Abstract
Arabidopsis HY5 is a bZIP transcription factor that promotes photomorphogenesis. Previous studies suggested that COP1, a negative regulator of photomorphogenesis, directly interacts with nuclear HY5 and targets it for proteasome-mediated degradation. Light negatively regulates the nuclear level of COP1 and thus permits HY5 accumulation. Here we report that HY5 abundance peaks in early seedling development, consistent with its role in promoting photomorphogenesis. HY5 acts exclusively within a complex and exists in two isoforms, resulting from phosphorylation within its COP1 binding domain by a light- regulated kinase activity. Unphosphorylated HY5 shows stronger interaction with COP1, is the preferred substrate for degradation, has higher affinity to target promoters and is physiologically more active than the phosphorylated version. Therefore, HY5 phosphorylation provides an added level of light-mediated regulation of HY5 stability and activity besides nuclear COP1 levels. Regulated HY5 phosphorylation not only provides abundant and physiologically more active unphosphorylated HY5 in the light, but also helps to maintain a small pool of less active phosphorylated HY5 in the dark, which could be essential for a rapid initial response during dark-to-light transition.
Keywords: CKII/COP1/HY5/phosphorylation/protein degradation
Introduction
Recent years have seen considerable progress in dissecting the molecular pathways sensing the intensity, composition, photoperiod and direction of light in Arabidopsis (Deng and Quail, 1999; Nagy and Schaefer, 2000; Neff et al., 2000). An interesting aspect of light signaling is the initial switch from dark-adapted development (skotomorphogenesis) to light-adapted development (photomorphogenesis). Skotomorphogenesis is a heterotrophic growth mode in which the seedling concentrates its resources to escape from darkness (such as underneath the soil). This is achieved by elongation of the hypocotyl towards the light, while cotyledon development is paused. Upon illumination, the seedling switches to photomorphogenesis, resulting in inhibition of hypocotyl elongation, opening and expansion of the cotyledons, and commencement of photosynthesis. A number of genes essential for this process have been identified and their activity is regulated by light signals perceived by multiple photoreceptors (Deng and Quail, 1999; Nagy and Schafer, 2000; Neff et al., 2000). Two important components in this developmental switch are encoded by the HY5 and COP1 genes of Arabidopsis (Osterlund et al., 1999).
HY5 encodes a basic leucine zipper (bZIP) transcription factor and has a role in the promotion of photomorphogenesis as well as in the regulation of lateral root development (Oyama et al., 1997). The activity of HY5 is largely controlled by COP1, a repressor of photomorphogenesis whose activity is abrogated by light. HY5 and COP1 interact both genetically and physically (Ang and Deng, 1994; Ang et al., 1998), and this interaction negatively regulates HY5 activity. Recently we demonstrated that the abundance of HY5 protein inversely correlates with light intensity and is a critical regulatory point for HY5 activity (Osterlund et al., 2000). The cellular HY5 level is regulated via a darkness-dependent degradation, probably mediated by the 26S proteasome. Degradation of HY5 in darkness requires its COP1 interaction domain, supporting the hypothesis that COP1 targets HY5 for degradation. Since COP1 displays a light-mediated nucleo-cytoplasmic repartitioning (von Arnim and Deng, 1994), enriched in the nucleus in the dark and depleted from the nucleus in the light, light ultimately regulates the abundance of HY5 by regulating nuclear COP1 levels. However, it is not known whether there are additional built-in biochemical mechanisms to regulate the COP1 and HY5 interaction within the nucleus specifically.
The activities of several plant transcription factors implicated in light-regulated gene expression are modulated by phosphorylation (Sarokin and Chua, 1992; Harter et al., 1994; Marechal et al., 1999). In particular, some Arabidopsis factors appear to be regulated by casein kinase II (CKII) (Klimczak et al., 1995; Sugano et al., 1998, 1999), a ubiquitous tetrameric serine/threonine kinase composed of two catalytic and two regulatory subunits (Klimczak et al., 1992). Recently, the influence of CKII on light-regulated gene expression has been demonstrated more directly. Overexpression of a regulatory subunit known to interact with circadian clock regulators disturbed the light period-driven oscillation of their target genes (Sugano et al., 1999). Antisense inhibition of the catalytic subunit of CKII likewise disturbed light-regulated transcription of well characterized light-responsive genes (Lee et al., 1999). Since a well conserved putative CKII phosphorylation site is located in the N-terminus of HY5 (Oyama et al., 1997), it is of interest to evaluate a possible role of phosphorylation in regulating HY5 abundance and activity. In this manuscript, we provide evidence that HY5 is indeed a likely target of CKII. We show that HY5 is part of a protein complex in vivo, and exists in two differentially phosphorylated isoforms. Our data suggest that HY5 phosphorylation indeed plays a key role in this light-regulated developmental switch.
Results
HY5 abundance peaks at the early seedling stage
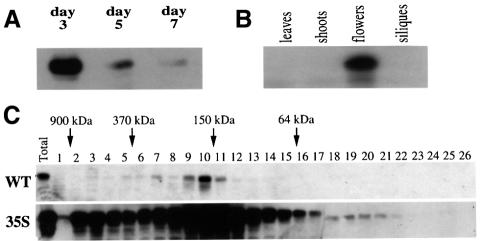
We sought to gain further insight into the role of HY5 in Arabidopsis photomorphogenesis by examination of the expression of HY5 protein at different developmental stages. While HY5 accumulates very little in dark grown seedlings, its level is increased 15- to 20-fold in the light (Osterlund et al., 2000). Interestingly, when Arabidopsis seedlings were grown in constant light, HY5 abundance exhibited its highest level 2–3 days after germination and was then followed by a steady decrease (Figure 1A). In extracts from vegetative aerial tissues and old seedlings, HY5 protein was hardly detectable (Figure 1B). The high abundance of HY5 during early seedling development is consistent with its prominent role in promoting commitment to photomorphogenesis. Interestingly, HY5 accumulation finally resumed in inflorescences, reflecting a possible requirement for HY5 activity at this developmental stage.
Fig. 1. Analysis of HY5 protein abundance in different tissues and in vivo conformation. (A) HY5 protein accumulation during seedling development in the light. Protein extracts were prepared from seedlings at the indicated age after germination and equal amounts of protein were loaded and examined by western blot analysis. Similar results were obtained if loading was based on an equal number of seedlings (not shown). (B) Tissue-specific expression of HY5 protein throughout Arabidopsis development. Protein extract was prepared from different aerial vegetative tissues and equal amounts of protein were loaded and examined by western blot analysis. (C) Gel filtration analysis of protein extract from Arabidopsis seedlings. Protein extracts were prepared from 3-day-old seedlings and 0.5 ml fractions (1–26) were collected and analyzed for the presence of HY5 by western blot analysis. Top: wild-type seedlings. Bottom: HY5 overexpressing line (35S::HY5). Note that HY5 is detected in a protein complex peaking at 150 kDa.
