteins of the adaptive immune system (e.g. major histocompatibility complex class I [MHCI] molecules and MHCI-binding immunoreceptors and their components (e.g., PIRB, Ly49, DAP12, CD3ζ) (for a review see (Boulanger, 2009)), C factors are now thought to also have nonimmune functions in the brain. Complement proteins were found to promote proliferation and regeneration in various tissues (reviewed in (Ricklin et al., 2010)) and may exert similar functions in the CNS, as neuronal stem cells differentiate and migrate in response to C. C3a-C3aR interactions were found to be a positive regulator of adult neurogenesis (Bogestal et al., 2007;Shinjyo et al., 2009).
Recent studies have also shown that C activation products can modulate synapse formation during brain development (Stevens et al., 2007;Chu et al., 2010). Neurons isolated from the developing eye were found to express high levels of C1q mRNA. Using C1q and C3 knock-out mice, it was shown that whereas relay neurons in wild type (wt) mice are innervated by one or two axons, relay neurons in C deficient (C1q −/−, C3 −/−) mice have four or more functional inputs. This lead to the conclusion that C1q and C3 may tag synapses for elimination, leading to remodelling of synaptic connections in the developing visual system (Stevens et al., 2007). In a subsequent study, a complete genetic deficiency of C1q resulted in enhanced circuitry that led to epileptogenesis in mouse models (Chu et al., 2010). C1q, both alone and in conjunction with C3, can facilitate microglial clearance of misfolded proteins, apoptotic neurons and damaged cells such as neuronal blebs (Fraser et al., 2010;Trouw et al., 2008) and modulate cytokine profiles to subdue potentially neurotoxic inflammatory gene expression (Fraser et al., 2010). Thus, depending on the timing and local environment, the C cascade can facilitate proper neuronal development or accelerate chronic inflammatory response contributing to neurodegeneration (see below).
Proteins related to C1q, cerebellins (Cbln) (Yuzaki, 2010) and C1ql (Bolliger et al., 2011) have been found to be expressed in the cerebellum, as well as other brain regions of developing and mature brain. Cbln members may serve as regulators of synapse development and synaptic plasticity through regulation of the post synaptic endocytosis pathway of AMPA receptors (Yuzaki, 2008), and C1ql proteins have recently been shown to interact with a neuronal surface receptor BAI3 also involved in regulation of synapse formation and/or maintenance (Bolliger et al., 2011). As C1q is expressed by neurons in the hippocampus and temporal cortex ((Afagh et al., 1996;Rozovsky et al., 1994) and Veerhuis, unpublished, and reviewed in (Alexander et al., 2008)) of injured brain, it is tempting to speculate that C1q in the neocortex may serve a function similar to that of the Cbln proteins in the cerebellum. In addition, in vitro C1q enhances neuronal survival and is neuroprotective in response to certain toxic agents, such as fibrillar amyloid and serum amyloid P (Pisalyaput and Tenner, 2008). Whether these BAI3-C1ql interactions are influenced by C1q itself (which has been shown to influence neuron survival and neurite outgrowth in vitro (Benoit and Tenner, 2011;Pisalyaput and Tenner, 2008)) remains to be seen. Interestingly, half of more than 50 genes encoding putative Cregs predicted in the mouse genome, are expressed in the CNS, consistent with at least some of the uncharacterized C control protein domain (CCP)-bearing proteins in mammals may be involved in synapse organization (Gendrel et al., 2009).
4. Complement during acute brain injury
Acute brain injuries including infections, brain trauma, ischemic and hemorrhagic stroke and subsequent reperfusion injuries are to date associated with a limited repertoire of effective treatments and high morbidity. Neurodegeneration and death in these acute conditions can be via necrosis or via apoptosis. For example, apoptosis was recently shown to be dominant in the peri-infarct area after ischemic stroke in humans (Sairanen et al., 2006). Most likely neuronal death following acute conditions occurs via a combination of both necrosis and apoptosis.
4.1 Brain infections
Various pathogens including bacteria, virus and fungi can invade the CNS and cause life- threatening diseases. In the immune privileged brain C functionality can be crucial to fight off and kill invading microbes. However, C activation and regulation needs to be delicately balanced as excessive C activation might be detrimental to bystander cells. Intriguingly, several CNS invading microorganisms have developed mechanisms to avoid the destructive actions of C and in fact even to use C to their advantage. One of these mechanisms is mimicry of human Cregs which enables control and down-regulation of C activation against invading microbes (Cooper and Nemerow, 1989). Further strategies to circumvent C include the use of membrane-bound C receptors and Cregs to enter the host cell and acquisition of Cregs during budding from the membranes of the host cell or by binding to soluble Cregs (reviewed in (Speth et al., 2002)). For example the meningitis causing bacteria Neisseria meningitidis invades the CNS through the nasopharyngeal mucosa and uses the membrane bound Creg CD46 which interacts with bacterial pili, to cross the blood-brain-barrier (Johansson et al., 2003). Also, gram-negative Escherichia coli K1 avoids C killing by binding to C4bp and promoting degradation of C3b and C4b (Wooster et al., 2006). Similar to bacteria, several virus strains have developed protective strategies to avoid C (recently reviewed in (Stoermer and Morrison, 2011)) . The herpes virus Epstein-Barr (EBV), which can cause encephalitis and aseptic meningitis, uses CR2 for viral entry by binding to the receptor at the same location as the C3 fragment C3dg (Carel et al., 1989). HIV-1, detectable in the brains of >85% patients who died with AIDS (Johnson et al., 1996), acquires Cregs CD46, CD55 and CD59 upon budding from the host cell (Frank et al., 1996) and binding to soluble Creg fH (Stoiber et al., 1995) thereby avoiding C mediated lysis of the virion particles. In addition, recent studies indicate that CNS invading fungi also have developed C evasion mechanisms (reviewed in Speth and colleagues (Speth et al., 2008)). Although resident brain cells including astrocytes, neurons, oligodendrocytes, but to a lesser extent microglia, produce highly increased levels of C1q, C4 and C3 in response to fungus infection, as in the case of cerebral aspergillosis, fungal hyphae can limit surface deposition of C3 and thereby interfere with C-mediated phagocytosis of this pathogen (Rambach et al., 2008;Speth et al., 2008). Taken together, these examples illustrate that C plays various roles in brain infections and that the C evasion strategies by microbial pathogens invading the CNS may be a target for therapeutic intervention.
4.2 Trauma, stroke and reperfusion injuries
The diverse roles played by the C system in acute brain disorders are not fully elucidated, however a growing body of evidence suggests an important role in secondary brain damage (Stahel et al., 1998). During some conditions of acute brain damage the BBB integrity is disrupted allowing influx of plasma proteins, including C proteins, and immune cells from the periphery, whereas in others CNS injuries C synthesis is induced by CNS insults (including oxidative stress). Activation of the CP in human brain following traumatic brain injury has been shown by increased immunoreactivity for C1q, C3b, C3d and C5b-9in the immediate vicinity of neurons in the penumbra area of the cerebral contusion (Bellander et al., 2001). Further, C3 mRNA and upregulation of clusterin was found in the penumbra, indicating local de novo synthesis of C and Cregs following injury. The authors suggested that an unknown component, possibly in the debris from injured neurons or myelin breakdown products, might be able to trigger C activation and formation of the following brain contusions. Investigation of brain tissue of patients with acute brain ischaemia or ischaemic stroke further revealed deposition of C1q, C3c and C4d in all ischaemic lesions, further supporting activation of the CP. In necrotic zones of the brains from the same patients, C9, C-reactive protein and IgM were found. The possibility of uncontrolled C activation following ischaemic insults, which might be harmful was underlined by the findings of virtually absent CD59 and CD55 in ischaemic lesions (Pedersen et al., 2009). Consistent with a detrimental role of a fully activated C cascade, in an animal model of traumatic brain injury the C5a receptor antagonist (CD88-specific) was shown to reduce disease activity (Sewell et al., 2004) suggesting that the generation of the chemotactic C5a activation fragment contributes to the detrimental consequences of C activation in this model.
4.3 Role of C5a in Neuroinflammation
The role of the activation fragment C5a in the brain has recently been reviewed (Woodruff et al., 2010) and thus will not be extensively discussed here. However, it should be noted that seemingly contradictory results of the influence of the C component C5 on inflammation have been reported. In contrast to the detrimental effect of C5a mentioned above in the traumatic brain injury models (and below in chronic neurodegenerative disorders), C5a, when given with kainic acid intraventricularly or 24 hours prior to glutamate treatment in neuronal mouse cultures, was shown to be neuroprotective against glutamate mediated caspase-3 activation (Osaka et al., 1999). It was subsequently hypothesized that the C5a mediated protection may be dependent on the modulation of Ca2+ and MAP-kinase activity (Mukherjee and Pasinetti, 2000;Mukherjee and Pasinetti, 2001). In other systems, C5a, as well as C3a, provided direct neuroprotection (Van Beek et al., 2001;Mukherjee and Pasinetti, 2001;O'Barr et al., 2001); however, these were cell lines and/or neurons perhaps at different stages of maturation which may align with the studies of Fontaine and colleagues in newborn rat brain. These researchers demonstrate that in the developing cerebellar cortex brain, C5aR stimulation triggered increased BrdU incorporation by granule neurons, and a C3aR agonist promoted migration of cells to their proper location (Jauneau et al., 2006;Benard et al., 2004;Benard et al., 2008). However, since defects in cerebellum have not been reported in C3, C3aR, C5 or C5aR deficient animals, further study will be necessary to determine if these systems are redundant, residual or involved in facilitating survival and development during infection. The underlying basis for the differences in outcome due to C5a/C3a engagement of their receptors are likely different differentiation states of the cells and/or the cell signaling resulting from mixed cell interaction. Another example of the complexity of these responses is the report that C5a (but not C3a) upregulates expression of microglial (but not astrocyte) glutamate receptor (GLT-1) which should provide increased glutamate uptake and thus protect neurons in the environment against glutamate toxicity (Humayun et al., 2009). Since there are two C5aR (CD88 and C5L2) and there are suggestions that these receptors may “cooperate” with other receptors (reviewed in (Klos et al., 2009), it is possible that a diverse, but precise, set of responses to a changing environment could be orchestrated depending on the repertoire of interacting receptors available in the sensing cell. Clearly a systematic approach using carefully characterized reagents with defined cells and differentiation states is needed to clarify these pathways and identify potential targets for therapeutic interventions.
