Abstract
In primates and rodents, trophoblast cells synthesize and secrete into the maternal circulation a family of proteins known as pregnancy specific glycoproteins (PSG). The current study was undertaken to characterize the receptor for two members of the murine PSG family, PSG17 and PSG23. Binding of recombinant PSG17 and PSG23 to CHO-K1 and L929 cells and their derived mutants was performed to determine whether these proteins bound to cell surface proteoglycans. We also examined binding of these proteins to cells transfected with syndecans and glypican-1 by flow cytometry. The interaction with glycosaminoglycans was confirmed in solid phase assays. Our results show that PSG17 binds to CD9 and to cell surface proteoglycans while PSG23 binds only to the latter. We found that the amino acids involved in CD9 binding reside in the region of highest divergence between the N1-domains of murine PSGs. For both proteins, the N-terminal domain (designated as N1) is sufficient for binding to cells and the ability to bind cell surface proteoglycans is affected by the cell line employed to generate the recombinant proteins. We conclude that while substantially different at the amino acid level, some murine PSGs share with human PSG1 the ability to bind to cell surface proteoglycans and that at least one PSG binds to more than one type of molecule on the cell surface.
Keywords: pregnancy specific glycoproteins, proteoglycans, receptor, CD9
1. Introduction
Pregnancy specific glycoproteins are secreted members of the carcinoembryonic antigen family expressed by the placenta [1]. PSG-related genes have been found in human, non-human primates and rodents [2,3]. In mice there are seventeen PSG genes (Psg16–Psg32) synthesized by giant cells and the spongiotrophoblast [4]. PSGs have been identified as early markers of human trophoblast differentiation with their expression preceding syncytialization and beta hCG expression [5]. Reduced levels of PSGs measured during the first trimester of pregnancy were reported in adverse pregnancy outcomes including small-for-gestational age fetuses and preterm delivery [6,7]. In addition, PSG levels were found to be below the normal range in late pregnancy in cases of fetal growth restriction [8,9].
PSGs have only been found in species with haemochorial placentation, therefore, it has been proposed that they play a role in suppression of the maternal immune system [2]. Indeed, we and others have found that human and murine members of the PSG family have immunoregulatory functions due to their ability to induce the secretion of anti-inflammatory cytokines such as TGFβ1 and IL-10 by monocyte/macrophages and regulate T-cell function [10–13]. We have also indicated that some members of the murine and human PSG family may be involved in placental angiogenesis. PSG1 induces the formation of tubes by endothelial cells and members of the human and murine PSG induce the secretion of VEGF-A [14–16].
As part of our interest in understanding the function of PSGs, we have worked on the identification of their receptors. We found that human PSG1 binds to cell surface proteoglycans and hypothesized that murine PSGs may use the same receptors [16]. The experiments described in this report show that PSG23, one of the highest expressed murine PSG family members [4], and PSG17 bind to glycosaminoglycans chains presented in syndecans and glypican-1. In addition, we determined that binding to these cell surface proteoglycans is mediated through the N1-domain of these proteins. We have previously shown that PSG17 and PSG19 bind to the tetraspanin CD9 while we did not observe binding of murine PSG23 [15,17,18]. Mutagenesis was performed to identify the amino acids in PSG17 which are required for CD9 binding. We found that the amino acids in PSG17 involved in CD9 binding, map to the area of highest divergence between the N1-domains of murine PSGs. Primate and rodent PSGs have undergone independent gene family expansions and structural diversification [19]. Our results indicate that some members of the murine and human PSG family could share biological functions despite their sequence divergence as they bind to the same molecules on cells. Same receptor usage could explain the previously observed species cross-reactivity and validates the use of murine models to better understand PSG function in vivo.
2. Materials and Methods
2.1. Generation of recombinant proteins
The cDNA for PSG17N and 23N were synthesized by GenScript Corporation (Piscataway, NJ). The cDNAs encode the leader peptide and the N1-domain. In the PSG17N cDNA, a nucleotide was changed to destroy an internal EcoRI site without resulting in an amino acid change. In addition, three new restriction enzyme sites to allow for the generation of hybrid molecules were added in the cDNAs resulting in no amino acid changes. To introduce a KpnI site in PSG17, a nucleotide change was required which resulted in a K to R substitution. The cDNAs were subcloned into the pFuse-IgG1 e3-Fc1 vector (InvivoGen, San Diego, CA). The proteins obtained are denoted PSG17N-Fc and PSG23N-Fc. The control protein FLAG-Fc and PSG23N1AHisFLAG were previously described [15].
