Abstract
Aims
The primary aim of this study was to compare the efficacy of smoking cessation treatment using a combination of nicotine patch and bupropion vs. nicotine patch and placebo bupropion. A secondary aim was to investigate whether the efficacy of bupropion is moderated by belief about whether one is receiving active or placebo medication.
Methods
Participants were recruited from a residential substance abuse treatment program and the community. We randomly assigned 148 smokers with between two and twelve months of alcohol abstinence to nicotine patch plus bupropion or nicotine patch plus placebo. All participants also received seven counseling sessions.
Results
At follow up, differences between medication conditions were not significant. Seven-day point prevalence quit rates in the patch plus bupropion vs. patch plus placebo conditions at week 24 were 6% and 11%, respectively. Differences between groups on prolonged abstinence and time to first smoking lapse were also not significant. However, among participants who received bupropion, those who accurately “guessed” that they were receiving bupropion were more likely to remain abstinent than those who incorrectly believed they were receiving placebo.
Conclusions
Findings do not support combining nicotine patch and bupropion for smoking cessation in this population. However, findings support previous studies suggesting the importance of assessing the blind in smoking cessation studies and its possible moderating effect on medication efficacy. Future directions for enhancing smoking cessation outcome in these smokers include investigations of intensive behavioral and pharmacological interventions, including studies of potential interactions between individual genetic differences and medication efficacy.
Keywords: tobacco dependence, smoking cessation, alcohol dependence
1. Introduction
People in recovery from alcohol problems have high rates of smoking (Kalman et al., 2005), are more likely to smoke heavily compared to smokers in the general population (Hughes, 1995), and are more likely to die from a smoking- than an alcohol-caused disease (Hurt et al., 1995). Although lifetime rates of quitting among smokers in alcohol recovery are lower than among smokers without a history of alcohol problems (Hughes and Kalman, 2006), studies indicate that successful smoking cessation on any given quit attempt is similar among smokers in long-term alcohol recovery and their nonalcoholic counterparts (Kalman et al., 2006; Prochaska et al., 2004).
By contrast, smokers in early recovery have great difficulty quitting. In a randomized clinical trial of smokers in alcohol recovery, smoking cessation at 6-month follow up was achieved by 28% of smokers with greater than one year of sobriety, but only 10% of smokers with less than a year (Kalman et al., 2006). In a meta-analysis of eight clinical trials of smokers in treatment for a substance use disorder (SUD), the mean quit rate at follow up for both intervention and control conditions was 7% (Prochaska et al., 2004). While smoking cessation outcomes have been disappointing, substantial evidence shows that treatment for tobacco dependence does not jeopardize alcohol/other drug abstinence, including among newly sober alcoholics (Kalman et al., 2010; Prochaska et al., 2004).
Most studies of smokers in alcohol recovery have investigated the efficacy of a single medication for smoking cessation. However, a recent study of smokers in early alcohol recovery showed promising results for the incremental efficacy of adding nicotine gum to nicotine patch (Cooney et al., 2009). To our knowledge, only one other study has investigated combination pharmacotherapy for smokers in early alcohol recovery. In a small placebo-controlled study (n=58), Grant et al. (2007) did not find any evidence for the incremental efficacy of adding bupropion to the nicotine patch. However, as the authors acknowledge, the study should be considered preliminary because of the small sample. In a recent meta-analysis of clinical trials that did not include smokers with histories of alcohol problems, Fiore et al. (2008) found cessation outcomes to be significantly higher with the combination of bupropion and the nicotine patch vs. the patch alone (estimated odds ratio = 1.3. The neurobiological actions of bupropion (a non-competitive antagonist of nACh receptors and weak inhibitor of dopamine uptake) and nicotine replacement (a competitive agonist of nACh receptors) also suggest that the combination may be more efficacious than either alone. The effects of bupropion on mesolimbic dopamine during the postquit period may be particularly important for smokers in early alcohol recovery because of the functional dopamine depletion associated with withdrawal from chronic, heavy alcohol and other drug use (Markianos et al., 2001).
The primary aim of this double-blind, placebo-controlled study was to investigate the incremental efficacy of bupropion added to transdermal nicotine in a sample of smokers in early alcohol recovery. We hypothesized that cessation outcomes would be higher in the group receiving nicotine patch + bupropion vs. nicotine patch + placebo. A secondary aim of this study was to investigate whether the efficacy of bupropion is moderated by belief about whether one is receiving active or placebo medication. Following Schnoll et al. (2008), we hypothesized that cessation outcomes would be higher among participants who received bupropion and believed they were receiving bupropion vs. those who believed they were receiving placebo. Finally, we investigated the effect of quitting smoking on self-perceived stress and the ability to remain alcohol abstinent. To our knowledge, this has never been investigated in this population of smokers.
2. Methods
2.1 Participants
Participants in the present study were recruited from a Veterans Administration Medical Center. To be eligible for the trial, participants must have (1) smoked at least 10 cigarettes per day, (2) had a history of alcohol abuse or dependence and (3) had between 2 and 12 months of abstinence from alcohol prior to enrollment. The Alcohol Use Disorders section of the Structured Clinical Interview for Diagnosis for DSM-IV (First et al., 1995) was administered to establish a diagnosis of alcohol use disorder. Exclusion criteria were: (1) older than age 70; (2) diagnosis of schizophrenia; (3) current psychotic episode; (4) cardiac problems in the past 3 months; (5) uncontrolled hypertension; (6) history of seizure; (7) history of head injury with neurological sequelae or prolonged loss of consciousness; and (8) use of medications that lower the seizure threshold. Participants were recruited between June 1, 2005 and November 30, 2009 and the follow-up assessment phase of the study was completed on April 1, 2010.