5. Complement during chronic conditions of brain injury
Substantial advances in understanding the effects of C in the brain comes from research in neurodegenerative diseases (ND) and subsequent studies with animal models for ND including C knock outs or genetically manipulated animals over expressing certain C factors bred to AD mouse models. Here we will focus on only a few diseases that demonstrate some of the mechanisms of disease acceleration and begin to provide insight on potential therapeutic targets. Recent reviews of the pathogenesis of age related macular degeneration (Charbel et al., 2010), as well as contributions of C to systemic lupus erythematosus and spinal cord injury (Alexander et al., 2008), and prion disease (Veerhuis et al., 2005) provide additional examples of a substantial role of C in neurodegenerative disease.
5.1 Complement in AD
Characteristic neuropathological changes seen in AD brain include synaptic and neuronal loss, neurofibrillary tangles (NFTs), extracellular senile plaques composed of amyloid (Aβ) protein deposits and evidence of inflammatory events (Querfurth and LaFerla, 2010). The relative contributions of these pathological markers to the cognitive dysfunction in AD remains controversial, but results from studies in both AD patients and transgenic mouse models of AD make it likely that multiple, overlapping processes contribute to the ultimate cognitive loss in this disorder. Evidence of neuroinflammation as a substantial component in the development of AD has been accumulating since the 1990’s and immune activation in the brain has been identified as a potential target for therapeutic intervention ((Craft et al., 2006;Hu et al., 2007) and reviewed in (Shaftel et al., 2008;Eikelenboom et al., 2011)), although the presence of beneficial as well as detrimental effects requires care in selection of targets (Lucin and Wyss-Coray, 2009;Gasparini et al., 2005).
The association of C factors with amyloid deposits in Alzheimer’s disease (AD) was first described in immunohistochemical studies in the early ‘80s (Eikelenboom and Stam, 1982;Ishii and Haga, 1984). The development of monoclonal antibodies improved the specific detection of C activation products and the use of component specific knockout mice to validate antibodies used in mouse models of neurodegeneration further strengthened the validity of the reports of C component association with fibrillar amyloid containing plaques, thus providing stronger evidence that amyloid plaques do activate complement in vivo, and suggesting a role for C in AD pathophysiology (Eikelenboom et al., 1989;Fonseca et al., 2004b;Rogers et al., 1992;McGeer et al., 1989).
Interestingly, when different neuropathological Braak stages, representing different stages of disease progression (from control to severe AD), are compared, C factors C1q, C4d and C3d were found in early AD stages in plaques, but later C factors such as C5b-9 were absent or much less prominent. In later AD stages along with more prominent immunostaining for C1q, C4d and C3d, and some C5b-9 is seen in neuritic plaques and on neurofibrillary tangles (NFT) (Fonseca et al., 2004a;Veerhuis et al., 2003;Webster et al., 1997;Zanjani et al., 2005;Veerhuis et al., 1995), suggesting a major role for CP activation and C3. In a post mortem study comparing young, middle aged and old Down syndrome (DS) cases, as a temporal model for studying the development of AD, similar results were obtained as in AD brain (Head et al., 2001;Stoltzner et al., 2000). The observed prominent presence of earlier activation products and relative absence of C5b-9 (Stoltzner et al., 2000;Zhan et al., 1995;Eikelenboom and Veerhuis, 1996;Zanjani et al., 2005) is in line with the results from a mouse study comparing APP23 Tg mice and wild type mice (Reichwald et al., 2009), and with in vitro data, showing lower than expected levels of C5b-9 upon activation of the C cascade by Aβ (Cadman and Puttfarcken, 1997). Alternatively, the C5b-9 may be cleared since it associates either with membranes, clusterin or vitronectin (“S Protein”) (Itagaki et al., 1994;McGeer et al., 1992;Verbeek et al., 1998) rather than becoming covalently linked to the more long lived plaque as occurs with C4b/d and C3b/d.
Strong immunostaining for C1q and C activation products C4b/c/d and C3b/c/d is observed in the majority of highly fibrillar, dense-cored and primitive neuritic plaques in the temporal cortex of AD cases (Loeffler et al., 2008;Veerhuis et al., 1996) and in mouse models of AD and/or neurodegeneration (Fan et al., 2007;Zhou et al., 2008;Loeffler et al., 2008) (Figure 2). In contrast, the C1 subcomponents C1r and C1s are only occasionally observed in neuritic plaques in AD (Veerhuis et al., 1996), undoubtedly due to their dissociation from the activator-bound C1q by C1-Inh (Ziccardi and Cooper, 1979). Some positive C immunostaining has been seen in thioflavine-negative, cognitively normal brains, (Lue et al., 2001;Zanjani et al., 2005) but to a far lesser extent (Zhan et al., 1995). Further indications that C1q binding to Aβ depends on the degree of Aβ fibril formation, came from an immunohistochemical study in a preclinical familial AD case with only diffuse Aβ plaques, where in contrast to advanced AD cases, immunostaining for C1q was only seen in neurons (Fonseca et al., 2004a). In vitro studies in which interactions of purified C1q and synthetic Aβ peptides were investigated (Tacnet-Delorme et al., 2001;Snyder et al., 1994;Velazquez et al., 1997), validated the activation of C by beta sheet amyloid fibrils and identified candidate amino acids on both the amyloid and the C1q molecule that are involved in the interaction (Velazquez et al., 1997;Jiang et al., 1994;Tacnet-Delorme et al., 2001).
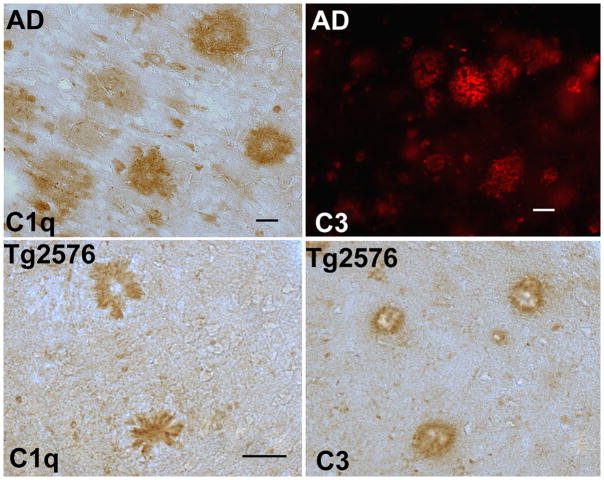
Figure 2. Complement proteins C1q and C3 are associated with plaque structures in human AD and in transgenic mouse models of AD.
C1q immunostaining (brown) in hippocampus of an AD case (90 years old) (top left) and in cortex of 20 mo Tg2576 (bottom left) using anti human (Dako) and anti mouse (1151) C1q antibodies respectively. Activated C3 immunostaining in frontal cortex of an 68 year old AD case (Dako, red, top right) and in cortex of an 18m Tg2576 (brown, bottom right) using an anti human C3d and an anti mouse C3b/iC3b/C3c (2/11, Hycult) antibody respectively. Scale bar: 50 um. Photomicrographs courtesy of Dr. M.I. Fonseca, UC, Irvine.
Predominantly CP C activation products were found to co-localize with most cerebral Aβ deposits in AD brain, as well as extracellular neuronal tangles, although AP components have been found associated with amyloid plaques in both human AD ((Strohmeyer et al., 2000) and reviewed in (Veerhuis, 2011)) and in murine models of AD (Fonseca et al., 2011). Additional in vitro studies have shown that Aβ can activate C via the AP pathway ((Bradt et al., 1998), reviewed in (Alexander et al., 2008;Veerhuis, 2011)). In addition, in C1q−/− AD mouse models, while there was essentially no CP deposition, cleaved C3 products and properdin were prominently present on the fibrillar amyloid plaques (Zhou et al., 2008;Fonseca et al., 2011).
The observed presence of CP products up to iC3b, and limited further C activation (C5b-9 and AP amplification loop) seen in AD, could be due to the presence of Cregs fH (Strohmeyer et al., 2002) and C4bp (Trouw et al., 2008;Zhan et al., 1995) that accumulate in Aβ deposits associated with C activation and covalently bound C4b and C3b. Factor H and C4bp enhance the conversion of C3b into iC3b, thereby preventing further C activation and enhancing Aβ uptake by microglia via CR3 and CR4 (Sjoberg et al., 2009;Strohmeyer et al., 2002). Clusters of activated microglia that express the β2-integrin C receptors CR3 and CR4, can be found surrounding fibrillar amyloid plaques (Rozemuller et al., 1989;Akiyama and McGeer, 1990;Kobayashi et al., 1998) suggesting that these phagocytes may be trying to ingest complement-tagged plaque material.
However, clearly the role of the C system in AD pathogenesis and progression is complex, as in animal models both C-dependent detrimental and protective effects have been observed. When AD mouse models were made C3 deficient or overexpressing Crry, pathology was enhanced relative to the C3 sufficient mice or to mice with normal levels of Crry suggesting a protective contribution of C3 (Wyss-Coray et al., 2002;Maier et al., 2008). Enhanced pathology in these mice was likely due to the loss of the opsonic effect of C3b for amyloid and/or cellular debris. This protective role of early components of C is also consistent with the recent report demonstrating a correlation between the induction of C1q and C3 and the suppression of Aβ deposition in the TgCRND8 AD mouse model (Chakrabarty et al., 2010). However, deletion of C1q in the Tg2576 and APPPS1 models of AD suggested a detrimental role for C activation since the Tg2576C1q−/− and APPPS1C1q−/− mice showed less reactive glia surrounding plaques and increased synaptophysin than the C1q-sufficient Tg2576 or APPPS1 (Fonseca et al., 2004b). The protection given by the lack of C1q was substantial (~50%) but not complete, suggesting that the AP and/or other non C mediated events contribute to the inflammatory reaction around the plaques. Consistent with a role for C in contributing to the rate of progression of the disease, the development of pathology was accelerated in the 3×Tg AD mouse model on BUB background (a strain with higher serum hemolytic activity in vitro) (Fonseca et al., 2011) .
