Abstract
Dense deposit disease (DDD) is an orphan disease that primarily affects children and young adults without sexual predilection. Studies of its pathophysiology have shown conclusively that it is caused by fluid-phase dysregulation of the alternative pathway of complement, however the role played by genetics and autoantibodies like C3 nephritic factors must be more thoroughly defined if we are to make an impact in the clinical management of this disease. There are currently no mechanism-directed therapies to offer affected patients, half of whom progress to end stage renal failure disease within 10 years of diagnosis. Transplant recipients face the dim prospect of disease recurrence in their allografts, half of which ultimately fail. More detailed genetic and complement studies of DDD patients may make it possible to identify protective factors prognostic for naïve kidney and transplant survival, or conversely risk factors associated with progression to renal failure and allograft loss. The pathophysiology of DDD suggests that a number of different treatments warrant consideration. As advances are made in these areas, there will be a need to increase healthcare provider awareness of DDD by making resources available to clinicians to optimize care for DDD patients.
Introduction
Dense deposit disease is a glomerular pathology characterized by intramembranous electron-dense change within the glomerular basement membrane (GBM). DDD is associated with deposition of complement C3 within the glomeruli with little or no staining for immunoglobulin. The presence of C3 without significant immunoglobulin suggested to early investigators that DDD was due to abnormal activation of the complement alternative pathway (AP). There is now strong evidence that DDD is caused by uncontrolled AP activation (reviewed in Appel et al., 2005; Smith et al., 2007). DDD was renamed membranoproliferative glomerulonephritis type II (MPGN2), a term that is inappropriate because: 1) it implies a relationship with MPGN1 and MPGN3, which unlike DDD are immune complex diseases; and 2) it implies that the membrano-proliferative pattern of injury is characteristic when in fact it is present in only 25% of DDD patients (Smith et al., 2007; Walker et al., 2007). Mild mesangial cell hypercellularity is most common (45%), but crescentic (18%) and acute proliferative-exudative (12%) patterns of injury also occur (Habib et al., 1975; Walker et al., 2007).
The densities in DDD, which are implicit in its name, appear in the GBM by light microscopy as elongated but brightly eosinophilic, variably refractile deposits. By electron microscopy, they are ‘sausage-shaped’ homogeneous densities within the lamina densa (Walker et al., 2007). Mass spectrometry on laser micro-dissected glomeruli isolated from paraffin-embedded tissue of DDD cases has confirmed that the diseased glomeruli contain components of the AP and terminal complement complex (TCC), consist with fluid-phase AP dysregulation (Sethi et al., 2009).
We will first summarize the clinical manifestations of DDD. We will then discuss the role of genetic factors and autoantibodies in DDD with particular emphasis on recent advances. Finally we will speculate on treatment strategies that are under development or warrant consideration. Understanding complement biology is a prerequisite for understanding DDD pathophysiology. Therefore we will briefly overview complement biology.
Complement Activation and Regulation
The complement system is the cornerstone of innate immunity. As one of the first lines of host defense, it plays a major role in microbial killing, immune complex handling, apoptotic cell clearance, tissue homeostasis and modulation of adaptive immunity (Volonakis and Frank, 1998; Walport, 2001a, 2001b). Critical to these functions is the sequential triggering of a series of cascades that result in the formation of metastable protease complexes which can culminate in formation of membrane attack complex (MAC). In the broadest terms, complement activation occurs in five sequential steps the first of which is its initiation by one of three independent pathways – the classical (CP), the lectin (LP) or the alternative (AP). Once activated, the second step is the formation of C3 convertase, which exponentially amplifies the initial triggering pathway (step 3) and provides the protein complex from which C5 convertase is generated (step 4). C5 convertase triggers the TCC with generation of MAC and the potent anaphylatoxin, C5a (step 5).
During complement activation, damage to self surfaces may occur. This is limited in vivo by a complex group of proteins that regulate complement activation at many steps in the cascade. These proteins modulate the generation and breakdown of the C3 and C5 convertases both in the circulation (‘fluid-phase’) and on cell surfaces and extra-cellular membranes (‘surface-phase’). Many complement regulatory proteins are also involved in other activities (e.g. cell adhesion and extracellular matrix interactions) (Zipfel and Skerka, 2009). Examples of fluid-phase regulators include: complement factor H (CFH) and complement factor I (CFI), which down regulate the AP; C1 inhibitor (C1INH), which down regulates the CP and LP; and C4 binding protein (C4BP), which down regulates the CP. Fluid-phase regulators of the TCC include clusterin and vitronectin. Relatively recently, complement factor H-related protein 1 has been demonstrated to down regulate C5 activation (Fritsche et al., 2010).
Several of these proteins including CFH, CFHL1, C4BP, CFHR1, clusterin and vitronectin also attach to cell surfaces and biomembranes (like the glomerular basement membrane (GBM) and Bruch's membrane) (Ferreira and Pangburn, 2007; Manuelian et al., 2003; Sanchez-Corral et al., 2004). This attachment adds a protective layer known as the ‘surface zone’ to limit formation of active complement products (Zipfel and Skerka, 2009). Examples of membrane-bound complement regulators include CR1 (complement receptor 1, CD35), CD55 (decay-accelerating factor, DAF), CD46 (membrane cofactor protein, MCP), CD59 and the complement receptor of the immunoglobulin superfamily (CRIg, also known as VSIG4 (V-set and Ig domain-containing 4)) (He et al., 2008; Isaak et al, 2006; Khera and Das, 2009; Kimberley et al., 2007; Roozendaal and Carroll, 2007; Seya and Atkinson, 1989; Spendlove et al., 2006; Wiesmann C et al., 2006). Their expression and distribution vary from cell type to cell type, which has important implications for complement-related diseases. An important distinction between fluid-phase and membrane-bound regulators is that while membrane-bound convertase regulators control the three initiating pathways by inactivating both C3 and C4 (CR1 and CD46, for example), fluid-phase regulators are pathway specific and control the AP, CP or LP by acting exclusively on either C3 or C4 (Zipfel and Skerka, 2009).
To understand the progress that has been made with respect to the pathophysiology of DDD it is important to understand the activation and regulation of the complement AP. Essential to the activation of the AP is cleavage of C3 to C3b, a change that is accompanied by a dramatic rearrangement of the domains of C3b (Gros et al., 2008; Janssen et al., 2005; Janssen et al., 2006). For example, migration and rotation of the thioester-containing domain (TED) of C3b exposes the thioester to particles, basement membranes and cell surface, facilitating the attachment of C3b to these sites (Morgan et al., 2011). Complement factor B (CFB) then complexes with C3b to form C3bB, which is cleaved by complement factor D into two factors, Ba and Bb, the latter remaining bound to C3b. C3bBb is the AP C3 convertase and generates additional C3bBb by cleaving C3. This self-propagation results in exponential amplification of the AP, necessitating tight control in the fluid phase and on self-surfaces.
