Abstract
Left ventricular torsion is increased and cardiac energetics are reduced in uncomplicated type 1 diabetes mellitus (T1DM). Our aim was to determine the relationships of these abnormalities to cardiovascular autonomic neuropathy (CAN) in subjects with T1DM. A cross-sectional study was conducted in 20 subjects with T1DM free of known coronary heart disease attending an outpatient clinic. Cardiovascular autonomic neuropathy was assessed using heart rate variability studies and the continuous wavelet transform method. Left ventricular function was determined by speckle tracking echocardiography. Magnetic resonance spectroscopy and stress magnetic resonance imaging were used to measure cardiac energetics and myocardial perfusion reserve index, respectively. Twenty subjects (age, 35 ± 8 years; diabetes duration, 16 ± 9 years; hemoglobin A1c, 8.0% ± 1.1%) were recruited. Forty percent of the subjects exhibited definite or borderline CAN. Log peak radial strain was significantly increased in subjects with CAN compared with those without (1.56 ± 0.06 vs 1.43 ± 0.14, respectively; P = .011). Data were adjusted for log duration of diabetes, and log left ventricular torsion correlated (r = 0.593, P = .01) with log low-frequency to high-frequency ratio during the Valsalva maneuver. Log isovolumic relaxation time correlated significantly with log Valsalva ratio and log proportion of differences in consecutive RR intervals of normal beats greater than 50 milliseconds during deep breathing. However, CAN did not correlate with cardiac energetics or myocardial perfusion reserve index. Spectral analysis of low-frequency to high-frequency ratio power during the Valsalva maneuver is associated with altered left ventricular torsion in subjects with T1DM. Parasympathetic dysfunction is closely associated with diastolic deficits. Cardiovascular autonomic neuropathy is not however the principal cause of impaired cardiac energetics. The role of CAN in the development of cardiomyopathy warrants further evaluation.
1. Introduction
Cardiovascular autonomic neuropathy (CAN) is a common complication of diabetes that confers considerable morbidity and mortality [1-3]. In type 1 diabetes mellitus, cardiovascular mortality is usually due to coronary artery disease (CAD), heart failure, or hypertension [4,5]. However, left ventricular (LV) dysfunction can occur without CAD or hypertension in diabetes [6], suggesting direct metabolic effects on the heart—metabolic heart disease [7]. Most reports have indicated that diastolic dysfunction precedes systolic dysfunction, but these findings may reflect the insensitivity of conventional systolic parameters [7]. Detection of early changes in the myocardium and identification of the contributing factors such as CAN are highly desirable to instigate preventative strategies.
Left ventricular twist, torsion, and strain provide information on regional and global cardiac tissue deformation [8]. Torsion reports the rotation of the apex with respect to the base along its long axis and is produced by contraction of the heart's obliquely spiraling myofiber layers. A period of rapid untwisting recoil follows maximal torsion and occurs largely during isovolumic relaxation [9]. The counterclockwise rotation of the apex during systole normally precedes the clockwise rotation of the base. Impairment of the function of the subendocardial fibers results in a delay and slowing in apical rotation. This has the paradoxical effect of increasing twist and untwist by moving apical and basal rotation into synchrony. Increased LV torsion and strain may provide sensitive and timely information on the early phases of LV systolic dysfunction in diabetes. Tagged magnetic resonance imaging (MRI) has identified increased torsion in subjects with both type 1 and type 2 diabetes mellitus with normal ejection fractions (EFs) [8,10]. Speckle tracking echocardiography (STE) is a novel angle-independent method of measuring LV strain and strain rates, which can measure LV rotation on grayscale 2-dimensional images [11,12]. We have previously used STE to demonstrate a significant increase in LV torsion in healthy subjects with type 1 diabetes mellitus compared with healthy controls [13] that correlated with the deficits of myocardial microvascular perfusion. This suggests a role for microvascular disease in the development of LV torsion.
Impaired cardiac energetic status may also play an important role in the development of heart failure [14]. We have previously demonstrated using magnetic resonance spectroscopy (MRS) that cardiac energetics are impaired in asymptomatic type 1 diabetes mellitus, a finding which is unrelated to diabetes duration or coronary microvascular function [15].
In summary, increased LV torsion, impaired cardiac energetics, and CAN often complicate type 1 diabetes mellitus. The aim of our study was therefore to determine the interrelationships of these functional and metabolic deficits.