Perhaps the most compelling evidence that cleavage of C5 plays a substantial detrimental role in AD progression was the decrease in pathology and the suppression of behavioral deficits in AD mice treated with a C5a receptor antagonist, PMX205 (Fonseca et al., 2009). It has been demonstrated that receptors for C5a are expressed in the brain (recently reviewed in (Klos et al., 2009)) and that CNS cells do respond to C5a (Sayah et al., 2003). The genetic deficiency of C5 has been shown to be one of a limited number of genetic differences that are associated with decreased amyloid deposition in DBA/2J mice vs. C57Bl6 mice transgenic for the human APP gene (Ryman et al., 2008) and more recently, the AD mouse model 3×Tg was shown to lack pathology when crossed onto the C5-deficient FVB strain for 6 generations (Morrissette, 2009). Recent studies have demonstrated that C5a-C5aR signaling synergizes with other receptor signaling, including TLR (Zhang et al., 2007) and P2Y6 (Flaherty et al., 2008), in multiple tissues including the brain. Some of these receptors, specifically TLR2 and TLR4 (Jana et al., 2008;Jin et al., 2008;Udan et al., 2008) have been shown to mediate detrimental effects of β-amyloid. Thus, in addition to recruiting glia to the site of plaque deposition, inflammation generated in response to Aβ interactions (or other proinflammatory signals) with receptors on C5a-recruited glial cells (Tahara et al., 2006) may be significantly enhanced by the binding of C5a to C5aR, thus accelerating pathology and/or neuronal dysfunction. Thus, developing inhibitors of C5a activation of myeloid cells, such as receptor antagonists of C5a, should be further investigated as a therapeutic strategy in human AD as this would specifically inhibit the detrimental consequences of complete activation of the C cascade but leave the beneficial effects of C1q and C3 intact.
Finally, recent reports of the potential polymorphisms in CR1 and clusterin associated with human AD also suggests a point of control of C activation (Lambert et al., 2009). While CR1 is a critical regulator of C3 convertase activity in humans, it is expressed predominantly in the periphery. Clusterin (Apo J) is a soluble inhibitor highly expressed in brain. How these regulators influence disease progression remains to be investigated.
5.2 Complement in other dementias and neurodegenerative diseases
Although in Parkinson’s disease (PD) C activation was described to be associated with Lewy bodies (the intraneuronal inclusion bodies consisting of aggregates of α-synuclein) as well as axonal spheroids in the substantia nigra (Yamada et al., 1992), no indications for C activation were found in another study, investigating cortical Lewy bodies in the cingulated gyrus (Rozemuller et al., 2000). Whether this is due to region specific forms of α-synuclein, or to regional differences in expression of C proteins and Cregs, remains to be determined. In Pick’s disease neuronal inclusions can be found in the frontal and temporal cortex. Pick’s bodies consisting of filaments of tau protein and can evoke inflammatory reactions and C activation. Complement activation products including C1q, C4, C2, C3, C5, C6, C8, but not C9 or C5b-9 were seen co-localized with astrocytes, cytoplasmic ballooned neurons and Pick bodies. Fluid phase Cregs vitronectin and clusterin, as well as the membrane bound regulatory protein CD59, but not other Cregs as CR1, DAF and MCP, were also found at these sites, suggesting sufficient protection to TCC mediated cell lysis. The intracellular localization of many C factors in ballooned neurons and the Pick bodies, was suggested to be caused by the internalization C targeted cell membranes (Singhrao et al., 1996). While animal models of these diseases are far from perfect, the reports of disease reduction (pathology and in some cases behavior) by treatment with C5a receptor antagonist of models of amyotrophic lateral sclerosis (ALS) (Woodruff et al., 2008) and Huntington-like neurodegeneration (Woodruff et al., 2006) warrant further investigation.
In other neurodegenerative diseases such as in familial British and Danish chromosome 13 dementia cases, termed ABri and ADan respectively, amyloid deposits were immunopositive for CP activation products C1q, C4d and also C5b-9 (Rostagno et al., 2002). When aggregated synthetic ABri and ADan, the peptides that form cerebral amyloid deposits in chromosome 13 dementia, were incubated with human serum, sC5b-9 was generated of which 25% could be attributed to AP activation (Rostagno et al., 2002). Taken together these findings suggest that C activation can be a general reaction to a number of proteins of different etiology that form highly fibrillar aggregates with specific motifs that interact with and activate the C cascade (Velazquez et al., 1997). Whether the neurtoxicity is the direct (primary) result of the aggregates or the result from the secretion of neurotoxic factors by glial cells activated by the fibrillar protein deposits or both, remains to be determined.
5.3 Multiple sclerosis
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system (CNS), resulting in progressive loss of motor and sensory function. Focal areas (lesions or plaques) of myelin and partial axonal loss within the CNS parenchyma are hallmarks of MS (Bo et al., 2003). Deposition of C and IgG in white matter MS lesions was reported by a number of groups. While the diffuse presence of C activation products probably results from leakage through a damaged BBB (Gay and Esiri, 1991), C activation products (C1q, C3d, and C5b-9) and IgG are also detectable in capillary walls in active MS lesions, and, although less consistently, on myelin sheaths (Lumsden, 1971) and on degraded myelin as well as in microglia /macrophages containing myelin (Compston et al., 1989;Brink et al., 2005;Barnett et al., 2009;Storch et al., 1998), all of which is consistent with the possible involvement of C in myelin degradation in MS. Based on observed differences in occurrence of C deposition between cases, a classification with 4 pathological subtypes of MS was proposed (Lucchinetti et al., 2000). However in subsequent studies, the heterogeneous presence of IgG and of C activation products was found to be due to different stages in evolution of lesions within cases, rather than heterogeneity between cases (Barnett et al., 2009;Breij et al., 2008;Barnett and Prineas, 2004).
C activation in MS probably is lesion and location dependent. In viturally all white matter lesions C3d and C4d were prominently present on myelin sheath and C3d, C1q and C5b-9 are on disrupted myelin and in macrophage / microglia, astrocytes and vessel walls (Compston et al., 1989;Brink et al., 2005;Prineas et al., 2001;Breij et al., 2008). C3d and C4d probably are covalently bound to the myelin, in contrast to other factors that are rapidly turned over, which may explain the inability to detect C1q and C5b-9 on myelin sheaths in many studies (Prineas et al., 2001;Brink et al., 2005;Compston et al., 1989;Breij et al., 2008). In mixed white and grey matter lesions much less frequent C activation was seen, with C3d and C4d on myelin sheaths on the border of the lesions, and C3d in blood vessel walls only. Moreover, in pure grey matter lesions the extent of C deposition was found to be extremely low (Brink et al., 2005). While not much is known concerning the source of C proteins in MS lesions, enhanced expression of mRNAs for C1q and to a lesser extent C3 in MS lesions demonstrates again that in response to injury at least part of the C proteins in areas of active demyelination are produced locally. Astrocytes in all lesion areas were immunopositive for C proteins, but C immunoreactive myeloid cells were restricted to inflammatory demyelinating areas, suggesting that macrophages are responsible for enhanced local production of C1q and C3 (Lock et al., 2002;Breij et al., 2008;Brink et al., 2005). Additional roles for complement uncovered in the murine model for MS, experimental autoimmune encephalomyelitis (EAE) have been recently reviewed by Alexander and colleagues (Alexander et al., 2008).
5.4 Disturbed protease / protease inhibitor balance in AD and other ND
Neurons and astrocytes express a number of serine protease inhibitors, including C1-Inh (Veerhuis et al., 1998), thrombin inhibitors, such as protease nexin 1 (PN-1) (Choi et al., 1995) and inhibitors of plasminogen activation (including PAI-1 (Soeda et al., 2008)), neuroserpin (Osterwalder et al., 1998) and alpha2-macroglobulin (α2M) (Bauer et al., 1991) However, in neurodegenerative diseases like AD, expression levels of several regulatory proteins including C1-Inh (Veerhuis et al., 1998;Yasojima et al., 1999a) and AP Cregs fH and fI (Strohmeyer et al., 2000) remain low or are decreased (PN-1) (Choi et al., 1995) which may lead to uncontrolled actions of the proteases. Functions of some regulatory proteins can be taken over by others, as many proteases and protease inhibitors act in the C, the coagulation, the kallikrein-kinin, as well as in fibrinolytic systems. Examples are α2M, which regulates thrombin, plasmin and kallikrein activation, PN-1 which inhibits thrombin and also forms complexes with activated C1s (Van Nostrand et al., 1988), and especially C1-Inh, that except for being the only known physiological regulator of C1 activation, it is a major inhibitor of MASP2 of the lectin pathway and of the contact system of coagulation (kallikrein-kinin system) (Beinrohr et al., 2008). In AD Aβ can initiate the C cascades and the kallikrein-kinin system (Bergamaschini et al., 1998). Therefore, Aβ-induced activation of one system may lead to a disturbed protease - protease inhibitor balance in another system, especially when simultaneously the synthesis of the proteases (thrombin, C1s,C1r, and other C factors) increase, as is seen in AD (Yasojima et al., 1999b;Veerhuis et al., 1999). Such disturbed protease – protease inhibitor balances may then initiate subsequent steps in neurodegenerative processes in AD, including APP metabolism, maintenance of BBB integrity and neuritic outgrowth. In an attempt toward a therapeutic strategy, administration of C1-Inh was found to restrict infarct size in experimental models (Storini et al., 2005). A recombinant form was shown to have a much wider time window of efficacy compared to plasma purified C1-Inh when applied in transient and permanent cerebral ischemia studies in mice. This difference probably is due to the selective binding of the recombinant protein to MBL (Gesuete et al., 2009). However, getting C1Inh into the brain in cases of an intact BBB is currently problematic. Another approach is to enhance C1-Inh functioning with low molecular weight heparin, which was found to be effective in reducing Aβ plaque load, as well as to reduce the number of activated astrocytes and activation of C and contact systems in an AD model (Bergamaschini et al., 2004)
Summary and Future Directions
In summary, various cell types in the CNS were shown to synthesize C factors, and the synthesis rates of many factors increase during development and within the injured brain. Data from human immunohistochemistry, animal models of diseases, and in vitro studies suggest that the role of C in AD is complex, with evidence for both detrimental and beneficial functions, presumably dependent on location, timing, and environmental signals. The potential disease associated polymorphisms of C factors also suggests that control of C activation may have substantial effect on the rate of progression of neurodegenerative diseases. As a result, with precise understanding of the interrelationships between these processes in the CNS in health and disease, C proteins and Cregs can be targeted for therapeutic intervention. The use of inhibitors of selective events downstream of potentially beneficial C cascade events would avoid interfering with these beneficial consequences of C activation (Fonseca et al., 2009). Some therapeutic approaches utilizing large recombinant molecules may work only when the BBB is compromised, but small molecule drugs, such as known receptor antagonists and low molecular weight heparin, are candidates for chronic disorders that may maintain an intact BBB. Indeed, while challenges of specificity and balance of multiple coincident cascades cannot be over emphasized, the cocktail approach of both promoting beneficial effects and preventing detrimental activities is an attractive and realistic goal for developing treatments for human neurological disorders. .