CFH is the key regulator of C3 activation through the AP (Figure 1A). Several elegant studies have clarified the mechanism of its interaction with C3b and C3 convertase, offering insight into normal complement region and its perturbation in association with disease-related mutations (Janssen et al., 2006; Morgan et al., 2011; Schmidt et al., 2008; Wu et al., 2009). Only the first four domains (also called short consensus repeats (SCRs) or complement control protein domains (CCPs)) of CFH are necessary for fluid-phase AP regulation (Schmidt et al., 2008). These SCRs bind to C3b in an extended configuration that spans multiple domains of C3b including the α′NT, MG1, MG2, MG6, MG7, CUB and TED (Wu et al., 2009). This extensive interface is necessary because binding affinity of CFH for C3b is low. It also provides an explanation for how CFH blocks the interaction of CFB and promotes decay-acceleration activity (DAA) and cofactor activity.
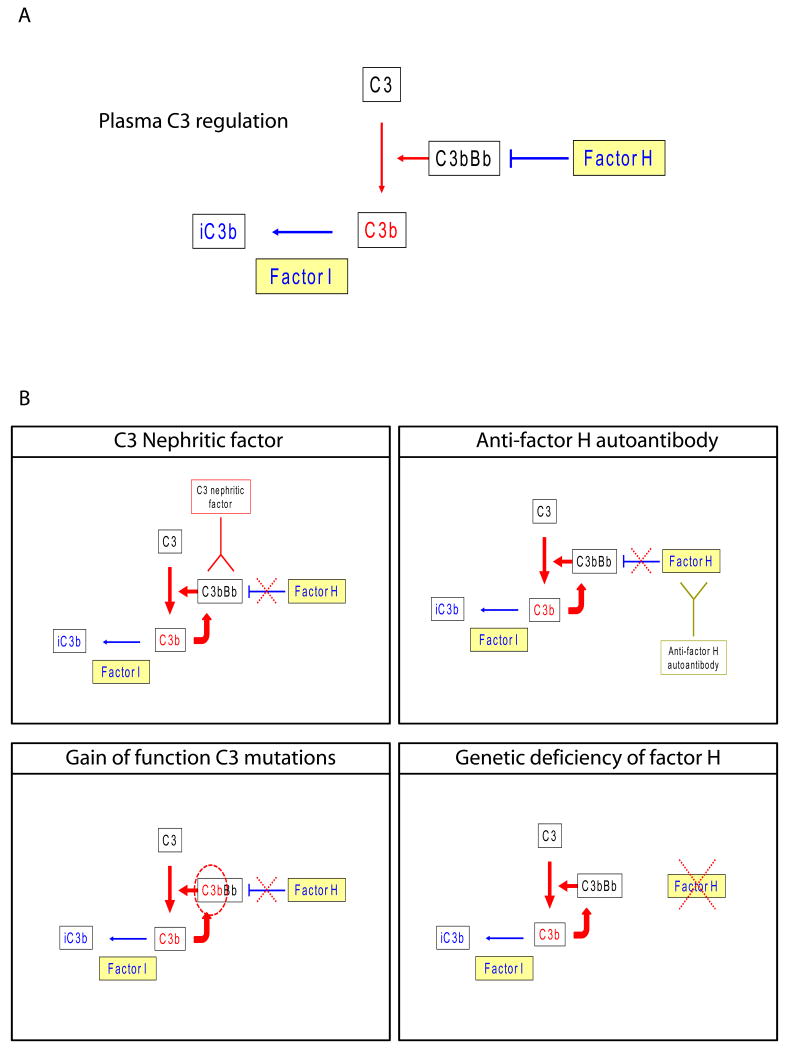
Figure 1.
A. Schematic showing cleavage of C3 by C3 convertase (C3bBb) to generate C3b. The generation of C3b is controlled by tight regulation of C3 convertase activity by a number of different proteins. In the fluid phase, the most important controlling protein is CFH (Factor H). C3b can be inactivated (iC3b) by CFI (Factor I). B. DDD is caused by fluid-phase dysregulation of the C3 convertase. The dysregulation can be caused by a number of different mechanisms. Illustrated are: C3 nephritic factors, which bind to and stabilize the C3 convertase, increasing its half-life from a few seconds to minutes or hours; CFH autoantibodies, which bind to the N-terminal SCRs of this protein and prevent CFH-mediated fluid-phase regulation of C3 convertase; gain-of-function mutations in C3, which render the mutant C3 convertase resistant to normal regulatory mechanisms; and genetic deficiency of CFH, which results in deficiency of CFH and C3 convertase control. The functional consequence of these different pathologies is consumption of plasma C3 and generation of vast amounts of C3 breakdown products, which are deposited in the GBMs and appear as the dense deposits. This observation suggests that therapies to prevent C3 convertase activity or to sequester iC3b in plasma should be evaluated as treatment possibilities for DDD.
DAA is mediated by SCRs 1 and 2 of CFH. These domains bind α′NT, MG2, MG6 and MG7 of C3b, and probably dissociate Bb from C3b by a combination of electrostatic repulsion and steric hindrance (Wu et al., 2009). Cofactor activity with CFI, another important function of CFH, is facilitated by the shape of CFH, which provides a contact interface for CFI to associate with the CFH-C3b complex by binding to SCRs 1-3 of CFH. CFH also stabilizes C3b so that CFI can sequentially cleave the scissile bonds C3b to produce iC3b and the C3f fragment (Wu et al., 2009).
The complex nature of these interactions make CFH, C3b and C3 convertase prone to functional interference with even small modifications in amino acid sequence, as has been illustrated by variations in AP activity associated with common polymorphisms of CFH and C3 (Abeleda et al., 2011; Heurich et al., 2010; Tortajada et al., 2009). These studies also provide a basis for understanding how DDD-associated variations and mutations can lead to dysregulation of the C3 convertase and uncontrolled AP activity. In addition, the importance of CFH and CFI in AP regulation is evident from the complement C3 levels seen in these respective deficiency states (reviewed in Botto et al., 2009). In each case, unregulated AP activation results in severe secondary C3 depletion.
Clinical Manifestations
DDD primarily affects children and young adults without sexual predilection (Lu et al., 2007; Lu et al., 2011). A recent review by Lu and colleagues of 98 DDD patients reported a median age-at-diagnosis of 14 years. At presentation, 90% of these patients had proteinuria, 84% had hematuria and over 50% were hypertensive (Lu et al., 2011).