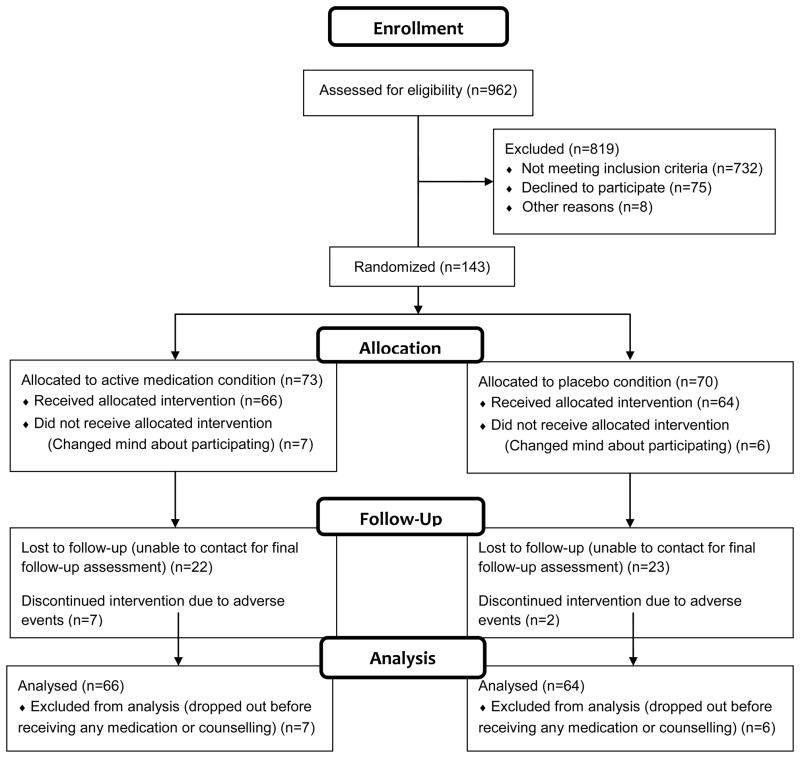
Nine hundred and sixty-two smokers were screened for the study (Figure 1). Seven hundred and thirty-two smokers did not meet study criteria. The most common reasons for exclusion were: currently uses alcohol and/or other drugs (n=243), no reported problem with alcohol use (n=164), reported more than 12 months of alcohol and other drug abstinence (n=128), history of head injury or seizure (n=116), current depression (n=60). Eighty-seven smokers who met study criteria decided not to enroll because they were not interested or were unable to meet the scheduling requirements of the study.
Figure 1.
Participant Flow Diagram
One hundred and forty-three participants signed an informed consent form. All participants provided written informed consent, and the study was approved by the Institutional Review Boards of the University of Massachusetts Institutional Review Board and the Edith Nourse Rogers Veterans Administration Hospital.
2.2 Medication
Participants were randomly assigned to bupropion or placebo for eight weeks. Participants began study medication (bupropion 150 mg SR tablets or placebo) one week prior to their quit day. Active and placebo medications were identical in appearance. Participants were instructed to take one tablet per day for three days and then one 150-mg tablet twice per day for the remainder of the treatment phase of the study. They were instructed to quit smoking one week after they began study medication. In addition, all participants received the nicotine patch for seven weeks starting on their quit day. They received the 21-mg patch for four weeks, the 14-mg patch for two weeks and the 7-mg patch for one week.
2.3 Randomization
Urn randomization was used to allocate 144 participants to medication condition (Stout et al., 1994). Four variables were included in the urn randomization: (1) gender; (2) severity of nicotine dependence (high versus low); (3) depressive symptoms (high versus low); and (4) substance use history (alcohol dependence only versus alcohol dependence plus at least one other drug dependence). Time to first cigarette in the morning (within 5 minutes of waking and more than 5 minutes after waking) was used to operationalize severity of nicotine dependence. This categorization differentiates smokers on a number of parameters, including carbon monoxide and cotinine levels and difficulty abstaining (Heatherton et al., 1989). Depressive symptoms and gender are often related to smoking cessation outcome (Covey, 1998; Wetter et al., 1999). A cutoff score of 16 on the Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977) was used to classify participants with “high” versus “low” depressive symptoms. The Structured Clinical Interview for Diagnosis for the DSM-IV was used to classify participants on the substance use history (First et al., 1997).
2.4 Counseling
All participants received eight weekly counseling sessions starting one week prior to their quit day. The first author wrote the counseling manual for the study and also provided the counseling. The manual described cognitive-behavioral and motivational techniques with demonstrated efficacy for smoking cessation (Fiore et al., 2008) (manual available upon request). Major elements of the counseling protocol involved the development of an individualized coping plan for preventing smoking in high-risk situations, practicing coping strategies prior to quitting (e.g., delaying stress-related smoking by thinking about one’s reasons for quitting and/or engaging in a distracting task), monitoring and addressing anxiety and ambivalence about quitting, and provision of counseling support prior to, and after, the quit day. The counseling protocol also included discussions of issues such as guarding against rationalizations, managing lapses, proactive vs. reactive coping, and developing a non-smoker identity.
2.5 Measures
Baseline Assessment
A sociodemographic questionnaire was used to collect data for age, gender and race/ethnicity. A smoking history questionnaire was used to collect data for cigarettes per day. The Fagerstrom Test of Nicotine Dependence (FTND) (Heatherton et al., 1991) was used to assess nicotine dependence. The CES-D (Radloff, 1977) was used to assess depressive symptomatology. Participants also indicated the number of weeks of alcohol/other drug abstinence at the time of enrollment.
Assessment of Participants’ Beliefs about their Assignment to Medication Condition
At the conclusion of treatment, participants were asked the following question, “Do you believe you are taking the real medication (Zyban)?” Response options were “yes” and “no.” Participants were also asked to indicate their confidence in their belief on a 10-point scale (0 = completely uncertain; 10 = completely certain).
Assessments of Smoking Status
Seven-day point prevalence smoking abstinence was determined at week 7 (end of treatment), week 11 and week 24. At week 7, smoking abstinence was defined via self report (complete abstinence during the 7 days prior to the time of assessment) and biochemical verification (CO reading of < 8ppm). At week 11 and week 24, smoking abstinence was defined via self report as above and biochemical verification (salivary cotinine levels of less than or equal to 15 ng/ml; (Hughes et al., 2003). In addition, we conducted assessments of prolonged abstinence which we defined as no smoking after the first two weeks of a participant’s scheduled quit day (Hughes et al., 2003). Finally, we determined the number of days between a participant’s scheduled quit day and his/her first cigarette. Participants lost to follow up were counted as smokers. In every case, these participants were smoking at last contact.