Acknowledgments
The work in the authors’ lab reported here was supported by NIH grants NS35144 and AG 00538 (AT), Stchting Dioraphte, Hersenstichting Nederland and Internationale Stichting Alzheimer Onderzoek (06-517) (RV) and Demensfonden and Alzheimerfonden (HN). The authors thank Dr. Maria I. Fonseca (University of California, Irvine) for Figure 2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robert Veerhuis, Email: R.Veerhuis@vumc.nl.
Henrietta M. Nielsen, Email: Henrietta.Nielsen@med.lu.se.
Andrea J. Tenner, Email: atenner@uci.edu.
Reference List
- 1.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Afagh A, Cummings BJ, Cribbs DH, Cotman CW, Tenner AJ. Localization and cell association of C1q in Alzheimer's disease brain. Exp Neurol. 1996;138:22–32. doi: 10.1006/exnr.1996.0043. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama H, McGeer PL. Brain microglia constitutively express B-2 integrins. J Neuroimmunol. 1990;30:81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- 4.Alexander JJ, Anderson AJ, Barnum SR, Stevens B, Tenner AJ. The complement cascade: Yin-Yang in neuroinflammation--neuro-protection and -degeneration. J Neurochem. 2008;107:1169–1187. doi: 10.1111/j.1471-4159.2008.05668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austen KF, Fearon DT. A molecular basis of activation of the alternative pathway of human complement. Adv Exp Med Biol. 1979;120B:3–17. [PubMed] [Google Scholar]
- 6.Barnett MH, Parratt JD, Cho ES, Prineas JW. Immunoglobulins and complement in postmortem multiple sclerosis tissue. Ann Neurol. 2009;65:32–46. doi: 10.1002/ana.21524. [DOI] [PubMed] [Google Scholar]
- 7.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 8.Bauer J, Strauss S, Schreiter-Gasser U, Ganter U, Schlegel P, Witt I, Yolk B, Berger M. Interleukin-6 and alpha-2-macroglobulin indicate an acute-phase state in Alzheimer's disease cortices. FEBS Lett. 1991;285:111–114. doi: 10.1016/0014-5793(91)80737-n. [DOI] [PubMed] [Google Scholar]
- 9.Beinrohr L, Dobo J, Zavodszky P, Gal P. C1, MBL-MASPs and C1-inhibitor: novel approaches for targeting complement-mediated inflammation. Trends Mol Med. 2008;14:511–521. doi: 10.1016/j.molmed.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Bellander BM, Singhrao SK, Ohlsson M, Mattsson P, Svensson M. Complement activation in the human brain after traumatic head injury. J Neurotrauma. 2001;18:1295–1311. doi: 10.1089/08977150152725605. [DOI] [PubMed] [Google Scholar]
- 11.Benard M, Gonzalez BJ, Schouft MT, Falluel-Morel A, Vaudry D, Chan P, Vaudry H, Fontaine M. Characterization of C3a and C5a receptors in rat cerebellar granule neurons during maturation. Neuroprotective effect of C5a against apoptotic cell death. J Biol Chem. 2004;279:43487–43496. doi: 10.1074/jbc.M404124200. [DOI] [PubMed] [Google Scholar]
- 12.Benard M, Raoult E, Vaudry D, Leprince J, Falluel-Morel A, Gonzalez BJ, Galas L, Vaudry H, Fontaine M. Role of complement anaphylatoxin receptors (C3aR, C5aR) in the development of the rat cerebellum. Mol Immunol. 2008;45:3767–3774. doi: 10.1016/j.molimm.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 13.Benoit ME, Tenner AJ. Complement Protein C1q-Mediated Neuroprotection Is Correlated with Regulation of Neuronal Gene and MicroRNA Expression. J Neurosci. 2011;31:3459–3469. doi: 10.1523/JNEUROSCI.3932-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergamaschini L, Parnetti L, Pareyson D, Canziani S, Cugno M, Agostoni A. Activation of the contact system in cerebrospinal fluid of patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12:102–108. doi: 10.1097/00002093-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Bergamaschini L, Rossi E, Storini C, Pizzimenti S, Distaso M, Perego C, De Luigi A, Vergani C, De Simoni MG. Peripheral treatment with enoxaparin, a low molecular weight heparin, reduces plaques and beta-amyloid accumulation in a mouse model of Alzheimer's disease. J Neurosci. 2004;24:4181–4186. doi: 10.1523/JNEUROSCI.0550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bo L, Vedeler CA, Nyland HI, Trapp BD, Mork SJ. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62:723–732. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]
- 17.Bogestal YR, Barnum SR, Smith PL, Mattisson V, Pekny M, Pekna M. Signaling through C5aR is not involved in basal neurogenesis. J Neurosci Res. 2007;85:2892–2897. doi: 10.1002/jnr.21401. [DOI] [PubMed] [Google Scholar]
- 18.Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44:33–43. doi: 10.1016/j.molimm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Bolliger MF, Martinelli DC, Sudhof TC. The cell-adhesion G protein-coupled receptor BAI3 is a high-affinity receptor for C1q-like proteins. Proc Natl Acad Sci U S A. 2011;108:2534–2539. doi: 10.1073/pnas.1019577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonifati DM, Kishore U. Role of complement in neurodegeneration and neuroinflammation. Mol Immunol. 2007;44:999–1010. doi: 10.1016/j.molimm.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Bradt BM, Kolb WP, Cooper NR. Complement-dependent proinflammatory properties of the Alzheimer's disease beta-peptide. J Exp Med. 1998;188:431–438. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breij EC, Brink BP, Veerhuis R, van den Berg C, Vloet R, Yan R, Dijkstra CD, Van der Valk P, Bo L. Homogeneity of active demyelinating lesions in established multiple sclerosis. Ann Neurol. 2008;63:16–25. doi: 10.1002/ana.21311. [DOI] [PubMed] [Google Scholar]
- 24.Brink BP, Veerhuis R, Breij EC, Van der Valk P, Dijkstra CD, Bo L. The pathology of multiple sclerosis is location-dependent: no significant complement activation is detected in purely cortical lesions. J Neuropathol Exp Neurol. 2005;64:147–155. doi: 10.1093/jnen/64.2.147. [DOI] [PubMed] [Google Scholar]
- 25.Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp Neurol. 1993;120:245–263. doi: 10.1006/exnr.1993.1059. [DOI] [PubMed] [Google Scholar]
- 26.Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. Synantocytes: the fifth element. J Anat. 2005;207:695–706. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cadman ED, Puttfarcken PS. Beta-amyloid peptides initiate the complement cascade without producing a comparable effect on the terminal pathway in vitro. Exp Neurol. 1997;146:388–394. doi: 10.1006/exnr.1997.6540. [DOI] [PubMed] [Google Scholar]
- 28.Canova C, Neal JW, Gasque P. Expression of innate immune complement regulators on brain epithelial cells during human bacterial meningitis. J Neuroinflammation. 2006;3:22. doi: 10.1186/1742-2094-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carel JC, Frazier B, Ley TJ, Holers VM. Analysis of epitope expression and the functional repertoire of recombinant complement receptor 2 (CR2/CD21) in mouse and human cells. j immunol. 1989;143:923–930. [PubMed] [Google Scholar]
- 30.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 31.Chakrabarty P, Ceballos-Diaz C, Beccard A, Janus C, Dickson D, Golde TE, Das P. IFN-gamma promotes complement expression and attenuates amyloid plaque deposition in amyloid beta precursor protein transgenic mice. J Immunol. 2010;184:5333–5343. doi: 10.4049/jimmunol.0903382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charbel IP, Victor CN, Scholl HP. The significance of the complement system for the pathogenesis of age-related macular degeneration -current evidence and translation into clinical application. Graefes Arch Clin Exp Ophthalmol. 2010 doi: 10.1007/s00417-010-1568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi BH, Kim RC, Vaughan PJ, Lau A, Van Nostrand WE, Cotman CW, Cunningham DD. Decreases in protease nexins in Alzheimer's disease brain. Neurobiol Aging. 1995;16:557–562. doi: 10.1016/0197-4580(95)00060-r. [DOI] [PubMed] [Google Scholar]
- 34.Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, Prince DA. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci U S A. 2010;107:7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole DS, Morgan BP. Beyond lysis: how complement influences cell fate. Clin Sci (Lond) 2003;104:455–466. doi: 10.1042/CS20020362. [DOI] [PubMed] [Google Scholar]
- 36.Compston DAS, Morgan BP, Campbell AK, Wilkins P, Cole G, Thomas ND, Jasani B. Immunocytochemical localization of the terminal complement complex in multiple sclerosis. Neuropathol Appl Neurobiol. 1989;15:307–316. doi: 10.1111/j.1365-2990.1989.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 37.Cooper NR, Nemerow GR. Complement and infectious agents: A tale of disguise and deception. Comple and Inflam. 1989;6:249–258. doi: 10.1159/000463100. [DOI] [PubMed] [Google Scholar]