2. Methods
Consecutive type 1 diabetes mellitus subjects who met the inclusion criteria and provided written informed consent were recruited from general diabetes clinics in the Heart of England NHS Foundation Trust and University Hospital Birmingham NHS Foundation Trust, Birmingham, UK. All investigations were undertaken in the University of Birmingham with approval from the Multicenter Regional Ethics Committee, Birmingham. Left ventricular torsion and LV torsion rate values for healthy controls with no history of diabetes were derived from data that we have previously published, with baseline characteristics including age, sex, body mass index, lipid profile, and blood pressure included in Table 1 [13].
Table 1.
Baseline characteristics comparing study population with type 1 diabetes mellitus and historical normal controls [13]
| Variable | Patients with T1DM | Historical controls |
|---|---|---|
| Age (y) | 34.8 ± 8 | 30 ± 8 |
| Male/female (n [%]) | 12/8 (60%/40%) | 22/10 (69%/31%) |
| Duration of diabetes (y) | 16.1 ± 9.0 | – |
| HbA1c (%) | 8.0 ± 1.12 | – |
| BMI (kg/m2) | 24.7 ± 3.2 | 25 ± 3.0 |
| Cholesterol (mmol/L) | 4.4 ± 0.8 | 4.9 ± 0.9 |
| HDL (mmol/L) | 1.7 ± 0.4 | 1.7 ± 0.6 |
| Microalbuminuria (ACR ≥2.5 in men or ≥3.5 in women) | 7 Patients (35%) | – |
| Retinopathy (any stage) | 13 Patients (65%) | – |
| Systolic blood pressure | 119 ± 15 | 112 ± 10 |
| Diastolic blood pressure | 77 ± 9 | 70 ± 10 |
Data are expressed as mean ± 1 standard deviation if not otherwise specified. HbA1c indicates hemoglobin A1c; BMI, body mass index; HDL, high-density lipoprotein; ACR, albumin to creatinine ratio.
2.1. Patients
We recruited 20 subjects with type 1 diabetes mellitus without evidence of CAD or heart failure based on history, 12-lead electrocardiogram, a normal EF on echocardiography, and metabolic exercise testing. Subjects fasted overnight, and blood samples were taken on the morning of the study. Subjects underwent exercise testing to rule out CAD and echocardiography, cardiac MRS, and stress MRI as previously reported [13]. Cardiovascular autonomic neuropathy was assessed on a separate visit, and there were no episodes of hypoglycemia during the assessment of CAN.
2.2. Exercise testing
Subjects underwent a symptom-limited erect treadmill exercise testing to exclude CAD.
2.3. Echocardiography
Echocardiography was performed with a Vivid 7 (GE Vingmed, GE Healthcare, USA) echocardiographic unit and a 2.5-MHz transducer. Standard echocardiographic views were obtained from parasternal and apical windows with the patient in left lateral decubitus position and breath held in end expiration. Left ventricular EF was calculated with the Simpson rule. Other cardiac parameters assessed included isovolumic relaxation time (IVRT), the ratio of early to late diastolic filling (E/A ratio) measured at the mitral valve, peak radial strain, and peak longitudinal strain, measured using speckle tracking. The IVRT and E/A ratio are measures of diastolic dysfunction, whereas radial strain and longitudinal strain reflect systolic function.
2.4. 31P cardiac MRS
In vivo myocardial energetics were measured using MRS via a 3T Philips Achieva scanner (Philips Healthcare, The Netherlands) as previously validated and described by our group [16]. Java magnetic resonance user interface version 3.0 was used for analysis [17]. Phosphocreatine (PCr) and γ-adenosine triphosphate (γATP) peaks in the distal septum were used to determine the PCr/γATP ratio. Data were analyzed blinded to the participants' clinical status. Cramér-Rao ratios were used to assess signal to noise ratio [16].
2.5. Stress MRI
Cardiac MRI was performed on a 3T Philips Achieva MRI scanner as previously described [13]. Survey images were followed by first-pass images after gadolinium contrast injection (0.1 mL/kg body weight, 4 mL/s). For perfusion images, a single shot turbo field echo SENSE pulse sequence was used with 3 slices per heartbeat. After a 20-minute gap, adenosine infusion was started at a rate of 140 µg/(kg min). At 3 minutes of infusion, stress first-pass images were obtained following injection of further gadolinium contrast (0.1 mL/kg).