2.6. Statistical Considerations
In a modified intent-to-treat analysis, data were analyzed for the 130 participants who received at least one dose of study medication. Fourteen participants who dropped out before receiving any study medication were not included in the analyses. Completion rates at the 7-, 11- and 24-week follow-ups were 86%, 74% and 65%, respectively. Chi-square analyses were used to determine the association between medication condition and point prevalence smoking abstinence at each follow up. To examine the effect of treatment across time within the context of other covariates, we ran repeated measures analyses using Generalized Estimating Equations (GEE) (Liang and Zeger, 1986) with point prevalence abstinence at each follow up as the dependent variable. GEE analyses were also conducted to examine (1) the effect of medication condition on prolonged abstinence and (2) whether the efficacy of bupropion was moderated by belief about whether one was receiving active or placebo medication. Analyses were conducted in SAS’s PROC GENMOD (SAS Institute Inc.) with the logit link function and an unstructured correlation matrix specified. Finally, Cox proportional hazards models were used to predict time to first smoking lapse.
Power to detect significant effects of bupropion in the GEE models analyzing point prevalence abstinence across 3 time points was modest. Using power analysis for GEE models (Rochon, 1998), we calculated that 348 subjects would have been needed to have power of .80 to obtain a significant effect of bupropion if its true effect size was equal to an odds ratio of 2.0. With the sample size of 130, power was .80 to detect effects of bupropion equal to or greater than an odds ratio of 3.0, which is approximately equal to the effect size found for varenicline at the higher dose (Fiore et al., 2008). Thus, the sample size provided adequate power only to detect relatively large effects of bupropion.
3. Results
3.1. Sample Characteristics
Mean age of participants was 42 (SD = 9.6), 83% were male and 70% were Caucasian. They smoked a mean of 20.8 (SD = 11.2) cigarettes per day and 35.9 (SD = 24.4) pack years. Mean FTND score was 5.9 (SD = 1.9) and mean CES-D score was 18.8 (SD = 8.7). Participants had a mean of 3.7 (SD = 2.6) months of alcohol and other drug abstinence at the time of enrollment. Eighty-seven percent of participants met DSM-IV-TR criteria for severe alcohol dependence (i.e., met five or more diagnostic criteria). Table 1 describes the sample by medication condition. There were no differences between the two conditions on any variables.
Table 1.
Baseline characteristics of participants by medication condition
| Placebo (n=70) | Bupropion (n=73) | |
|---|---|---|
| Variable | Mean (SD) or % | Mean (SD) or % |
| Age | 49.2 (7.5) | 47.8 (10.5) |
| Race | ||
| White | 62.3 | 72.6 |
| Black (other than Hispanic) | 30.4 | 21.9 |
| Hispanic | 7.3 | 4.1 |
| Other | 0.0 | 1.4 |
| Severity of nicotine dependence | 5.9 (1.91) | 5.8 (1.9) |
| Cigarettes per Day | 21.7 (12.8) | 20.1 (9.1) |
| Number of Alcohol Dependence | ||
| Symptoms | 5.1 (1.2) | 5.1 (1.3) |
| Months since last drink | 4.3 (2.9) | 4.1 (3.0) |
| Severity of depressive symptoms | 19.2 (7.8) | 19.7 (9.2) |
Note. Severity of nicotine dependence measured with the Fagerstrom Test for Nicotine Dependence (Fagerstrom and Schneider, 1989); severity of depressive symptoms measured with the Center for Epidemiological Studies Depression Scale (Radloff, 1977); alcohol symptom count based on DSM-IV-R symptom list (American Psychiatric Association, 2000). Medication groups did not differ on any variables (all p’s > .20).
3.2 Adherence to Treatment
We assessed adherence to the medication and counseling protocols. Among participants in the active vs. placebo condition, mean number of days of medication use were 33.8 (SD = 15.8) and 32.0 (SD = 21.0), respectively (p = .58). Mean number of days of patch use across groups was 25.2 (SD = 18.3), and the difference between groups (bupropion vs. placebo) on patch use was nonsignificant (p = .38). Mean number of counseling sessions across groups was 4.8 (SD = 2.0); again, the difference between groups was nonsignificant (p = .89).
3.3 Medication Effects
Seven-day point prevalence abstinence rates at each follow up are shown in Table 2. There were no significant differences in abstinence rates between groups at any assessment point (all p values > .20).
Table 2.
Abstinence rates by medication condition (N=130)
| Week 7 | Week 11 | Week 24 | |
|---|---|---|---|
| Point prevalence abstinence rates | |||
| Bupropion | 19% | 12% | 6% |
| Placebo | 21% | 9% | 11% |
| Prolonged Abstinence | |||
| Bupropion | 13% | 9% | 3% |
| Placebo | 11% | 5% | 5% |
Note. For both 7-day point prevalence and prolonged abstinence, differences between groups at each assessment were not significant.
We then examined the effect of treatment within the context of other covariates using GEE with point prevalence abstinence at 7, 11, and 24 weeks post-quit as the dependent variable. In the initial model, nonsignificant variables were: gender, race, FTND, and time since last alcohol/other drug use. The effect of time was significant, odds ratio (OR) = 0.57 (95% Confidence Interval [CI] = 0.41, 0.80), reflecting the fact that the odds of abstinence decreased across follow-up assessments. CES-D score was also significant (OR = 0.96, CI = 0.92, 0.99). Higher scores were associated with a lower likelihood of abstinence. In the second step of the hierarchical analysis, treatment group was added to the model. The effect was nonsignificant (OR = 0.94, 95% CI = 0.43, 2.01, p > .20). In an exploratory analysis, the interaction term for FTND score and treatment condition was entered in the third step. The interaction was nonsignificant (OR = 1.34, CI = 0.84, 2.13, p > .20). We then removed this term from the model and entered the interaction term for CES-D and treatment condition which was also nonsignificant (OR = 0.96, CI = 0.90, 1.04, p >.20). Similarly, for prolonged abstinence at follow-ups, the main effect of bupropion was nonsignificant (OR = 1.62, 95% CI=0.55–4.79; see also Table 2). Cox proportional hazards models predicting time to first smoking lapse also indicated nonsignificant effects of bupropion in reducing the risk of lapsing compared to placebo (hazards ratio = 0.82, 95% CI = 0.58–1.16).