- 38.Craft JM, Watterson DM, Van Eldik LJ. Human amyloid beta-induced neuroinflammation is an early event in neurodegeneration. Glia. 2006;53:484–490. doi: 10.1002/glia.20306. [DOI] [PubMed] [Google Scholar]
- 39.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 40.Eikelenboom P, Hack CE, Rozemuller JM, Stam FC. Complement activation in amyloid plaques in Alzheimer's dementia. Virchows Archiv B Cell Pathol. 1989;56:259–262. doi: 10.1007/BF02890024. [DOI] [PubMed] [Google Scholar]
- 41.Eikelenboom P, Stam FC. Immunoglobulins and complement factors in senile plaques. An immunoperoxidase study. Acta Neuropathol. 1982;57:239–242. doi: 10.1007/BF00685397. [DOI] [PubMed] [Google Scholar]
- 42.Eikelenboom P, Veerhuis R. The role of complement and activated microglia in the pathogenesis of Alzheimer's disease. Neurobiol Aging. 1996;17:673–680. doi: 10.1016/0197-4580(96)00108-x. [DOI] [PubMed] [Google Scholar]
- 43.Eikelenboom P, Veerhuis R, Van Exel E, Hoozemans JJ, Rozemuller AJ, van Gool WA. The Early Involvement of the Innate Immunity in the Pathogenesis of Alzheimer's Disease: Neuropathological, Epidemiological and Genetic Evidence. Curr Alzheimer Res. 2011 doi: 10.2174/156720511795256080. [DOI] [PubMed] [Google Scholar]
- 44.Erdei A, Isaak A, Torok K, Sandor N, Kremlitzka M, Prechl J, Bajtay Z. Expression and role of CR1 and CR2 on B and T lymphocytes under physiological and autoimmune conditions. Mol Immunol. 2009;46:2767–2773. doi: 10.1016/j.molimm.2009.05.181. [DOI] [PubMed] [Google Scholar]
- 45.Familian A, Eikelenboom P, Veerhuis R. Minocycline does not affect amyloid beta phagocytosis by human microglial cells. Neurosci Lett. 2007;416:87–91. doi: 10.1016/j.neulet.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 46.Fan R, DeFilippis K, Van Nostrand WE. Induction of complement proteins in a mouse model for cerebral microvascular A beta deposition. J Neuroinflammation. 2007;4:22. doi: 10.1186/1742-2094-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flaherty P, Radhakrishnan ML, Dinh T, Rebres RA, Roach TI, Jordan MI, Arkin AP. A dual receptor crosstalk model of G-protein-coupled signal transduction. PLoS Comput Biol. 2008;4:e1000185. doi: 10.1371/journal.pcbi.1000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fonseca MI, Ager RR, Chu SH, Yazan O, Sanderson SD, LaFerla FM, Taylor SM, Woodruff TM, Tenner AJ. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performancein murine models of Alzheimer's disease. J Immunol. 2009;183:1375–1383. doi: 10.4049/jimmunol.0901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fonseca MI, Chu SH, Berci AM, Benoit ME, Peters DG, Kimura Y, Tenner AJ. Contribution of complement activation pathways to neuropathology differs among mouse models of Alzheimer's disease. J Neuroinflammation. 2011;8:4. doi: 10.1186/1742-2094-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fonseca MI, Kawas CH, Troncoso JC, Tenner AJ. Neuronal localization of C1q in preclinical Alzheimer's disease. Neurobiol Dis. 2004a;15:40–46. doi: 10.1016/j.nbd.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Fonseca MI, Zhou J, Botto M, Tenner AJ. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer's disease. J Neurosci. 2004b;24:6457–6465. doi: 10.1523/JNEUROSCI.0901-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frank I, Stoiber H, Godar S, Stockinger H, Steindl F, Katinger HW, Dierich MP. Acquisition of host cell-surface-derived molecules by HIV-1. AIDS. 1996;10:1611–1620. doi: 10.1097/00002030-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Fraser DA, Laust AK, Nelson EL, Tenner AJ. C1q differentially modulates phagocytosis and cytokine responses during ingestion of apoptotic cells by human monocytes, macrophages, and dendritic cells. J Immunol. 2009;183:6175–6185. doi: 10.4049/jimmunol.0902232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fraser DA, Pisalyaput K, Tenner AJ. C1q enhances microglial clearance of apoptotic neurons and neuronal blebs, and modulates subsequent inflammatory cytokine production. J Neurochem. 2010;112:733–743. doi: 10.1111/j.1471-4159.2009.06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gasparini L, Ongini E, Wilcock D, Morgan D. Activity of flurbiprofen and chemically related anti-inflammatory drugs in models of Alzheimer's disease. Brain Res Brain Res Rev. 2005;48:400–408. doi: 10.1016/j.brainresrev.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 56.Gasque P, Fontaine M, Morgan BP. Complement expression in human brain. Biosynthesis of terminal pathway components and regulators in human glial cells and cell lines. J Immunol. 1995;154:4726–4733. [PubMed] [Google Scholar]
- 57.Gasque P, Ischenko A, Legoedec J, Mauger C, Schouft MT, Fontaine M. Expression of the complement classical pathway by human glioma in culture. A model for complement expression by nerve cells. J Biol Chem. 1993;268:25068–25074. [PubMed] [Google Scholar]
- 58.Gasque P, Thomas A, Fontaine M, Morgan BP. Complement activation on human neuroblastoma cell lines in vitro: route of activation and expression of functional complement regulatory proteins. J Neuroimmunol. 1996;66:29–40. doi: 10.1016/0165-5728(96)00015-x. [DOI] [PubMed] [Google Scholar]
- 59.Gay D, Esiri M. Blood-brain barrier damage in acute multiple sclerosis plaques. An immunocytological study. Brain. 1991;114 ( Pt 1B):557–572. doi: 10.1093/brain/114.1.557. [DOI] [PubMed] [Google Scholar]
- 60.Gendrel M, Rapti G, Richmond JE, Bessereau JL. A secreted complement-control-related protein ensures acetylcholine receptor clustering. Nature. 2009;461:992–996. doi: 10.1038/nature08430. [DOI] [PubMed] [Google Scholar]
- 61.Gesuete R, Storini C, Fantin A, Stravalaci M, Zanier ER, Orsini F, Vietsch H, Mannesse ML, Ziere B, Gobbi M, De Simoni MG. Recombinant C1 inhibitor in brain ischemic injury. Ann Neurol. 2009;66:332–342. doi: 10.1002/ana.21740. [DOI] [PubMed] [Google Scholar]
- 62.Griffiths MR, Gasque P, Neal JW. The multiple roles of the innate immune system in the regulation of apoptosis and inflammation in the brain. J Neuropathol Exp Neurol. 2009;68:217–226. doi: 10.1097/NEN.0b013e3181996688. [DOI] [PubMed] [Google Scholar]
- 63.Head E, Azizeh BY, Lott IT, Tenner AJ, Cotman CW, Cribbs DH. Complement association with neurons and beta-amyloid deposition in the brains of aged individuals with Down Syndrome. Neurobiol Dis. 2001;8:252–265. doi: 10.1006/nbdi.2000.0380. [DOI] [PubMed] [Google Scholar]
- 64.Hosokawa M, Klegeris A, Maguire J, McGeer PL. Expression of complement messenger RNAs and proteins by human oligodendroglial cells. Glia. 2003;42:417–423. doi: 10.1002/glia.10234. [DOI] [PubMed] [Google Scholar]
- 65.Hu W, Ranaivo HR, Roy SM, Behanna HA, Wing LK, Munoz L, Guo L, Van Eldik LJ, Watterson DM. Development of a noveltherapeutic suppressor of brain proinflammatory cytokine up-regulation that attenuates synaptic dysfunction and behavioral deficits. Bioorg Med Chem Lett. 2007;17:414–418. doi: 10.1016/j.bmcl.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Humayun S, Gohar M, Volkening K, Moisse K, Leystra-Lantz C, Mepham J, McLean J, Strong MJ. The complement factor C5a receptor is upregulated in NFL−/− mouse motor neurons. J Neuroimmunol. 2009 doi: 10.1016/j.jneuroim.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 67.Ishii T, Haga S. Immuno-electron-microscopic localization of complement in amyloid fibrils of senile plaques. Acta Neuropathol. 1984;63:296–300. doi: 10.1007/BF00687336. [DOI] [PubMed] [Google Scholar]
- 68.Itagaki S, Akiyama H, Saito H, McGeer PL. Ultrastructural localization of complement membrane attack complex (MAC)-like immunoreactivity in brains of patients with Alzheimer's disease. Brain Res. 1994;645:78–84. doi: 10.1016/0006-8993(94)91640-3. [DOI] [PubMed] [Google Scholar]
- 69.Jana M, Palencia CA, Pahan K. Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer's disease. J Immunol. 2008;181:7254–7262. doi: 10.4049/jimmunol.181.10.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jauneau AC, Ischenko A, Chatagner A, Benard M, Chan P, Schouft MT, Patte C, Vaudry H, Fontaine M. Interleukin 1b and anaphylatoxins exert a synergistic effect on NGF expression by astrocytes. J Neuroinflammation. 2006;3:8. doi: 10.1186/1742-2094-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang H, Burdick D, Glabe CG, Cotman CW, Tenner AJ. β-amyloid activates complement by binding to a specific region of the collagen-like domain of the C1q A chain. J Immunol. 1994;152:5050–5059. [PubMed] [Google Scholar]