Plasmids encoding the wild type or mutated proteins were transfected into CHO-K1, HeLa or HEK 293T cells with Lipofectamine 2000 (Invitrogen, CA). Five hours post-transfection, the media was replaced for OPTI-MEM I (Invitrogen) and harvested 48 hours later. The collected supernatant was passed through a HiTrap protein A column in an AKTA-Plus system (GE Healthcare, NJ) and proteins were eluted with 0.1M glycine [pH 2.8]. Fractions containing the protein were pooled, concentrated and buffer exchanged with PBS in an Amicon Utra-10K centrifugal filter unit (Millipore). For quantitation purposes the proteins were separated on 4–20% NuPAGE Bis-Tris gels (Invitrogen) at different dilutions next to known amounts of BSA, used as standards (Thermo Scientific). The gels were stained with GelCode Blue (Thermo Scientific) and the proteins quantitated by densitometry.
2.2. Cell lines
Cells were cultured in 5% CO2 humidified incubator at 37°C. Chinese hamster ovary epithelial (CHO-K1 and CHO-pgsA-745), baby hamster kidney (BHK-21), human epithelial (HeLa), human embryonic kidney (HEK 293T), and mouse fibroblast (L929) cells were obtained from the American Type Culture Collection (Manassas, VA). Sog9 fibroblasts were a gift of Drs. Frank Tufaro and Gary Cohen and were sent to us by Dr. K. Spindler (University of Michigan, Ann Arbor). The human B lymphocyte (Namalwa) transfectants have been previously described [20]. To generate Sog9 cell expressing murine CD9, cells were transfected with the Amaxa nucleofector with the CD9 cDNA cloned into pEF6/V5-His (Invitrogen). Cells were selected with 2 ug/ml of blasticidin and the expression of CD9 was verified by FACS. The brightest population was sorted in a BD FACSAria and propagated. Bone marrow derived macrophages (BMDM) from wild type and CD9-null mice were obtained and maintained as previously reported [21].
2.3. Antibodies
Biotin-conjugated anti-mouse CD9 antibody and the isotype control were from BD Biosciences. Heparan sulfate expression was detected with FITC-conjugated 10E4 antibody (US Biological, Swampscott, MA). Biotin-labeled anti-FLAG M2 and HRP-conjugated anti-FLAG M2 mAb were obtained from Sigma and streptavidin-APC was from Invitrogen.
2.4. FACS analysis
Adherent cells were detached with Accutase (Innovative Cell Technologies, San Diego, CA) and 1×106 were resuspended in FACS buffer (2%BSA-0.02% NaN3 in PBS) containing the indicated proteins or antibodies for 1 hour on ice. Excess protein and antibody were removed by washing between steps. Namalwa or BMDM were first incubated with Fc block or mouse Fc block. Cells were analyzed in the BD LSRII and fifty thousand total events were collected using BD FACS Diva software. The FlowJo software (Tree Star, Inc., Ashland, OR) was used for post acquisition analysis.
2.5. Solid phase binding assays
To determine whether PSG17N and PSG23N bind to immobilized heparin, 100 μg of protein in loading buffer (10mM Sodium Phosphate, pH 7.0) were passed through a 1ml heparin column in the AKTA-Plus system (GE Healthcare). The column was washed with 10 column volumes of loading buffer and bound proteins were eluted using a gradient of NaCl. Eluted samples were separated on a 4–12% NuPAGE Bis-Tris gel followed by immunoblot analysis. Binding of PSGs to coated plates was performed on 96-well ELISA plates incubated overnight at 4°C with 200 μg of heparin, heparan or chondroitin sulfate or BSA in quadruplicate. The coated plates were washed and blocked with PBS-0.5% BSA. Purified recombinant proteins (200 ng/well) were applied to the coated wells and incubated overnight at 4°C. After three washes with PBS-0.05% Tween 20, the proteins were detected with 1 ng/ml of HRP-conjugated anti-Fcγ Ab (Thermo Scientific) followed by the addition of TMB substrate. The reaction was stopped with sulfuric acid and the plate was read at 450 nm. Statistical significance between protein control and PSG-treated wells coated with GAGs was determined by Student’s t test with a P value of <0.05 as a cut-off.