3.4 Moderating Effect of Belief on Medication Efficacy
Judgment Accuracy
Participants were asked to judge whether they were assigned to placebo or bupropion at quit date and at end of treatment. At each time point, both medication groups performed at the chance level in judging mediation assignment, quit date, χ2 (1) = 1.48, p = 0.22; end of treatment, χ2 (1) = 0.67, p = 0.41.
Judgment Confidence
Participants were asked to rate how confident they were in their medication judgment. At each time point, both medication groups were similarly confident in their judgments (quit date: t(98) = −0.64, p = 0.52, M = 6.34 SD = 2.63; end of treatment: t(87) = −0.43, p = 0.67, M = 7.33, SD = 2.49).
Judgment Accuracy and Abstinence
In order to evaluate the association between medication judgment accuracy and abstinence, we conducted a GEE logistic regression analysis, including medication assignment, time of follow up, and judgment accuracy at end of treatment. In addition, a term for the interaction between medication assignment and medication judgment accuracy was included. A main effect of medication assignment on abstinence was observed, χ2 (1) = 4.89, p = 0.0271, OR = 5.41, 95% C.I. (1.21, 24.2). In this more complex model, placebo-treated subjects had a slightly higher abstinence rates (placebo, 23.8%; bupropion, 20.4%). However, this main effect should be interpreted with caution in light of the significant interaction (see Figure 2). There was no main effect of judgment accuracy on abstinence, χ2 (1) = 1.75, p = 0.1860. However, judgment accuracy significantly moderated the effect of medication assignment on abstinence, χ2 (1) = 6.05, p = 0.0139, OR = 9.35, 95% C.I. (1.57, 55.5). Among those receiving bupropion, those who accurately judged being on bupropion were significantly more likely to be abstinent (26.1% abstinent) than bupropion-treated subjects who misjudged themselves to be receiving placebo (8.3% abstinent), χ2 (1) = 3.73, p = 0.05. Judgment accuracy did not influence abstinence rates in placebo-treated subjects although the rates were in the expected direction (placebo, correct, abstinence rate = 16.7%; placebo, incorrect, abstinence rate = 30.3%), χ2 (1) = 1.79, p = 0.18. We also explored the possibility that participant judgments were influenced by perceived medication-related adverse events. Rates of adverse events were computed by dividing the number of times an adverse event was endorsed by the number of times the participant completed the adverse events questionnaire. A formal profile analysis (a special case of MANOVA) was conducted to determine if the adverse events profiles of the four groups differed, and if the elevation of each profile differed. The shapes of the profiles (i.e., line plots) did not differ, Wilks’ Lambda = 0.87, F(15, 254.37) = 0.81, p = 0.6663. In addition the elevations of the profiles did not differ, F(3, 96) = 1.88, p = 0.1386.
Figure 2.
The moderating effect of participants’ beliefs about medication they received on medication efficacy
*p
Note. One week after starting study medication, participants were asked, “What medication do you think you are receiving?” White bar on far left represents the percentage of participants in the placebo condition who incorrectly believed they received active medication. The black bar on far left represents the percentage of participants in the placebo condition who correctly believed they received placebo medication. White bar on right represents the percentage of participants in the bupropion condition who incorrectly believed they received active medication. The black bar on right represents the percentage of participants in the bupropion condition who correctly believed they received bupropion medication. Outcome data on the Y axis are the percent abstinent at end of treatment based on 7-day point prevalence. Judgement accuracy significantly moderated the effect of medication assignment on abstinence for participants in the bupropion condition, χ2 (1) = 5.07, p = 0.02, OR = 7.01, 95% C.I. (1.29, 38.20).
3.5 The Effect of Quitting Smoking on Self-Reported Stress and Ability to Stay Sober
One week after their quit day, participants were asked, “What effect has trying to quit smoking had on your stress level?” Forty-one percent reported that trying to quit smoking increased their stress level. However, only 7% reported that it increased their stress a lot; the remainder said it increased their stress a little. In addition, participants were asked “What effect has trying to quit smoking had on your ability to stay clean and sober?” Only 6% said that trying to quit smoking made it more difficult to stay clean and sober. The remaining participants said it either had no effect or made it easier (Figure 3). A chi square analysis revealed no significant difference in self-reported difficulty maintaining sobriety according to quit status at week one postquit χ2 (1, N = 105) = 0.134, p = .52.
Figure 3.
The effect of trying to quit smoking on ability to stay clean and sober
3.6 Adverse Events
T-tests were conducted to determine whether there were significant differences between medication groups for adverse effects reported to be associated with the use of bupropion: insomnia, anxiety, headache, dizziness, dry mouth, and nausea. None of the differences were statistically significant, although there was a trend for participants in the bupropion condition to be more likely to report insomnia (p = .09).
Fifteen participants discontinued medication
Seven participants assigned to bupropion discontinued their medication due to adverse events (five for insomnia) that were likely to be related to study medication. Two participants assigned to placebo medication discontinued, one complained of severe dry mouth and one experienced a sudden rise in systolic blood pressure. Two participants discontinued patch use due to itching. Four participants discontinued participation due to adverse events (e.g., receiving a diagnosis of cancer) unrelated to study participation.