- 72.Jin JJ, Kim HD, Maxwell JA, Li L, Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model ofAlzheimer's disease. J Neuroinflammation. 2008;5:23. doi: 10.1186/1742-2094-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johansson L, Rytkonen A, Bergman P, Albiger B, Kallstrom H, Hokfelt T, Agerberth B, Cattaneo R, Jonsson AB. CD46 in meningococcal disease. Science. 2003;301:373–375. doi: 10.1126/science.1086476. [DOI] [PubMed] [Google Scholar]
- 74.Johnson RT, Glass JD, McArthur JC, Chesebro BW. Quantitation of human immunodeficiency virus in brains of demented and nondemented patients with acquired immunodeficiency syndrome. Ann Neurol. 1996;39:392–395. doi: 10.1002/ana.410390319. [DOI] [PubMed] [Google Scholar]
- 75.Kemper C, Hourcade DE. Properdin: Newroles in pattern recognition and target clearance. Mol Immunol. 2008;45:4048–4056. doi: 10.1016/j.molimm.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kobayashi K, Muramori F, Aoki T, Hayashi M, Miyazu K, Fukutani Y, mukai m, Koshino F. KP-1 is a marker for extraneuronal neurofibrillary tangles and senile plaques in Alzheimer diseased brains. Dement Geriatr Cogn Disord. 1998;9:13–19. doi: 10.1159/000017015. [DOI] [PubMed] [Google Scholar]
- 78.Kolev MV, Ruseva MM, Morgan BP, Donev RM. Targeting neural-restrictive silencer factor sensitizes tumor cells to antibody-based cancer immunotherapy in vitro via multiple mechanisms. j immunol. 2010;184:6035–6042. doi: 10.4049/jimmunol.1000045. [DOI] [PubMed] [Google Scholar]
- 79.Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fievet N, Barberger-Gateau P, Engelborghs S, De DP, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, dePancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossu P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanche H, Dartigues JF, Tzourio C, Gut I, Van BC, Alperovitch A, Lathrop M, Amouyel P. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 80.Lampert-Etchells M, Pasinetti GM, Finch CE, Johnson SA. Regional localization of cells containing complement C1q and C4 mRNAs in the frontal cortex during Alzheimer's disease. Neurodegeneration. 1993;2:111–121. [Google Scholar]
- 81.Levi-Strauss M, Mallat M. Primary cultures of murine astrocytes produce C3 and Factor B, two components of the alternative pathway of complement activation. J Immunol. 1987;139:2361–2366. [PubMed] [Google Scholar]
- 82.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 83.Loeffler DA, Camp DM, Bennett DA. Plaque complement activation and cognitive loss in Alzheimer's disease. J Neuroinflammation. 2008;5:9. doi: 10.1186/1742-2094-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 85.Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lue LF, Rydel R, Brigham EF, Yang LB, Hampel H, Murphy GM, Jr, Brachova L, Yan SD, Walker DG, Shen Y, Rogers J. Inflammatory repertoire of Alzheimer's disease and nondemented elderly microglia in vitro. Glia. 2001;35:72–79. doi: 10.1002/glia.1072. [DOI] [PubMed] [Google Scholar]
- 87.Lumsden CE. The immunogenesis of the multiple sclerosis plaque. Brain Res. 1971;28:365–390. doi: 10.1016/0006-8993(71)90052-7. [DOI] [PubMed] [Google Scholar]
- 88.Maier M, Peng Y, Jiang L, Seabrook TJ, Carroll MC, Lemere CA. Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci. 2008;28:6333–6341. doi: 10.1523/JNEUROSCI.0829-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McGeer PL, Akiyama H, Itagaki S, McGeer EG. Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neurosci Lett. 1989;107:341–346. doi: 10.1016/0304-3940(89)90843-4. [DOI] [PubMed] [Google Scholar]
- 90.McGeer PL, Kawamata T, Walker DG. Distribution of clusterin in Alzheimer brain tissue. Brain Res. 1992;579:337–341. doi: 10.1016/0006-8993(92)90071-g. [DOI] [PubMed] [Google Scholar]
- 91.Morrissette DA. Effects of mouse genetic background strain on Alzheimer-like pathology and behavior in the triple transgenic mouse model of Alzheimer Disease. University of California; Irvine: 2009. [Google Scholar]
- 92.Mukherjee P, Pasinetti GM. The role of complement anaphylatoxin C5a in neurodegeneration: implications in Alzheimer's disease. J Neuroimmunol. 2000;105:124–130. doi: 10.1016/s0165-5728(99)00261-1. [DOI] [PubMed] [Google Scholar]
- 93.Mukherjee P, Pasinetti GM. Complement anaphylatoxin C5a neuroprotects through mitogen-activated protein kinase-dependent inhibition of caspase 3. J Neurochem. 2001;77:43–49. doi: 10.1046/j.1471-4159.2001.00167.x. [DOI] [PubMed] [Google Scholar]
- 94.Nielsen HM, Mulder SD, Belien JA, Musters RJ, Eikelenboom P, Veerhuis R. Astrocytic A beta 1–42 uptake is determined by A beta-aggregation state and the presence of amyloid-associated proteins. Glia. 2010;58:1235–1246. doi: 10.1002/glia.21004. [DOI] [PubMed] [Google Scholar]
- 95.Nielsen HM, Veerhuis R, Holmqvist B, Janciauskiene S. Binding and uptake of A beta1–42 by primary human astrocytes in vitro. Glia. 2009;57:978–988. doi: 10.1002/glia.20822. [DOI] [PubMed] [Google Scholar]
- 96.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cellsare highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 97.O'Barr SA, Caguioa J, Gruol D, Perkins G, Ember JA, Hugli T, Cooper NR. Neuronal expression of a functional receptor for the C5a complement activation fragment. J Immunol. 2001;166:4154–4162. doi: 10.4049/jimmunol.166.6.4154. [DOI] [PubMed] [Google Scholar]
- 98.Osaka H, Mukherjee P, Aisen PS, Pasinetti GM. Complement-derived anaphylatoxin C5a protects against glutamate-mediated neurotoxicity. J Cell Biochem. 1999;73:303–311. [PubMed] [Google Scholar]
- 99.Osterwalder T, Cinelli P, Baici A, Pennella A, Krueger SR, Schrimpf SP, Meins M, Sonderegger P. The axonally secreted serine proteinase inhibitor, neuroserpin, inhibits plasminogen activators and plasmin but not thrombin. J Biol Chem. 1998;273:2312–2321. doi: 10.1074/jbc.273.4.2312. [DOI] [PubMed] [Google Scholar]
- 100.Pedersen ED, Aass HC, Rootwelt T, Fung M, Lambris JD, Mollnes TE. CD59 efficiently protects human NT2-N neurons against complement-mediated damage. Scand J Immunol. 2007;66:345–351. doi: 10.1111/j.1365-3083.2007.01959.x. [DOI] [PubMed] [Google Scholar]
- 101.Pedersen ED, Loberg EM, Vege E, Daha MR, Maehlen J, Mollnes TE. In situ deposition of complement in human acute brain ischaemia. Scand J Immunol. 2009;69:555–562. doi: 10.1111/j.1365-3083.2009.02253.x. [DOI] [PubMed] [Google Scholar]
- 102.Piddlesden SJ, Storch MK, Hibbs M, Freeman AM, Lassmann H, Morgan BP. Soluble recombinant complement receptor 1 inhibits inflammation and demyelination in antibody-mediated demyelinating experimental allergic encephalomyelitis. j immunol. 1994;152:5477–5484. [PubMed] [Google Scholar]
- 103.Pisalyaput K, Tenner AJ. Complement component C1q inhibits beta-amyloid-and serum amyloid P -induced neurotoxicity via caspase-and calpain -independent mechanisms. J Neurochem. 2008;104:696–707. doi: 10.1111/j.1471-4159.2007.05012.x. [DOI] [PubMed] [Google Scholar]
- 104.Prineas JW, Kwon EE, Cho ES, Sharer LR, Barnett MH, Oleszak EL, Hoffman B, Morgan BP. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646–657. doi: 10.1002/ana.1255. [DOI] [PubMed] [Google Scholar]
- 105.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 106.Rambach G, Maier H, Vago G, Mohsenipour I, Lass-Florl C, Defant A, Wurzner R, Dierich MP, Speth C. Complement induction and complement evasion in patients with cerebral aspergillosis. Microbes Infect. 2008;10:1567–1576. doi: 10.1016/j.micinf.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 107.Reichwald J, Danner S, Wiederhold KH, Staufenbiel M. Expression of complement system components during aging and amyloid deposition in APP transgenic mice. J Neuroinflammation. 2009;6:35. doi: 10.1186/1742-2094-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, Styren SD, Civin WH, Brachova L, Bradt B, Ward P, Lieberburg I. Complement activation by beta-amyloid in Alzheimer disease. Proc Natl Acad Sci. 1992;89:10016–10020. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rostagno A, Revesz T, Lashley T, Tomidokoro Y, Magnotti L, Braendgaard H, Plant G, Bojsen-Moller M, Holton J, Frangione B, Ghiso J. Complement activation in chromosome 13 dementias. Similarities with Alzheimer's disease. J Biol Chem. 2002;277:49782–49790. doi: 10.1074/jbc.M206448200. [DOI] [PubMed] [Google Scholar]
- 111.Rozemuller AJ, Eikelenboom P, Theeuwes JW, Jansen Steur EN, de Vos RA. Activated microglial cells and complement factors are unrelated to cortical Lewy bodies. Acta Neuropathol. 2000;100:701–708. doi: 10.1007/s004010000225. [DOI] [PubMed] [Google Scholar]