2.6. CAN assessment
Heart rate (HR) variability was analyzed using the continuous wavelet transform methods to generate numerical and graphical data, instead of conventional fast Fourier transform for frequency domain analysis (ANX-3.0 software; ANSAR, Philadelphia, PA). The heartbeat intervals are recorded, and the HR variability is plotted in the frequency domain to separate the high-frequency (respiratory-frequency area, 0.15-0.4 Hz) from the low-frequency (low-frequency area, 0.04-0.15 Hz) components by spectral analysis.
3. Analysis
3.1. Speckle tracking echocardiography
Left ventricular rotation was measured using a commercially available speckle tracking system in an ECHOPAC (version 4.2.0) workstation (GE Healthcare, USA) as we have described previously [13]. Of the 20 subjects in the study, 19 (95%) subjects had both adequate LV basal and apical images for speckle tracking to complete analysis of all LV rotational parameters.
3.2. Cardiac autonomic neuropathy
The ANX-3.0 software was used to calculate low-frequency area (analogous to low-frequency power) and respiratory-frequency area (analogous to high-frequency power). The ratio of low-frequency power (LF) to high-frequency power (HF) is an estimate of the sympathetic/parasympathetic balance and was determined at baseline, deep breathing, Valsalva maneuver (VM), and standing. For the definition of CAN, standardized ratios were calculated during deep breathing (expiration-inspiration [E/I] ratio), during VM (the Valsalva ratio), and in response to standing (30/15 ratio) and postural drop in systolic blood pressure. Age-specific normal values were derived from published data [1,18,19]. We defined 1 or at least 2 abnormal test results as being consistent with borderline or definite CAN, respectively, out of the 3 ratios and postural drop in systolic blood pressure. Time domain indices including standard deviation of the RR interval from normal to normal beats (sdNN), root mean square of successive differences (rmsSD), and proportion of differences in consecutive RR intervals of normal beats greater than 50 milliseconds (pNN50) were also calculated for each of the 4 stages of the test; and correlations with LV torsion, parameters of diastolic function, and cardiac energetic as well as myocardial perfusion reserve index (MPRI) were done.
3.3. Statistics
SPSS 16.0 software (SPSS, Chicago, IL) was used for analysis. Continuous variables are expressed as means ± standard deviation. The means of independent groups were compared using the independent t test. A P value of < .05 was considered to indicate statistical significance. Data were checked for normality using the Shapiro-Wilk test. Non-normally distributed data were log transformed, and Pearson correlation coefficient (r) was used to describe the relationship between log-transformed variables.
4. Results
Baseline characteristics are described in Table 1. The mean age was 35 ± 8 years with 60% male, mean hemoglobin A1c of 8% ± 1.1%, and mean diabetes duration of 16 ± 9 years. Thirty-five percent of subjects had retinopathy (background, preproliferative, or proliferative), and 65% had microalbuminuria (albumin to creatinine ratio ≥2.5 for men and ≥3.5 for women). Seventy-five percent of the subjects in this study had retinopathy, microalbuminuria, or both, whereas 25% of the subjects had no evidence of either. Baseline characteristics of historical nondiabetic controls (NCs) [13] used to derive normal LV torsion values are also included in Table 1.
4.1. Cardiac autonomic neuropathy
The E/I ratio was abnormal in 3 (15%) of the 20 patients, the Valsalva ratio was abnormal in 7 (35%) of the 20 patients, and the 30/15 ratio was abnormal in 2 (11%) of the 18 patients. One patient had a more than 20–mm Hg drop in systolic blood pressure on standing. Based on the above CAN tests, 4 subjects (20%) had 2 or more abnormalities and were classified as definite CAN; and 4 subjects (20%) had 1 abnormality and were classified as borderline CAN.
4.2. LV torsion and CAN
Left ventricular torsion was significantly increased in the subjects with type 1 diabetes mellitus compared with values in our database of age-matched healthy NCs that we have previously reported (2.0°/cm ± 0.7°/cm vs 1.4°/cm ± 0.7°/cm, P = .01) [13]. There was a trend toward significant increase in LV torsion rate during systole compared with NCs (13.7°/[cm s] ± 6.0°/[cm s] vs 10.9°/[cm s] ± 4.8°/[cm s]), P = .07) [13].
