Abstract
Introduction
This study aims to evaluate the pharmacokinetic, behavioral, and motor effects of a liposomal preparation of hydromorphone hydrochloride (LE-hydro) in rhesus monkeys. We administered either 2 mg/kg of LE-hydro (n=8) subcutaneous (s.c.) or 0.1 mg/kg of standard pharmaceutical hydromorphone HCl (hydro) preparation either intravenous (i.v.; n=4) or s.c. (n=5).
Materials and methods
Serial blood samples were drawn after injection and analyzed for serum hydro concentration by liquid chromatography/mass spectrometry. Following s.c. injection of 0.1 mg/kg hydro or 2 mg/kg LE-hydro, behavioral evaluations were conducted in groups of rhesus monkeys (n=10/group) in the presence of a compatible stimulus animal and motor skills were also evaluated (n=10/group). The motor skills test consisted of removing a food reward (carrot ring) from either a straight peg (simple task) or a curved peg (difficult task).
Results
LE-hydro (MRT0-INF = 105.9 h) demonstrated extended-release pharmacokinetics compared to hydro when administered by either i.v. (MRT0-INF =1.1 h) or s.c. (MRT0-INF =1.3 h) routes. Hydro did not affect motor performance of the simpler task, but the monkeys’ performance deteriorated on the more difficult task at 0.5 and 1 h after injection. LE-hydro had no effect on motor skills in either the simpler or more difficult task.
Conclusions
The results of these studies indicate that LE-hydro has a pharmacokinetic and behavioral side effects profile consistent with an analgesic that could be tested for surgical use in animals. Our studies also expand the use of rhesus monkeys as a translational behavioral pharmacodynamics model for testing extended-release opioid medication.
Keywords: Liposome, Extended release, Hydromorphone, Pain, Analgesia
Introduction
The initial evaluation of a new drug requires pharmacokinetic studies in animals. Evaluation for a new formulation of an already well-characterized drug may begin with pharmacokinetics, but can also include a more thorough assessment of the physiologic and behavioral effects of the preparation. The latter approach is especially true for opioid drugs, which have well-documented pharmacokinetics that have already been determined for multiple compounds in multiple species, including human beings, domestic animals such as the dog, and nonhuman primates (Hawk and Leary 2005; Jaffe and Matin 1985). Rhesus monkeys are particularly good models for evaluation of novel drug formulations because they are more closely genetically related to human beings, their normal behavior has been extensively studied, and many test paradigms have been developed for the evaluation of specific behaviors, such as fine motor coordination (Goodwin et al. 2009; Weerts et al. 1998). In the current study, we determined the pharmacokinetics, effects on normal social behavior, and motor skills of a liposomal formulation of the commonly used opioid drug, hydromorphone (hydro).
Extended-release opioid drugs have several advantages over immediate release formulations, including more stable serum concentrations, a more favorable side-effects profile, and still provide high enough concentrations in the CNS to provide analgesia to the patient (Krugner-Higby, accepted for publication; KuKanich et al. 2008). Extended duration liposomal opioids suitable for use as surgical and post-surgical analgesics would be expected to have a rapid onset and a high enough peak to provide pain control during the first 24 h after surgery when pain would be expected to be most severe, but taper off rapidly enough to have minimal sedative effects later, when post-surgical pain begins to wane. A liposomal formulation of morphine sulfate is currently marketed for the treatment of post-surgical pain in human beings (Viscusi et al. 2005, 2006). This formulation is delivered as an epidural injection and provides durable analgesia for up to 48 h after administration. Liposomes with a similar composition to Depodur® have been administered subcutaneous (s.c.) in animal studies, but the commercial product is only licensed for epidural use (Kim et al. 1993; Smith et al. 2003). The liposomal hydro formulation used in the current study is administered s.c. instead of epidurally as its primary route of administration, and has been characterized with respect to its pharmacokinetics (Smith et al. 2008), side-effect profile and efficacy as a post-surgical analgesic in dogs undergoing ovariohysterectomy (OVH). A single s.c. dose of 2 mg/kg liposome-encapsulated hydromorphone (LE-hydro) provided robust analgesia with an acceptable side effects profile in dogs undergoing OVH (Krugner-Higby, accepted for publication). But this formulation has not previously been studied in nonhuman primates.
The current study evaluated hydromorphone encapsulated in multilamellar liposomes prepared by the freeze–thaw method (LE-hydro) and compared their effects to those of the standard pharmaceutical formulation of hydromorphone (hydro; Smith et al. 2008). The pharmacokinetics of hydro and LE-hydro were determined, behavior was evaluated in a playroom setting using compatible animals as a social stimulus, and the effects of both drug preparations on motor skills were evaluated using two tests of graded difficulty for a food reward.
Materials and methods
Preparation of liposome-encapsulated hydromorphone, using modified dehydration-rehydration vesicles
Liposomes containing hydromorphone HCl were prepared from dipalmitoylphosphatidylcholine and cholesterol, in a molar ratio of 2:1. A mixture of 80 μmol and DPPC 40 μmol cholesterol was dried from chloroform solution in a 160×20 mm screw capped tube, dissolved in 1 ml of sterile tert-Butanol, 99+% A.C.S. by heating to 55°C in a water bath, frozen in dry ice–isopropanol, and lyophilized for 24 h. The dried lipid was swollen in 1 ml of 40 mg/mL hydromorphone HCl in 10 mM citrate buffer, pH 4.0, for 60 min in a 55°C water bath. The liposomes were frozen in dry ice–isopropanol for 2 min, and stored at −20°C overnight. The liposomes were thawed at room temperature, diluted to 5 ml in sterile saline for irrigation, placed in 5 mL sterile ultraclear centrifuge tubes and sedimented at 100,000×g for 30 min at 4°C in a Beckman Model L8-M Ultracentrifuge. The supernatant was removed from the tube using a sterile 5 mL disposable pipette and the liposome pellet was resuspended in 10 mM sodium acetate buffer, pH 4.0. Liposome-encapsulated hydromorphone was quantified spectrophotometrically, and the suspension was stored in a dark cabinet at 4°C for no more than 7 days prior to use. Immediately prior to injection into an animal, the preparation was gently agitated and then slowly drawn up into a syringe using a 22-gage needle.
Animal groups and instrumentation
The University of Wisconsin School of Veterinary Medicine Research Animal Care and Use Committee approved all experimental procedures and studies.
Animals
Subjects were rhesus macaques (Macaca mulatta) of Indian origin. These monkeys were well-acclimated members of a long-established breeding colony at the Harlow Center for Biological Psychology. Monkeys used for pharmacokinetics studies were males or females over 3 years of age (n=4–5 for hydro, n=10 for LE-hydro). All of the animals used for playroom behavioral studies were females over 3 years of age (n=10/group), currently living in a compatible pair, so they could be placed with their familiar conspecific in the extended cage without physical aggression. The monkeys ranged in weight from between 5 and 10 kg. The monkeys were healthy and apparently free of infectious disease at the time of experimental assignment.
Drug administration and sample collection
Based upon the results of previous pharmacokinetics studies in rats and dogs, the pharmacokinetics of a single 2 mg/kg dose of subcutaneous (s.c.) LE-hydro was compared to 0.1 mg/kg of standard hydro administered s.c. or intravenous (i.v.) in adult rhesus macaques (Smith et al. 2006, 2008). A single dose of a liposomal drug is higher than a single dose of an immediate release drug, but the cumulative dose over the extended time period needs to reach blood concentrations that are high enough to be analgesic. Liposomal opioid drugs are typically administered at dosages that are at least ten times the parenteral dose for the free drug. The dosages in milligram per kilogram were based on similar metabolic characteristics and body weight ranges between the rhesus monkeys used in the present study and the beagle dogs used for the initial pharmacokinetic studies (Smith et al. 2008). Hydro was administered as an i.v. injection into a saphenous vein by an experienced person (BS). Subcutaneous injections were made into the loose skin caudal to the scapula. All injections were made with a 3-mL syringe attached to a 22-gage 1″ needle. Blood samples were collected from conscious monkeys in a squeeze restrainer apparatus either from the saphenous or femoral vein into serum separator tubes (Vacutainer SST 5 mL; Becton-Dickson, Franklin Lakes, NJ, USA). The serum was separated after centrifugation at 1,000×g for 15 min at 10° C. Sera were stored frozen at −70° C until analysis. Samples from monkeys administered hydro were collected prior to drug administration and at 5, 10, 15, 20, 30, and 45 min and 1, 1.5, 2, 4, 6, and 8 h after i.v. or s.c. drug administration. Blood samples were collected from eight monkeys receiving 2 mg/kg LE- hydro prior to drug administration and once daily for 144 h, and two monkeys were sampled to 456 h in order to make sure that there was no drug remaining in the serum.