HY5 acts exclusively as a component of a complex in vivo
It has been shown that in vivo, many transcription factors act as part of larger protein complexes (Wolberger, 1998). To determine whether this is also the case for HY5, we analyzed protein extract from wild-type seedlings by gel filtration chromatography (Figure 1C). This experiment revealed that HY5 is present in a protein complex of ∼150 kDa. Interestingly, no monomeric HY5 was detected, even in a 35S::HY5 overexpression line (Ang et al., 1998). Rather, some excess HY5 formed high molecular weight aggregates in the latter case. It has been shown that HY5 monomer is predicted to be a monomer of ∼18 kDa, but migrates at 30 kDa in SDS–PAGE (Osterlund et al., 2000); thus a HY5 dimer could migrate in the range of 40–60 kDa depending on the shape of the dimer in gel filtration. In any event, a HY5 dimer would be much smaller than the observed 150 kDa size. Thus it seems likely that HY5 associates with other factors, which must be abundant in the cell. Further examination revealed that HY5 is also present in the same size complex in dark-grown seedlings (data not shown). Therefore, HY5 probably functions exclusively as part of a protein complex.
HY5 protein is a target for phosphorylation by a light-regulated kinase activity
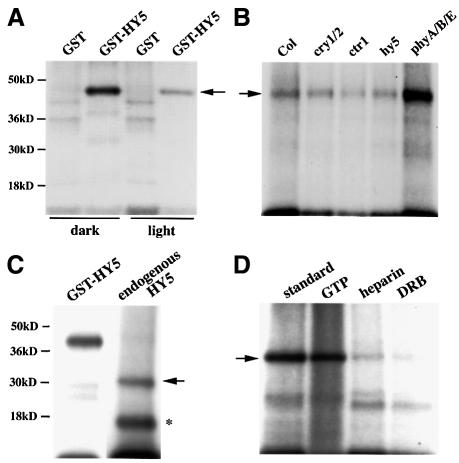
Phosphorylation by CKII modulates the activities of several transcription factors implicated in light-dependent gene expression (e.g. Klimczak et al., 1995; Sugano et al., 1999). As a first step to investigate whether phosphorylation also plays a role in the regulation of HY5, we used bacterially expressed glutathione S-transferase (GST)–HY5 fusion protein (GSTHY5) as a substrate in in vitro kinase assays in the presence of radioactively labeled ATP. While no autophosphorylation potential was observed in these assays (data not shown), GSTHY5, unlike GST control protein, was a good substrate for phosphorylation by Arabidopsis protein extract (Figure 2A). Interestingly, the responsible kinase activity was higher in extract from dark-grown seedlings than from light-grown seedlings. We also observed elevated kinase activity in the extract from a phyA/phyB/phyE triple mutant (Devlin et al., 1998) grown in continuous white light (Figure 2B). However, in the same experiment, no striking difference to wild type in kinase activity was observed in extracts from a double mutant for the blue light receptors (cry1/cry2) (Mockler et al., 1999), an unrelated kinase mutant (ctr1) (Kieber et al., 1993) or hy5 itself. Thus, the light-dependent reduction of kinase activity directed towards HY5 seems to be mediated primarily by the phytochromes.
Fig. 2. Characterization of an Arabidopsis kinase activity for HY5 in vitro. (A) Bacterially expressed GSTHY5 is a substrate for phosphorylation in vitro. GST or GSTHY5 protein was purified from E.coli and tested as a substrate in in vitro kinase assays by adding equal amounts of protein extract from seedlings in the presence of radioactively labeled ATP. GST or GSTHY5 was then collected from the assays using glutathione–Sepharose beads and run on SDS–polyacrylamide gels. Autoradiography detects a signal at the expected size of GSTHY5 (arrow), while no significant phosphorylation of the GST moiety alone is observed. Equal amounts of extracts (based on total protein) from light- and dark-grown seedlings were compared. (B) Light regulation of the kinase activity toward GSTHY5. The level of kinase activity towards GSTHY5 (arrow) was compared between equal amounts of protein extract from seedlings, grown in continuous white light, of the wild type (Col, Columbia ecotype), the cry1/cry2 blue light receptor double mutant, the ctr1 kinase mutant, the hy5 mutant and the phyA/phyB/phyE triple mutant. (C) Phosphorylation of the endogenous HY5. The kinase containing fraction from a heparin–Sepharose column also contains endogenous HY5. Endogenous HY5 was immunoprecipitated after a kinase assay with this fraction without added substrate (right lane). The precipitated HY5 became labeled due to phosphorylation (arrow, right lane). A protein cross-hybridizing with the HY5 antibody (asterisk) is also phosphorylated. The same protein fraction was also used for a kinase activity assay for GSTHY5 recombinant protein (left lane), as shown in (A). (D) Characteristics of the kinase activity enriched in the heparin–Sepharose fraction. GSTHY5 (arrow) was used as a substrate in assays using GTP as an alternative phosphate donor, or containing the non-specific kinase inhibitor heparin (10 µM) or the casein kinase inhibitor DRB (10 µM).
To determine whether endogenous HY5 is likewise phosphorylated, we enriched HY5 through heparin–Sepharose column chromatography. This HY5 fraction also contained enriched kinase activity. After incubation of the fraction under kinase assay conditions, endogenous HY5 was immunoprecipitated using HY5 antibody (Figure 2C). Again, endogenous HY5 was phosphorylated, as was a smaller protein that cross-reacts with the HY5 antibody (Osterlund et al., 2000).
Since the CKII activity involved in light-dependent gene expression can be enriched via heparin–Sepharose chromatography (Klimczak et al., 1992), similar to the enrichment of HY5 and the kinase activity as described above, we examined whether CKII activity is responsible for the HY5 phosphorylation. Indeed, the HY5-specific kinase activity fulfills several criteria characteristic of CKII (Klimczak et al., 1992; Figure 2D): the efficient use of GTP as a phosphate donor instead of ATP, inhibition by low amounts of heparin, and inhibition by the CKII specific inhibitor dichlororibofuranosylbenzimidazole (DRB). Thus, a CKII activity is probably responsible for phosphorylating HY5.
Unphosphorylated HY5 interacts more strongly with COP1 in vitro
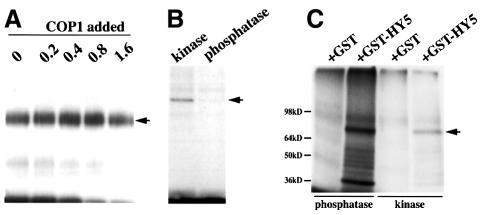
Since COP1 has been shown to physically interact with HY5 (Ang et al., 1998), we sought to determine whether COP1 possibly influences HY5 activity by interfering with HY5 phosphorylation. To test this, increasing amounts of bacterially expressed maltose binding protein–COP1 fusion protein (MBPCOP1) were added to the established in vitro kinase assays. In these experiments, no significant influence of COP1 on HY5 phosphorylation was observed (Figure 3A), indicating that COP1–HY5 interaction does not alter the rate of HY5 phosphorylation in vitro.