2.6. Panning of transfected cells on protein-coated dishes
Panning of transfected cells on PSG17N and PSG23N coated dishes was performed as described in [17] with minor modifications. Briefly, Petri dishes were coated with rabbit anti-goat IgG (KPL) after which the plates were blocked with 1% BSA prior to the addition of PSG17N-Fc, PSG23N-Fc or FLAG-Fc. The following day, the plates were rinsed with PBS and the different cells (empty transfected or mCD9 transfected BHK-21) were added to the dishes. BHK-21 cells were transfected with a plasmid encoding murine CD9 or the same plasmid with no insert using Lipofectamine LTX (Invitrogen). Forty eight hours post-transfection, cells were harvested with Accutase and resuspended to 2.5 × 106 cells/ml in PBS with 2% BSA (wash buffer). Expression of mCD9 was confirmed by FACS. Two milliliters of cells were added to the protein-coated Petri dishes which were placed in the tissue culture incubator. After 60 minutes, the buffer was aspirated and the plates were rinsed extensively. The presence of cells was determined by examination under the microscope and the cells in three representative fields per dish were counted.
3. Results
3.1 Recombinant murine PSG17 and 23 bind to cell surface proteoglycans via their first domain
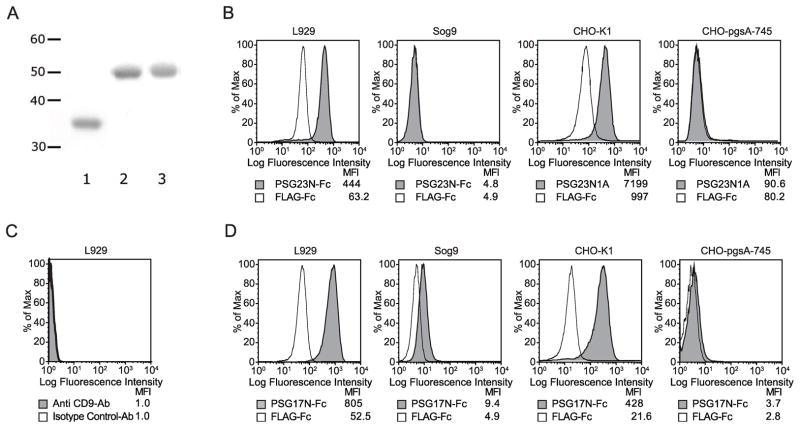
To determine whether murine PSGs bind to cell surface proteoglycans and to investigate whether the binding was mediated through the N1-domain, we generated fusion proteins comprised of the N1-domain and the Fc tag. As a control for binding studies, we utilized a protein with the Fc fused to the FLAG tag (Fig. 1A). We performed binding experiments in mouse L929 cells and its derivative Sog9, which lacks the ability to synthesize heparan sulfate and has chondroitin sulfate of reduced length [22]. As showed in Fig. 1B, PSG23N-Fc bound to the L929 cells while it did not bind to Sog9 cells. To verify the importance of cell surface proteoglycans for PSG23 binding, we also tested whether these proteins showed differential binding to CHO-K1 and its derived heparan and chondroitin sulfate null mutant, CHO-pgsA-745 [23]. PSG23N1A bound to CHO-K1 cells while it did not bind to CHO-pgsA-745 (Fig. 1B). Identical results were found when testing binding of PSG23N-Fc to these cells (data not shown).
Figure 1.
Protein characterization, binding of PSGs to different cell lines, and analysis of CD9 expression in L929 cells. (A) Coomassie stained SDS-PAGE gel showing the recombinant proteins after purification. Lane 1: FLAG-Fc, lane 2: PSG17N-Fc, and lane 3: PSG23N-Fc. The molecular weight marker on the left indicates the sizes in kilodaltons. L929, CHO-K1, Sog9 or CHO-pgsA-745 cells were incubated with PSG23N-Fc or PSG23N1AHisFLAG or FLAG-Fc as indicated (B) or with the control protein and PSG17N-Fc (D) followed by PE-conjugated anti-human Fcγ for the Fc-tagged proteins or anti-FLAG biotin followed by streptavidin APC for proteins with the FLAG tag. (C) L929 cells were incubated with biotin-labeled anti-CD9 mAb or biotin-labeled isotype control followed by streptavidin-APC. The median fluorescence intensity (MFI) of each treatment is shown.