Serum drug analysis
Serum samples were analyzed for hydromorphone (m/z 286→185) by liquid chromatography with tandem mass spectrometry (Acquity TQD, Waters Corporation, Milford, MA, USA). The internal standard (IS) was hydro d6 (m/z 292→185). Sodium borate buffer (0.1 M) was added (200 μL) to the serum (200 μL) and vortexed. Solid-phase extraction (Varian Bond Elut C18, Varian Inc. Palo Alto, CA, USA) was used to extract drug from serum. The solid-phase extraction (SPE) cartridges were conditioned with 1 mL methanol followed by 1 mL of deionized water. The sample (400 μL total volume of serum, buffer, and IS) was loaded, the SPE cartridges were washed with 3 mL deionized water, and the drug eluted with 1 mL methanol. The eluate was evaporated to dryness and then reconstituted with 200 μL of 0.1% formic acid. The mobile phase consisted of A: ammonium acetate (5 mM, pH 3.5) and B: acetonitrile using a linear gradient from 0 min (98A:2B) to 3 min (5A:95B) to 3.6 min (98A:2B) with a flow rate of 0.3 mL/min. A C18 column achieved separation (BEH C18, 2.1×50 mm, 1.7 μm, Waters Corporation, Milford, MA, USA). The lower limit of quantification of the assay was 0.5 ng/mL. The accuracy (mean±SD) was within 6±7% of the actual concentration and the coefficient of variation was 3±2% on replicates of five each at 0.5, 15, and 150 ng/mL.
Pharmacokinetic analysis
Pharmacokinetic parameters were estimated with computer software (WinNonlin, Pharsight Corporation, Mountain View, CA, USA) using non-compartmental analysis. The estimated variables included the area under the curve from time 0 to infinity (AUC0-∞) and the area under the curve from 0 to the last time point above the LOQ of the assay (AUC0-LAST) using the linear trapezoidal rule. The area under the first moment curve from time 0 to infinity (AUMC0-∞), area under the first moment curve from time 0 to the last time point above the LOQ of the assay (AUMC0-LAST), serum clearance (Cl), serum clearance per fraction of the dose absorbed (Cl/F), apparent volume of distribution at steady state (Vdss), apparent volume of distribution (area method; Vdarea), apparent volume of distribution (area method) per fraction of the dose absorbed (Vdarea/F), first-order rate constant (λz), terminal half-life (t½ λz), biologic half-life (T½Biologic = 0.693*MRT0-LAST), mean residence time extrapolated to infinity (MRT0-∞), mean residence time from 0 to the last measured time point (MRT0-LAST), maximum serum concentration (CMAX), and time to maximum serum concentration (TMAX) were also estimated. The CMAX per dose (CMAX/D) was calculated by dividing the CMAX by the actual dose administered as hydromorphone base. The concentration at time 0 (C0) was calculated by log-linear regression utilizing the first two time points. Serum drug concentrations below the LOQ of the assay were not used in the determination of the pharmacokinetic variables.
Playroom social interaction and behavioral analysis
Monkeys were presented with a familiar conspecific between 0800 and 1200 hours in an extended enclosure 304×71×79 cm. The monkeys were allowed to interact in the enclosure for 1 h, during which they were observed and scored using a standard ethographic program designed for rhesus macaques (Hypercard, 2.3) on a lap top computer (Apple, iBook G4). This ethogram has been used previously in our laboratory and similar ethograms have been used to study the behavioral toxicology of lead exposure (Ferguson et al. 1993; Krugner-Higby et al. 2009). Behaviors were scored for frequency and duration if applicable. Observations were done unblinded by BS and four other observers. Liposomal preparations, including the LE-hydro evaluated in this study, are white, require refrigeration, are drawn into syringes at the time of administration, and are therefore difficult to disguise. Training of observers and validation was done as previously described (Krugner-Higby et al. 2009). Monkeys were observed in the playroom daily (three baseline values) for 1 h between 0800 and 1200 hours prior to any drug administration. Monkeys were baseline tested in the weeks prior to drug administration. Monkeys were introduced to the extended cage on at least three and up to 11 occasions prior to testing. Monkeys were never tested for baseline or post-treatment, after having been in the extended cage the same day. This ensured that monkeys were never in the extended cage for more than 1 h/day. Each member of a pair was tested on separate occasions. Testing was done immediately after subcutaneous injection with either 0.1 mg/kg hydro or 2.0 mg/kg LE-hydro subcutaneously (0 h), and then for the next 4 days after drug administration. Monkeys administered LE-hydro were tested for 4 days, until the serum concentration of drug would be expected to decline below the concentrations considered therapeutic in humans (approximately 4 ng/mL; Smith et al. 2008). Ethographic data were analyzed using Wilcoxon's signed–ranks test run on SPSS (Carey, NC, USA). Significance was inferred at p<0.05 (one-tailed probability).
Motor skills testing Monkeys: were at least 1.5 years old or older, 10/group (four males and six females for hydro and two males and eight females for LE-hydro), weighing between 4.5 and 10.5 kg. Paired monkeys were separated during training and testing with a mesh divider. Drugs were administered as described for playroom testing and testing was performed at 0, 0.5, and 1 h and then daily for 5 days after injection. Equipment: Peg boards consisted of a straight and curved metal peg, attached to a clear Plexiglas board with an opening to allow the monkey's hand to easily reach the pegs. The board slides over the opening of the cage door, basically replacing the door, boards were secured by bungee cords. Carrots were sliced into rings and holes were cut to allow them to easily slide on the metal pegs. Training: Monkeys were initially screened in order to make sure all were comfortable with the puzzle board. Those who showed signs of nervousness, apprehension, or lack of interest in taking food off the puzzle board were not included. Only three monkeys were dropped for failure to perform the motor skills task during the training period. No monkeys were dropped during the testing period. Training was done at any time of the day between 0900 and 1700 hours. Monkeys were not food-deprived. Training initially began by placing a carrot on the shelf of the puzzle board; this was done simply to ensure the monkey was comfortable with putting his/her hand through the hole before moving on to the peg tasks. Once comfortable with retrieving items off the board, the straight peg training started. Monkeys were given unlimited time to explore and retrieve the carrot off the peg. Once comfortable and consistent on the straight peg, training on the curved peg began. Again, monkeys were given unlimited time to explore and retrieve the carrot from the curved peg. At anytime during training, if a monkey failed to complete the task within 10 min, testing would stop for the day. If the monkey failed only at the curved peg, we discontinued the curved peg for the day and more trials were done with the straight peg to encourage further participation and reward. Once monkeys were able to retrieve the carrot quickly (within a few seconds) and consistently from both pegs, they moved onto the testing phase. Training was completed in two to five sessions.
Testing
Baseline measurements were taken during one morning, between the hours of 9 and 11a.m. Baselines were done 1–5 days prior to testing. Food was withheld the morning of testing. Monkeys were briefly removed from their home cage for the injection and returned immediately. Testing consisted of at least six successful trials of both the straight and curved pegs. The pegs alternated between the straight and curved. If a monkey was uninterested in taking the carrots, we would attempt to encourage participation by putting a carrot on the base of the board (no skill required), or by putting a carrot on both the pegs and giving the monkey a choice. If the monkey failed to do either peg over the course of 10 consecutive minutes, the trial for that time point was ended. Time points included 30, 60 min, and 24, 48, 72, 96, and 120 h post-injection. Trials were videotaped and reviewed at a later time to determine the exact time of each trial. Reviewers were not blinded to treatment condition. Start time began when the monkey's fingertips passed through the opening; end time was when the monkey's fingertips passed back through the opening. A total of six trials were averaged for each animal at each time point. Trials where the monkey would not perform the task for either peg over 10 consecutive minutes were considered “timed out”. Testing sessions for which the monkey timed out required an estimation of the missing data points. The highest average for a completed testing session of six trials for each of the data sets (straight or curved) was used as the estimate as this provided a more conservative analysis than using 600 s (10 min). Analysis for differences within and between groups was done using Wilcoxon's signed-ranks test run on run on SPSS (Carey, NC, USA). Significance was inferred at p<0.05.