Log peak radial strain was significantly increased in type 1 diabetes mellitus subjects with borderline and/or definite CAN compared with subjects without abnormalities of autonomic function (1.56 ± 0.06 vs 1.43 ± 0.14, respectively; P = .011). No other differences of LV function were however identified.
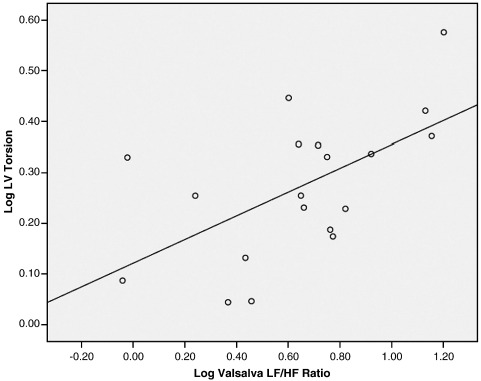
There was a significant positive correlation between log LF/HF ratio during the VM and log LV torsion (r = 0.58, P = .009) (Fig. 1). This correlation was still significant after adjusting for log duration of diabetes (r = 0.593, P = .01) (Table 2). The correlation between log LF/HF ratio during the VM and log LV torsion remained significant (r = 0.594, P = .019) even after adjusting for age and systolic and diastolic blood pressure, in addition to log duration of diabetes. Analysis of the 15 subjects who had retinopathy, microalbuminuria, or both showed that the correlation between log LF/HF ratio during the VM and log LV torsion remained similar (r = 0.584, P = .036) when adjusted for log duration of diabetes. Log LV torsion did not correlate with any of the other CAN tests.
Fig. 1.
Scatter plot of log LV torsion and log Valsalva LF/HF ratio.
Table 2.
Significant correlations of echocardiographic findings with parameters of HR variability, after adjusting for log duration of diabetes
| Correlation | Correlation coefficient (r) | Significance (P value) |
|---|---|---|
| Log LV torsion (°/cm) with log Valsalva LF/HF ratio | r = 0.593 | P = .01 |
| Log IVRT (ms) with log Valsalva ratio | r = 0.502 | P = .029 |
| Log IVRT (ms) with log deep breathing pNN50 | r = 0.483 | P = .042 |
| Log E/A ratio with log baseline pNN50 | r = 0.564 | P = .015 |
| Log radial strain (%) with log E/I ratio | r = −0.607 | P = .006 |
| Log radial strain (%) with log Valsalva ratio | r = −0.509 | P = .026 |
| Log longitudinal strain (cm/s) with log deep breathing LF/HF ratio | r = −0.456 | P = .05 |
4.3. Diastolic function and CAN
Correlations of log-transformed data were performed after adjustment for log duration of diabetes, and the significant correlations are presented in Table 2. Log IVRT was significantly positively correlated with log pNN50 during deep breathing and with log E/I ratio. Log E/A ratio was significantly positively correlated with log pNN50 at baseline. Log radial strain was significantly negatively correlated with log E/I ratio and log Valsalva ratio, whereas log longitudinal strain was significantly negatively correlated with log LF/HF ratio during deep breathing.
4.4. Cardiac energetics and CAN
Overall, the PCr/γATP ratio, a measure of cardiac energetics, was reduced in type 1 diabetes mellitus compared with our database of healthy controls (1.6 ± 0.2 vs 2.1 ± 0.5, P = .003) [15]. Log PCr/γATP ratio was not different in subjects with borderline and/or definite CAN compared with those with normal autonomic function (0.19 ± 0.09 vs 0.19 ± 0.06). No correlation of log PCr/γATP ratio was detected with any of the time or frequency domain parameters of CAN (data not shown).
4.5. MPRI and CAN
The MPRI was calculated using stress MRI, and log MPRI showed no correlation with any of the time or frequency domain parameters of CAN (data not shown).
5. Discussion
Left ventricular torsion, which contributes significantly to energy-efficient ejection during systole [20], is increased in asymptomatic subjects with type 1 diabetes mellitus. The etiology of this deficit is poorly understood. We therefore explored the relationships of LV torsion and cardiac energetic deficits to CAN in these subjects. Log peak radial strain was significantly increased in subjects with CAN compared with subjects with normal autonomic function test results. Spectral analysis of LF/HF power during the VM (a measure of sympathovagal balance) was associated with altered LV torsion. This correlation remained significant after adjustment for log duration of diabetes, age, and systolic and diastolic blood pressure. A similar correlation remained when only the 15 subjects with retinopathy, microalbuminuria, or both were included. In contrast, parasympathetic dysfunction was more closely correlated with diastolic deficits in these subjects [21].