Fig. 3. Relationship between HY5 phosphorylation and HY5–COP1 interaction. (A) COP1 does not significantly interfere with HY5 phosphorylation in vitro. An increasing amount of MBP–COP1 fusion protein (given in micrograms) purified from bacteria was added to in vitro kinase assays (see Figure 2) using 0.2 µg of the heparin–Sepharose fraction with 0.1 µg of GSTHY5 (arrow) as substrate. (B) Phosphorylation of GSTHY5 (arrow) with radioactively labeled ATP in a kinase assay (kinase lane) can be reversed by a successive treatment with the non-specific lambda protein phosphatase (phosphatase lane). (C) Unphosphorylated GSTHY5 interacts stronger with COP1 protein in an in vitro protein interaction assay. GST or GSTHY5 was pretreated in a kinase assay and then half of the sample was dephosphorylated using the non-specific lambda protein phosphatase. The preparations were then compared with respect to their capacity to pull down a bacterially purified, radioactively labeled FLAG–COP1 fusion protein (arrow) in an in vitro protein interaction assay (see Materials and methods). Lower bands represent degradation products of the COP1 fusion protein, which have been observed previously (Ang et al., 1998).
We also have previously shown that enhancement of HY5 degradation in darkness depends on its interaction with the COP1 protein (Osterlund et al., 2000). Thus, we were interested to test whether phosphorylation influences HY5’s interaction with COP1. To this end, the GSTHY5 protein was first phosphorylated by the kinase-containing fraction of enriched seedling extract in an in vitro kinase assay. The non-specific lambda protein phosphatase was then used to dephosphorylate GSTHY5 (Figure 3B). To test interactions, the capacity of the phosphorylated GSTHY5 (generated by kinase treatment) and dephosphorylated GSTHY5 (generated by a successive lambda protein phosphatase treatment) to pull down bacterially expressed and radioactively labeled FLAG-tagged COP1 in an in vitro protein interaction assay (Ang et al., 1998) was compared. Interestingly, the de-phosphorylated GSTHY5 interacted about four times stronger with COP1 than the phosphorylated protein, as determined by total radioactivity associated with the COP1 band (Figure 3C). As the in vitro phosphorylation and dephosphorylation of GSTHY5 may not be 100% efficient, the observed difference in COP1 binding capacity between the phosphorylated and dephosphorylated forms of GSTHY5 is very likely to be an underestimation.
A consensus CKII site is located in the COP1 interaction domain of HY5
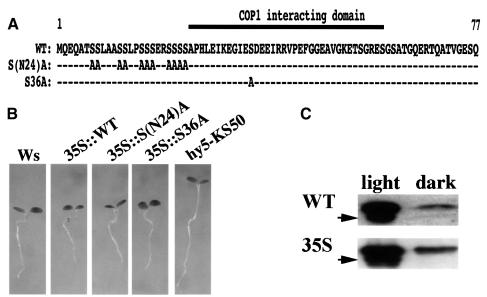
The clear effect of phosphorylation on HY5’s ability to interact with COP1 prompted us to examine whether the phosphorylation site overlaps with the COP1 binding domain. It has been shown in yeast two-hybrid assays that the COP1 interaction domain resides within the N-terminal 77 amino acids of HY5 (Ang et al., 1998). A putative CKII phosphorylation site is present in this region (ESDEE, amino acids 35–39; Oyama et al., 1997). We carried out further deletions on both ends of the 77 amino acid fragment and examined their ability to interact with COP1 by yeast two-hybrid assays (Figure 4A). This enabled us to narrow down further the COP1 interaction domain to a 36 amino acid stretch between residues 25 and 60, which includes the putative CKII site. On the other hand, we have shown previously that a deletion of the first 40 amino acids of HY5, which just removes the putative CKII site, completely abolished its ability to interact with COP1 (Ang et al., 1998). Together, our results suggest that the CKII site is within the COP1-interactive domain of HY5.
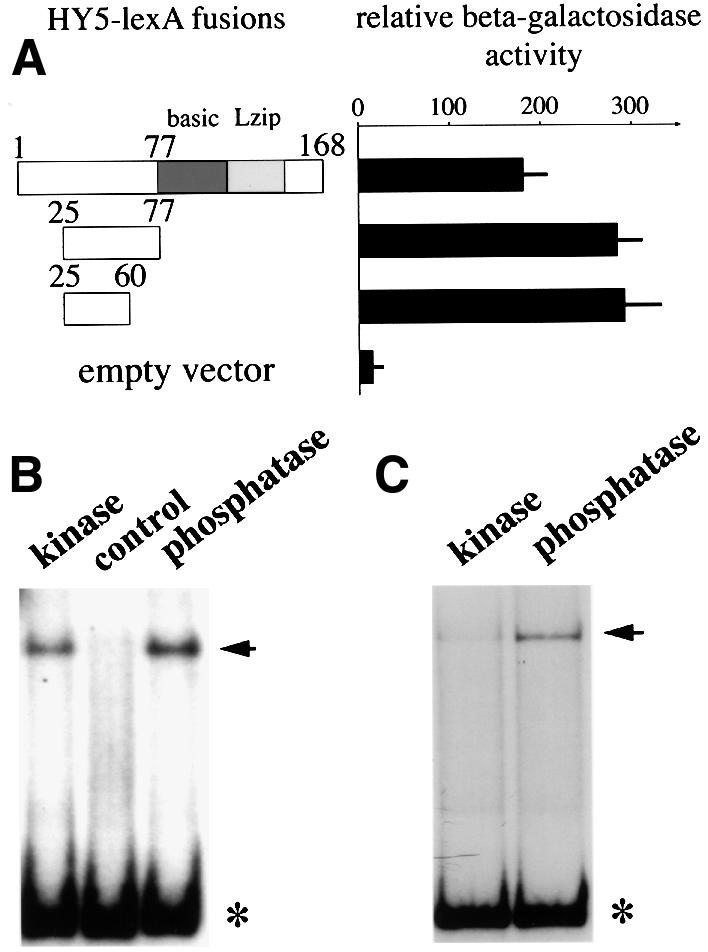
Fig. 4. Delineation of the COP1-interactive domain in HY5 and the effect of phosphorylation on HY5’s ability to bind light-responsive promoter elements. (A) Yeast two-hybrid interaction assays with HY5 and COP1. Interaction of activation domain-tagged COP1 with LexA fusions of a 53 (aa 25–77) and a 36 (aa 25–60) amino acid fragment within the N-terminus of HY5 was compared with a fusion with full-length HY5 in yeast two-hybrid assays. Relative β-galactosidase units are given. The empty vector control (LexA without any fusion) had <15 U of activity. In each interacting pair, a total of 24 independent clones were used for the assay and the standard deviations are shown as error bars. (B) EMSAs with the minimal light-responsive element of the CHS1 promoter. Phosphorylated (kinase) and unphosphorylated (phosphatase) GSTHY5 were compared with respect to their ability to bind to radioactively labeled promoter DNA fragment (bound probe marked by arrow) in gel shift experiments (free probe marked by asterisk). The kinase/HY5 fraction used to phosphorylate the GSTHY5 (see Figure 2C) is shown as a control, demonstrating that the native HY5 in this fraction is not sufficient to cause a significant shift. In addition, no shifting was observed with GST alone (not shown). (C) Same as in (B) except the minimal light responsive element of the Rbcs1a promoter was used as a probe.