Results
Pharmacokinetic analysis
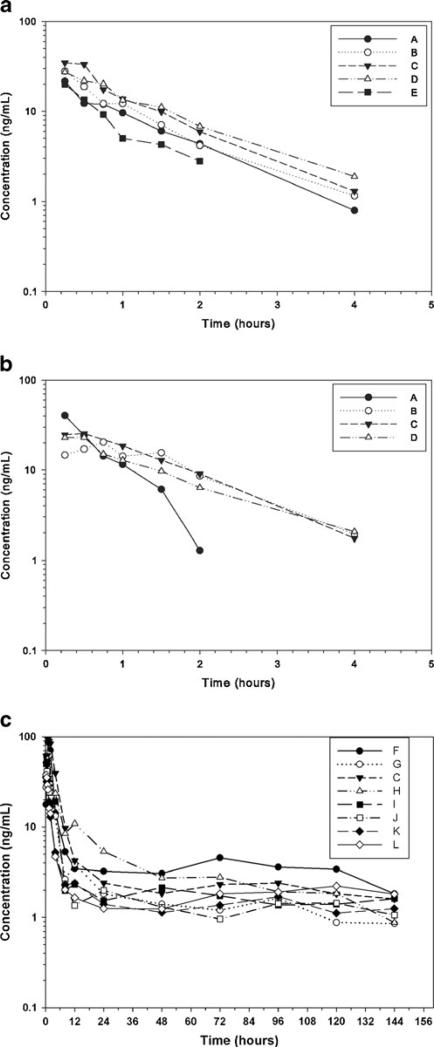
Intravenous administration of 0.1 mg/kg hydro to rhesus monkeys produced a pharmacokinetic profile that included a mean half-life (T½Biologic) of 0.6 h and a mean residence time (MRT 0-INF) of 1.1 h (Table 1, Fig. 1a). The pharmacokinetic profile of the same dose of hydro administered s.c. was very similar, and included T½Biologic of 0.7 h and a mean residence time (MRT 0-INF) of 1.3 h (Table 2, Fig. 1b). These parameters differed when monkeys were administered 2 mg/kg LE-hydro s.c. The T½Biologic was 30.4 h and mean residence time (MRT 0-INF) was 105.9 h. (Table 3, Fig. 1c). The peak concentration in the LE-hydro monkeys was larger (CMAX=55.3 ng/mL; Table 3) than in the s.c. hydro monkeys (CMAX=26.4 ng/mL; Table 2) or the group of monkeys administered i.v. hydro (C0=35.6 ng/mL; Table 1), but none of the animals in the three treatment groups were ever visibly sedated.
Table 1.
Pharmacokinetics of hydromorphone (0.1 mg/kg hydromorphone HCl) i.v. to rhesus (n=5)
| Parameter | Low | Median | High | Geometric mean | |
|---|---|---|---|---|---|
| AUCExtrapolated | % | 3.2 | 4.2 | 14.9 | 5.3 |
| AUC0-LAST | h ng mL–1 | 19.1 | 34.4 | 44.6 | 32.5 |
| AUC0-INF | h ng mL–1 | 22.4 | 35.9 | 46.3 | 34.8 |
| AUMC0-INF | h hng mL–1 | 21.7 | 39.4 | 61.6 | 38.7 |
| AUMC0-LAST | h hng mL–1 | 11.1 | 31.3 | 46.1 | 28.4 |
| C0 | ng mL–1 | 29.7 | 35.9 | 41.5 | 35.6 |
| Cl | mL min–1 kg–1 | 32.1 | 41.3 | 66.3 | 42.7 |
| T ½ λ z | h | 0.8 | 0.9 | 1.0 | 0.9 |
| λ z | h–1 | 0.669 | 0.798 | 0.841 | 0.777 |
| MRT0-INF | h | 1.0 | 1.1 | 1.4 | 1.1 |
| MRT0-LAST | h | 0.6 | 0.9 | 1.1 | 0.9 |
| Vss | L kg–1 | 2.1 | 2.7 | 3.9 | 2.8 |
| Vz | L kg–1 | 2.4 | 3.3 | 4.7 | 3.3 |
| T ½ Biologic | h | 0.4 | 0.7 | 0.8 | 0.6 |
AUC0-INF the area under the curve from time 0 to infinity, AUC0-LAST area under the curve from 0 to the last time point, AUCExtrapolated percent of the AUC extrapolated to infinity, AUMC0-INF area under the first moment curve from time 0 to infinity, AUMC0-LAST area under the first moment curve from time 0 to the last measured time point, Cl serum clearance, Vss apparent volume of distribution at steady state, Vz apparent volume of distribution of the area during the elimination phase, λz first-order rate constant, T½ λz terminal half-life, MRT0-INF mean residence time extrapolated to infinity, MRT0-last mean residence time from 0 to the last measured time point, C0 the concentration extrapolated to time 0, CMAX maximum serum concentration, TMAX time to maximum serum concentration, Vz F–1 Vz per fraction of the dose absorbed
Fig. 1.
Serum concentration of hydromorphone after 0.1 mg kg−1 hydromorphone HCl i.v. to rhesus monkeys (n=5; a). Serum concentration of hydromorphone after 0.1 mg kg−1 hydromorphone HCl s.c. to rhesus monkeys (n=4; b). Serum concentrations of hydromorphone after s.c. administration of 2 mg/kg in rhesus monkeys (n=8; c). Letters data from individual monkeys
Table 2.
Pharmacokinetics of hydromorphone (0.1 mg/kg hydromorphone HCl) s.c. to rhesus (n=4)
| Parameter | Units | Low | Median | High | Geometric mean |
|---|---|---|---|---|---|
| AUCExtrapolated | % | 2.1 | 5.3 | 8.8 | 4.8 |
| AUC0-LAST | h ng mL–1 | 28.7 | 36.9 | 44.5 | 36.3 |
| AUC0-INF | h ng mL–1 | 28.0 | 39.8 | 46.7 | 37.9 |
| AUMC0-INF | h hng mL–1 | 19.2 | 64.0 | 66.5 | 47.8 |
| AUMC0-LAST | h hng mL–1 | 17.8 | 48.3 | 54.9 | 38.7 |
| Cl F–1 | mL min–1 kg–1 | 31.8 | 37.4 | 52.9 | 39.1 |
| C MAX | ng mL–1 | 20.4 | 24.2 | 40.4 | 26.4 |
| T½ λz | h | 0.3 | 0.9 | 1.1 | 0.7 |
| λ z | h–1 | 0.61 | 0.80 | 2.19 | 0.96 |
| MRT0-INF | h | 0.7 | 1.5 | 1.6 | 1.3 |
| MRT0-LAST | h | 0.6 | 1.2 | 1.4 | 1.1 |
| T MAX | h | 0.3 | 0.5 | 0.8 | 0.5 |
| Vz F–1 | L kg–1 | 1.5 | 2.5 | 3.8 | 2.4 |
| T ½ Biologic | h | 0.5 | 0.9 | 1.0 | 0.7 |
| F | 0.84 | 0.97 | 1.15 | 0.98 |
AUC0-INF the area under the curve from time 0 to infinity, AUC0-LAST area under the curve from 0 to the last time point, AUCExtrapolated percent of the AUC extrapolated to infinity, AUMC0-INF area under the first moment curve from time 0 to infinity, AUMC0-LAST area under the first moment curve from time 0 to the last measured time point, Cl serum clearance, Vss apparent volume of distribution at steady state, Vz apparent volume of distribution of the area during the elimination phase, λz first-order rate constant, T½ λz terminal half-life, MRT0-INF mean residence time extrapolated to infinity, MRT0-last mean residence time from 0 to the last measured time point, C0 the concentration extrapolated to time 0, CMAX maximum serum concentration, TMAX time to maximum serum concentration, Vz F–1 Vz per fraction of the dose absorbed
Table 3.