4. Discussion
The current study investigated the incremental efficacy of bupropion in combination with the nicotine patch in smokers with two to twelve months of alcohol abstinence. No significant differences in abstinence rates were found between individuals receiving bupropion and nicotine patch versus individuals receiving nicotine patch and placebo at any time point. Interaction effects between medication and tobacco dependence and medication and depressive symptoms were also nonsignificant. While statistical power to detect a main effect was modest (see Statistical Methods section above) and power was low to detect an interaction effect, these results suggest that individuals in early alcohol recovery may not benefit from the addition of bupropion when already using transdermal nicotine for smoking cessation.
These results are consistent with findings from a laboratory study we conducted as part of this trial in which there were no differences between medication groups on any subjective effect measure of smoking and had little effect on a purchase task used to model demand elasticity (Madden and Kalman, 2010). In addition, two of the three clinical trials that were included in the Public Health Service meta-analyses (Fiore et al., 2008) to investigate this combination of medication did not support the addition of bupropion to nicotine patch treatment. In their study of 244 smokers, Simon and colleagues (Simon et al., 2004) reported quit rates of 22% in the bupropion group and 28% in the placebo group at six-month follow up. In their study of active duty sailors, Swanson and colleagues (Swanson et al., 2003) reported that zero of 30 smokers versus three of 30 smokers achieved six-month abstinence in the bupropion plus patch and bupropion plus placebo conditions, respectively. By contrast, in the third study, which was also the largest of the three clinical trials, quit rates at 6-month follow up were 39% and 21%, respectively, in the combination versus patch only conditions (Jorenby et al., 1999). Findings for the incremental efficacy of bupropion added to nicotine patch are also mixed in psychiatric populations of smokers. While a significant incremental effect was found in a small study of smokers with schizophrenia (George et al., 2008), no incremental effect for bupropion was found in a study of smokers who met criteria for a lifetime depressive disorder, (Evins et al., 2008). In addition, as already noted, Grant and colleagues did not find an incremental effect for bupropion in their study of smokers in alcohol recovery (Grant et al., 2004). A comparison of the characteristics of these studies does not seem to suggest an explanation for these disparate findings. However, substantial heterogeneity of findings across studies is not uncommon (Ioannidis, 2005).
As noted earlier, the effects of bupropion on mesolimbic dopamine during the postquit period may be particularly important for smokers in early alcohol recovery because of the functional dopamine depletion often observed during withdrawal from chronic, heavy alcohol and other drug use (Markianos et al., 2001). However, there appear to be important genetic differences on the effect of withdrawal from chronic alcohol consumption on midbrain dopamine levels. Several studies have found increases in basal dopamine levels in rats selectively bred for high alcohol preference (Thielen et al., 2004). Most participants in the present study met criteria for severe alcohol dependence, a condition which has a strong genetic influence (Leggio et al., 2009). While speculative, it is possible that these participants did not experience the neuroadaptations that can lead to deficiencies in the functioning of the midbrain dopamine system with chronic alcohol exposure. If this were the case, then a critical action of bupropion that is believed to mediate its efficacy in smoking cessation is less likely to benefit smokers with severe alcohol dependence. While speculative, the lack of incremental efficacy we observed for bupropion may be related to the effect chronic alcohol consumption has on brain neurotransmitter systems, particularly the midbrain dopamine system. For example, chronic alcohol consumption alters the firing activity of dopamine neurons in the ventral tegmental area (Morikawa and Morrisett, 2010).
As already noted, there is substantial heterogeneity of findings across studies for the combination of bupropion and the nicotine patch. Stronger support currently exists for the use of a combination of a passive and ad libitum medication. For example, Cooney and colleagues (2009) recently reported a 6-month quit rate of 20% for smokers who received nicotine patch plus nicotine gum versus 12% for those receiving active patch plus placebo gum; quit rates for participants receiving the combination treatment dropped to 13% at 12 months, however. Additional support for this combination derives from the Public Health Service meta-analyses (Fiore et al., 2008). In these analyses, the estimated odds ratio of achieving abstinence with the nicotine patch plus nicotine gum versus patch alone was 1.9 percent. Recent support has also been found for the combination of patch and nicotine lozenge (Piper et al., 2009). Smith and colleagues (2009) and Piper and colleagues (2009) also found support for the combination of bupropion and nicotine lozenge versus.
The six-month point prevalence rate for the two medication groups combined (8.5%) in the present study is very similar to what we reported in a previous study (Kalman et al., 2006). It is also similar to the follow-up abstinence rate reported by Prochaska et al. (2004) in their meta-analysis of eight trials of smokers in addictions treatment. Interestingly, however, the end-of treatment point prevalence abstinence rate for the two medication groups combined (20%) in the present study is considerably higher than both the rate we obtained in our previous study (9%) and Prochaska et al. (2004) reported (11.7%) in their meta-analysis. One possible explanation is the intensity of the counseling. In the present study, participants received seven postquit counseling sessions. By contrast, in our previous study, participants received only three postquit counseling sessions. Support for this explanation derives from meta-analyses demonstrating an association between counseling intensity and outcome (Fiore et al., 2008). In addition, Burling et al. (2001) reported a 12-month quit rate of 19% for smokers who received more frequent counseling (several days per week) following a quit attempt during residential treatment for alcoholism. Further, the relatively high relapse rate we observed between the end-of-treatment and the six-month follow up assessment suggests that these smokers may benefit from extended counseling. Hall et al. (2004) found some of the highest quit rates ever reported at 1 year post-cessation (approximately 50% abstinence) when they treated smokers using an extended-duration (12 month) counseling protocol.