Unphosphorylated HY5 interacts more strongly with target promoters in vitro
For some transcription factors, it has been shown that phosphorylation by CKII affected their affinities for their DNA targets in vitro (Klimczak et al., 1995; Ciceri et al., 1997). The G-boxes in the promoters of the RBCS1a and CHS1 genes are well defined targets of the HY5 protein (Ang et al., 1998; Chattopadhyay et al., 1998). We thus tested whether phosphorylation had any influence on HY5’s affinity to these binding sites. To this end, recombinant GSTHY5 protein was again first phosphorylated by treatment with the kinase activity fraction. This phosphorylated GSTHY5 (generated by kinase treatment) and dephosphorylated GSTHY5 (generated by a successive lambda protein phosphatase treatment) were used to test the binding capacity to the G-box containing promoter fragments in electrophoretic mobility shift assays (EMSAs). We observed that for both the CHS1 and the RBCS1a minimal light inducible promoter fragments, dephosphorylation enhanced binding of HY5 to its DNA target in vitro ∼3- and 5-fold (as determined from scintillation counting of the retarded bands), respectively (Figure 4B and C). This result indicates that unphosphorylated HY5 is more effective in binding to these promoters.
Both phosphorylated and unphosphorylated isoforms of HY5 exist in vivo
To examine the in vivo phosphorylation status of HY5, we first tested the presence of phosphorylated amino acids in immunoprecipitated HY5 from seedling extract. This was done by western blotting with commercially available monoclonal antibodies against specific phospho-amino acids. We obtained a signal using anti-phosphoserine antibody, but not anti-phosphothreonine or anti-phosphotyrosine antibodies (data not shown). This finding is consistent with the serine in the CKII site of HY5 being a phosphorylation site in vivo. However, outside the putative CKII site, a stretch of serines in the N-terminus of HY5 could also be a target for phosphorylation. To test the role of these residues in phosphorylation, we constructed transgenic plants overexpressing wild-type HY5 (35S:: WT) and mutant versions driven by the 35S cauliflower mosaic virus promoter in a hy5 null mutant background (Figure 5A). One construct expressed a mutant protein in which all serines within the N-terminal 24 amino acids of HY5 were replaced by alanines [35S::S(N24)A], while in another the serine in the suspected CKII site at position 36 was replaced by an alanine (35S::S36A). The alanine substitutions should abolish any phosphorylation of the respective residues.
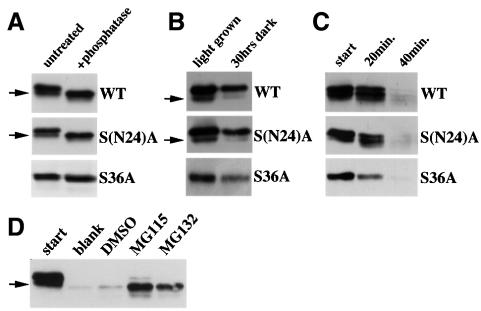
Fig. 5. Effect of selected serine residue mutations on HY5 function and isoform formation in vivo when ectopically overexpressed in Arabidopsis. (A) Sequence of the 77 N-terminal amino acids of the HY5 protein and the two mutated versions (serine to alanine substitutions). The location of the COP1 interaction domain as determined in yeast two-hybrid assays (Figure 4A) is indicated. Transgenes of full-length HY5 forms (wild type, WT) and two mutants, [S(N24)A and S36A] under control of the 35S promoter were transformed into a hy5 null mutant background (hy5-KS50). The serine altered in the 35S::S36A transgene corresponds to the target residue of a putative CKII site (ESDEE). (B) All three transgenes were able to rescue the phenotype of a hy5 null mutant (hy5-KS50). Transgenic seedlings were compared with mutant and wild-type seedlings 3 days after germination in white light. All transgenes rescue the long hypocotyl phenotype of the hy5 mutant as compared with wild-type seedlings of the Wassilewskaja (Ws) ecotype, which is the background line. (C) Comparison between protein extract from light- and dark-grown seedlings. Extracts from wild-type (WT) or HY5 overexpressor (35S) seedlings was run on high percentage (>15%) SDS–PAGE gels and HY5 was detected by western blot analysis. Two closely migrating isoforms are observed in the extract from light-grown seedlings. The lower sized band (arrow) could not be detected in dark-grown extract from both wild-type and HY5 overexpressor lines even after extended exposure.
All transgenes were able to rescue the hy5 mutant phenotype, indicating functionality of the overexpressed mutant proteins (Figure 5B). In line with this observation, we found that the S36A protein is again exclusively present in the HY5 protein complex (data not shown). We had noticed that in wild type, the HY5 antibody lights up a second band on high percentage SDS–polyacrylamide gels, running at a slightly smaller size (Figure 5C). This HY5 doublet band was also present in western blots of both the 35S::WT and 35S::S(N24)A transgenic lines. However, no doublet was observed in the 35S::S36A plants. To correlate further the observed isoforms to phosphorylation, we treated transgenic protein extracts with the lambda protein phosphatase and compared these samples to untreated ones (Figure 6A). We found that both the WT and S(N24)A proteins shifted to a single band, similar in size to the lower band of the doublet in untreated extract. In contrast, only a very minor shift, if any, was observed for the S36A protein. It is of interest to note that the S36A mutant protein did not migrate precisely according to either of the two bands in the wild-type doublet in our SDS–PAGE. This may be due to the different effect of the serine and alanine at position 36 on the migration behavior of the HY5 protein. However, since all versions of HY5 run as single bands at ∼18 kDa in strongly denaturing SDS–PAGE containing 7% urea (data not shown), we conclude that the unusual migration behavior of HY5 wild-type and mutant proteins is not caused by major covalent modifications, but rather by subtle changes in conformation or charge.
Fig. 6. Phosphorylation and stability of the overexpressed wild-type and mutant versions of HY5 in Arabidopsis seedlings. (A) Comparison of protein extract from transgenic overexpressor lines before (untreated) and after (+phosphatase) treatment with a non-specific protein phosphatase (see Figure 3B). For the wild-type (WT) and the S(N24)A mutant proteins, a shift of all HY5 protein to the fast-migrating (lower) band (arrow) is observed after treatment. Only a single band is observed for the S36A mutant protein, which is not significantly changed after the phosphatase treatment. (B) Comparison of in vivo HY5 protein forms from light-grown seedlings and the light-grown seedlings subjected to a 30 h dark adaptation. The lower migrating band (arrow), presumably representing an unphosphorylated isoform of HY5 (A), seems to disappear in darkness. Only a single band is detected in the 35S::S36A lines. Equal amounts of protein extract were loaded in each lane. (C) The in vitro degradation characteristics of the overexpressed HY5 protein forms. Protein extracts were prepared from transgenic seedlings in an in vitro degradation buffer system (Osterlund et al., 2000). Equal volumes of extract were taken out at the indicated time points and the assay was stopped by addition of sample buffer. The HY5 proteins were examined by western blot. (D) Presence of proteasome inhibitors in the in vitro HY5 degradation assay results in accumulation of the unphosphorylated HY5 form. Extract was prepared from 35S::WT seedlings (start) and incubated in the cell-free degradation assay as in (C) for 2 h. Equal amounts of aliquot were supplemented with nothing (blank), 2% DMSO or 40 µM of the proteasome inhibitors MG115 or MG132, and compared by western blot analysis.
Taken together, our data suggest that the serines located within the 24 N-terminal amino acids seem to be irrelevant for the observed HY5 isoforms, since the same pattern as in wild type is seen for the S(N24)A mutant protein. However, substitution of the serine located within the predicted CKII site in the 35S::S36A lines abolishes the doublet as well as the shift upon dephosphorylation. Thus, we conclude that phosphorylation of this residue is responsible for the upper band observed in the wild-type doublet, which seems to represent a phosphorylated isoform of HY5.