DDD patients also develop drusen – electron-dense deposits in the retina between the collagenous layer of Bruch's membrane and the retinal pigmented epithelial cells – which carries a ∼10% risk for long-term visual problems (Chadha and Wright, 2009; Ritter et al., 2010). In some patients (less than 5% of cases in our series), DDD is seen with acquired partial lipodystrophy (APL), a disease characterized by the loss of fat from the face, extending to involve the neck, shoulders, arms, forearms and thorax. Renal disease can either precede or follow the loss of fat (Appel et al. 2005; Smith et al., 2007).
Although few families report multiple affected persons, which is consistent with DDD being a complex disease, it is striking that in 16% of DDD families there is at least one family member with type 1 diabetes (T1D) (Lu et al., 2011). This occurrence is far greater than expected based on the 1.4:1,000 familial prevalence of T1D in the general US population as reported by the Centers for Disease Control (2008) (Lu et al., 2011).
Once diagnosed, DDD interminably progresses to end-stage renal failure (ESRF) with a mean renal survival time of 10.24 years (Lu et al., 2011). ESRF is the more likely outcome if DDD is diagnosed in childhood, and of children, females have a more aggressive disease course. Transplantation is associated with histological disease recurrence in virtually all cases, resulting in a 5-year graft failure rate of 50% (Braun et al., 2005; Lu et al., 2011).
Unfortunately we are currently unable to predict who will progress to ESRF. Neither can we determine which DDD transplant recipients will lose their grafts. A focused effort to identify these persons is an important goal to improve disease management.
Genetics
Genetic dysregulation of the complement AP is associated with the development of DDD (reviewed in Smith et al., 2007). An extreme example of such dysregulation is homozygous deficiency of CFH, as described by Levy and colleagues in two Algerian siblings who had electron microscopic evidence of DDD in association with undetectable CFH levels (Levy et al., 1986). The affected individuals also had undetectable complement hemolytic activity and markedly reduced plasma C3 and terminal pathway components. The genetic defect was characterized as a homozygous cysteine-to-serine change in SCR7 of CFH (Dragon-Durey et al., 2004).
In another family reported by Licht and colleagues, two DDD-affected siblings of consanguineous parentage were homozygous for CFH delLys224 (Licht et al., 2006). This lysine is in SCR4. Its amide group forms a hydrogen bond with the carboxylate of C3b Glu1138 in TED, while its lysine side chain forms a salt bridge with CFH Glu213. Deletion of this residue is therefore predicted to alter the local structure of SCR4, with diminished binding of CFH to C3b and loss of its regulatory function (Wu et al., 2011). Consistent with these predictions, both siblings had markedly reduced complement hemolytic activity and plasma C3 levels. Circulating C3d levels (consistent with uncontrolled plasma C3 activation) were raised.
Although genetic deficiency of CFH is also associated with non-DDD renal pathologies such as thrombotic microangiopathy (Thompson and Winterborn, 1981), collagen type III glomerulopathy (Vogt et al., 1995) and fibrillary glomerulopathy (Bircan et al., 2004), the association between CFH deficiency and DDD has been corroborated by animal studies. A serendipitous event was the identification of a strain of pigs with spontaneous renal disease due to complete CFH deficiency. The Norwegian Yorkshire piglets were healthy at birth but developed rapidly progressive glomerulonephritis that inevitably lead to death at about one month of life (Jansen et al., 1998). The molecular basis for this outcome was a point mutation in CFH (Ile1166Arg), which prevented extracellular release of CFH effectively resulting in a CFH null phenotype (Hegasy et al., 2002). The renal lesion was analogous to human DDD. Consistent with the studies in this pig strain, gene-targeted CFH-deficient mice also develop spontaneous renal disease characterized by abnormal accumulation of C3 fragments within the glomeruli and the development of electron-dense deposits within the glomerular basement membrane (Pickering et al., 2002).
The association between DDD and CFH is further strengthened by the description of DDD in individuals with autoantibodies to CFH (discussed below), and demonstrates that both genetic and acquired CFH ‘loss-of-function’ is linked to DDD. From these data and the longstanding association between C3 nephritic factors (C3Nefs) and DDD (discussed below), the paradigm that DDD was due to uncontrolled AP C3 activation emerged.
Martínez-Barricarte and colleagues recently characterized a highly informative DDD pedigree (Martínez-Barricarte et al., 2010). The disease-segregating mutation was a two amino-acid deletion in MG7 of C3 (Δ923-924AspGly) that was the predominant C3 protein in the plasma of mutation carriers. It circulated as non-activated C3 and was resistant to activation to C3b. Interaction with CFH was also impaired, although a mutant C3Δ923DG convertase was formed by normal ‘tickover’ hydrolysis. The net consequence of these functional changes was a mutant fluid-phase C3 convertase (C3Δ923DG convertase) that, unlike a normal C3 convertase, could not be regulated by CFH. The mutation also disrupted the binding of C3Δ923DG to C3Bb, preventing cleavage of C3Δ923DG by the C3 convertase, explaining why serum C3 levels in affected persons in this family were reduced by only ∼50% (C3Δ923DG remained in the serum while C3 (wild-type) was consumed) (Martínez-Barricarte et al., 2010). Because both accelerated decay of the C3Δ923DG convertase by CD55 (DAF) and CD46 (MCP) cofactor activity for CFI were unaffected, cell-surface control of the AP was not impaired.
The demonstration by Martinez-Barricarte and colleagues that a ‘gain-of-function’ in the AP activating protein, C3, is associated with DDD further supports the paradigm that DDD is associated with uncontrolled C3 activation in the fluid phase. This result is also consistent with an earlier report that described C3 convertases resistant to inactivation by CFH in familial DDD (Linshaw et al., 1987). Critically, normal inhibition of the mutant C3 convertase by the cell surface regulators, CD55 (DAF) and CD46 (MCP), provides conclusive evidence that DDD in the Martínez-Barricarte pedigree results exclusively from fluid-phase AP dysregulation (Martínez-Barricarte et al., 2010).
Additional studies have searched for mutations in other complement genes in DDD patients. To our knowledge, there have not been reports of gain-of-function mutations in factor B in DDD or mutations in CFI, the enzyme responsible for the conversion of C3b to iC3b. The latter is of interest since CFI deficiency is associated with secondary C3 deficiency due to uncontrolled AP activation. Mutations have been reported in C3aR1, CR1 and ADAM19 genes but the functional significance of these findings remains unclear (Abrera-Abeleda et al., 2011).