Consistent with many other studies (Burgess et al., 2002; Kenford et al., 2002; Zelman et al., 1992), elevated depressive symptoms were positively associated with a lower likelihood of smoking abstinence. Shiffman et al. (2000) found that bupropion significantly decreased elevated negative affect associated with withdrawal when compared to placebo. Lerman et al. (2002) found evidence that the effect of bupropion on outcome was partially mediated by bupropion’s effect on depressive symptoms. However, the effect was small and other studies provide mixed support for the role of depressive symptoms or, more generally, negative affect in mediating the effect of bupropion on smoking cessation (Brown et al., 2007; Kodl et al, 2008; Lerman et al., 2004; Mooney and Sofuoglu, 2006; Strong et al., 2009). Thus, in our analysis testing moderation, the lack of an interaction between bupropion and depressive symptoms was not unexpected, although this finding should be interpreted cautiously because of low statistical power to detect a significant interaction. The association between nicotine dependence and outcome was nonsignificant as was the interaction between nicotine dependence and medication condition. By contrast, in a large-scale study by Baker et al. (2007) the FTND score was strongly associated with cessation outcome (Fagerstrom and Schneider, 1989). However, in their analysis of data from 505 heavy smokers from three smoking cessation clinical trials, Kenford et al. (2002) found that variables associated with the affective component of dependence were significantly more strongly associated with outcome than variables associated with the physical component. The FTND taps the latter. Based upon their findings, they argue that “only the affective constituents of the [dependence] syndrome have motivational significance” (p. 224) (see also Baker et al., 2004 and Piasecki et al., 2000). Our findings for the CES-D and the FTND are consistent with this argument.
It was also hypothesized that cessation outcomes would be higher among participants who received bupropion and believed they were receiving bupropion versus those who believed they were receiving placebo. While both groups performed at chance level in judging medication assignment, the results indicated that among participants who received bupropion, those who accurately “guessed” that they were receiving the active medication were more likely to remain abstinent than those who incorrectly believed they were receiving placebo. Similarly, Schnoll et al. (2008) found that quit rates for participants who correctly guessed they were receiving bupropion were almost double that for participants who incorrectly guessed that they were receiving placebo; the 6-month quit rates were 36% and 19%, respectively. Only four nicotine patch studies have examined whether blindness failure moderated medication efficacy. Three studies found no evidence of moderation (Hall et al., 1987; Hughes et al., 1989; Tonnesen et al., 1991). In the fourth study, which investigated whether the nicotine patch helped smokers to reduce their smoking, Dar et al. (2005) found a significantly greater reduction in smoking among participants in both the active and placebo conditions who guessed that they received active medication. The main effect of medication condition was no longer significant after the assessment of the integrity of the blind was taken into consideration. Taken together, these studies and our own provide strong support for including such assessments in clinical trials (for further discussion, see Mooney et al., 2004; Perkins et al., 2003).
Finally, most participants (94%) revealed that smoking cessation did not have a negative effect on their ability to remain alcohol abstinent. To our knowledge, this is the first smoking cessation study to investigate the effect of quitting smoking on self-perceived ability to remain alcohol abstinent. Previous studies assessed participants’ beliefs about the effect that trying to quit smoking would have on their ability to remain alcohol abstinent (Monti et al., 1995; Rohsenow et al., 2005). Importantly, findings from these studies are consistent with our own (these smokers do not believe that trying to quit will jeopardize their sobriety), leading us to conclude that alcoholic smokers who try to quit smoking early in recovery neither expect that the quit attempt will jeopardize their sobriety nor conclude that it does upon quitting. Findings pertain only to alcoholic smokers who are motivated to try to quit smoking, however. Alcoholic smokers in early recovery unselected for their motivation to quit smoking are more likely to believe that trying to quit will jeopardize their sobriety (Rohsenow et al., 2005); however, even in this sample, over 60% said they did not believe trying to quit smoking would jeopardize their sobriety. Finally, participants in our study reported that making a quit attempt increased perceived stress. It is noteworthy, however, that only 7% reported that trying to quit smoking increased their stress “a lot.” It is also noteworthy that participants did not believe that trying to quit jeopardized their sobriety even though many reported that it increased their stress. Perhaps these smokers are prepared to return to smoking if they discover that the stress of trying to quit does, indeed, affect their ability to maintain sobriety. However, the fact that they do not expect quitting to have this effect undermines this explanation.
The present study has important strengths, including sample size, assessment of the blind and its effect on outcome, and post-cessation assessment of the effect of a quit attempt on self-perceived difficulty maintaining abstinence. Limitations include the fact that almost all participants had a history of severe alcohol dependence (e.g., caution should be exercised when generalizing findings to less severely dependent and hazardous drinkers), the somewhat lower than optimal long-term follow-up rates, and low statistical power to detect potential moderating effects of other variables (e.g., tobacco dependence) on medication condition. In addition, nicotine patch treatment is typically eight weeks, whereas, it was seven-weeks in the present study. Future research should continue to investigate intensive smoking cessation treatments for this population. As noted, there is some support for the use of intensive counseling interventions for this population (Burling et al., 2001). Building on the findings by Hall and colleagues (2002), future research should also investigate the use of extended treatment protocols for this population. These finding have potentially important implications for smokers in early alcohol recovery. Regarding pharmacotherapy, in addition to further investigations of combination treatments, there is a need to test medications, such as varenicline and topiramate, that have also shown promise for the treatment of alcohol dependence (George and Weinberger, 2007; Johnson et al., 2005; McKee et al., 2009). Finally, studies are needed that investigate potential interactions between individual genetic differences and medication efficacy (Furberg et al., 2010).