The unphosphorylated isoform of HY5 is a preferred substrate for degradation
Since the lower, unphosphorylated isoform of HY5 is hardly ever observed in darkness, even in overexpression lines (Figure 5C), we wanted to investigate whether the different isoforms of HY5 are differentially degraded. Indeed, when light-grown seedlings expressing the 35S::WT or 35S::S(N24)A transgene were shifted into darkness, we observed preferential disappearance of the unphosphorylated HY5 isoform (Figure 6B). In contrast, in the 35S::S36A lines again only a single band was observed, whose degradation was faster compared with the phosphorylated isoforms in the other two lines.
In a previously established in vitro assay (Osterlund et al., 2000), in which HY5 is rapidly degraded, we observed both isoforms fading over time (Figure 6C). This might be due to a dynamic equilibrium resulting in oscillation between the two isoforms in this assay. However, again only a single band was observed in the S36A mutant protein, and it seemed to get degraded slightly faster than either the wild-type or S(N24)A proteins. Moreover, when degradation of wild-type HY5 in this assay is blocked by the addition of proteasome inhibitors, the faster migrating isoform of HY5 accumulates after an extended time period of 2 h (Figure 6D). This result suggests that degradation is preceded by a conversion into the unphosphorylated form of HY5, which seems to be a better substrate for proteasome-mediated degradation.
Both the in vitro and in vivo data suggest that the unphosphorylated isoform of HY5 is the preferred substrate for degradation. This is consistent with the observation that the unphosphorylated HY5 also has higher affinity for COP1. Thus, the absence of the unphosphorylated isoform of HY5 in dark-grown seedlings is very likely to reflect it being a preferred target for degradation of the nuclear localized COP1.
A HY5 S36A transgene is physiologically more active than the wild-type gene
The fact that unphosphorylated HY5 has higher affinity for the light-responsive promoter elements implied that the mutant S36A HY5 protein might be a more active form in promoting photomorphogenesis than the wild-type HY5. However, we failed to detect any consistent phenotypic or physiological differences, such as in hypocotyl length, between seedlings of the different transgenic overexpressor lines [35S::S36A versus 35S::WT or 35S: S(N24)A] under several light regimes examined. As these ectopic overexpression lines driven by the 35S promoter contain ∼50- to 100-fold higher levels of the HY5 complex in seedlings (Figure 1C and data not shown), this vast elevation in HY5 abundance could probably mask any difference in the physiological activity of distinct HY5 forms. It is also possible that expression timing and cell-type specificity may be a problem with the 35S promoter. Thus, it is critical to determine the activity of the S36A mutant protein expressed in its normal cell types and developmental stages, and at physiological levels. To this end, a mutant S36A HY5 protein was expressed from its native promoter and terminator context (HY5::S36A). Again, the construct was introduced into a hy5 mutant background.
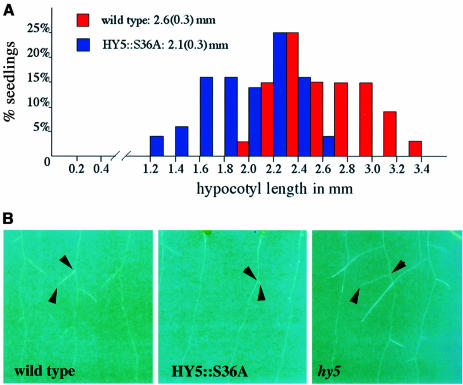
Analogous to the 35S::S36A transgenic lines, HY5:: S36A lines were able to rescue the hy5 mutant phenotype. Moreover, in all four transgenic lines examined in detail, we were able to detect overcompensated phenotypic traits influenced by HY5 activity as compared with the wild type. Two prominent defects observed in a hy5 null mutant are the inability to suppress hypocotyl elongation in the light and the enhancement of the initiation and elongation of lateral roots (Oyama et al., 1997). Upon close examination, we found that both of these aspects are affected in the four selected independent HY5::S36A lines. HY5:: S36A plants display a shorter hypocotyl in the light than wild type (Figure 7A). Moreover, they show a considerable delay in the initiation of lateral roots (Figure 7B). Thus, both of these traits are opposite to the mutant phenotype and exaggerated as compared with wild-type seedlings or hy5 mutant seedlings expressing a transgenic wild-type HY5 under its own promoter context. Our western blot analysis of the four transgenic lines indicated that their HY5 protein level was similar to the wild-type seedlings. Thus, the data confirmed the prediction that the unphosphorylated isoform of HY5 is indeed the physiologically more active isoform in vivo.
Fig. 7. Functional analysis of the S36A mutant protein expressed in the native HY5 promoter and terminator context in a hy5 null mutant background. (A) Hypocotyl length distribution of wild-type and a representative HY5::S36A transgenic line. Hypocotyl lengths of seedlings grown in white light were determined 7 days after germination. A statistically significant difference in hypocotyl length was observed between the two populations. The average hypocotyl lengths are: wild type 2.6(0.3) mm, n = 34; HY5::S36A 2.1(0.3) mm, n = 51; hy5 (not shown) 5.6(0.9) mm, n = 28. (B) Comparison of lateral root initiation in light-grown seedlings. The corresponding portions of the roots of wild-type, HY5::S36A and hy5 mutant seedlings grown in white light for 10 days were compared. The roots of representative seedlings are oriented from top to bottom in each picture. Arrowheads point out the starting point and tip of a representative lateral root in each panel.
Discussion
Modulation of HY5 by a light-regulated kinase activity
Phosphorylation of transcription factors is a common modification that can influence their cell biological properties, such as multimerization or nucleocytoplasmic partitioning. In our studies, we demonstrated that unphosphorylated HY5 binds DNA of its target promoters, like the G-box in the RBCS1a or CHS1 genes, stronger than phosphorylated HY5. Interestingly, this corresponds with the observation that RBCS transcription is enhanced in transgenic lines expressing antisense RNA of the catalytic α-subunit of CKII (Lee et al., 1999). This remarkable influence of CKII on light-dependent gene expression correlates with its ability to modulate several of the Arabidopsis transcription factors involved (e.g. Klimczak et al., 1995; Sugano et al., 1999). Notably, phosphorylation by CKII has been shown to affect the DNA binding capacity of transcription factors in several cases (Klimczak et al., 1995; Sugano et al., 1998). Thus, a network of transcription factors might exist whose (antagonistic) activities, as well as possible interactions, might be partly regulated by CKII phosphorylation. More complexity is added to this by the existence of three different non-catalytic β-subunits of CKII in Arabidopsis, which could have distinct protein interaction properties and expression patterns. It is quite possible that the higher CKII activity toward HY5 observed in dark-grown extract might reflect oscillation of a HY5-specific regulatory β-subunit rather than overall CKII activity. However, while our data point to CKII as the most likely candidate kinase regulating HY5, future experiments involving purified CKII should be carried out to provide a more definite proof. In any event, our observed regulation of HY5 by a kinase activity is similar to an excellent precedent for a plant bZIP protein that was reported in maize. Opaque 2 (O2), like HY5, is a likely target of CKII (Ciceri et al., 1997). For O2, diurnal oscillation between hyper- and hypophosphorylated isoforms results in accumulation of phosphorylated isoforms, whose DNA binding potential is diminished, in darkness.