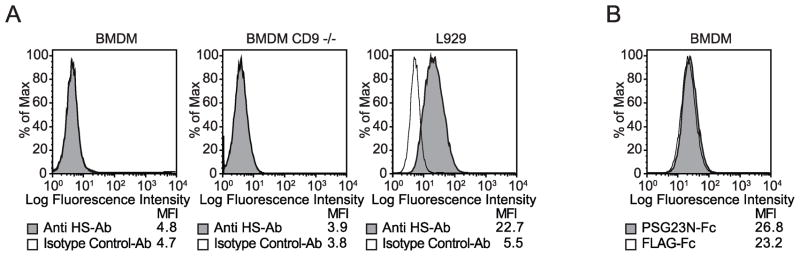
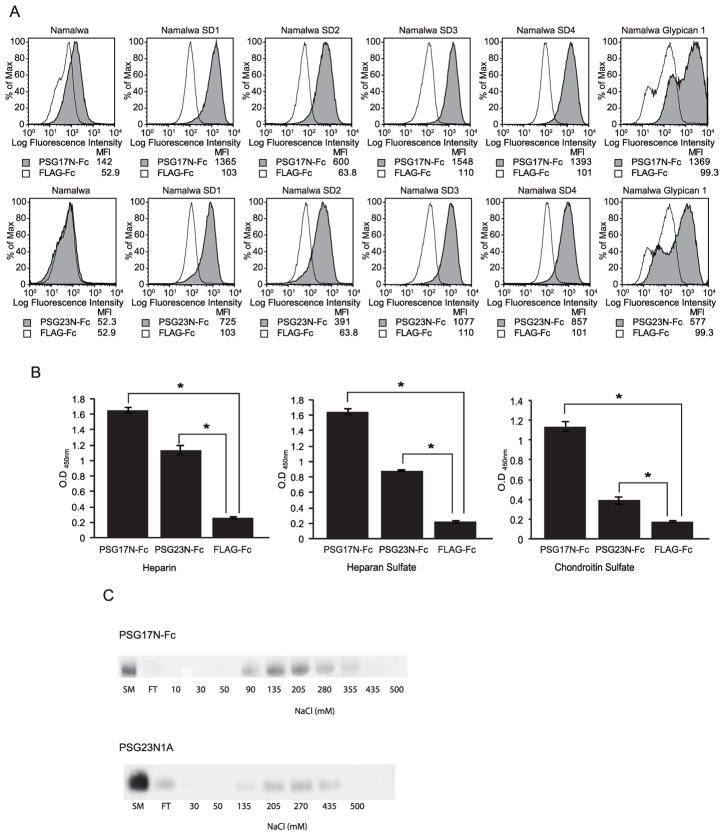
While L929 cells do not express CD9 (Fig. 1C), PSG17N-Fc bound to this cell line (Fig. 1D). This indicated that CD9 is not the only receptor for PSG17. We hypothesized that PSG17 may also bind to cell surface proteoglycans. Figure 1D shows that PSG17N bound significantly better to L929 and CHO-K1 than to Sog9 and CHO-pgsA-745. Because we previously reported that PSG17 did not bind to bone-marrow derived macrophages (BMDM) generated from CD9-null mice while it bound to the cells derived from wild type mice[17], we wondered whether BMDM lack HSPG expression. Fig. 2A shows that BMDM from CD9-null or wild type mice do not express detectable levels of HS, compared to L929, which were used as positive control. This is in agreement with the observed lack of binding of PSG23N to BMDM (Fig. 2B) or PSG23N1A (data not shown), which use proteoglycans as their only receptor. Therefore, PSG17 binds to cells expressing only CD9 or cell surface proteoglycans and these interactions are mediated through the N1-domain. To confirm that murine PSG17N and 23N bind to cell surface proteoglycans and to determine if the nature of the core protein affects the interaction, we performed binding assays in cells stably transfected with syndecans 1–4 or with glypican-1. Expression of the proteoglycans was confirmed with specific antibodies (data not shown). Namalwa cells express very low levels of HSPGs but their membrane expression is greatly increased in the transfected cells [16]. PSG17N and PSG23N bound significantly more to cells expressing syndecans or glypican-1 than to untransfected cells (Fig. 3A). Identical results were obtained whether the binding experiments were performed with the PSGs comprised of the N1-domain or with proteins containing the N1 and the A-domain (data not shown).
Figure 2.
Expression of heparan sulfate in bone marrow derived macrophages (BMDM) and binding of PSG23N-Fc to these cells. (A) BMDM derived from wild type or CD9-null mice and L929 cells were incubated with 1 μg of FITC-labeled anti-heparan sulfate 10E4 (anti-HS Ab) or FITC-labeled isotype control. (B) BMDM cells were incubated with 30 μg/ml of PSG23N-Fc or FLAG-Fc followed by anti-FLAG biotin and streptavidin APC.
Figure 3.