Pharmacokinetics of DPPC hydromorphone (2 mg/kg hydromorphone HCl) s.c. to rhesus (n=8)
| Parameter | Units | Low | Median | High | Geometric mean |
|---|---|---|---|---|---|
| AUCExtrapolated | % | 9.8 | 22.5 | 57.1 | 25.4 |
| AUC0-LAST | h ng mL–1 | 270.9 | 383.1 | 705.2 | 424.7 |
| AUC0-INF | h ng mL–1 | 357.2 | 618.1 | 874.3 | 613.2 |
| AUMC0-INF | h hng mL–1 | 32,258.2 | 71,234.6 | 241,291.8 | 64,921.7 |
| AUMC0-LAST | h hng mL–1 | 13,067.1 | 18,073.1 | 34,950.9 | 18,644.6 |
| Cl F–1 | mL min–1 kg–1 | 33.9 | 49.1 | 83.1 | 48.4 |
| C MAX | ng mL–1 | 35.6 | 54.8 | 93.4 | 55.3 |
| T½ λz | h | 54.6 | 79.6 | 213.3 | 82.5 |
| λ z | h–1 | 0.0033 | 0.0087 | 0.0127 | 0.0084 |
| MRT0-INF | h | 54.6 | 86.2 | 277.3 | 105.9 |
| MRT0-LAST | h | 32.5 | 47.2 | 61.9 | 43.9 |
| T MAX | h | 0.3 | 0.6 | 1.0 | 0.6 |
| Vz F–1 | L kg–1 | 176.9 | 394.0 | 629.3 | 345.4 |
| T ½ Biologic | h | 22.5 | 32.7 | 42.9 | 30.4 |
AUC0-INF the area under the curve from time 0 to infinity, AUC0-LAST area under the curve from 0 to the last time point, AUCExtrapolated percent of the AUC extrapolated to infinity, AUMC0-INF area under the first moment curve from time 0 to infinity, AUMC0-LAST area under the first moment curve from time 0 to the last measured time point, Cl serum clearance, Vss apparent volume of distribution at steady state, Vz apparent volume of distribution of the area during the elimination phase, λz first-order rate constant, T½ λz terminal half-life, MRT0-INF mean residence time extrapolated to infinity, MRT0-last mean residence time from 0 to the last measured time point, C0 the concentration extrapolated to time 0, CMAX maximum serum concentration, TMAX time to maximum serum concentration, Vz F–1 Vz per fraction of the dose absorbed
Playroom social interaction and behavioral analysis
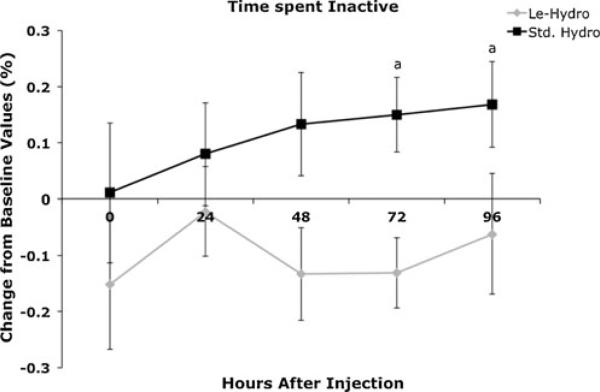
Many behaviors, including most social–interactive behaviors, alimentation, and elimination behaviors did not change between monkeys administered 0.1 mg/kg hydro s.c. and monkeys administered 2 mg/kg LE-hydro s.c. (Table 4). The behaviors that differed between the two groups usually differed only at a single time point; and usually at 24–96 h after injection, when the hydro monkeys would be expected to have minimal plasma concentrations of hydromorphone. Most of the behaviors that were different between the two groups were either environmentally or self-directed (Table 5). Only one behavior, “inactive” differed between the two groups at two time points. This behavior was decreased in monkeys administered LE-hydro at 72 and 96 h after injection (Fig. 2).
Table 4.
List of behaviors scored on the ethogram that did not differ at any time point between monkeys administered hydro or LE-hydro
| General Category | Specific Behavior | Hours Post Injection Mean ± SD (p value) |
|||||
|---|---|---|---|---|---|---|---|
| Drug | 1 | 24 | 48 | 72 | 96 | ||
| Alimentation | Eat | Hydro | 3.0±9.9 | 84±26 | 24±76 | 219±691 | 162±503 |
| LE-Hydro | 1.4±6.2 (0.143) | 1.2±2.5 (0.400) | -0.41±0.75 (0.167) | -0.12±0.64 (0.480) | 0.42±1.9 (0.222) | ||
| Drink | Hydro | -0.17±1.2 | -0.29±0.65 | -0.18±1.0 | -0.59±0.45 | 0.18±1.4 | |
| LE-Hydro | -0.77±0.42 (0.084) | 2.78±10.4 (0.444) | 2.1±8.8 (0.477) | 0.30±2.1 (0.119) | 3.8±12.4 (0.433) | ||
| Social | Groom | Hydro | 1.2±4.4 | 0.31±2.1 | 0.59±1.8 | -0.30±0.48 | -0.30±0.48 |
| LE-Hydro | 0.55±2.1 (0.353) | 31±2.1 (0.102) | -0.70±0.64 (0.174) | -0.2±0.42 (0.282) | -0.05±0.37 (0.129) | ||
| Non-specific contact | Hydro | 5.9±21 | 32±102 | 62±191 | 19±54 | 8.0±23 | |
| LE-Hydro | 2.4±10 (0.130) | 3.6±9.9 (0.384) | 2.1±6.2 (0.254) | 14±41 (0.288) | 15±41 (0.254) | ||
| Hostility | Hydro | -0.20±0.42 | -0.20±0.42 | -0.20±0.42 | 0.0±0.47 | 0.60±2.0 | |
| LE-Hydro | -0.10±3.2 (0.282) | 0.0±0.47 (0.207) | -0.10±0.32 (0.282) | 0.04±0.13 (0.382) | -0.10±0.32 (0.207) | ||
| Lip smack | Hydro | -0.4±0.52 | 0.20±1.0? | -0.40±0.52 | 0.3±1.1 | 0.5±1.8 | |
| LE-Hydro | -0.20±0.42 (0.159) | 0.3±1.7 (0.393) | -0.1±0.57 (0.129) | 0.3±1.7 (0.298) | 0.13±0.83 (0.358) | ||
| Threat | Hydro | -0.10±0.32 | -0.10±0.32 | -0.10±0.32 | -0.10±0.32 | 1.3±4.4 | |
| LE-Hydro | 0.0±0.0 (0.159) | 0.0±0.0 (0.159) | 0.0±0.0 (0.159) | 0.0±0.0 (0.159) | 0.0±0.0 (0.330) | ||
| Submit | Hydro | -0.10±0.32 | 0.05±0.16 | -0.03±0.08 | -0.03±0.08 | 0.0±0.47 | |
| LE-Hydro | -0.10±0.32 (0.500) | -0.10±0.32 (0.090) | -0.10±0.32 (0.330) | -0.10±0.32 (0.330) | -0.10±0.32 (0.282) | ||
| Fear Grimace | Hydro | -0.10±0.32 | -0.10±0.32 | -0.10±0.32 | 0.20±0.63 | 0.10±0.74 | |
| LE-Hydro | -0.10±0.32 (0.500) | -0.10±0.32 (0.500) | 0.0±0.47 (0.282) | -0.10±0.32 (0.090) | 0.10±0.74 (0.500) | ||
| Environmentally-directed | Environment Explore | Hydro | 0.12±3.1 | 4.7±1.6 | 11.3±36 | 0.30±1.9 | 3.6±11.2 |
| LE-Hydro | -0.78±0.48 (0.368) | 0.11±1.4 (0.254) | 0.41±1.3 (0.400) | 0.06±1.2 (0.439) | 0.10±1.1 (0.480) | ||
| Misjudge | Hydro | 0.9±3.2 | 0.0±0.47 | -0.10±0.31 | 0.15±0.34 | -0.10±0.32 | |
| LE-Hydro | 0.0±0.0 (0.328) | 0.0±0.0 (0.500) | 0.0±0.0 (0.159) | 0.0±0.0 (0.090) | 0.0±0.0 (0.159) | ||
| Self-directed | Self-Clasp | Hydro | -0.64±0.60 | 1.6±5.8 | 2.3±6.9 | 1.2±2.6 | -0.44±0.87 |
| LE-Hydro | -0.50±0.52 (0.137) | -0.35±0.58 (0.104) | 15.2±39 (0.288) | -0.48±0.51 (0.064) | -0.50±0.53 (0.500) | ||
| Self-Mouth | Hydro | -0.06±0.66 | 0.20±0.92 | -0.20±0.63 | -0.30±0.48 | -0.30±0.48 | |
| LE-Hydro | -0.20±0.42 (0.207) | 0.30±1.7 (0.500) | 0.43±1.2 (0.066) | -0.10±0.57 (0.207) | 0.0±0.82 (0.207) | ||
| Scratch | Hydro | 8.8±11.6 | 0.26±1.4 | 2.8±8.1 | 1.6±5.6 | 2.0±5.4 | |
| LE-Hydro | 12.6±16.5 (0.143) | 5.8±9.0 (0.055) | 1.9±3.0 (0.430) | 1.1±2.6 (0.430) | 4.6±8.5 (0.167) | ||
| Salute | Hydro | -0.07±0.22 | -0.05±0.16 | 0.051±0.28 | -0.71±0.22 | 0.007±0.02 | |
| LE-Hydro | -0.20±0.42 (0.138) | -0.20±0.42 (0.138) | -0.10±0.57 (0.291) | 0.21±0.75 (0.143) | -0.12±0.31 (0.055) | ||
| Elimination | Defecate | Hydro | -0.70±0.48 | 0.55±3.7 | 0.25±2.8 | -0.03±1.2 | -0.40±0.97 |
| LE-Hydro | -0.60±0.52 (0.282) | -0.36±0.74 (0.417) | -0.50±0.53 (0.259) | -0.50±0.71 (0.140) | -0.06±1.3 (0.231) | ||
| Urinate | Hydro | -0.80±0.33 | -0.13±1.1 | -0.18±1.1 | -0.11±0.96 | -0.43±0.95 | |
| LE-Hydro | -0.38±1.1 | -0.64±0.96 (0.088) | -0.24±0.61 (0.500) | -0.42±0.83 (0.407) | -0.02±1.1 (0.147) | ||
Table 5.