Footnotes
The trial is registered with ClinicalTrials.gov (NCT00304707)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ascher J, Cole J, Colin J, Feighner J, Ferris R, Fibiger H, Golden R, Martin P, Potter W, Richelson E. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395. [PubMed] [Google Scholar]
- Baker T, Piper M, McCarthy D, Bolt D, Smith S, Kim S, Colby S, Conti D, Giovino G, Hatsukami D. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9:S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T, Piper M, McCarthy D, Majeskie M, Fiore M. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Brown R, Niaura R, Lloyd-Richardson E, Strong D, Kahler C, Abrantes A, Abrams D, Miller I. Bupropion and cognitive–behavioral treatment for depression in smoking cessation. Nicotine Tob Res. 2007;9:721–730. doi: 10.1080/14622200701416955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess E, Brown R, Kahler C, Niaura R, Abrams D, Goldstein M, Miller I. Patterns of change in depressive symptoms during smoking cessation: who’s at risk for relapse? J Consult Clin Psychol. 2002;70:356–361. doi: 10.1037//0022-006X.70.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burling T, Burling A, Latini D. A controlled smoking cessation trial for substance-dependent inpatients. J Consult Clin Psychol. 2001;69:295–304. doi: 10.1037//0022-006x.69.2.295. [DOI] [PubMed] [Google Scholar]
- Cooney N, Cooney J, Perry B, Carbone M, Cohen E, Steinberg H, Pilkey D, Sevarino K, Oncken C, Litt M. Smoking cessation during alcohol treatment: a randomized trial of combination nicotine patch plus nicotine gum. Addiction. 2009;104:1588–1596. doi: 10.1111/j.1360-0443.2009.02624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J, Bruijnzeel A, Skjei K, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 2003;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Dar R, Stronguin F, Etter J. Assigned versus perceived placebo effects in nicotine replacement therapy for smoking reduction in Swiss smokers. J Consult Clin Psychol. 2005;73:350–353. doi: 10.1037/0022-006X.73.2.350. [DOI] [PubMed] [Google Scholar]
- Evins EA, Culhane MA, Alpert JE, Pava J, Liese BS, Farabaugh A, Fava M. A controlled trial of bupropion added to nicotine patch and behavioral therapy for smoking cessation in adults with unipolar depression. J Clin Psychol. 2008;28:660–66. doi: 10.1097/JCP.0b013e31818ad7d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom K, Schneider N. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Fiore M, Jaén C, Baker T, Bailey W, Benowitz N, Curry S, Dorfman S, Froelicher E, Goldstein M, Healton C. Clinical practice guideline. US Department of Health and Human Services, Public Health Service 5; Rockville, MD: 2008. Treating tobacco use and dependence: 2008 update. [Google Scholar]
- First MB, Spitzer RL, Williams JB, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Access I Disorders, Clinical Version. Columbia University Press; New York: 1997. [Google Scholar]
- Furberg H, Ostroff J, Lerman C, Sullivan P. The public health utility of genome-wide association study results for smoking behavior. Genome Med. 2010;2:26. doi: 10.1186/gm147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Sacco KA, Weinberger AH, Dudas MM, Allen BA, Creeden BS, Potenza MN, Feingold A, Jatlow PI. A placebo-controlled trial of bupropion combined with nicotine patch for smoking cessation in schizophrenia. Biol Psychiatry. 2008;63:1092–96. doi: 10.1016/j.biopsych.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George T, Weinberger A. Monoamine oxidase inhibition for tobacco pharmacotherapy. Clin Pharmacol Ther. 2007;83:619–621. doi: 10.1038/sj.clpt.6100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant K, Kelley S, Smith L, Agrawal S, Meyer J, Romberger D. Bupropion and nicotine patch as smoking cessation aids in alcoholics. Alcohol. 2007;41:381–391. doi: 10.1016/j.alcohol.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, Humfleet G, Reus V, Munoz R, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. Am J Psychiatry. 2004;161:2100. doi: 10.1176/appi.ajp.161.11.2100. [DOI] [PubMed] [Google Scholar]
- Hall S, Tunstall C, Ginsberg D, Benowitz N, Jones R. Nicotine gum and behavioral treatment: a placebo controlled trial. J Consult Clin Psychol. 1987;55:603–605. doi: 10.1037/0022-006X.55.4.603. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Johnston J. Measuring the heaviness of smoking: using self-reported time to first cigarette of the day and number of cigarettes per day. Br J Addict. 1989;84:791–800. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski L, Frecker R. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes J. Clinical implications of the association between smoking and alcoholism. In: Fertig JB, Allen JP, editors. Alcohol and Tobacco: From Basic Science to Clinical Practice. NIAAA Res. Mon. 30. NIH Pub. No. 95–3931; Washington, DC: 1995. [Google Scholar]
- Hughes J, Gust S, Keenan R, Fenwick J, Healey M. Nicotine vs. placebo gum in general medical practice. JAMA. 1989;261:1300. [PubMed] [Google Scholar]
- Hughes J, Kalman D. Do smokers with alcohol problems have more difficulty quitting? Drug Alcohol Depend. 2006;82:91–102. doi: 10.1016/j.drugalcdep.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Hughes J, Keely J, Niaura R, Ossip-Klein D, Richmond R, Swan G. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–26. [PubMed] [Google Scholar]
- Hurt R, Dale L, Offord K, Croghan I, Hays J, Gomez-Dahl L. Nicotine patch therapy for smoking cessation in recovering alcoholics. Addiction. 1995;90:1541–1546. doi: 10.1046/j.1360-0443.1995.9011154112.x. [DOI] [PubMed] [Google Scholar]
- Ioannidis JPA. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005;294:218–28. doi: 10.1001/jama.294.2.218. [DOI] [PubMed] [Google Scholar]
- Johnson B, Ait-Daoud N, Akhtar F, Javors M. Use of oral topiramate to promote smoking abstinence among alcohol-dependent smokers: a randomized controlled trial. Arch Intern Med. 2005;165:1600–05. doi: 10.1001/archinte.165.14.1600. [DOI] [PubMed] [Google Scholar]
- Jorenby D, Leischow S, Nides M, Rennard S, Johnston J, Hughes A, Smith S, Muramoto M, Daughton D, Doan K. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340:685. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Kalman D, Kahler CW, Garvey AJ, Monti PM. High-dose nicotine patch therapy for smokers with a history of alcohol dependence: 36-week outcomes. J Subst Abuse Treat. 2006;30:213–217. doi: 10.1016/j.jsat.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Kalman D, Kim S, DiGirolamo G, Smelson D, Ziedonis D. Addressing tobacco use disorder in smokers in early remission from alcohol dependence: the case for integrating smoking cessation services in substance use disorder treatment programs. Clin Psychol Rev. 2010;30:12–24. doi: 10.1016/j.cpr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14:106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: contrasting affective and physical models of dependence. J Consult Clin Psychol. 2002;70:216–227. [PubMed] [Google Scholar]