An interesting aspect of these genetic studies has been the identification of polymorphic variants in complement genes that associate with DDD (DDD ‘at risk’ alleles). Four SNPs in CFH and C3 – namely CFH p.Tyr402His, CFH p.Val62Ile, C3 p.Arg102Gly and C3 p.Pro314Leu – are associated with DDD (Abrera-Abeleda et al, 2011). The presence of two or more of these risk alleles increases the odds ratio of developing disease and defines a DDD complement haplotype or complotype (Abrera-Abeleda et al, 2011). The best studied of these variants is the CFH Tyr402His polymorphism: CFH His402 is preferentially found in DDD patients. This polymorphism may determine differential binding to glycosaminoglycans (GAGs) and as a consequence differential protection of surfaces (Holz et al., 2004; Laine et al., 2007; Skerka et al., 2007).
The finding of a DDD complotype is consistent with the concept that variants in multiple interacting complement proteins have a combinatorial effect on AP control (Abrera-Abeleda et al., 2011; Heurich et al., 2010). Abrera-Abeleda and colleagues confirmed this hypothesis by showing that AP activity is increased in normal controls carrying DDD-associated genetic variants. The presence of two or more of these DDD risk alleles increases the odds ratio for developing DDD and defines a DDD complement haplotype or complotype (Abrera-Abeleda et al, 2011). In further support of this finding, functional analyses of the age-related macular degeneration (AMD)-associated polymorphisms in C3 (p.Arg102Gly), CFB (p.Arg32Gln) and CFH (p.Val61Ile) have shown that these variants also directly influence AP activity and that their effects are additive (Montes et al., 2009; Tortajada et al, 2009; Heurich et al. 2010). These data illustrate how an individual's complotype can influence susceptibility to diseases driven by dysregulation of the AP.
Copy number variations (CNVs) are an extremely important cause of complex disease and have not been adequately studied in DDD patients. We have looked at CNVs over the CFH-CFHR5 genomic interval using multiplex-ligation probe amplification (MLPA) in a large cohort of DDD patients. While homozygosis for del(CFHR3-CFHR1) was present in about 3% of controls and 15% of atypical hemolytic uremic (aHUS) patients, it was not seen in any of 68 DDD patients. This finding warrants further study as it suggests that deletion of both copies of CFHR3-CFHR1 is protective against DDD, a finding consistent with data demonstrating a protective role for this same genotype against AMD (Fritsche et al., 2010; Hageman et al., 2006; Zipfel et al., 2010).
At this time our understanding of the genetics of DDD is not satisfactory. Detailed studies must be completed in a large DDD population targeting for sequence analysis either the entire exome or all complement and complement-related genes (and genes in pathways that interact with complement), and for CNV analysis the entire genome. These data should be carefully correlated with clinical course to identify, if possible, genetic predictors of disease outcome in patients with native kidneys and following transplantation.
Autoantibodies and DDD
In 1969, Spitzer and colleagues described in serum of patients with glomerulonephritis “a substance that combines with a normal serum cofactor in the presence of magnesium ions to specifically cleave the third component of complement” (Spitzer et al., 1969). This substance was later characterized as an autoantibody to the AP C3 convertase that caused loss of control by stabilization of C3bBb (Davis et al., 1977; Daha and van Es, 1976 and 1981). These autoantibodies, generically termed C3 nephritic factors (C3NeF), have been extensively studied over nearly four decades and have been shown to be associated with various glomerular nephropathies and some other diseases.
C3NeF are detected in approximately 80% of DDD patients and are also described in patients with APL, many of whom develop DDD (Misra et al., 2004). However, a fundamental unanswered question is whether the associated NeF itself triggers disease, or whether NeF is a consequence of the disease process that then acts to exacerbate disease pathology. While NeFs are strongly associated with DDD, they are also occasionally found in normal individuals; indeed, it has been suggested that NeF is part of the normal immune repertoire based upon the observation that artificially activated mononuclear cells can produce high affinity NeF perhaps held in check by anti-idiotypic antibodies (Spitzer et al., 1990, 1992). For this reason, and also because levels of NeF do not always correlate with plasma C3 consumption and disease severity (Schena et al., 1982; Cameron et al., 1983; Schwertz et al., 2001), NeF is considered by some investigators to be an epiphenomenon. Hence their precise role in disease pathogenesis remains unclear.
In most cases, binding of NeF stabilizes the C3 convertase, increasing its half-life from a few seconds to minutes or even hours (Daha and van Es, 1981; Daha et al, 1976). NeF may also prevent decay accelerators, including CFH, CR1 and CD55 (DAF), from binding to C3 convertase to accelerate its disruption (Fischer et al., 1984; Ito et al., 1989; Weiler et al, 1976). Either mechanism results in fluid-phase dysregulation of the AP producing activated C3 fragments. Some NeFs appear to be dependent on the presence of properdin bound to the convertase and also cause marked terminal pathway activation (C3Nef:P) (Tanuma et al., 1990). While most NeFs bind to neoepitopes on C3b, Bb or epitopes shared between C3b and Bb in the C3bBb complex (Daha and van Es, 1981), there are reports that some autoantibodies can also bind native protein. An autoantibody which binds to native CFB and Bb has recently been described in a DDD patient (Strobel et al., 2010). This autoantibody stabilized the C3 convertase and enhanced C3 consumption, but had an inhibitory effect on the C5 convertase, decreasing TCC formation and target cell lysis. Autoantibodies to CFH that bind to the four amino-terminal SCRs essential for CFH-C3b interaction also cause fluid-phase dysregulation by preventing control of AP ‘tickover’ (Jokiranta et al., 1999). The relationship between uncontrolled AP activation and C3NeFs, ‘gain-of-function’ C3 mutations, CFH autoantibodies and CFH deficiency is illustrated schematically in Figure 1B.
Treatment
Disease-specific therapy for DDD is not currently available. About 80% of patients are placed on angiotensin II type 1 receptor blockers (ARBs) or angiotensin-converting enzyme (ACE) inhibitors, both first line agents used to improve renal dynamics, decrease proteinuria, control blood pressure and limit glomerular leukocyte infiltration. Over 50% of patients also self-report treatment with steroids, the efficiency of which is questionable (Appel et al., 2005; Lu et al., 2011; Smith et al., 2007). Concerted efforts to develop effective therapies are needed as 50% of patients progress to ESRF and face the dismal reality that DDD recurs in nearly all allografts and leads to graft failure in half (Smith et al., 2007).