List of behaviors that differed at a single time point (p<0.05) between monkeys administered hydro or LE-hydro
| Hours Post Injection Mean ±SD SD (p value) |
|||||||
|---|---|---|---|---|---|---|---|
| General Category | Specific Behavior | Drug | 1 | 24 | 48 | 72 | 96 |
| Social-Interactive | Retreat | Hydro | -0.15±0.34 | -0.15±0.34 | -0.14±0.33 | -0.15±0.34 | -0.03±0.48 |
| LE-Hydro | 0.20±0.42 (0.051)* | 0.20±0.63 (0.090) | 0.0±0.0 (0.090) | 0.0±0.0 (0.090) | 0.0±0.0 (0.393) | ||
| Vocalize | Hydro | -0.28±0.88 | 0.059±1.1 | -0.21±0.71 | 0.04±1.2 | 0.16±1.4 | |
| LE-Hydro | -0.72±0.41 (0.070) | -0.71±0.25 (0.021)* | -0.61±0.65 (0.111) | 0.04±1.8 (0.121) | -0.32±1.1 (0.101) | ||
| Social-explore | Hydro | 0.50±1.3 | -0.10±0.32 | -0.1±0.32 | 0.25±0.42 | 0.0±0.47 | |
| LE-Hydro | 0.0±0.0 (0.090) | 0.0±0.0 (0.159) | 0.0±0.0 (0.159) | 0.0±0.0 (0.051)* | 0.0±0.0 (0.500) | ||
| Environmentally-directed | Locomotion | Hydro | -0.09±0.97 | -0.02±0.72 | -0.18±0.63 | -0.069±0.89 | -0.04±0.91 |
| LE-Hydro | -0.292±0.72 (0.361) | -0.45±0.41 (0.019)* | -0.077±0.56 (0.419) | 0.02±0.85 (0.288) | -0.04±0.77 (0.480) | ||
| Visual Explore | Hydro | -0.54±1.2 | 6.3±19 | 1.2±5.6 | 0.17±2.1 | -0.14±0.88 | |
| LE-Hydro | 0.52±3.7 (0.173) | 7.6±13 (0.020)* | 1.1±4.2 (0.220) | 1.7±6.9 (0.361) | 3.5±6.3 (0.047) | ||
| Watch | Hydro | 1.2±3.8 | 0.41±1.8 | 0.25±1.3 | 0.15±1.7 | 0.50±1.6 | |
| LE-Hydro | 0.17±1.9 (0.480) | 0.58±1.9 (0.254) | 3.5±7.9 (0.167) | 0.79±1.7 (0.047)* | 1.8±5.7 (0.400) | ||
| Self-directed | Yawn | Hydro | 0.15±1.2 | 0.30±0.95 | -0.30±0.48 | 0.30±0.95 | 0.75±2.2 |
| LE-Hydro | 0.0±0.0 (0.500) | 0.0±0.0 (0.138) | 0.0±0.0 (0.042)* | 0.0±0.0 (0.138) | 0.13±0.41 (0.297) | ||
| Self-Groom | Hydro | 7.7±25 | 5.6±13 | 2.7±6.7 | 17±27 | 27±34 | |
| LE-Hydro | 8.6±19 (0.433) | 12.1±17 (0.164) | 0.57±2.1 (0.337) | 3.2±5.6 (0.104) | 2.3±5.6 (0.037)* | ||
| Stereotypic Locomotion | Hydro | 0.45±2.2 | -1.4±0.91 | 0.16±1.4 | -0.07±1.1 | -0.27±0.82 | |
| LE-Hydro | 1.4±2.2 (0.121) | 2.2±3.9 (0.047)* | 0.88±1.3 (0.193) | 0.96±1.5 (0.102) | 0.84±1.6 (0.085) | ||
| Huddle | Hydro | -0.19±0.41 | 1.77±6.0 | -0.16±0.51 | 2.8±9.1 | 15±43 | |
| LE-Hydro | -0.60±0.52 (0.017)* | 0.11±2.3 (0.104) | -0.12±1.2 (0.117) | 1.22±5.9 (0.062) | 2.7±10 (0.104) | ||
p≤0.05
Fig. 2.
Effect of 2.0 mg/kg LE-hydro (diamonds) or 0.1 mg/kg hydro (squares) administered s.c. on the amount of time that rhesus monkeys spent inactive in the test cage. Monkeys administered LE-hydro were significantly less inactive (spent more time moving around) than monkeys administered 0.1 mg/kg hydro at 72 and 96 h post-injection. Significance indicator: a significant between groups (p<0.05)
Motor skills testing
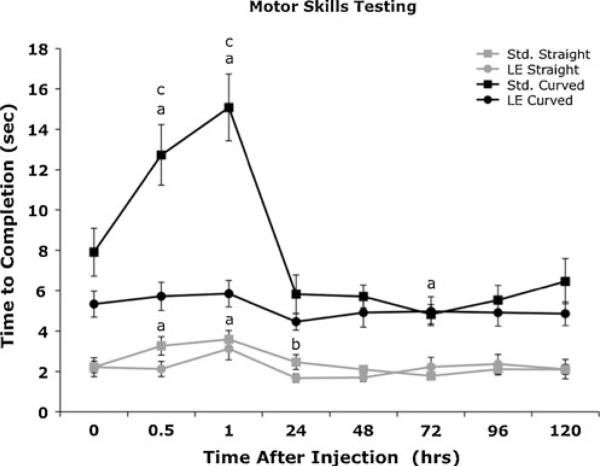
No significant differences were found between the performance of female and male monkeys on the task. Motor skills tests for the more difficult task using the curved peg were the most affected by administration of standard hydro. Task performance was not significantly different from baseline for monkeys administered LE-hydro for either the straight or curved peg. Time to completion for the straight peg task was significantly (p<0.05) longer compared to baseline for the hydro group at 0.5 and 1 h after injection, and was significantly longer than the performance of LE-hydro monkeys at 24 h after drug injection. Time to completion for the curved peg task in the hydro group was longer than baseline at 0.5 and 1 h after injection and was shorter compared to baseline values at 72 h after injection. Monkeys administered 0.1 mg/kg hydro took significantly longer to complete the curved peg task than monkeys administered 2 mg/kg LE-hydro at 0.5 and 1.0 h after injection (Fig. 3).