The kinase activity responsible for HY5 phosphorylation is regulated by light. Examination of cryptochrome- and phytochrome-deficient Arabidopsis strains indicated that it is the phytochromes, rather than the cryptochromes, that are responsible for mediating this light regulation. We have established previously that multiple light receptors can mediate the light regulation of COP1 nuclear depletion (Osterlund and Deng, 1998; Osterlund et al., 1999). Therefore, there are evidently two parallel light signaling processes that affect HY5 abundance. One is the regulation of the availability of COP1 in the nucleus to regulate HY5 and the other is the specific phosphorylation of HY5, which modulates HY5’s ability to interact with COP1. Both modulations contribute to COP1-targeted degradation of HY5 and thus HY5 abundance.
HY5 acts in a complex in vivo
Our finding that HY5 is exclusively present in a protein complex in vivo supports the idea that other (transcription) factors are required to function together with HY5. As no monomeric HY5 is detected, it is likely that HY5 associates with components which are ubiquitous to the cell and present at fairly abundant levels. Thus, HY5 might be a rate-limiting factor either for the formation of this complex or its activity. Recently, an increasing number of higher order transcriptional complexes (e.g. Kim and Maniatis, 1997; Kelley et al., 1998) have been implicated in regulatory processes associated with cell differentiation from yeast to mammals (Sieweke and Graf, 1998; Wolberger, 1998). The HY5 complex will add to this growing list.
The fact that the S36A mutant HY5 protein was still found in the protein complex rules out the possibility that phosphorylation regulates assembly of HY5 into the complex. Rather, it seems that the phosphorylation might influence the DNA binding capacity of HY5 and thereby of its associated factors. Since HY5 does not seem to have a notable transactivation potential of its own (Ang et al., 1998), it could be that directing a transcriptional complex to its target sites is the primary function of HY5 in this context.
Preferred degradation of unphosphorylated HY5
We have previously shown that the abundance of HY5 protein is positively correlated with light intensity and regulated by targeted degradation, probably mediated by the 26S proteasome (Osterlund et al., 2000). The rate of degradation is enhanced in darkness, which coincides with nuclear enrichment of COP1. Moreover, the COP1 interaction domain of HY5 is essential for its degradation in the dark. Thus, our finding that unphosphorylated HY5 interacts more strongly with COP1 in vitro is in line with the observation that the S36A mutant protein is degraded faster than wild-type protein in our assays. Furthermore, the finding that the unphosphorylated isoform accumulates in vitro upon treatment with proteasome inhibitors suggests that an isomerization to this HY5 version is preceding degradation. More importantly, our in vivo results, showing that the unphosphorylated HY5 isoform is hardly detected in extract from dark-grown plants and selectively disappears in light-to-dark shifts of light-grown seedlings, suggest that indeed the unphosphorylated isoform is the preferred substrate for COP1-mediated degradation. Our result is in line with many reported studies where phosphorylation events have also been implicated in the regulation of protein abundance. A well studied example is the proteasome-mediated degradation of the inhibitory factor cactus after phosphorylation, which is partly mediated by Drosophila CKII (Liu et al., 1997). Also, direct control of degradation and activity of the bZIP factor Jun by phosphorylation has been extensively documented (Karin et al., 1997).
A model for the regulation of HY5 activity
Our results support a model in which HY5 exists in two different phosphorylation states. In darkness, COP1 enriches in the nucleus, targeting the unphosphorylated isoform for degradation. At the same time, elevated CKII activity ensures maintenance of a small pool of phosphorylated HY5, which is less susceptible to degradation and physiologically less active. Upon light stimulus, COP1 is excluded from the nucleus and HY5-related CKII activity is reduced. This results in an increase of physiologically more active unphosphorylated HY5 in the light, and activation of its light-inducible target genes. Together, the parallel regulation of HY5 by nuclear COP1 levels and phosphorylation extends the range for the fine tuning and the magnitude of HY5 activity. For example, a 20-fold difference in total HY5 abundance, together with a 5-fold difference in the DNA-binding affinity for the phosphorylated and unphosphorylated HY5 forms, could potentially multiply into a 100-fold difference in the HY5 DNA-binding capacity.
From a developmental point of view, this mechanism provides some advantages to the plant. By maintaining a pool of rapidly activatable HY5 even in darkness, the plant can respond to a light stimulus with minimal delay. This should be of great advantage in a natural environment, where the sessile seedling has to respond quickly to changes in light condition. In fact, the primary function of HY5 could be to initiate and promote photomorphogenesis rapidly during the early seedling development, because the hy5 null mutants are perfectly viable and relatively normal in adulthood. Moreover, expression of HY5 fades towards undetectable levels in the light at later stages of development. Thus, it seems like HY5 might not have a prominent role in post-seedling development at all, but is specific to the seedling stage.
Materials and methods
Plant materials and growth conditions
Plant growth conditions were essentially as described (Ang et al., 1998). Seeds were surface sterilized (5% bleach, 0.1% Tween 20) for 15 min and washed three times with sterile water before spreading onto 0.7% agar plates containing half-strength Murashige and Skoog basal salt mixture (Sigma) and 0.3% sucrose. The plates were vernalized for 3 days in darkness at 4°C and then placed in the light. Most Arabidopsis materials were in the Wassilewskaja ecotype background unless stated otherwise. The lines used in Figure 2B were the cry1-304/cry2-1 double mutant (Mockler et al., 1999), the ctr1-2 mutant (Kieber et al., 1993), the hy5-215 mutant (Oyama et al., 1997) and the phyA-201/phyB-5/phyE triple mutant (Devlin et al., 1998).
Western blot analysis
Western blots were performed according to standard procedures. For detection of the primary antibodies, a peroxidase coupled anti-rabbit-IgG antibody (Boehringer Mannheim) was used in conjunction with an enhanced chemiluminescence detection system (Amersham). The antibody used to detect HY5 protein has been described previously (Osterlund et al., 2000). Antibodies against phospho-amino acids were purchased from Sigma.
Gel filtration chromatography
Three-day-old light-grown seedlings (∼100) were ground in liquid nitrogen and the powder was resuspended in 200 µl of buffer (400 mM sucrose, 50 mM Tris–HCl pH 7.5, 2.5 mM EDTA, 10 mM phenylmethylsulfonyl fluoride). The extract was cleared by microcentrifugation first, and subsequently filtered through a 0.2 µm syringe filter. The cleared extract was then passed through a Superdex 200 (Pharmacia) gel filtration column with elution buffer (0.203 g/l NaH2PO4, 1.15 g/l Na2HPO4, 8.5 g/l NaCl, 2 mM MgCl2, 0.1% Tween). After a 7 ml void volume, 26 fractions of 0.5 ml each were collected. The protein in the fractions was concentrated using Strataresin (Stratagene) and resuspended in a final volume of 50 µl of sample buffer.