Spectral analysis of LF/HF power during VM has, to our knowledge, not previously been reported. During the VM, forced expiration and the resultant increased intrathoracic pressure and reduced venous return subsequently stimulate the arterial baroreceptors to create a sympathetic drive in late phase II [22]. In phase IV, an increase in blood pressure stimulates lung mechanoreceptors to elicit parasympathetic predominance, resulting in bradycardia [22]. Therefore, complex, coordinated changes in sympathetic and parasympathetic activity and balance are elicited during the VM that, when expressed as log LF/HF power, correlate with increased log LV torsion. These data therefore implicate a role for altered autonomic balance in the development of abnormal LV systolic function.
We have previously reported using positron emission tomography (PET) and the sympathetic neurotransmitter analogue [11C]meta-hydroxyephedrine [23] that microvascular complications in type 1 diabetes mellitus are associated with increased cardiac sympathetic tone and adrenergic hyperresponsiveness. Increased LV torsion may therefore be the early consequence of cardiac sympathetic activation, thereby leading to myocardial injury [23]. In more advanced CAN, there is heterogeneous LV sympathetic denervation [24]. Left ventricular torsion is greatest at the apex of the LV; thus, regional LV sympathetic denervation may be mechanistically important. Future studies using meta-hydroxyephedrine–positron emission tomography will need to directly characterize sympathetic innervation/LV function relationships.
Alternatively, sympathetic activation and consequent increased torsion may be compensatory for a subclinical reduction in LV long-axis function and impaired early diastolic filling secondary to subendocardial ischemia and fibrosis. Longitudinal (long-axis) contraction of the LV is dependent on the integrity of longitudinal subendocardial myocardial fibers that are more susceptible to ischemia and fibrosis than circumferential fibers that are responsible for radial (short-axis) contraction [25]. In diabetes, increased radial contractility may compensate for reduced longitudinal contractility [26]. We have previously reported that LV torsion correlates with impaired MPRI in type 1 diabetes mellitus consistent with a role for myocardial ischemia [13]. However, this interpretation is complicated by evidence that sympathetic activation per se can impair myocardial perfusion in diabetes [23].
Our data demonstrated a robust correlation of CAN with a diverse spectrum of diastolic abnormalities. Diastolic dysfunction is an early marker of heart failure and has been demonstrated using radionuclide assessment as well as echocardiography in patients with diabetes, especially in those with CAN [27,28]. After maximal LV torsion occurs during systole, the heart experiences a clockwise recoil of twist (untwists) during the period of isovolumic relaxation of the LV. This release of restoring forces contributes to diastolic suction that is largely responsible for early LV diastolic filling. Increased LV torsion is associated with increased late peak LV untwisting rate and delayed time to late peak untwisting rate. Increased LV torsion is associated with early relaxation abnormalities in the LV, and the left atrial contribution to LV filling is increased [15]. We found a robust correlation of log-transformed IVRT and the E/A ratio with multiple frequency and time domain parameters in our subjects consistent with a role for parasympathetic deficits in diastolic dysfunction.
We did not detect a correlation of cardiac energetics with any parameter of CAN in our subject population. In the diabetic heart, free fatty acids (FFA) contribute to more than 90% of the myocardial oxygen consumption [29]. However, fatty acids are inefficient as an energy source in terms of oxygen requirement and may contribute to mitochondrial uncoupling, programmed cell death, fibrosis, and arrhythmogenesis [30]. Sympathetic activation increases plasma FFAs and can increase mitochondrial FFA uptake and oxidation [31]. The lack of correlation of CAN with cardiac energetics in our subjects is therefore somewhat surprising but may reflect either the small sample size particularly with regard to subjects with advanced CAN or that CAN does not significantly further exacerbate the diabetes-mediated perturbations of cardiac fatty acid and glucose metabolism.
A limitation of our study was the small sample size, which may limit our ability to detect significant correlations, and the indirect nature of the cardiac sympathetic assessment. We are also aware that a history of frequent severe hypoglycemia or degree of antecedent long-term diabetes control can affect the development of complications in diabetes, and these data were not available in our study. However, our data are consistent with the construct that LF/HF ratio during the VM may deserve consideration as a potential screening test for LV torsion. Prospective studies in which direct characterization of cardiac sympathetic tone is compared with LV torsion are currently in progress.