Fig. 3.
Effect of 2.0 mg/kg LE-hydro (circles) or 0.1 mg/kg hydro (squares) administered s.c. on performance in a motor skills task (n=10/group). The straight peg (gray lines) was the simpler task, the curved peg (black lines) was more difficult. Significance indicators: a significant for hydro within groups, b significant for straight peg between groups, c significant for curved peg between groups (p<0.05)
Discussion
Pharmacokinetics
The pharmacokinetics of hydro in rhesus monkeys determined by LC/MS analysis in this study agrees well with previous studies in dogs (Guedes et al. 2008; KuKanich et al. 2008) and with previous studies in rhesus monkeys using C-11 radiolabeled hydromorphone (Hartvig et al. 1989). The half-life of 0.1 mg/kg hydro administered i.v. in dogs is consistent with 60 min of analgesic efficacy, based on the assumption that plasma/serum drug concentrations must be at least 4 ng/mL (Coda et al. 1997; KuKanich et al. 2008; Reidenberg et al. 1988). However, this same dose produced 120 min of analgesia in a dog analgesiometry model, so it is possible that serum drug concentrations lower than 4 ng/mL may be clinically useful in dogs (Guedes et al. 2008). A lag from the decrease in serum drug concentrations to loss of effect (hysteresis) may also occur with hydromorphone in dogs, similar to that of morphine in dogs (KuKanich et al. 2007) which may explain the duration of effect lasting longer than serum drug concentrations.
Nonhuman primates have thresholds to noxious stimuli and opioid receptor systems that are similar to human beings (Ko et al. 2009; Kuhar et al. 1973). It might be expected that data on opioid dose response, side effects, and analgesia would be readily extrapolated between nonhuman primates and human beings. But for both human beings and nonhuman primates, how much hydromorphone is required for effective analgesia? Serum or plasma concentrations of hydromorphone ≥4 ng/mL have been associated with adequate analgesia in human beings with severe chronic pain. Hydromorphone doses in these patients were very high, ranging from 24 to 96 mg/day with a median of 48 mg/day for patients with good pain control (Reidenberg et al. 1988). Analgesic serum concentrations of hydromorphone in healthy human volunteers administered electrical tooth pulp stimulation ranged from approximately 0.4 to 6.0 ng/mL, considerably lower than for severe chronic pain (Coda et al. 1997). This result is consistent with studies in post-operative patients, where doses of 0.9–1.2 mg per person were found to provide equivalent analgesia to 10 mg of morphine (Mahler and Forrest 1975). Using tail withdrawal from 50° C water in rhesus monkeys, the ED50 value for hydromorphone was 1.56±0.18 mg/kg administered i.v. Plasma kinetics data for hydromorphone alone were not determined in this study, only the plasma concentration of hydromorphone produced by biotransformation after a 3.2 mg/kg dose of hydrocodone, so the analgesiometric data was not directly referable to plasma drug concentration (Lelas et al. 1999). Additional analgesiometric studies would be necessary to determine the duration of analgesic efficacy of LE-hydro in primates.
Previous studies in our laboratory determined that 2 mg/kg LE-hydro administered s.c. in dogs produces serum drug concentrations exceeding 4 ng/mL for approximately 96 h, which is associated with analgesic efficacy for people (Coda et al. 1997; KuKanich et al. 2008). LE-hydro in rhesus monkeys gives values for T½Biologic and MRT that were similar to the values determined for dogs (KuKanich et al. 2008). The serum concentrations were lower than in dogs by 24-h post-injection, but higher doses could be administered if needed. There is also evidence from studies in dogs that serum concentrations lower than 4 ng/mL are analgesic (Guedes et al. 2008). It is also important to note that the intensity of pain may also affect the effective concentration (or dose); less painful conditions may require lower concentrations for effect, whereas greater intensity of pain would require higher drug concentrations for analgesic effect. A single dose of 2 mg/kg LE-hydro s.c. provided durable analgesia in dogs undergoing ovariohysterectomy (OVHX) (Krugner-Higby, unpublished data). Analgesiometric studies in primates will be necessary to determine the optimal dosages of LE-hydro for clinical pain control in monkeys. Our previous studies in dogs indicated that LE-hydro could be used as a surgical analgesic for OVHX. There was no indication that any of the dogs administered LE-hydro developed tolerance in the immediate post-operative period (Krugner-Higby, accepted for publication).
Behavior
Previous behavioral studies in rhesus monkeys compared the effects of ammonium gradient-loaded liposomal oxymorphone (ASG-LE-oxy). This formulation lasts for more than 3 weeks in vivo, and would be suitable for use in treating chronic pain (Krugner-Higby et al. 2009). These behavior studies were repeated using LE-hydro made using the freeze–thaw method. There were fewer differences between hydro and LE-hydro than there were between oxymorphone (oxy) and ASG-LE-oxy in the previous study. The differences were less durable, only one was significantly different at more than two time points after injection (“inactive”: Fig. 3) whereas there were several behaviors that differed between monkeys administered ASG-LE-oxy and oxy at two to three time points, including “watch”, “locomotion”, “misjudge”, “self-clasp”, “urination”, and “defecation” (Krugner-Higby et al. 2009).
The reasons for this difference across assessments could derive either from variation in drug sensitivity in the animals studied, the drug itself or from variation in the drug formulation. The differences did not likely derive from the animals. The current monkeys were from the same colony used in the previous study, which has a circumscribed set of adult breeders, and the same personnel evaluated the test animals. Oxy and hydro may have some subtle differences in their behavioral and analgesic effects in a given species. Oxy has ten times the analgesic potency of morphine, and hydro has been reported to have five to seven times morphine's potency using this standard (Jaffe and Matin 1985). However, both drugs demonstrated equivalent effects on clinical pain in dogs and cats in a blinded study, when administered equipotent dosages (Bateman et al. 2008). Behavioral actions and adverse effects of opioid drugs may be more variable and subtle. Rhesus monkeys will learn to discriminate hydromorphone from saline vehicle and will show physical dependence to this drug (Ternes et al. 1985). In contrast, another macaque species, the cynomolgus monkey will not show dependence toward hydromorphone, but will to morphine (Aceto and Harris 1985; Ternes et al. 1983). Similarly, some human patients will not respond to hydromorphone, but will get adequate analgesia from other opioid drugs (Jaffe and Matin 1985).
Some of the behavioral differences noted in the rhesus monkeys in this study appeared to be associated with documented opioid side effects. There was an increased amount of scratching at 24 h, in the monkeys administered LE-hydro (Table 5), possibly due to opioid pruritus. Pruritus is a recognized adverse effect of this class of drugs in human beings (Reich and Szepietowski 2010). Scratching behavior has been used to selectively quantify the duration of central mu opioid activation in monkeys (Ko et al. 2004; Ko et al. 2009). The LE-hydro group also yawned more often at 48 h, an effect observed in people during opioid withdrawal and a behavior that is often associated with tension and arousal-related stereotypies (Fontenot et al. 2006; Gowing et al. 2009). Both LE-oxy and LE-hydro showed behavioral evidence of anxiolytic activity. Several self-directed behaviors were also decreased in monkeys administered LE-hydro (salute, self-groom, and self-explore, Table 5). Animals in a novel situation may use self-directed behaviors to cope with anxiety, and a decrease in self-directed behaviors would be expected for anxiolytic drugs (Krugner-Higby et al. 2009; Winslow et al. 2007). Monkeys administered LE-hydro and ASG-LE-oxy both had increased outward-directed behaviors, which could also be interpreted as an expression of decreased anxiety directed towards a social or environmental stimulus (watch, visual explore, Table 5; Krugner-Higby et al. 2009). The experiments in the current study were done using very broad general behavioral assessments. Experiments to specifically address the anxiolytic effects of hydro and LE-hydro would have to use behavioral paradigms that test for anxiety in nonhuman primates, such as assessment of snake fear (Kalin et al. 2004).