PSG17 and PSG23 bind to Syndecans 1–4, Glypican-1 and to immobilized glycosaminoglycans. (A) Namalwa and Namalwa cells stably transfected with syndecans 1–4 or glypican-1 were incubated with 30 μg/ml of PSG17N-Fc, PSG23N-Fc or FLAG-Fc followed by PE-conjugated anti-human Fcγ. The median fluorescence intensity (MFI) of each treatment is shown. (B) 96-well NUNC plates were coated overnight with 200 μg/well of heparin, heparan sulfate, or chondroitin sulfate. After blocking, proteins were added to the wells. Following extensive washing, bound proteins were detected with HRP-conjugated anti-Fcγ Ab. All treatments were performed in quadruplicate and data is representative of three independent experiments. Statistical significance between the protein control and the PSG17N and 23N-treated wells was determined by two-tailed Student t-test and error bars represent the S.E.M. (C) PSG17N-Fc or PSG23N1AHisFLAG were applied to a 1 ml heparin-Sepharose column, after washing, the proteins were eluted with a NaCl gradient. Aliquots of each eluted fraction as well as the starting material (SM) and flow through (FT) were assessed by immunoblot analysis with HRP-conjugated anti-Fcγ Ab for PSG17N-Fc and HRP-conjugated anti-FLAG M2 mAb for PSG23N1AHisFLAG.
3.2 PSGs bind to immobilized glycosaminoglycans
The results reported above suggest that PSGs bind to the heparan and/or chondroitin sulfate of syndecans and glypican-1 rather than to the core proteins. We tested whether PSG17N and PSG23N could bind to heparin, HS and CS. As shown in Fig. 3B, PSG17N-Fc and PSG23N-Fc bound to all three GAG-coated wells over background binding represented by the control protein. We consistently observed that PSG17N bound better to the GAG-coated wells than PSG23N, which may indicate a difference of binding affinity between the proteins. The ability of PSGs to bind to heparin was also tested by chromatography using a heparin-Sepharose column. Both proteins bound to the heparin column (Fig. 3C). Small differences in the elution profiles were found when using the N1-domain-Fc tagged proteins rather than the N1, A-domain, His-Flag tagged proteins, which eluted at higher NaCl concentration than the Fc counterparts (data not shown).
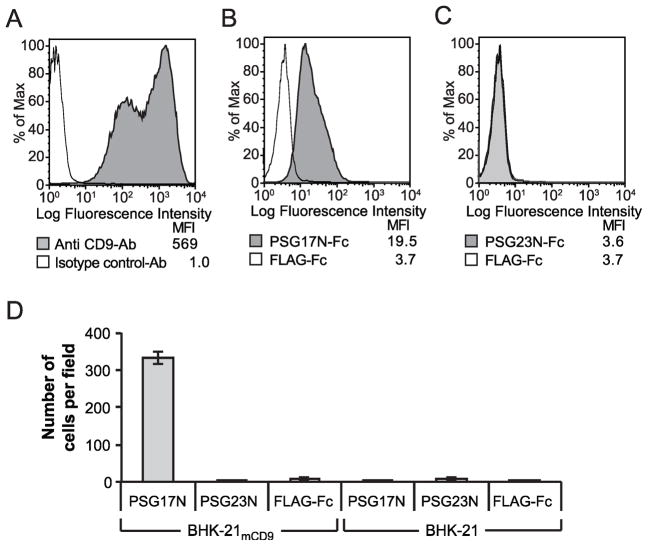
3.3 PSG23 does not bind to CD9
We have previously shown that PSG23 binds equally to peritoneal macrophages derived from wild type or CD9-null mice [15]. In addition, as described below, PSG23N does not bind to Sog9 cells expressing CD9, strongly suggesting that it does not bind to CD9. Besides PSG17, we found that PSG19 binds to CD9. The CD9-PSG19 interaction was demonstrated by panning but was not detected by FACS [18]. Therefore, to further investigate a possible interaction between CD9 and PSG23N, we performed a panning experiment. Fig. 4D shows that BHK-21 cells transfected with the murine CD9 bound to PSG17N-coated plates in significantly higher numbers than to the control protein coated dish, as expected [17]. Few cells transfected with empty plasmid were found in plates coated with any of the proteins. CD9 transfected cells did not attach in higher number than empty plasmid transfected cells to the PSG23N-coated dishes.
Figure 4.
PSG17N binds to Sog9 cells expressing CD9 (Sog9-CD9) while PSG23 does not. (A)Sog9 cells were stably transfected with a plasmid encoding murine CD9. Expression of CD9 in the sorted cells was verified with anti-CD9 mAb. Sog9-CD9 cells were incubated with PSG17N-Fc (B) or PSG23N-Fc (C). Protein binding was detected with PE-conjugated anti-human Fcγ. (D) BHK-21 cells transfected with mCD9 or empty plasmid were added to Petri dishes coated with PSG17N, PSG23N or FLAG-Fc. After several washes, the cells bound to the dish were counted in three separate fields.