In summary, spectral analysis of LF/HF power during the VM is associated with altered LV torsion in healthy subjects with type 1 diabetes mellitus. Parasympathetic dysfunction is closely associated with diastolic deficits. Cardiovascular autonomic neuropathy is not however the principal cause of impaired cardiac energetics. The role of CAN in the development of cardiomyopathy warrants further evaluation.
Acknowledgment
The authors would like to thank British Heart Foundation for funding this project. Prof Martin Stevens is supported by Eli Lilly. Rodica Pop-Busui is supported by the American Diabetes Association Grant 1-08-CR-48, the Juvenile Diabetes Research Foundation Grant 1-2008-1025, and the National Institute of Health NIH/NHLBI 1R01HL102334-01. Dr Abd Tahrani is a research training fellow supported by the National Institute for Health Research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health.
Footnotes
MKP contributed to data collection, writing the manuscript, and contributing to the discussion. GNS contributed to data collection, writing the manuscript, and contributing to the discussion. AAT contributed to data collection, contributing to the discussion, and reviewing the manuscript. KD contributed to data collection. KA contributed to data collection. TTP contributed to data collection. PN contributed to data collection. RPB contributed to the discussion. MF contributed to data collection, contributed to the discussion, and reviewed the manuscript. MJS contributed to data collection, contributed to the discussion, and helped write the manuscript.
References
- 1.Boulton A.J., Vinik A.I., Arezzo J.C. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien I.A., McFadden J.P., Corrall R.J. The influence of autonomic neuropathy on mortality in insulin-dependent diabetes. Q J Med. 1991;79:495–502. [PubMed] [Google Scholar]
- 3.Sampson M.J., Wilson S., Karagiannis P. Progression of diabetic autonomic neuropathy over a decade in insulin-dependent diabetics. Q J Med. 1990;75:635–646. [PubMed] [Google Scholar]
- 4.Stone P.H., Muller J.E., Hartwell T. The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. The MILIS Study Group. J Am Coll Cardiol. 1989;14:49–57. doi: 10.1016/0735-1097(89)90053-3. [DOI] [PubMed] [Google Scholar]
- 5.Torffvit O., Lovestam-Adrian M., Agardh E. Nephropathy, but not retinopathy, is associated with the development of heart disease in type 1 diabetes: a 12-year observation study of 462 patients. Diabet Med. 2005;22:723–729. doi: 10.1111/j.1464-5491.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- 6.Rubler S., Dlugash J., Yuceoglu Y.Z. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 7.Marwick T.H. Diabetic heart disease. Heart. 2006;92:296–300. doi: 10.1136/hrt.2005.067231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung J., Abraszewski P., Yu X. Paradoxical increase in ventricular torsion and systolic torsion rate in type I diabetic patients under tight glycemic control. J Am Coll Cardiol. 2006;47:384–390. doi: 10.1016/j.jacc.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 9.Dong S.J., Hees P.S., Siu C.O. MRI assessment of LV relaxation by untwisting rate: a new isovolumic phase measure of tau. Am J Physiol Heart Circ Physiol. 2001;281:H2002–H2009. doi: 10.1152/ajpheart.2001.281.5.H2002. [DOI] [PubMed] [Google Scholar]
- 10.Fonseca C.G., Dissanayake A.M., Doughty R.N. Three-dimensional assessment of left ventricular systolic strain in patients with type 2 diabetes mellitus, diastolic dysfunction, and normal ejection fraction. Am J Cardiol. 2004;94:1391–1395. doi: 10.1016/j.amjcard.2004.07.143. [DOI] [PubMed] [Google Scholar]
- 11.Helle-Valle T., Crosby J., Edvardsen T. New noninvasive method for assessment of left ventricular rotation speckle tracking echocardiography. Circulation. 2005;112:3149–3156. doi: 10.1161/CIRCULATIONAHA.104.531558. Am Heart Assoc. [DOI] [PubMed] [Google Scholar]