The pathophysiology of DDD suggests that a number of different treatments warrant consideration. In the CFH-deficient mouse the abnormal accumulation of C3 fragments within the glomeruli is the first pathological abnormality to develop – it precedes the development of GBM abnormalities and glomerular inflammation. Importantly, renal C3 deposition and its depletion in plasma are rapidly reversed when either mouse or human CFH is administered to the CFH-deficient animals (Fakhouri et al., 2010; Paixao-Cavalcante et al., 2009). This outcome suggests that in DDD patients with CFH mutations resulting in either intracellular CFH retention or non-functional but excreted CFH, CFH replacement therapy would be expected to restore the underlying defect and correct the disease. Importantly CFH preparations may be available for therapeutic use in the future (Buttner-Mainik et al., 2010; Schmidt et al., 2011). Whether administration of exogenous CFH to DDD patients without CFH mutations would be therapeutically successful is not clear. Certainly this type of treatment would not be an appropriate approach in DDD patients with the C3Δ923DG mutation (or functionally analogous C3 mutations) reported by Martínez-Barricarte and colleagues since this mutant C3 protein formed a C3 convertase that was CFH resistant (Martínez-Barricarte et al., 2010). However, demonstration of differential regulation of dysfunctional convertases by CFH and other regulators (as is the case with the C3Δ923DG convertase) may enable treatment with non-CFH therapies, such as soluble forms of CD55 (DAF).
In the CFH-deficient mouse model, spontaneous renal disease did not occur when AP activation was prevented. In this animal, the AP was controlled by introducing genetic deficiency of CFB to prevent formation of C3 convertase (Pickering et al. 2002). If this observation is relevant to human DDD then one theoretical approach would be to inhibit AP activation by targeting CFB or CFD. An unexpected insight from murine studies was that the introduction of CFI deficiency prevented the accumulation of C3 along the glomerular basement membrane in the CFH-deficient mouse (Rose et al., 2008). The absence of CFI prevented the conversion of C3b to iC3b in this model, suggesting that it is the production of excessive amounts of iC3b in plasma, which then accumulates along the GBM, that triggers the renal pathology in CFH deficiency. This observation leads to the hypothesis that therapies that sequester iC3b in plasma may be beneficial in DDD.
In DDD patients with autoantibodies to CFH or CFB or with the C3NeFs, one can speculate that removal of the antibody (or reducing its titer in plasma) might ameliorate C3 dysregulation and renal pathology. B-cell depletion with agents such as rituximab could be considered although we are unaware of informative data on this type of approach.
In summary, DDD-specific treatment options that should be considered would restore C3 convertase control, impair C3 convertase activity or remove C3 breakdown products from the circulation (Smith et al., 2007). The benefit of targeting MAC is more difficult to assess. Presently, eculizumab, a monoclonal antibody that blocks C5 activation, is licensed for the treatment of anemia in patients with paroxysmal nocturnal hemoglobinuria. The role of C5 activation in the pathogenesis of DDD is unclear. In the CFH-deficient mouse model, the introduction of genetic C5 deficiency prevented neither the abnormal accumulation of C3 fragments along the GBM or the abnormal electron dense changes within it (Pickering et al., 2006). However, the spontaneous glomerular inflammation was reduced. Furthermore, CFH-deficient animals are hypersensitive to experimentally-induced renal disease including heterologous serum nephrotoxic nephritis (Pickering et al., 2006). This hypersensitivity is dependent on the ability to activate C5 suggesting that, whilst eculizumab would not be expected to influence either plasma C3 activation or the abnormal deposition of C3 within the GBM, it may be a beneficial intervention during episodes of glomerular inflammation. Its use in DDD needs to be considered carefully. It seems unlikely that patients with stable disease with little evidence of glomerular inflammation on renal biopsy would gain much benefit from C5 inhibiting strategies. However during episodes of renal decline associated with glomerular inflammation, C5 inhibition may be very beneficial.
Conclusion
DDD is an orphan disease. While our understanding of its pathophysiology has improved, its genetics and the role of autoantibodies in its progression must be explored more thoroughly to understand their association with clinical outcome. Integrating these data may make it possible to identify protective factors prognostic for naïve kidney and transplant survival, or conversely risk factors associated with progression to ESRF and allograft loss. There is also an opportunity for the development of mechanism-directed therapies for affected patients. As advances are made in these areas, there will be a need to increase healthcare provider awareness of DDD by making resources available to clinicians to optimize care for DDD patients.
Acknowledgments
We are grateful to those patients with DDD whose participation makes this research possible. This work was supported in part by NIH grant DK074409 to RJHS and MRC grant G0701298 to CLH. MCP is a Wellcome Trust Senior Fellow in Clinical Science (WT082291MA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrera-Abeleda MA, Nishimura C, Frees K, Jones M, Maga T, Katz LM, Zhang Y, Smith RJH. Allele variants of complement genes associated with dense deposit disease. J Am Soc Nephol. 2011 doi: 10.1681/ASN.2010080795. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel GB, Cook T, Hageman G, Jennette C, Kashgarian M, Kirschfink M, Lambris JD, Lanning L, Lutz HU, Meri S, Rose NR, Salant DJ, Sethi S, Smith RJH, Smoyer W, Tully HF, Tully SP, Walker P, Welsh M, Würzner R, Zipfel PF. Membranoproliferative glomerulonephritis type II (Dense Deposit Disease): An update. J Am Soc Nephrol. 2005;16:1392–1403. doi: 10.1681/ASN.2005010078. [DOI] [PubMed] [Google Scholar]
- Bircan Z, Toprak D, Kilicaslan I, Solakoglu S, Uysal V, Ponard D, Turker G. Factor H deficiency and fibrillary glomerulopathy. Nephrol Dial Transplant. 2004;19:727–730. doi: 10.1093/ndt/gfg605. [DOI] [PubMed] [Google Scholar]