3.4 Mapping of the amino acids in the N1- domain of PSG17 required for CD9 binding
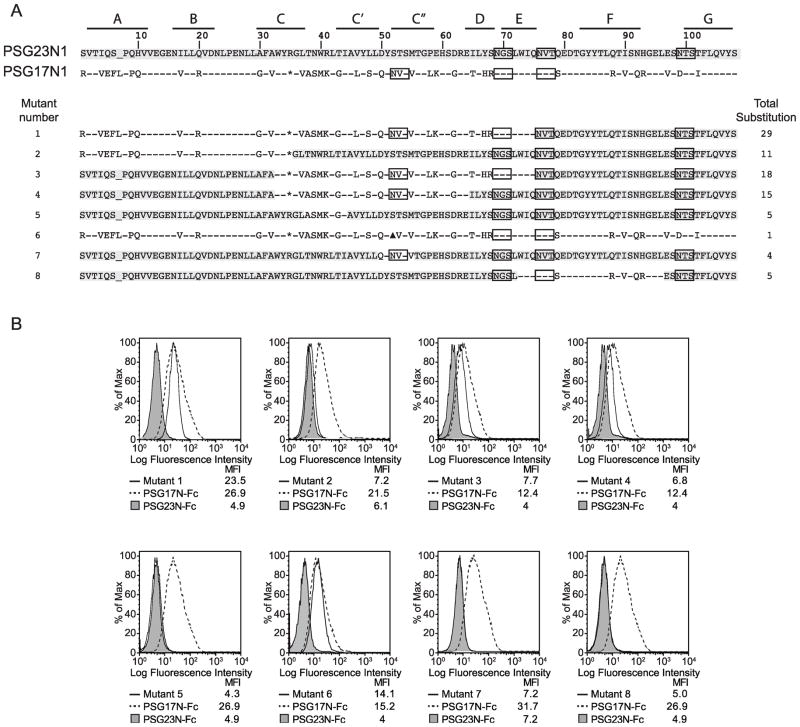
We previously showed that the N1-domain of PSG17 binds amino acids SFQ (173–175) of CD9 [24]. To investigate which amino acids in PSG17 are involved in the interaction with CD9, we generated a series of PSG23/PSG17 hybrid proteins. Our strategy was to substitute amino acids in PSG23 for the ones present in PSG17 at the same positions and determine which substitutions would confer to the mutated PSG23 the ability to bind CD9 (Fig. 5A). To perform the binding studies, we generated Sog9 cells stably transfected with CD9 (Fig. 4A). As anticipated, PSG17 bound to these cells while PSG23 did not (Fig. 4B and C). All mutants had the predicted molecular weight (data not shown). When the first 75 amino acids of PSG23 were replaced for the corresponding ones in PSG17, resulting in 29 amino acid substitutions (mutant 1 in Fig. 5A), the chimeric protein bound to Sog-mCD9 cells (Fig. 5B). To narrow down the number of amino acids in PSG17 which are essential for CD9 binding, mutant 2 and 3 were constructed. Mutant 2 did not bind to Sog9-mCD9 cells while substitution of amino acids 37 to 68 (mutant 3) conferred binding (Fig. 5A and 5B). These results indicated that the amino acids which include the C, C′ and C″ β-sheets and the loops between them are likely involved in the PSG17-CD9 interaction. The importance of the C, C′ and C″ region was confirmed by the results obtained after the generation of mutant 4 (amino acids 37 to 62), which has fifteen amino acid substitutions and showed binding. Figure 5A and 5B shows that when attempting to further narrow down the CD9 binding region from amino acids 37 to 62 by constructing mutant 5 and 7, the capacity of the protein to interact with CD9 was lost.
Figure 5.
PSG-mutants and mapping of the CD9-binding site in the N1-domain of PSG17. (A) Identity between amino acids in the N1 domains of PSG17 and PSG23 is shown with a dash. Potential glycosylation sites are boxed. Highlighted amino acids indicate correspondence to the PSG23N1 sequence. “*” represents the K35 to R substitution in PSG17 required to introduce a restriction site and “▲” indicates the N52 to A mutation in mutant 6, which deletes one of the potential N-linked glycosylation sites in PSG17. The β-strands (A to G) are indicated with lines on the upper part of the figure. Total substitution refers to the number of amino acids changed from the wild type sequence. (B) Sog9 expressing murine CD9 were treated with 30 μg/ml of the mutants shown in part A, PSG17N-Fc or PSG23N-Fc followed by PE-conjugated anti-human Fcγ.