Kinase assays
Assays were done in 50 mM HEPES pH 7.3, 1 mM MgCl2, 0.2 mM CaCl2, 0.1 mM EGTA and 6.7 µM of [γ-32P]ATP (3000 mCi/mmol). GST or GSTHY5 (1 µg) was added to 5 µg of plant extract (extracted in 50 mM Tris–HCl pH 7.5, 1 mM EDTA) and incubated at room tempera ture for 30 min. Glutathione–Sepharose beads (25 µl; Pharmacia), washed once with 1× PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.3), were added and samples were incubated for another 30 min with occasional mixing. Beads were recovered by centrifugation and washed four times in 1× PBS. Samples were resuspended in 30 µl of protein sample buffer and run on 10% SDS–polyacrylamide gels. For endogenous HY5, 8 µg of protein from the heparin–Sepharose-column fraction (see below) was incubated in a kinase assay, and HY5 was immunoprecipitated with antibody coupled to protein A beads (Pharmacia). Inhibitors used in Figure 2D were purchased from Calbiochem.
Chromatography
Extract from 2.5 g fresh weight of seedlings grown for 3–5 days in the light was resuspended in 10 ml of buffer (100 mM NaCl, 20 mM Tris–HCl pH 8.0, 2 mM MgCl2, 8% glycerol, 0.1% Tween), cleared by centrifugation and loaded onto a 20 ml heparin–Sepharose column (Pharmacia). The column was washed with 10 vol buffer before bound protein was eluted in 26 fractions of 5 ml each with a 0.2–1.0 M NaCl gradient. Fractions containing HY5 were determined by western blot analysis.
Immunoprecipitation
For immunoprecipitations, 9 vol ice-cold acetone were added to plant extract or assays, respectively, and total protein was precipitated by centrifugation. The protein pellet was resuspended in 1 vol TBS–TS (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.1% Triton X-100, 0.1% SDS) and then 9 vol TBS (TBS-TS lacking Triton and SDS) were added. Insoluble material was spun down and the supernatant was transferred into another tube. Protein A–Sepharose beads (25 µl; Pharmacia) coupled to anti-HY5 antibody were added and the suspension was nutated for 6 h at 4°C. Beads were recovered by centrifugation and washed three times in TBS-TS. Finally, 50 µl of 1× protein sample buffer was added and the samples were boiled for 5 min before loading onto an SDS–polyacrylamide gel.
In vitro protein interaction
Fusion proteins were purified from Escherichia coli as described previously (Ang et al., 1998). GST (1 µg) or 0.5 µg of GSTHY5 was incubated with 8 µg protein from the kinase containing heparin–Sepharose fraction in kinase buffer and 1 mM ATP for 30 min at room temperature. One of two parallel sets of samples was then treated with 400 U of lambda protein phosphatase for another 30 min. GST or GSTHY5 was then coated to glutathione–Sepharose beads (Pharmacia) and washed four times with 1× PBS. FLAG-COP1 (800 ng) containing a heart muscle kinase (HMK) site was radioactively labeled in HMK buffer [20 mM Tris–HCl pH 7.5, 1 mM dithiothreitol (DTT), 100 mM NaCl, 12 mM MgCl2] with 6.7 µM of [γ-32P]ATP (3000 mCi/mmol) and 20 U of HMK (Sigma) for 1 h at 37°C in a 100 µl reaction. Labeled COP1 (25 µl) and 200 µl of interaction buffer (0.5% BSA, 20 mM Tris–HCl pH 7.5, 4 mM MgCl2, 1 mM DTT, 0.5% tergitol, 100 mM NaCl) were then added to the GST- or GSTHY5-coated beads. Reactions were nutated overnight at 4°C, and beads were washed four times with 1× NTE buffer (500 mM NaCl, 10 mM Tris–HCl pH 7.5, 1 mM EDTA). Finally, the beads were resuspended in 30 µl of protein sample buffer and boiled for 5 min. The samples were then run on a 10% SDS–polyacrylamide gel. Quantification of bands was performed by scintillation counting.
EMSAs
Approximately 80 ng of GSTHY5 were preincubated with 320 ng protein of the kinase containing heparin–Sepharose fraction in kinase buffer and 0.5 mM ATP at room temperature for 30 min. The sample was split in half and one half was treated with 320 U of lambda protein phosphatase. GSTHY5 (40 ng) in a 6 µl volume was then added to 19 µl of a mix containing 5 µl of 5× binding buffer (75 mM HEPES pH 7.5, 175 mM KCl, 5 mM EDTA, 30% glycerol, 5 mM DTT, 1 mM MgCl2), 20 000 c.p.m. of 32P-labeled (1.65 × 107 d.p.m.) promoter fragment (Ang et al., 1998; Chattopadhyay et al., 1998) and 1 µg of dI-dC (Pharmacia). Reactions were incubated at room temperature for 20 min and run on a 5% strength non-denaturing polyacrylamide gel in 0.25× TBE buffer. Quantification of bands was performed by scintillation counting.
Yeast two-hybrid assays
Interaction trap assays in yeast were performed as described previously (Ang et al., 1998). Truncated versions of the COP1 interacting domain of HY5 were amplified by PCR using Pfu polymerase (Stratagene). These were cloned into the vector pEG202 in frame with the lexA DNA binding domain via restriction sites introduced into the primers. Constructs were verified for their integrity by sequencing. Yeast transformants were grown on selective medium containing glucose overnight. To induce expression of the activation domain fusion protein, cells were washed twice in water and then resuspended in selective medium containing galactose as a carbon source. After 10 h of growth under inductive conditions, liquid β-galactosidase assays were performed and the activity in relative units was calculated according to Ausubel et al. (1999).
Transgenic plants
HY5 cDNAs for transgenic overexpression constructs were designed by site-directed mutagenesis and cloned into a derivative of pGPTV-BAR (Becker et al., 1992) for expression under control of the full-length 35S cauliflower mosaic virus promoter and the nopaline synthase terminator. For expression under its own promoter, the cDNA giving rise to a S36A mutant protein was cloned between the native HY5 promoter and terminator (Oyama et al., 1997). Transgenic plants were obtained by Agrobacterium-mediated transformation of the hy5 allele KS50 (Oyama et al., 1997; Clough and Bent, 1998). Homozygous lines were then established from primary transformants. The transgenes were amplified from the transformants by PCR and the PCR products were directly sequenced to confirm the integrity of the constructs. For analysis of the HY5 protein in the overexpressors, two or more independent transgenic lines per construct were analyzed. For the transgenics expressing the S36A HY5 gene under its own promoter, four single insertion lines were selected for more detailed analysis and similar results were obtained.
Dephosphorylation of plant protein extract
Protein was extracted from light-grown seedlings in lambda protein phosphatase buffer (New England Biolabs), cleared by centrifugation and incubated at room temperature for 20 min with or without 400 U of phosphatase. An equal volume of 2× protein sample buffer was added and the reactions were boiled for 5 min and compared on 15% SDS–polyacrylamide gels.
In vitro degradation assay
The cell-free degradation assay was performed as described (Osterlund et al., 2000). Protein extract was prepared from 3-day-old light-grown seedlings and resuspended in buffer (25 mM Tris pH 7.5, 10 mM MgCl2, 5 mM DTT, 10 mM NaCl, 10 mM ATP). The samples were then incubated at room temperature and aliquots were taken at the time points indicated. The reaction was stopped by the addition of an equal volume of 2× sample buffer. For the inhibitor study, the extract aliquots were incubated for 2 h in the presence of the supplements indicated (Calbiochem). HY5 was then detected using western blot analysis of equal amounts of sample.