- Botto M, Kirschfink M, Macor P, Pickering MC, Würzner R, Tedesco F. Complement in human diseases: Lessons from complement deficiencies. Mol Immunol. 2009;46:2774–83. doi: 10.1016/j.molimm.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Braun MC, Stablein DM, Hamiwka LA, Bell L, Bartosh SM, Strife CF. Recurrence of membranoproliferative glomerulonephritis type II in renal allografts: The north american pediatric renal transplant cooperative study experience. JASN. 2005;16:2225–2233. doi: 10.1681/ASN.2005020175. [DOI] [PubMed] [Google Scholar]
- Büttner-Mainik A, Parsons J, Jérôme H, Hartmann A, Lamer S, Schaaf A, Schlosser A, Zipfel PF, Reski R, Decker EL. Production of biologically active recombinant human factor H in Physcomitrella. Plant Biotechnol J. 2010 doi: 10.1111/j.1467-7652.2010.00552.x. In Press. [DOI] [PubMed] [Google Scholar]
- Cameron JS, Turner DR, Heaton J, Williams DG, Ogg CS, Chantler C, Haycock GB, Hicks J. Idiopathic mesangiocapillary glomerulonephritis. Comparison of types I and II in children and adults and long-term prognosis. Am J Med. 1983;74:175–192. doi: 10.1016/0002-9343(83)90606-x. [DOI] [PubMed] [Google Scholar]
- Chadha V, Wright M. Small margin excision of periocular basal cell carcinomas. Br J Ophthalmol. 2009;93:803–806. doi: 10.1136/bjo.2008.151183. [DOI] [PubMed] [Google Scholar]
- Daha MR, Fearon DT, Austen KF. C3 nephritic factor (C3NeF): stabilization of fluid phase and cell-bound alternative pathway convertase. J Immunol. 1976;116:1–7. [PubMed] [Google Scholar]
- Daha MR, van Es LA. Further evidence for the antibody nature of C3 nephritic factor (C3NeF) J Immunol. 1979;123:755–758. [PubMed] [Google Scholar]
- Daha MR, van Es LA. Stabilization of homologous and heterologous cell-bound amplification convertases, C3bBb, by C3 nephritic factor. J Immunol. 1981;43:33–38. [PMC free article] [PubMed] [Google Scholar]
- Davis AE, Ziegler JB, Gelfand EW, Rosen FS, Alper CA. Heterogeneity of nephritic factor and its identification as an immunoglobulin. Proc Natl Acad Sci U S A. 1977;74:3980–3983. doi: 10.1073/pnas.74.9.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragon-Durey MA, Frémeaux-Bacchi V, Loirat C, Blouin J, Niaudet P, Deschenes G, Coppo P, Herman Fridman W, Weiss L. Heterozygous and homozygous factor h deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J Am Soc Nephrol. 2004;15:787–795. doi: 10.1097/01.asn.0000115702.28859.a7. [DOI] [PubMed] [Google Scholar]
- Fakhouri F, de Jorge EG, Brune F, Azam P, cook HT, Pickering MC. Treatment with human complement factor H rapidly reverses renal complement deposition in factor H-deficient mice. Kidney Int. 2010;78:279–286. doi: 10.1038/ki.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira VP, Pangburn MK. Factor H mediated cell surface protection from complement is critical for the survival of PNH erythrocytes. Blood. 2007;110:2190–2192. doi: 10.1182/blood-2007-04-083170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E, Kazatchkine MD, Mecarelli-Halbwachs L. Protection of the classical and alternative complement pathway C3 convertases, stabilized by nephritic factors, from decay by the human C3b receptor. Eur J Immunol. 1984;14:1111–1114. doi: 10.1002/eji.1830141209. [DOI] [PubMed] [Google Scholar]
- Fritsche LG, Lauer N, Hartmann A, Stippa S, Keilhauer CN, Oppermann M, Pandey MK, Köhl J, Zipfel PF, Weber BH, Skerka C. An imbalance of human complement regulatory proteins CFHR1, CFHR3 and factor H influences risk for age-related macular degeneration (AMD) Hum Mol Genet. 2010;19:4694–4704. doi: 10.1093/hmg/ddq399. [DOI] [PubMed] [Google Scholar]
- Gros P, Milder FJ, Janssen BJC. Complement driven by conformational changes. Nat Rev Immunol. 2008;8:48–58. doi: 10.1038/nri2231. [DOI] [PubMed] [Google Scholar]
- Habib R, Gubler MC, Loirat C, Maiz HB, Levy M. Dense deposit disease: A variant of membranoproliferative glomerulonephritis. Kidney Int. 1975;7:204–215. doi: 10.1038/ki.1975.32. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Hancox LS, Taiber AJ, Gehrs KM, Anderson DH, Johnson LV, Radeke MJ, Kavanagh D, Richards A, Atkinson J, Meri S, Bergeron J, Zernant J, Merriam J, Gold B, Allikmets R, Dean M AMD Study Group. Extended haplotypes in the complement factor H (CFH) and CFH-related (CFHR) family of genes protect against age-related macular degeneration: characterization, ethnic distribution and evolutionary implications. Ann Med. 2006;38:592–604. [PMC free article] [PubMed] [Google Scholar]
- He JQ, Wiesmann C, van Lookeren Campagne M. A role of macrophage complement receptor CRIg in immune clearance and inflammation. Mol Immunol. 2008;45:4041–4047. doi: 10.1016/j.molimm.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Hegasy GA, Manuelian T, Hogasen K, Jansen JH, Zipfel PF. The molecular basis for hereditary porcine membranoproliferative glomerulonephritis type II. Am J Pathol. 2002;161:2027–2034. doi: 10.1016/S0002-9440(10)64481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen S, Hartmann A, Lauer N, Wiehl U, Dahse HM, Schirmer S, Gropp K, Enghardt T, Wallich R, Hälbich S, Mihlan M, Schlötzer-Schrehardt U, Zipfel PF, Skerka C. Factor H related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood. 2009;114:2363–2364. doi: 10.1182/blood-2009-02-205641. [DOI] [PubMed] [Google Scholar]
- Heurich M, Martinez-Barricarte R, Francis NJ, Roberts DL, de Cordoba SR, Morgan BP, Harris CL. The common C3F/S polymorphism, R102G, alters factor H regulation of the alternative pathway convertase and combines with other complement variants to influence complement activity in plasma. Mol Immunol. 2010;47:2257. [Google Scholar]
- Holz FG, Pauleikhoff D, Klein R, Bird AC. Pathogenesis of lesions in late age-related macular disease. Am J Ophthalmol. 2004;137:504–510. doi: 10.1016/j.ajo.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Ito S, Tamura N, Fujita T. Effect of decay-accelerating factor on the assembly of the classical and alternative pathway C3 convertases in the presence of C4 or C3 nephritic factor. J Immunol. 1989;68:449–452. [PMC free article] [PubMed] [Google Scholar]
- Isaak A, Prechl J, Gergely J, Erdei A. The role of CR2 in autoimmunity. Autoimmunity. 2006;39:357–366. doi: 10.1080/08916930600739001. [DOI] [PubMed] [Google Scholar]