The most N-terminal N-linked glycosylation site in PSG17, which is absent in PSG23 but is present in PSG19, was tested by mutating the N52 to an A (mutant 6 in Fig. 5). This mutation resulted in a protein of lower molecular weight when compared to the wild type, indicating that this site is most likely glycosylated in PSG17 (data not shown). Our results show that the first glycosylation site in PSG17 is not essential for the PSG17-CD9 interaction (Fig. 5B, mutant 6). To analyze the possible contribution of amino acids after 75, we constructed mutant 8 (Fig. 5A). This mutant has five amino acid substitutions and did not confer PSG23 the ability to bind CD9 (Fig. 5B).
3.5 PSG17N and PSG23N binding to cells is affected by the cell type used to produce the recombinant protein
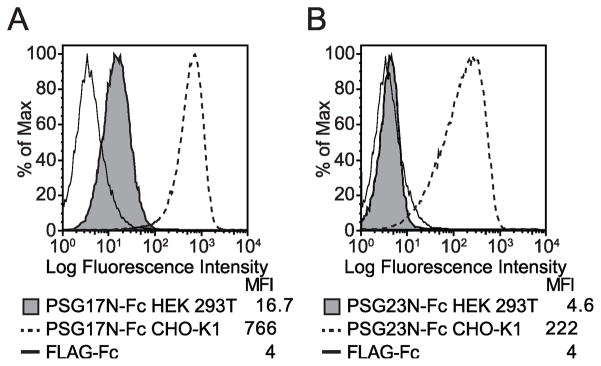
When exploring different expression systems, we generated PSG17N and PSG23N in CHO-K1, HeLa, and HEK 293T cells. Binding studies were performed and we observed that when PSG17N was generated in HEK 293T cells, it bound to L929 cells at a significantly lower level than when the protein was produced in CHO-K1 cells (Fig. 6A). The difference in binding was even more pronounced when PSG23N was analyzed. PSG23N generated in CHO-K1 cells bound to L929 cells while the same protein generated in HEK 293T cells did not bind (Fig. 6B). The same phenomena was observed when using the Namalwa cell stably transfected with glypican-1 and the recombinant proteins produced in HeLa cells behaved like the ones produced in CHO-K1 cells (data not shown). The difference in binding was not observed when comparing binding of PSG17N made in the three cell lines to Sog9-mCD9, which suggested that binding to CD9 was not affected by the cell type chosen to produce this protein (data not shown). PSGs do not have O-linked oligosaccharides but they are heavily N-linked glycosylated [25]. We have observed that PSG1 purified from human serum binds to syndecans (Dveksler, unpublished). Therefore, we believe that the CHO-K1 or HeLa produced PSGs mimic more closely the proteins secreted by the trophoblasts.
Figure 6.
Differential binding of recombinant PSGs produced in CHO-K1 or HEK 293T cells to L929 cells. L929 cells were incubated with 30 μg/ml PSG17N-Fc (A) or PSG23N-Fc (B), which were generated in transiently transfected CHO-K1 or HEK 293T cells. After washing, the cells were incubated with PE-conjugated anti-human Fcγ. The median florescence intensity (MFI) of each treatment is shown.
4. Discussion
Previous reports from our laboratory have shown that members of the human and murine PSG families have cross species reactivity [11,14,15]. In addition, we determined that the N1 Ig-variable like domain appeared sufficient for binding to cells and for the observed induction of TGFβ1 by PSG17 [17,21]. Recently we showed that PSG1 binds to cell surface proteoglycans including syndecan 1–4 and that this binding mediated the ability of this protein to induce capillary-like tube formation by endothelial cells [16]. We hypothesized that murine PSGs, while having limited amino acid sequence identity with human PSG1, may have conserved the ability to bind to the same molecules on the surface of cells. Our results show that this is indeed the case. Murine PSG17N and PSG23N bound to all four syndecans and to glypican-1. We found that PSG17 and PSG23 comprising the N1 and A-domain and the proteins consisting of just the N1-domain bound to glycosaminoglycans. The same result was obtained with recombinant PSG17 consisting of the N1 and N2 domains followed by the Fc tag and with full length PSG17 fused to secreted alkaline phosphatase (data not shown) [17]. Both recombinant proteins bound to CHO-K1 cells while they did not bind or bound slightly over the control proteins (FLAG-Fc and secreted alkaline phosphatase, respectively) to the heparan and chondroitin sulfate null mutant, CHO-pgsA-745. While we only tested PSG23N1A and PSG23N, we hypothesized that full length PSG23, like full length PSG17, will also bind to GAGs. In addition, our data strongly suggests that the core proteins do not play a critical role in this interaction as these proteins do not share significant homology at the amino acid level. Therefore it is likely that PSGs may interact with GAG chains on other glypicans or cell surface proteoglycans.