- 112.Rozemuller JM, Eikelenboom P, Stam FC, Beyreuther K, Masters CL. A4 protein in Alzheimer's disease: primary and secondary cellular events in extracellular amyloid deposition. J of Neuropathology and Experimental Neurology. 1989;48:674–691. doi: 10.1097/00005072-198911000-00009. [DOI] [PubMed] [Google Scholar]
- 113.Rozovsky I, Morgan TE, Willoughby DA, Dugichi-Djordjevich MM, Pasinetti GM, Johnson SA, Finch CE. Selective expression of clusterin (SGP-2) and complement C1qB and C4 during responses to neurotoxins in vivo and in vitro. Neuroscience. 1994;62:741–758. doi: 10.1016/0306-4522(94)90473-1. [DOI] [PubMed] [Google Scholar]
- 114.Ryman D, Gao Y, Lamb BT. Genetic loci modulating amyloid-beta levels in a mouse model of Alzheimer's disease. Neurobiol Aging. 2008;29:1190–1198. doi: 10.1016/j.neurobiolaging.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sairanen T, Karjalainen-Lindsberg ML, Paetau A, Ijas P, Lindsberg PJ. Apoptosis dominant in the periinfarct area ofhuman ischaemic stroke --a possible target of antiapoptotic treatments. Brain. 2006;129:189–199. doi: 10.1093/brain/awh645. [DOI] [PubMed] [Google Scholar]
- 116.Sayah S, Jauneau AC, Patte C, Tonon MC, Vaudry H, Fontaine M. Two different transduction pathways are activated by C3a and C5a anaphylatoxins on astrocytes. Brain Res Mol Brain Res. 2003;112:53–60. doi: 10.1016/s0169-328x(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 117.Schwab C, McGeer PL. Complement activated C4d immunoreactive oligodendrocytes delineate small cortical plaques in multiple sclerosis. Exp Neurol. 2002;174:81–88. doi: 10.1006/exnr.2001.7851. [DOI] [PubMed] [Google Scholar]
- 118.Scolding NJ, Morgan BP, Compston DA. The expression of complement regulatory proteins by adult human oligodendrocytes. J Neuroimmunol. 1998;84:69–75. doi: 10.1016/s0165-5728(97)00241-5. [DOI] [PubMed] [Google Scholar]
- 119.Sewell DL, Nacewicz B, Liu F, Macvilay S, Erdei A, Lambris JD, Sandor M, Fabry Z. Complement C3 and C5 play critical roles in traumatic brain cryoinjury: blocking effects on neutrophil extravasation by C5a receptor antagonist. J Neuroimmunol. 2004;155:55–63. doi: 10.1016/j.jneuroim.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shaftel SS, Griffin WS, O'Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shen Y, Li R, McGeer EG, McGeer PL. Neuronal expression of mRNAs for complement proteins of the classical pathway in Alzheimer brain. Brain Res. 1997;769:391–395. doi: 10.1016/s0006-8993(97)00850-0. [DOI] [PubMed] [Google Scholar]
- 122.Shinjyo N, Stahlberg A, Dragunow M, Pekny M, Pekna M. Complement-derived anaphylatoxin C3a regulates in vitro differentiation and migration of neural progenitor cells. Stem Cells. 2009;27:2824–2832. doi: 10.1002/stem.225. [DOI] [PubMed] [Google Scholar]
- 123.Singhrao SK, Neal JW, Gasque P, Morgan BP, Newman GR. Role of complement in the aetiology of Pick's disease? J Neuropathol Exp Neurol. 1996;55:578–593. doi: 10.1097/00005072-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 124.Singhrao SK, Neal JW, Rushmere NK, Morgan BP, Gasque P. Spontaneous classical pathway activation and deficiency of membrane regulators render human neurons susceptible to complement lysis. Am J Pathol. 2000;157:905–918. doi: 10.1016/S0002-9440(10)64604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sjoberg AP, Trouw LA, Blom AM. Complement activation and inhibition: a delicate balance. Trends Immunol. 2009;30:83–90. doi: 10.1016/j.it.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 126.Snyder SW, Wang GT, Barrett L, Ladror US, Casuto D, Lee CM, Krafft GA, Holzman RB, Holzman TF. Complement C1q does not bind monomeric β-amyloid. Exp Neurol. 1994;128:136–142. doi: 10.1006/exnr.1994.1121. [DOI] [PubMed] [Google Scholar]
- 127.Soeda S, Koyanagi S, Kuramoto Y, Kimura M, Oda M, Kozako T, Hayashida S, Shimeno H. Anti-apoptotic roles of plasminogen activator inhibitor-1 as a neurotrophic factor in the central nervous system. Thromb Haemost. 2008;100:1014–1020. doi: 10.1160/th08-04-0259. [DOI] [PubMed] [Google Scholar]
- 128.Speth C, Dierich MP, Gasque P. Neuroinvasion by pathogens: a key role of the complement system. Mol Immunol. 2002;38:669–679. doi: 10.1016/s0161-5890(01)00104-3. [DOI] [PubMed] [Google Scholar]
- 129.Speth C, Rambach G, Wurzner R, Lass-Florl C. Complement and fungal pathogens: an update. Mycoses. 2008;51:477–496. doi: 10.1111/j.1439-0507.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- 130.Stahel PF, Morganti-Kossmann MC, Kossmann T. The role of the complement system in traumatic brain injury. Brain Res Brain Res Rev. 1998;27:243–256. doi: 10.1016/s0165-0173(98)00015-0. [DOI] [PubMed] [Google Scholar]
- 131.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 132.Stoermer KA, Morrison TE. Complement and viral pathogenesis. Virology. 2011;411:362–373. doi: 10.1016/j.virol.2010.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stoiber H, Ebenbichler C, Schneider R, Janatova J, Dierich MP. Interaction of several complement proteins with gp120 and gp41, the two envelope glycoproteins of HIV-1. AIDS. 1995;9:19–26. doi: 10.1097/00002030-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 134.Stoltzner SE, Grenfell TJ, Mori C, Wisniewski KE, Wisniewski TM, Selkoe DJ, Lemere CA. Temporal accrual of complement proteins in amyloid plaques in Down's syndrome with Alzheimer's disease. Am J Pathol. 2000;156:489–499. doi: 10.1016/S0002-9440(10)64753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Storch MK, Piddlesden S, Haltia M, Iivanainen M, Morgan P, Lassmann H. Multiple sclerosis: in situ evidence for antibody-and complement -mediated demyelination. Ann Neurol. 1998;43:465–471. doi: 10.1002/ana.410430409. [DOI] [PubMed] [Google Scholar]
- 136.Storini C, Rossi E, Marrella V, Distaso M, Veerhuis R, Vergani C, Bergamaschini L, De Simoni MG. C1-inhibitor protects against brain ischemia-reperfusion injury via inhibition of cell recruitment and inflammation. Neurobiol Dis. 2005;19:10–17. doi: 10.1016/j.nbd.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 137.Strohmeyer R, Ramirez M, Cole GJ, Mueller K, Rogers J. Association of factor H of the alternative pathway of complement with agrin and complement receptor 3 in the Alzheimer's disease brain. J Neuroimmunol. 2002;131:135–146. doi: 10.1016/s0165-5728(02)00272-2. [DOI] [PubMed] [Google Scholar]
- 138.Strohmeyer R, Shen Y, Rogers J. Detection of complement alternative pathway mRNA and proteins in the Alzheimer's disease brain. Brain Res Mol Brain Res. 2000;81:7–18. doi: 10.1016/s0169-328x(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 139.Tacnet-Delorme P, Chevallier S, Arlaud GJ. Beta-amyloid fibrils activate the C1 complex of complement under physiological conditions: evidence for a binding site for A beta on the C1q globular regions. J Immunol. 2001;167:6374–6381. doi: 10.4049/jimmunol.167.11.6374. [DOI] [PubMed] [Google Scholar]
- 140.Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thomas A, Gasque P, Vaudry D, Gonzalez B, Fontaine M. Expression of a complete and functional complement system by human neuronal cells in vitro. Int Immunol. 2000;12:1015–1023. doi: 10.1093/intimm/12.7.1015. [DOI] [PubMed] [Google Scholar]
- 142.Tocco G, Musleh W, Sakhi S, Schreiber SS, Baudry M, Pasinetti GM. Complement and glutamate neurotoxicity. Genotypic influences of C5 in a mouse model of hippocampal neurodegeneration. Mol Chem Neuropathol. 1997;31:289–300. doi: 10.1007/BF02815131. [DOI] [PubMed] [Google Scholar]
- 143.Trouw LA, Nielsen HM, Minthon L, Londos E, Landberg G, Veerhuis R, Janciauskiene S, Blom AM. C4b-binding protein in Alzheimer's disease: binding to Abeta1–42 and to dead cells. Mol Immunol. 2008;45:3649–3660. doi: 10.1016/j.molimm.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 144.Udan ML, Ajit D, Crouse NR, Nichols MR. Toll-like receptors 2 and 4 mediate Abeta(1–42) activation of the innate immune response in a human monocytic cell line. J Neurochem. 2008;104:524–533. doi: 10.1111/j.1471-4159.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- 145.Van Beek J, Elward K, Gasque P. Activation of complement in the central nervous system: roles in neurodegeneration and neuroprotection. Ann N Y Acad Sci. 2003;992:56–71. doi: 10.1111/j.1749-6632.2003.tb03138.x. [DOI] [PubMed] [Google Scholar]
- 146.Van Beek J, Nicole O, Ali C, Ischenko A, MacKenzie ET, Buisson A, Fontaine M. Complement anaphylatoxin C3a is selectively protective against NMDA-induced neuronal cell death. NeuroReport. 2001;12:289–293. doi: 10.1097/00001756-200102120-00022. [DOI] [PubMed] [Google Scholar]