- Kodl MM, Fu SS, Willenbring AG, Gravely A, Nelson DB, Joseph AM. The impact of depressive symptoms on alcohol and cigarette consumption following treatment for alcohol and nicotine dependence. Alcohol Clin Exp Res. 2008;32:92–99. doi: 10.1111/j.1530-0277.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Leggio L, Kenna GA, Fenton M, Bonenfant E, Swift RM. Typologies of alcohol dependence: from Jellinick to genetics and beyond. Neuropsychol Rev. 2009;19:115–29. doi: 10.1007/s11065-008-9080-z. [DOI] [PubMed] [Google Scholar]
- Lerman C, Niaura R, Collins B, Wileyto P, Audrain-McGovern J, Pinto A, Hawk L, Epstein L. Effect of bupropion on depression symptoms in a smoking cessation clinical trial. Psychol Addict Behav. 2004;18:362–366. doi: 10.1037/0893-164X.18.4.362. [DOI] [PubMed] [Google Scholar]
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, Niaura R, Epstein L. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend. 2002;67:219–223. doi: 10.1016/s0376-8716(02)00067-4. [DOI] [PubMed] [Google Scholar]
- Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biom J. 1986;73:13. [Google Scholar]
- Madden G, Kalman D. Effects of bupropion on simulated demand for cigarettes and the subjective effects of smoking. Nicotine Tob Res. 2010;12:416–422. doi: 10.1093/ntr/ntq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markianos M, Lykouras L, Moussas G, Hatzimanolis J. Changes in dopamine receptor responsivity during alcohol detoxification may predict relapse. Drug Alcohol Depend. 2001;64:363–365. doi: 10.1016/s0376-8716(01)00139-9. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti P, Rohsenow DJ, Colby SM, Abrams DB. Smoking among alcoholics during and after treatment: implications for models, treatment strategies, and policy. In: Fertig JB, Allen JP, editors. Alcohol and Tobacco: From Basic Science to Clinical Practice, Research Monograph. Vol. 30. National Institute on Alcohol Abuse and Alcoholism; Washington, DC: 1995. pp. 187–206. [Google Scholar]
- Mooney M, Sofuoglu M. Bupropion for the treatment of nicotine withdrawal and craving. Expert Rev Neurother. 2006;6:965–981. doi: 10.1586/14737175.6.7.965. [DOI] [PubMed] [Google Scholar]
- Mooney M, White T, Hatsukami D. The blind spot in the nicotine replacement therapy literature: assessment of the double-blind in clinical trials. Addict Behav. 2004;29:673–684. doi: 10.1016/j.addbeh.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Morrisett R. Ethanol action on dopaminergic neurons in the ventral tegmental area:interaction with intrinsic ion channels and neurotransmitter inputs. Int Rev Neurobiol. 2010;91:235. doi: 10.1016/S0074-7742(10)91008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins K, Jetton C, Stolinski A, Fonte C, Conklin C. The consistency of acute responses to nicotine in humans. Nicotine Tob Res. 2003;5:877. doi: 10.1080/14622200310001614638. [DOI] [PubMed] [Google Scholar]
- Piasecki T, Niaura R, Shadel W, Abrams D, Goldstein M, Fiore M, Baker T. Smoking withdrawal dynamics in unaided quitters. J Abnorm Psychol. 2000;109:74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, Baker TB. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psych. 2009;66:1253–62. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska J, Delucchi K, Hall S. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol. 2004;72:1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385. [Google Scholar]
- Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Stat Med. 1998;17:1643–1658. doi: 10.1002/(sici)1097-0258(19980730)17:14<1643::aid-sim869>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Rohsenow D, Colby S, Martin R, Monti P. Nicotine and other substance interaction expectancies questionnaire: relationship of expectancies to substance use. Addict Behav. 2005;30:629–641. doi: 10.1016/j.addbeh.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Schnoll R, Epstein L, Audrain J, Niaura R, Hawk L, Shields P, Lerman C, Wileyto E. Can the blind see? Participant guess about treatment arm assignment may influence outcome in a clinical trial of bupropion for smoking cessation. J Subst Abuse Treat. 2008;34:234–241. doi: 10.1016/j.jsat.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Johnston J, Khayrallah M, Elash C, Gwaltney C, Paty J, Gnys M, Evoniuk G, DeVeaugh-Geiss J. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology (Berl) 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Simon JA, Duncan C, Carmody TP, Hudes ES. Bupropion for smoking cessation: a randomized trial. Arch Int Med. 2004;164:1797–1803. doi: 10.1001/archinte.164.16.1797. [DOI] [PubMed] [Google Scholar]
- Smith SS, McCarthy DE, Japuntich SJ, Christiansen B, Piper ME, Jorenby DE, Fraser DL, Fiore MC, Baker TB, Jackson TC. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Arch Int Med. 2009;169:2148–55. doi: 10.1001/archinternmed.2009.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong D, Kahler C, Leventhal A, Abrantes A, Lloyd-Richardson E, Niaura R, Brown R. Impact of bupropion and cognitive-behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine Tob Res. 2009;11:1142–1153. doi: 10.1093/ntr/ntp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol (Suppl) 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Swanson NA, Burroughs CC, Long MA, Lee RW. Controlled trail for smoking cessation in a Navy shipboard population using nicotine patch, sustained-release bupropion, or both. Mil Med. 2003;168:830–34. [PubMed] [Google Scholar]
- Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, Li TK, McBride WJ. Ethanol drinking and deprivation alter dopaminergic and serotinergic function in the nucleus accumbens of alcohol-preferring rats. J Pharmacol Exp Ther. 2004;309:216–25. doi: 10.1124/jpet.103.059790. [DOI] [PubMed] [Google Scholar]
- Tonnesen P, Norregaard J, Simonsen K, Sawe U. A double-blind trial of a 16-hour transdermal nicotine patch in smoking cessation. N Engl J Med. 1991;325:311. doi: 10.1056/NEJM199108013250503. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- Zelman D, Brandon T, Jorenby D, Baker T. Measures of affect and nicotine dependence predict differential response to smoking cessation treatments. J Consult Clin Psychol. 1992;60:943–952. doi: 10.1037//0022-006x.60.6.943. [DOI] [PubMed] [Google Scholar]