The largest behavioral differences between LE-hydro and the ASG-LE-oxy drug preparation used in our previous study were in locomotion and in urination and defecation. Monkeys administered LE-hydro had increased time spent in stereotypic locomotion (Table 5), but this was not found in monkeys treated with ASG-LE-oxy (Krugner-Higby et al. 2009). Increases in home cage activity were observed in pigtail macaques (Macaca nemistrina) after administration of morphine sulfate in the morning (Weed and Hienz 2006). The type of locomotor behavior was not identified as stereotypic or non-stereotypic in that study, but since activity was monitored in the home cage, it likely consisted of pacing: an activity that would have been scored by our ethogram as “stereotypic locomotion”. In our behavioral studies, all injections and testing were done in the morning between 0800 and 1000 hours, when increases in locomotion were likely to occur. The fact that increases in locomotion were observed with LE-hydro and not ASG-LE-oxy lends support to the idea that the difference was due to differences in the drug release and systemic exposure from liposomal preparations and not to differences in the drugs themselves.
There were persistent differences in observed urination and defecation between monkeys administered oxy and ASG-LE-oxy, but not between monkeys administered hydro and LE-hydro (Krugner-Higby et al. 2009). The pharmacokinetics of the preparation was most likely responsible for this observation. LE-hydro was made using freeze–thaw liposomes, and had pharmacokinetics that provided a higher peak and shorter duration of action (Table 3, Fig. 1c) than ASG-LE-oxy, which was made using ammonium sulfate gradient loaded liposomes and was present in serum for over 3 weeks (Krishna et al. 2001; Krugner-Higby et al. 2009; Webb et al. 2007). Thus, the behavioral effects of LE-hydro was more like those of hydro than those of ASG-LE-oxy were like oxy.
Motor skills
Both hydro and LE-hydro did not substantially affect performance on the motor skills task using the straight peg, and LE-hydro did not affect performance using the curved peg (Fig. 3). Hydro, however, significantly decreased the monkeys’ ability to perform the more difficult curved peg task at 0.5 and at 1 h after injection (Fig. 3). This result was consistent with what has been previously published. In human beings, graded tests of psychomotor function are used to determine the extent of impairment resulting from alcohol, drugs, and mental illness (Grootens et al. 2009; Kress and Kraft 2005; Leung and Starmer 2005). People will make more mistakes in a driving simulator when presented with a curved road than a straight one after consuming vodka in orange juice (Leung and Starmer 2005) and cancer patients on stable, long-term morphine analgesia performed no differently from controls on a battery of cognitive and psychomotor tests, but were significantly less able to balance with their eyes closed than healthy controls (Kress and Kraft 2005). People with recent-onset schizophrenia performed less well on psycho-motor tests as the tasks became more demanding (Grootens et al. 2009). Therefore, it was not surprising that monkeys administered hydro did not perform as well on the curved peg test. Their consistently better performance on the straight peg task when given 0.1 mg/kg hydro indicated that this was not the result of a lack of motivation for the food (carrot) reward.
Other investigators have performed tests of fine motor skills in baboons using a monometric task in which the animals retrieved raisins or shelled peanuts from six small cups. Using this test, increasing doses of zolpidem increased the time required to complete the task, and similar results were obtained using increasing doses of gamma-hydroxybutyrate, gamma-butyrolactone and 1,4-butanediol (Goodwin et al. 2009; Weerts et al. 1998). Using two tests of differential difficulty also allowed us to determine gradations in motor acuity, but without having to repeatedly dose monkeys with higher doses of hydro or LE-hydro.
In this study, the pharmacokinetics of LE-hydro and hydro were determined and effects of these two opioid preparations on behavior and motor skills investigated. The pharmacokinetics of LE-hydro was consistent with the same preparation in dogs. LE-hydro had mild behavioral effects without overt sedation, and minimal affects on motor skills. While there are studies that have been published on the behavioral and analgesic properties of opioids in primates, most experiments use bolus dosing rather than extended-release formulations (Aceto and Harris 1985; Ko et al. 2004, 2009; Lelas et al. 1999; Ternes et al. 1983, 1985). These studies make use of liposomal hydromorphone that can be used to study the effects of extended-release opioid medication and the basic biology of opioids in primates in general as a translational model for human beings. Additional studies will be necessary to evaluate LE-hydro as an analgesic for clinical pain, and the potential for opioid reward, tolerance, and hyperalgesia in rhesus monkeys.
Supplementary Material
Acknowledgments
This study was financially supported by a grant from the National Institutes of Health, National Center for Research Resources [2R01RR0188-02]. The authors would like to acknowledge Melissa Luck and the staff of the Harlow Center for Biological Psychology for their exemplary care of the monkeys used in this study. We would also like to thank Andrea Smetana and Laura Wunsch for their assistance in data collection, and Dr. Christopher Coe for use of the use of the motor skills testing equipment and review of the manuscript.
Abbreviations
- hydro
hydromorphone hydrochloride, standard pharmaceutical preparation
- LE-hydro
liposomal hydromorphone, freeze/thaw preparation
- oxy
oxymorphone hydrochloride, standard pharmaceutical preparation
- ASG-LE-oxy
ammonium sulfate gradient-loaded liposomal oxymorphone
- DPPC
dipalmitoyl phosphadityl choline
- LOQ
limit of quantification
- AUC0-INF
the area under the curve from time 0 to infinity
- AUC0-LAST
area under the curve from 0 to the last time point
- AUCExtrapolated
percent of the AUC extrapolated to infinity
- AUMC0-INF
area under the first moment curve from time 0 to infinity
- AUMC0-LAST
area under the first moment curve from time 0 to the last measured time point
- Cl
serum clearance
- Vss
apparent volume of distribution at steady state
- Vz
apparent volume of distribution of the area during the elimination phase
- λz
first-order rate constant
- T½ λz
terminal half-life
- MRT0-INF
mean residence time extrapolated to infinity
- MRT0-last
mean residence time from 0 to the last measured time point
- C0
the concentration extrapolated to time 0
- CMAX
maximum serum concentration
- TMAX
time to maximum serum concentration
- Vz F−1
Vz per fraction of the dose absorbed
- OVHX
Ovariohysterectomy
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-011-2239-y) contains supplementary material, which is available to authorized users.
Contributor Information
Lisa Krugner-Higby, Department of Surgical Sciences, School of Veterinary Medicine, University of Wisconsin, Madison, WI, USA; Research Animal Resource Center, 1710 University Ave, Madison, WI 53726-4089, USA.
Butch KuKanich, PharmCATS and the Department of Anatomy and Physiology, Kansas State University, Manhattan, KS, USA.
Brynn Schmidt, Department of Surgical Sciences, School of Veterinary Medicine, University of Wisconsin, Madison, WI, USA.
Timothy D. Heath, Division of Pharmaceutical Sciences, School of Pharmacy, University of Wisconsin, Madison, WI, USA
Carolyn Brown, Department of Surgical Sciences, School of Veterinary Medicine, University of Wisconsin, Madison, WI, USA.