- Jansen JH, Hogasen K, Harboe M, Hovig T. In situ complement activation in procine membranoproliferative glomerulonephritis type II. Kidney Int. 1998;53:331–349. doi: 10.1046/j.1523-1755.1998.00765.x. [DOI] [PubMed] [Google Scholar]
- Janssen BJC, Christodoulidou A, McCarthy A, Lambris JD, Gros P. Structure of C3b reveals conformational changes that underlie complement activity. Nature. 2006;444:213–216. doi: 10.1038/nature05172. [DOI] [PubMed] [Google Scholar]
- Janssen BJC, Huizinga EG, Raaijmakers HC, Roos A, Daha MR, Nilsson-Ekdahl K, Nilsson B, Gros P. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature. 2005;437:505–511. doi: 10.1038/nature04005. [DOI] [PubMed] [Google Scholar]
- Jokiranta TS, Solomon A, Pangburn MK, Zipfel PF, Meri S. Nephritogenic lambda light chain dimer: A unique human miniautoantibody against complement factor. H J Immunol. 1999;163:4590–4596. [PubMed] [Google Scholar]
- Khera R, Das N. Complement receptor 1: disease associations and therapeutic implications. Mol Immunol. 2009;46:761–772. doi: 10.1016/j.molimm.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberley FC, Sivasankar B, Paul Morgan B. Alternative roles for CD59. Mol Immunol. 2007;44:73–81. doi: 10.1016/j.molimm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Laine M, Jarva H, Seitsonen S, Haapasalo K, Lehtinen MJ, Lindeman N, Anderson DH, Johnson PT, Jarvela I, Jokiranta TS, Hageman GS, Immonen I, Meri S. Y402H polymorphism of complement factor H affects binding to C-reactive protein. 2007;178:3831–3836. doi: 10.4049/jimmunol.178.6.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Halbwachs-Mecarelli L, Gubler MC, Kohout G, Bensenouci A, Niaudet P, Hauptmann G, Lesavre P. H deficiency in two brothers with atypical dense intramembranous deposit disease. Kidney Int. 1986;30:949–956. doi: 10.1038/ki.1986.278. [DOI] [PubMed] [Google Scholar]
- Licht C, Heinen S, Jozsi M, Loschmann I, Saunders RE, Perkins SJ, Waldherr R, Skerka C, Kirschfink M, Hoppe B, Zipfel PF. Deletion of Lys224 in regulatory domain 4 of Factor H reveals a novel pathomechanism for dense deposit disease (MPGN II) Kidney Int. 2006;70:42–50. doi: 10.1038/sj.ki.5000269. [DOI] [PubMed] [Google Scholar]
- Linshaw MA, Stapleton FB, Cuppage FE, Forristal J, West CD, Schreiber RD, Wilson CB. Hypocomplementemic glomerulonephritis in an infant and mother. Evidence for an abnormal form of C3. Am J Nephrol. 1987;7:470–7. doi: 10.1159/000167525. [DOI] [PubMed] [Google Scholar]
- Lu D, McCarthy A, Lanning LD, Delaney C, Porter C. A descriptive study of individuals with membranoproliferative glomerulonephritis. Nephrol Nurs J. 2007;34:295–303. [PubMed] [Google Scholar]
- Lu D, Moon M, Lanning LD, McCarthy A, Smith RJH. A descriptive study of individuals with dense deposit disease (DDD) Pediatr Nephrol. 2011 submitted. [Google Scholar]
- Manuelian T, Hellwage J, Meri S, Caprioli J, Noris M, Heinen S, Jozsi M, Neumann HP, Remuzzi G, Zipfel PT. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J Clin Invest. 2003;111:1181–1190. doi: 10.1172/JCI16651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Barricarte R, Heurich M, Valdes-Cañedo F, Vazquez-Martul E, Torreira E, Montes T, Tortajada A, Pinto S, Lopez-Trascasa M, Morgan BP, Llorca O, Harris CL, Rodríguez de Córdoba S. Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest. 2010;120:3702–3712. doi: 10.1172/JCI43343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra A, Peethambaram A, Garg A. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy: report of 35 cases and review of the literature. Medicine (Baltimore) 2004;83:18–34. doi: 10.1097/01.md.0000111061.69212.59. [DOI] [PubMed] [Google Scholar]
- Montes T, Tortajada A, Morgan BP, de Cordoba SR, Harris CL. Functional basis of protection against age-related macular degeneration conferred by a common polymorphism in complement factor. B Proc Natl Acad Sci U S A. 2009;106:4366–71. doi: 10.1073/pnas.0812584106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan HP, Schmidt CQ, Guariento M, Blaum BS, Gillespie D, Herbert AP, Kavanagh D, Mertens HD, Svergun DI, Johansson CM, Uhrín D, Barlow PN, Hannan JP. Structural basis for engagement by complement factor H of C3b on a self surface. Nat Struct Mol Biol. 2011 doi: 10.1038/nsmb.2018. 2011 Feb 13 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paixao-Cavalcante D, Hanson S, Botto M, Cook HT, Pickering MC. Factor H facilitates the clearance of GBM bound iC3b by controlling C3 activation in fluid phase. Mol Immunol. 2009;46:1942–1950. doi: 10.1016/j.molimm.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtl IC, Kavanagh D, McIntosh N, Harris CL, Barlow PN. Disease-associated N-terminal complement factor H mutations perturb cofactor and decay-accelerating activities. J Biol Chem. 2011 doi: 10.1074/jbc.M110.211839. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, Botto M. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor. H Nat Genet. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- Pickering MC, Warren J, Rose KL, Carlucci F, Wang Y, Walport MJ, Cook HT, Botto M. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc Natl Acad Sci USA. 2006;103:9649–9654. doi: 10.1073/pnas.0601094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissner KT, Seiffert D. Role of vitronectin and its receptors in haemostasis and vascular remodeling. Thromb Res. 1998;89:1–21. doi: 10.1016/s0049-3848(97)00298-3. [DOI] [PubMed] [Google Scholar]
- Ritter M, Bolz M, Haidinger M, Deák G, Sacu S, Säemann M, Schmidt-Erfurth U. Functional and morphological macular abnormalities in membranoproliferative glomerulonephritis type II. Br J Ophthalmol. 2010;94:1112–1114. doi: 10.1136/bjo.2009.159475. [DOI] [PubMed] [Google Scholar]
- Roozendaal R, Carroll MC. Complement receptors CD21 and CD35 in humoral immunity. Immunol Rev. 2007;219:157–166. doi: 10.1111/j.1600-065X.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Rose KL, Paixao-Cavalcante D, Fish J, Manderson AP, Malik TH, Bygrave AE, Lin T, Sacks SH, Walport MJ, Cook HT, Botto M, Pickering MC. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest. 2008;118:608–618. doi: 10.1172/JCI32525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Corral P, Gonzalez-Rubio C, Rodriguez de Cordoba S, Lopez-Trascasa M. Functional analysis in serum from atypical hemolytic uremic syndrome patients reveals impaired protection of host cells associated with mutations in factor. H Mol Immunol. 2004;41:81–84. doi: 10.1016/j.molimm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Schena FP, Pertosa G, Stanziale P, Vox E, Pecoraro C, Andreucci VE. Biological significance of the C3 nephritic factor in membranoproliferative glomerulonephritis. Clin Nephrol. 1982;18:240–246. [PubMed] [Google Scholar]