- 12.Edvardsen T., Helle-Valle T., Smiseth O.A. Systolic dysfunction in heart failure with normal ejection fraction: speckle-tracking echocardiography. Prog Cardiovasc Dis. 2006;49:207–214. doi: 10.1016/j.pcad.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Shivu G.N., Abozguia K., Phan T.T. Increased left ventricular torsion in uncomplicated type 1 diabetic patients: the role of coronary microvascular function. Diabetes Care. 2009;32:1710–1712. doi: 10.2337/dc09-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abozguia K., Shivu G.N., Ahmed I. The heart metabolism: pathophysiological aspects in ischaemia and heart failure. Curr Pharm Des. 2009;15:827–835. doi: 10.2174/138161209787582101. [DOI] [PubMed] [Google Scholar]
- 15.Shivu G.N., Phan T.T., Abozguia K. Relationship between coronary microvascular dysfunction and cardiac energetics impairment in type 1 diabetes mellitus. Circulation. 2010;121:1209–1215. doi: 10.1161/CIRCULATIONAHA.109.873273. [DOI] [PubMed] [Google Scholar]
- 16.Shivu G.N., Abozguia K., Phan T.T. 31P magnetic resonance spectroscopy to measure in vivo cardiac energetics in normal myocardium and hypertrophic cardiomyopathy: experiences at 3T. Eur J Radiol. 2010;73:255–259. doi: 10.1016/j.ejrad.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Naressi A., Couturier C., Castang I. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31:269–286. doi: 10.1016/s0010-4825(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 18.Low P.A., Denq J.C., Opfer-Gehrking T.L. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997;20:1561–1568. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Gelber D.A., Pfeifer M., Dawson B. Cardiovascular autonomic nervous system tests: determination of normative values and effect of confounding variables. J Auton Nerv Syst. 1997;62:40–44. doi: 10.1016/s0165-1838(96)00107-5. [DOI] [PubMed] [Google Scholar]
- 20.Beyar R., Sideman S. Left ventricular mechanics related to the local distribution of oxygen demand throughout the wall. Circulation Research. 1986;58:664–677. doi: 10.1161/01.res.58.5.664. [DOI] [PubMed] [Google Scholar]
- 21.Poirier P., Bogaty P., Philippon F. Preclinical diabetic cardiomyopathy: relation of left ventricular diastolic dysfunction to cardiac autonomic neuropathy in men with uncomplicated well-controlled type 2 diabetes. Metabolism. 2003;52:1056–1061. doi: 10.1016/s0026-0495(03)00091-x. [DOI] [PubMed] [Google Scholar]
- 22.Looga R. The Valsalva manoeuvre—cardiovascular effects and performance technique: a critical review. Respir Physiol Neurobiol. 2005;147:39–49. doi: 10.1016/j.resp.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Pop-Busui R., Kirkwood I., Schmid H. Sympathetic dysfunction in type 1 diabetes. Association with impaired myocardial blood flow reserve and diastolic dysfunction. J Am Coll Cardiol. 2004;44:2368–2374. doi: 10.1016/j.jacc.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 24.Stevens M.J., Raffel D.M., Allman K.C. Cardiac sympathetic dysinnervation in diabetes: implications for enhanced cardiovascular risk. Circulation. 1998;98:961–968. doi: 10.1161/01.cir.98.10.961. [DOI] [PubMed] [Google Scholar]
- 25.Lumens J., Delhaas T., Arts T. Impaired subendocardial contractile myofiber function in asymptomatic aged humans, as detected using MRI. Am J Physiol Heart Circ Physiol. 2006;291:H1573. doi: 10.1152/ajpheart.00074.2006. [DOI] [PubMed] [Google Scholar]
- 26.Zhi You F., Leano R., Marwick T.H. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin Sci. 2004;106:53–60. doi: 10.1042/CS20030153. [DOI] [PubMed] [Google Scholar]
- 27.Kahn J.K., Zola B., Juni J.E. Radionuclide assessment of left ventricular diastolic filling in diabetes mellitus with and without cardiac autonomic neuropathy. J Am Coll Cardiol. 1986;7:1303–1309. doi: 10.1016/s0735-1097(86)80150-4. [DOI] [PubMed] [Google Scholar]
- 28.Ragonese P., Ferrazza A., Paolini A. Left ventricular diastolic filling in type I diabetes mellitus: a pulsed Doppler echocardiographic study. Eur J Med. 1992;1:69–74. [PubMed] [Google Scholar]
- 29.Herrero P., Peterson L.R., McGill J.B. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol. 2006;47:598–604. doi: 10.1016/j.jacc.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 30.Frustaci A., Kajstura J., Chimenti C. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 31.Stanley W.C., Lopaschuk G.D., McCormack J.G. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]