References
- Aceto MD, Harris LS. Cynomolgus monkeys and morphine tolerance and dependence. Drug Alcohol Depend. 1985;15:15–18. doi: 10.1016/0376-8716(85)90025-0. [DOI] [PubMed] [Google Scholar]
- Bateman SW, Haldane S, Stephens JA. Comparison of the analgesic efficacy of hydromorphone and oxymorphone in dogs and cats: a randomized blinded study. Vet Anesth Analg. 2008;35:341–347. doi: 10.1111/j.1467-2995.2007.00387.x. [DOI] [PubMed] [Google Scholar]
- Coda BA, Tanaka A, Jacobson RC, Donaldson G, Chapman CR. Hydromorphone analgesia after intravenous bolus administration. Pain. 1997;71:41–48. doi: 10.1016/s0304-3959(97)03336-8. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Medina RO, Bowman RE. Home cage behavior and lead treatment in rhesus monkeys: a comparison with open-field behavior. Neurotoxicol Teratol. 1993;15:145–149. doi: 10.1016/0892-0362(93)90073-w. [DOI] [PubMed] [Google Scholar]
- Fontenot MB, Wilkes MN, Lynch CS. Effects of outdoor housing on self-injurious behavior and stereotypic behavior in adult male rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci. 2006;45:35–43. [PubMed] [Google Scholar]
- Goodwin AK, Brown PR, Jansen EEW, Jacobs C, Gibson KM, Weerts EM. Behavioral effects and pharmacokinetics of gamma-hydroxybutyrate (GHB) precursors gamma-butyrolactone (GBL) and 1, 4-butanediol (1, 4-BD) in baboons. Psychopharmacology. 2009;204:465–476. doi: 10.1007/s00213-009-1477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing L, Ali R, White JM. Buprenorphine for the management of opioid withdrawal. Cochrane Database of Systematic Reviews 3. 2009 doi: 10.1002/14651858.CD002025.pub4. Art. No.: CD002025. doi:10.1002/14651858.CD0022025.pub4. [DOI] [PubMed] [Google Scholar]
- Grootens KP, Vermeeren L, Verke RJ, Baitelaar JK, Sabbe BGC, van Veelen N, Kahn RS, Hulstijn W. Psychomotor planning is deficient in recent-onset schizophrenia. Schizophr Res. 2009;107:294–302. doi: 10.1016/j.schres.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Guedes AGP, Papich MG, Rude EP, Rider MA. Pharmacokinetics and physiological effects of intravenous hydromorphone in conscious dogs. J Vet Pharmacol Ther. 2008;31:334–343. doi: 10.1111/j.1365-2885.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- Hartvig P, Neil A, Terenius L, Antoni G, Rimland A, Ulin J, Langstrom B. Brain and plasma kinetics of the opioid 11C-hydromorphone in two macaque species. Pharmacol Toxicol. 1989;65:214–216. doi: 10.1111/j.1600-0773.1989.tb01159.x. [DOI] [PubMed] [Google Scholar]
- Hawk C, Leary . Formulary for laboratory animals. 3dth edn. Iowa State University Press; Ames: 2005. [Google Scholar]
- Jaffe JH, Matin WR. Opioid analgesics and antagonists. In: Gilman AG, Rall TW, Murad F, editors. Goodman and Gilman's the pharmacological basis of therapeutics. Macmillan Publishing Company; New York: 1985. pp. 491–531. [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Kim J, Kim S. Extended-release formulation of morphine for subcutaneous administration. Cancer Chemother Pharmacol. 1993;33:187–190. doi: 10.1007/BF00686214. [DOI] [PubMed] [Google Scholar]
- Ko MCH, Song MS, Edwards T, Lee H, Naughton NN. The role of central opioid receptors in opioid-induced itch in primates. J Pharmacol Exp Ther. 2004;310:169–176. doi: 10.1124/jpet.103.061101. [DOI] [PubMed] [Google Scholar]
- Ko M-C, Woods JH, Fantegross WE, Galuska CM, Wichmann J, Prinssen EP. Behavioral effects of a synthetic agonist selective for nociception/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology. 2009;34:2088–2096. doi: 10.1038/npp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress HG, Kraft B. Opioid medication and driving ability. Eur J Pain. 2005;9:141–144. doi: 10.1016/j.ejpain.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Krishna R, Webb MS, St. Onge G, Mayer LD. Liposomal and nonliposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution properties. J Pharmacol Exp Ther. 2001;298:1206–1212. [PubMed] [Google Scholar]
- Krugner-Higby L, KuKanich B, Schmidt B, Heath TD, Brown C, Smith LJ. Pharmacokinetics and behavioral effects of an extended-release, liposome-encapsulated preparation of oxymorphone in rhesus macaques. J Pharmacol Exp Ther. 2009;330:135–141. doi: 10.1124/jpet.108.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ, Pert CB, Snyder SH. Regional distribution of opiate receptor binding in monkey and human brain. Nature. 1973;245:447–450. doi: 10.1038/245447a0. [DOI] [PubMed] [Google Scholar]
- KuKanich B, Hogan BK, Krugner-Higby LA, Smith LJ. Pharmacokinetics of hydromorphone hydrochloride in healthy dogs. Vet Anesth Analg. 2008;35:256–264. doi: 10.1111/j.1467-2995.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- KuKanich B, Lascelles BDX, Riviere JE, Papich MG. Pharmacokinetic–pharmacodynamics modeling of morphine in dogs. J Vet Intern Med. 2007;21:617. [Google Scholar]
- Lelas S, Wegert S, Otton SV, Sellers EM, France CP. Inhibitors of cytochrome P450 differentially modify discriminative-stimulus and antinociceptive effects of hydrocodone and hydromorphone in rhesus monkeys. Drug Alcohol Depend. 1999;54:239–249. doi: 10.1016/s0376-8716(98)00169-0. [DOI] [PubMed] [Google Scholar]
- Leung SY, Starmer GA. Gap-acceptance and risk-taking by young and mature drivers, both sober and alcohol-intoxicated, in a simulated driving task. Accid Anal Prev. 2005;37:1056–1065. doi: 10.1016/j.aap.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Mahler DL, Forrest WH. Relative analgesic potencies of morphine and hydromorphone in postoperative pain. Anesthesiology. 1975;42:602–607. doi: 10.1097/00000542-197505000-00021. [DOI] [PubMed] [Google Scholar]
- Reich A, Szepietowski JC. Opioid-induced pruritus: an update. Clin Exp Dermatol. 2010;35:2–6. doi: 10.1111/j.1365-2230.2009.03463.x. [DOI] [PubMed] [Google Scholar]
- Reidenberg MM, Goodman H, Erle H, Gray G, Lorenzo B, Leipzig RM, Meyer BR, Drayer DE. Hydromorphone levels and pain control in patients with severe chronic pain. Clin Pharmacol Ther. 1988;44:376–382. doi: 10.1038/clpt.1988.167. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Krugner-Higby L, Clark M, Wendland A, Heath TD. A single dose of liposome-encapsulated oxymorphone or morphine provides long-term analgesia in an animal model of neuropathic pain. Comp Med. 2003;53:280–287. [PubMed] [Google Scholar]
- Smith LJ, KuKanich B, Krugner-Higby L, Brown C, Heath TD. Pharmacokinetics of liposome-encapsulated hydromorphone in dogs. J Vet Pharmacol Ther. 2008;31:415–422. doi: 10.1111/j.1365-2885.2008.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LJ, Valenzuela JR, Krugner-Higby L, Brown C, Heath TD. A single dose of liposome encapsulated hydromorphone provides extended relief of hyperalgesia in a rodent model of neuropathic pain. Comp Med. 2006;56:487–492. [PubMed] [Google Scholar]
- Ternes JW, Ehrman R, O'Brien CP. Cynomolgus monkeys do not develop tolerance to opioids. Behav Neurosci. 1983;97:327–330. doi: 10.1037//0735-7044.97.2.327. [DOI] [PubMed] [Google Scholar]
- Ternes JW, Ehrman RN, O'Brien CP. Nondependent monkeys self-administer hydromorphone. Behav Neurosci. 1985;99:583–588. doi: 10.1037//0735-7044.99.3.583. [DOI] [PubMed] [Google Scholar]
- Viscusi ER, Kopacz D, Hartrick C, Martin G, Manvelian G. Single dose extended release epidural morphine for pain following hip arthroplasty. Am J Ther. 2006;13:423–431. doi: 10.1097/01.mjt.0000178903.72619.ee. [DOI] [PubMed] [Google Scholar]
- Viscusi ER, Martin G, Kopacz D, Hartrick C, et al. EREM Study Group Forty-eight hours of postoperative pain relief after total hip arthroplasty with a novel, extended release epidural morphine formulation. Anesthesiology. 2005;102:1014–1022. doi: 10.1097/00000542-200505000-00022. [DOI] [PubMed] [Google Scholar]
- Webb MS, Boman NL, Masin D, Yapp D, Ramsay E, Chiu GNC, Cullis PR, Bally MB. A cationic liposomal vincristine formulation with improved vincristine retention, extended circulation lifetime and increased anti-tumor activity. Lett Drug Des Discov. 2007;4:426–433. [Google Scholar]
- Weed MR, Hienz RD. Effects of morphine on circadian rhythms of motor activity and body temperature in pig-tailed macaques. Pharmacol Biochem Behav. 2006;84:487–496. doi: 10.1016/j.pbb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Ator NA, Grech DM, Griffiths RR. Zolpidem physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. J Pharmacol Exp Ther. 1998;285:41–53. [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Davis M. Modulation of fear-potentiated startle and vocalizations in juvenile rhesus monkeys by morphine, diazepam, and buspirone. Biol Psychiatry. 2007;61:389–395. doi: 10.1016/j.biopsych.2006.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.