- Schmidt CQ, Herbert AP, Hocking HG, Uhrín D, Barlow PN. Translational mini-review series on complement factor H: structural and functional correlations for factor. H Clin Exp Immunol. 2008;151:14–24. doi: 10.1111/j.1365-2249.2007.03553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CQ, Slingsby FC, Richards A, Barlow PN. Production of biologically active complement factor H in therapeutically useful quantities. Protein Expr Purif. 2011;76:254–263. doi: 10.1016/j.pep.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M, Spath L, Lux CA, Paprotka K, Torzewski M, Dersch K, Koch-Brandt C, Husmann M, Bhakdi S. Potential protective role of apoprotein J (clusterin) in atherogenesis: binding to enzymatically modified low-density lipoprotein reduces fatty acid-mediated cytotoxicity. Thromb Haemost. 2008;100:110–118. doi: 10.1160/TH07-12-0737. [DOI] [PubMed] [Google Scholar]
- Schwertz R, Rother U, Anders D, Gretz N, Scharer K, Kirschfink M. Complement analysis in children with idiopathic membranoproliferative glomerulonephritis: A long-term follow-up. Pediatr Allergy Immunol. 2001;12:166–172. doi: 10.1034/j.1399-3038.2001.012003166.x. [DOI] [PubMed] [Google Scholar]
- Sethi S, Gamez JD, Vrana JA, Theis JD, Bergen R, Zipfel PF, Dogan A, Smith RJH. Glumeruli of dense deposit disease contain components of the alternative and terminal complement pathway. Kidney Int. 2009;75:952–960. doi: 10.1038/ki.2008.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T, Atkinson JP. Functional properties of membrane cofactor protein of complement. Biochem J. 1989;264:581–538. doi: 10.1042/bj2640581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerka C, Lauer N, Weinberger AW, Keilhamer CN, Smith RJH, Schlotzer-Schrehardt U, Heinen S, Hartmann A, Weber BH, Zipfel PF. Defective complement control of factor H (Y402H) and FHL-1 in age-related macular degeneration. Mol Immunol. 2007;44:3398–3406. doi: 10.1016/j.molimm.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Smith RJH, Alexander J, Barlow PN, Botto M, Cassavant TL, Cook T, Rodriguez de Córdoba S, Hageman GS, Jokiranta TS, Kimberling WJ, Lambris JD, Lanning LD, Levidiotis V, Licht C, Lutz HU, Meri S, Pickering MC, Quigg RJ, Rops AL, Salant DJ, Sethi S, Thurman JM, Tully HF, Tully SP, van der Vlag J, Walker PD, Würzner R, Zipfel PF. New approaches to the treatment of dense deposit disease. J Am Soc Neprhol. 2007;18:2447–2456. doi: 10.1681/ASN.2007030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spendlove I, Ramage JM, Bradley R, Harris C, Durrant LG. Complement decay accelerating factor (DAF)/CD55 in cancer. Cancer Immunol Immunother. 2006;55:987–995. doi: 10.1007/s00262-006-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RE, Stitzel AE, Tsokos GC. Evidence that production of autoantibody to the alternative pathway C3 convertase is a normal physiologic event. J Pediatr. 1990;116:S103–108. doi: 10.1016/s0022-3476(05)82711-8. [DOI] [PubMed] [Google Scholar]
- Spitzer RE, Stitzel AE, Tsokos GC. On the origin of C3 nephritic factor (antibody to the alternative pathway C3 convertase): evidence for the Adam and Eve concept of autoantibody production. Clin Immunol Immunopathol. 1992;64:177–183. doi: 10.1016/0090-1229(92)90197-v. [DOI] [PubMed] [Google Scholar]
- Spitzer RE, Vallota EH, Forristal J, Sudora E, Stitzel A, Davis NC, West CD. Serum C′3 lytic system in patients with glomerulonephritis. Science. 1969;164:436–437. doi: 10.1126/science.164.3878.436. [DOI] [PubMed] [Google Scholar]
- Strobel S, Zimmering M, Papp K, Prechl J, Józsi M. Anti-factor B autoantibody in dense deposit disease. Mol Immunol. 2010;47:1476–1483. doi: 10.1016/j.molimm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Tanuma Y, Ohi H, Hatano M. Two types of C3 nephritic factor: properdin-dependent C3NeF and properdin-independent C3NeF. Clin Immunol Immunopathol. 1990;56:226–238. doi: 10.1016/0090-1229(90)90144-f. [DOI] [PubMed] [Google Scholar]
- Thompson RA, Winterborn MH. Hypocomplementaemia due to a genetic deficiency of beta 1H globulin. Clin Exp Immunol. 1981;46:110–119. [PMC free article] [PubMed] [Google Scholar]
- Tortajada A, Montes T, Martínez-Barricarte R, Morgan BP, Harris CL, de Córdoba SR. The disease-protective complement factor H allotypic variant Ile62 shows increased binding affinity for C3b and enhanced cofactor activity. Hum Mol Genet. 2009;18:3452–61. doi: 10.1093/hmg/ddp289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Wyatt RJ, Burke BA, Simonton SC, Kashtan CE. Inherited factor H deficiency and collagen type III glomerulopathy. Pediatr Nephrol. 1995;9:11–15. doi: 10.1007/BF00858956. [DOI] [PubMed] [Google Scholar]
- Volonakis JE, Frank MM. The Human Complement System in Health and Disease. Dekker; New York: 1998. [Google Scholar]
- Walker PD, Ferrario F, Joh K, Bonsib SM. Dense deposit disease is not a membranoproliferative glomerulonephritis. Mod Pathol. 2007;20:605–616. doi: 10.1038/modpathol.3800773. [DOI] [PubMed] [Google Scholar]
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- Weiler JM, Daha MR, Austen KF, Fearon DT. Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci USA. 1976;73(9):3268–72. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann C, Katschke KJ, Yin J, Helmy KY, Steffek M, Fairbrother WJ, McCallum SA, Embuscado L, DeForge L, Hass PE, van Lookeren Campagne M. Structure of C3b in complex with CRIg gives insights into regulation of complement activation. Nature. 2006;444:217–220. doi: 10.1038/nature05263. [DOI] [PubMed] [Google Scholar]
- Wu J, Wu YQ, Ricklin D, Janssen BJ, Lambris JD, Gros P. Structure of complement fragment C3b-factor H and implications for host protection by complement regulators. Nat Immunol. 2009;10:728–733. doi: 10.1038/ni.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF, Lauer N, Skerka C. The role of complement in AMD. Adv Exp Med Biol. 2010;703:9–24. doi: 10.1007/978-1-4419-5635-4_2. [DOI] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]