- 147.van Kooten C, Fiore N, Trouw LA, Csomor E, Xu W, Castellano G, Daha MR, Gelderman KA. Complement production and regulation by dendritic cells: molecular switches between tolerance and immunity. Mol Immunol. 2008;45:4064–4072. doi: 10.1016/j.molimm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 148.Van Nostrand WE, McKay LD, Baker JB, Cunningham DD. Functional and structural similarities between protease nexin I and C1 inhibitor. J Biol Chem. 1988;263:3979–3983. [PubMed] [Google Scholar]
- 149.Vastag M, Skopal J, Kramer J, Kolev K, Voko Z, Csonka E, Machovich R, Nagy Z. Endothelial cells cultured from human brain microvessels produce complement proteins factor H, factor B, C1 inhibitor, and C4. Immunobiology. 1998;199:5–13. doi: 10.1016/S0171-2985(98)80059-4. [DOI] [PubMed] [Google Scholar]
- 150.Veerhuis R. Histological and Direct Evidence for the Role of Complement in the Neuroinflammation of AD. Curr Alzheimer Res. 2011;8:34–58. doi: 10.2174/156720511794604589. [DOI] [PubMed] [Google Scholar]
- 151.Veerhuis R, Boshuizen RS, Familian A. Amyloid associated proteins in Alzheimer's and prion disease. Curr Drug Targets CNS Neurol Disord. 2005;4:235–248. doi: 10.2174/1568007054038184. [DOI] [PubMed] [Google Scholar]
- 152.Veerhuis R, Janssen I, De Groot CJ, Van Muiswinkel FL, Hack CE, Eikelenboom P. Cytokines associated with amyloid plaques in Alzheimer's disease brain stimulate human glial and neuronal cell cultures to secrete early complement proteins, but not C1-inhibitor. Exp Neurol. 1999;160:289–299. doi: 10.1006/exnr.1999.7199. [DOI] [PubMed] [Google Scholar]
- 153.Veerhuis R, Janssen I, Hack CE, Eikelenboom P. Early complement components in Alzheimer's disease brains. Acta Neuropathol (Berl ) 1996;91:53–60. doi: 10.1007/s004019570001. [DOI] [PubMed] [Google Scholar]
- 154.Veerhuis R, Janssen I, Hoozemans JJM, De Groot CJA, Hack CE, Eikelenboom P. Complement c1-inhibitor expression in Alzheimer's disease. Acta Neuropathol (Berl ) 1998;96:287–296. doi: 10.1007/s004010050896. [DOI] [PubMed] [Google Scholar]
- 155.Veerhuis R, Van Breemen MJ, Hoozemans JM, Morbin M, Ouladhadj J, Tagliavini F, Eikelenboom P. Amyloid beta plaque-associated proteins C1q and SAP enhance the Abeta(1–42 )peptide-induced cytokine secretion by adult human microglia in vitro. Acta Neuropathol (Berl) 2003;105:135–144. doi: 10.1007/s00401-002-0624-7. [DOI] [PubMed] [Google Scholar]
- 156.Veerhuis R, Van der Valk P, Janssen I, Zhan SS, VanNostrand WE, Eikelenboom P. Complement activation in amyloid plaques in Alzheimer's disease brains does not proceed further than C3. Virchows Arch. 1995;426:603–610. doi: 10.1007/BF00192116. [DOI] [PubMed] [Google Scholar]
- 157.Velazquez P, Cribbs DH, Poulos TL, Tenner AJ. Aspartate residue 7 in amyloid beta-protein is critical for classical complement pathway activation: implications for Alzheimer's disease pathogenesis. Nat Med. 1997;3:77–79. doi: 10.1038/nm0197-77. [DOI] [PubMed] [Google Scholar]
- 158.Verbeek MM, Otte-Holler I, Ruiter DJ, de Waal RM. Human brain pericytes as a model system to study the pathogenesis ofcerebrovascular amyloidosis in Alzheimer's disease. Cell Mol Biol (Noisy -le-grand) 1999;45:37–46. [PubMed] [Google Scholar]
- 159.Verbeek MM, Otte-Höller I, Veerhuis R, Ruiter DJ, De Waal RMW. Distribution of Aβ-associated proteins in cerebrovascular amyloid of Alzheimer's disease. Acta Neuropathol (Berl ) 1998;96:628–636. doi: 10.1007/s004010050944. [DOI] [PubMed] [Google Scholar]
- 160.Verkhratsky A, Parpura V. Recent advances in (patho)physiology of astroglia. Acta Pharmacol Sin. 2010;31:1044–1054. doi: 10.1038/aps.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Walker DG, Kim SU, McGeer PL. Complement and cytokine gene expression in cultured microglial derived from postmortem human brains. J Neurosci Res. 1995;40:478–493. doi: 10.1002/jnr.490400407. [DOI] [PubMed] [Google Scholar]
- 162.Walker DG, McGeer PL. Complement gene expression in human brain: comparison between normal and Alzheimer disease cases. Brain Res Mol Brain Res. 1992;14:109–116. doi: 10.1016/0169-328x(92)90017-6. [DOI] [PubMed] [Google Scholar]
- 163.Watabe K, Osborne D, Kim SU. Phagocytic activity of human adult astrocytes and oligodendrocytes in culture. J Neuropathol Exp Neurol. 1989;48:499–506. doi: 10.1097/00005072-198909000-00001. [DOI] [PubMed] [Google Scholar]
- 164.Webster S, Lue LF, Brachova L, Tenner AJ, McGeer PL, Terai K, Walker DG, Bradt B, Cooper NR, Rogers J. Molecular and cellular characterization of the membrane attack complex, C5b-9, in Alzheimer's disease. Neurobiol Aging. 1997;18:415–421. doi: 10.1016/s0197-4580(97)00042-0. [DOI] [PubMed] [Google Scholar]
- 165.Woodruff TM, Ager RR, Tenner AJ, Noakes PG, Taylor SM. The role of the complement system and the activation fragment C5a in the central nervous system. Neuromolecular Med. 2010;12:179–192. doi: 10.1007/s12017-009-8085-y. [DOI] [PubMed] [Google Scholar]
- 166.Woodruff TM, Costantini KJ, Crane JW, Atkin JD, Monk PN, Taylor SM, Noakes PG. The complement factor C5a contributes to pathology in a rat model of amyotrophic lateral sclerosis. J Immunol. 2008;181:8727–8734. doi: 10.4049/jimmunol.181.12.8727. [DOI] [PubMed] [Google Scholar]
- 167.Woodruff TM, Crane JW, Proctor LM, Buller KM, Shek AB, de VK, Pollitt S, Williams HM, Shiels IA, Monk PN, Taylor SM. Therapeutic activity of C5a receptor antagonists in a rat model of neurodegeneration. FASEB J. 2006;20:1407–1417. doi: 10.1096/fj.05-5814com. [DOI] [PubMed] [Google Scholar]
- 168.Wooster DG, Maruvada R, Blom AM, Prasadarao NV. Logarithmic phase Escherichia coli K1 efficiently avoids serum killing by promoting C4bp-mediated C3b and C4b degradation. Immunology. 2006;117:482–493. doi: 10.1111/j.1365-2567.2006.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wren DR, Noble M. Oligodendrocytes and oligodendrocyte/type-2 astrocyte progenitor cells of adult rats specifically susceptible to the effects of complement in absence of antibody. Proc Natl Acad Sci USA. 1989;86:9025–9029. doi: 10.1073/pnas.86.22.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wyss-Coray T, Yan F, Lin AH, Lambris JD, Alexander JJ, Quigg RJ, Masliah E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer's mice. Proc Natl Acad Sci U S A. 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yamada T, McGeer PL, McGeer EG. Lewy bodies in Parkinson's disease are recognized by antibodies to complement proteins. Acta Neuropathol. 1992;84:100–104. doi: 10.1007/BF00427222. [DOI] [PubMed] [Google Scholar]
- 172.Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010;17:6–10. doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yasojima K, McGeer EG, McGeer PL. Complement regulators C1 inhibitor and CD59 do not significantly inhibit complement activation in Alzheimer disease. Brain Res. 1999a;833:297–301. doi: 10.1016/s0006-8993(99)01514-0. [DOI] [PubMed] [Google Scholar]
- 174.Yasojima K, Schwab C, McGeer EG, McGeer PL. Up-regulated production and activation of the complement system in Alzheimer's disease brain. Amer J Path. 1999b;154:927–936. doi: 10.1016/S0002-9440(10)65340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yuzaki M. Cbln and C1q family proteins: new transneuronal cytokines. Cell Mol Life Sci. 2008;65:1698–1705. doi: 10.1007/s00018-008-7550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yuzaki M. Synapse formation and maintenance by C1q family proteins: a new class of secreted synapse organizers. Eur J Neurosci. 2010;32:191–197. doi: 10.1111/j.1460-9568.2010.07346.x. [DOI] [PubMed] [Google Scholar]
- 177.Zanjani H, Finch CE, Kemper C, Atkinson J, McKeel D, Morris JC, Price JL. Complement activation in very early Alzheimer disease. Alzheimer Dis Assoc Disord. 2005;19:55–66. doi: 10.1097/01.wad.0000165506.60370.94. [DOI] [PubMed] [Google Scholar]
- 178.Zhan SS, Veerhuis R, Kamphorst W, Eikelenboom P. Distribution of beta amyloid associated proteins in plaques in Alzheimer's disease and in the non-demented elderly. Neurodegeneration. 1995;4:291–297. doi: 10.1016/1055-8330(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 179.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhou J, Fonseca MI, Pisalyaput K, Tenner AJ. Complement C3 and C4 expression in C1q sufficient and deficient mouse models of Alzheimer's disease. J Neurochem. 2008;106:2080–2092. doi: 10.1111/j.1471-4159.2008.05558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ziccardi RJ, Cooper NR. Active Disassembly of the First Complement Component, C1, by C1 Inactivator. J Immunol. 1979;123:788–792. [PubMed] [Google Scholar]