Acknowledgments
Acknowledgements
We thank M.Holm, H.Okamoto and M.Terry for reading and commenting on this manuscript. Our work was supported by grants from the Human Frontier Science Program Organization (to X.W.D. and K.O.), and in part by grants from the NIH (to X.W.D.) and the Japanese Ministry of Education, Science, Culture and Sports, the Mitsubishi Foundation and the Sciences and Technology Agency of Japan (to K.O.). C.S.H. is a Human Frontier Science Program Organization postdoctoral fellow, M.T.O. is an NIH and Department of Education predoctoral trainee, and X.W.D. is an NSF Presidential Faculty Fellow.
References
- Ang L.H. and Deng,X.W. (1994) Regulatory hierarchy of photomorpho genic loci: allele-specific and light-dependent interaction between the HY5 and COP1 loci. Plant Cell, 6, 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang L.H., Chattopadhyay,S., Wei,N., Oyama,T., Okada,K., Batschauer,A. and Deng,X.W. (1998) Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol. Cell, 1, 213–222. [DOI] [PubMed] [Google Scholar]
- Ausubel F.M., Brent,R., Kingston,R.E., Moore,D.D., Seidman,J.G., Smith,J.A. and Struhl,K. (1999) Short Protocols in Molecular Biology. 4th edn. Wiley and Sons, New York, NY. [Google Scholar]
- Becker D., Kemper,E., Schell,J. and Masterson,R. (1992) New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol. Biol., 20, 1195–1197. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S., Ang,L.H., Puente,P., Deng,X.W. and Wei,N. (1998) Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell, 10, 673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceri P., Gianazza,E., Lazzari,G., Lippoli,G., Genga,A., Hoscheck,G., Schmidt,R.J. and Viotti,A. (1997) Phosphorylation of Opaque2 changes diurnally and impacts its DNA binding activity. Plant Cell, 9, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J. and Bent,A.F. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J., 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Deng X.W. and Quail,P.H. (1999) Signalling in light-controlled development. Semin. Cell. Dev. Biol., 10, 121–129. [DOI] [PubMed] [Google Scholar]
- Devlin P.F., Patel,S.R. and Whitelam,G.C. (1998) Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell, 10, 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter K., Kircher,S., Frohnmeyer,H., Krenz,M., Nagy,F. and Schaefer,E. (1994) Light-regulated modification and nuclear translocation of cytosolic G-box binding factors in parsley. Plant Cell, 6, 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Liu,Z.G. and Zandi,E. (1997) AP-1 function and regulation. Curr. Opin. Cell Biol., 9, 240–246. [DOI] [PubMed] [Google Scholar]
- Kelley C.M., Ikeda,T., Koipally,J., Avitahl,N., Wu,L., Georgopoulos,K. and Morgan,B.A. (1998) Helios, a novel dimerization partner of Ikaros expressed in the earliest hematopoietic progenitors. Curr. Biol., 8, 508–515. [DOI] [PubMed] [Google Scholar]
- Kieber J.J., Rothenberg,M., Roman,G., Feldmann,K.A. and Ecker,J.R. (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell, 72, 427–441. [DOI] [PubMed] [Google Scholar]
- Kim T.K. and Maniatis,T. (1997) The mechanism of transcriptional synergy of an in vitro assembled interferone-β enhanceosome. Mol. Cell, 1, 119–129. [DOI] [PubMed] [Google Scholar]
- Klimczak L.J., Collinge,M.A., Farini,D., Giuliano,G., Walker,J.C. and Cashmore,A.R. (1995) Reconstitution of Arabidopsis casein kinase II from recombinant subunits and phosphorylation of transcription factor GBF1. Plant Cell, 7, 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimczak L.J., Schindler,U. and Cashmore,A.R. (1992) DNA binding activity of the Arabidopsis G-box binding factor GBF1 is stimulated by phosphorylation by casein kinase II from broccoli. Plant Cell, 4, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Lloyd,A.M. and Roux,S.J. (1999) Antisense expression of the CK2 α-subunit gene in Arabidopsis. Effects on light-regulated gene expression and plant growth. Plant Physiol., 119, 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.P., Galindo,R.L. and Wasserman,S.A. (1997) A role for CKII phosphorylation of the cactus PEST domain in dorsoventral patterning of the Drosophila embryo. Genes Dev., 11, 3413–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marechal E., Hiratsuka,K., Delgado,J., Nairn,A., Qin,J., Chait,B.T. and Chua,N.H. (1999) Modulation of GT-1 DNA-binding activity by calcium-dependent phosphorylation. Plant Mol. Biol., 40, 373–386. [DOI] [PubMed] [Google Scholar]
- Mockler T.C., Guo,H., Yang,H., Duong,H. and Lin,C. (1999). Antagonistic actions of the Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development, 126, 2073–2082. [DOI] [PubMed] [Google Scholar]
- Nagy F. and Schaefer,E. (2000) Nuclear and cytosolic events of light-induced, phytochrome-regulated signaling in higher plants. EMBO J., 19, 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff M.M., Fankhauser,C. and Chory,J. (2000) Light: an indicator of time and place. Genes Dev., 14, 257–271. [PubMed] [Google Scholar]
- Osterlund M.T. and Deng,X.W. (1998) Multiple photoreceptors mediate the light-induced reduction of GUS-COP1 from Arabidopsis hypocotyl nuclei. Plant J., 16, 201–208. [DOI] [PubMed] [Google Scholar]
- Osterlund M.T., Ang,L.H. and Deng,X.W. (1999) The role of COP1 in repression of Arabidopsis photomorphogenic development. Trends Cell Biol., 9, 113–118. [DOI] [PubMed] [Google Scholar]
- Osterlund M.T., Hardtke,C.S., Wei,N. and Deng,X.W. (2000) Targeted destabilization of HY5 in light development of Arabidopsis. Nature, 405, 462–466. [DOI] [PubMed] [Google Scholar]
- Oyama T., Shimura,Y. and Okada,K. (1997) The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev., 11, 2983–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarokin L.P. and Chua,N.H. (1992) Binding sites for two novel phosphoproteins, 3AF5 and 3AF3, are required for rbcS-3A expression. Plant Cell, 4, 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieweke M.H. and Graf,T. (1998) A transcription factor party during blood cell differentiation. Curr. Opin. Genet. Dev., 8, 545–551. [DOI] [PubMed] [Google Scholar]
- Sugano S., Andronis,C., Ong,M.S., Green,R.M. and Tobin,E.M. (1999) The protein kinase CK2 is involved in regulation of circadian rhythms in Arabidopsis. Proc. Natl Acad. Sci. USA, 96, 12362–12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano S., Andronis,C., Green,R.M., Wang,Z.Y. and Tobin,E.M. (1998) Protein kinase CK2 interacts with and phosphorylates the Arabidopsis circadian clock-associated 1 protein. Proc. Natl Acad. Sci. USA, 95, 11020–11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arnim A.G. and Deng,X.W. (1994) Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell-specific regulation of its nucleocytoplasmic partitioning. Cell, 79, 1035–1045. [DOI] [PubMed] [Google Scholar]
- Wolberger C. (1998) Combinatorial transcription factors. Curr. Opin. Genet. Dev., 8, 552–559. [DOI] [PubMed] [Google Scholar]