Syndecan expression was observed in cells undergoing trophoblast giant cell differentiation and in differentiated giant cells, which produce PSGs [12,26,27]. Syndecan-4 is expressed in the microvasculature of mouse embryos and placenta and deficiency of this proteoglycan results in increased degeneration of fetal vessels [28]. In addition to syndecan-4, syndecan-1 is expressed in endothelial cells in the gestation 8.5 pregnant uteri [29] and syndecan 1-null embryos are smaller than their wild type siblings [30]. These observations, together with the inability of heparinase-treated endothelial cells to form tubes in response to PSG1 [16], suggests that the interaction between PSGs and syndecans could play an important role in the establishment of the vasculature during pregnancy. PSG23 was found associated with maternal endothelial cells and we propose that this association is likely mediated by the presence of HSPG such as syndecans in these cells [27]. Studies in our laboratory are in progress to determine whether murine PSGs like PSG1, induce endothelial tube formation.
Our previous report showed that PSG17 binds to CD9 and in the cells examined there was no evidence for a second receptor or binding site [17]. Interestingly, as shown in Fig. 2A, the cells employed in those studies expressed undetectable levels of HS on their cell surface. Significant PSG17 binding is demonstrated in L929 which do not express CD9 but express HSPG. Therefore we conclude that PSG17 binds independently to cell surface proteoglycans and to CD9. The biological significance of the CD9-PSG17 and cell surface proteoglycans-PSG17 interactions remains to be elucidated. CD9 and members of the syndecan family are co-expressed in some cell types in the placenta such as trophoblast giant cells and endothelial cells [27,31]. Different syndecans as well as CD9 have been reported to participate in the regulation of the immune response and in angiogenesis [32–35]. Interestingly, adeno-associated virus type 2 can bind to cells either using CD9 or HSPG, as we here report for PSG17 [36]. Which signaling mechanisms may be triggered upon binding of PSG17 to one or the other receptor remains to be investigated, but both syndecans and CD9 have been reported to associate with different integrins and modulate their functions [34,37–41].
In an attempt to be able to predict whether a particular PSG could potentially bind to CD9, we sought to investigate the amino acids in PSG17 involved in this interaction. The crystal structure of the N-domain of mouse CEACAM1 has been utilized to predict the structure of the N-domain of human and murine PSGs [42]. There is 21.3% identity at the amino acid level between the N1-domains of murine PSGs. The region corresponding to the predicted β-sheet forming CFG face is the most variable region in this domain [19]. It is in the CFG face that pathogens such as mouse hepatitis virus and Neisseria bind to CEACAM family members [43–45]. We found that amino acids 37 to 62, which are in the CGF face, are required for the interaction of PSG17 with CD9 and that this region is also likely involved in the ability of PSG17 to bind with higher affinity to GAGs when compared to PSG23 (data not shown). While PSG23 mutants 3 and 4, containing amino acids 37 to 62 of PSG17 bound to CD9, these mutants did not result in the same level of binding as PSG17N or mutant 1. Therefore, a contribution of the first 32 amino acids of PSG17 in the interaction of this protein with CD9 cannot be excluded. We speculated that PSG17 and PSG19 binding to CD9 requires the presence of carbohydrates at position 52, but we found that CD9 binding does not segregate with the presence of this glycosylation site.
PSGs made in HEK 293T cells showed lower binding to proteoglycans when compared to CHO-K1 or HeLa made proteins. This is likely due to the differences in the ability of HEK 293T cells to add complexed glycans bearing sialic acid, which will impart a more negative charge to the proteins affecting their ability to bind to a negatively charged cell surface molecule such as a proteoglycan [46]. Therefore, as previously observed for other recombinant glycoproteins, the expression system selected to generate recombinant PSGs should be carefully considered as differences in binding and/or activity may be observed.
In conclusion, we found that murine PSG17 and 23, like human PSG1, bind to the GAG chains on cell surface proteoglycans. Whether all PSGs share the ability to interact with these molecules remains to be determined. It is possible that during gene expansion some PSGs have evolved to bind to other receptors such as we observed for PSG17. Whether this dual binding capacity is observed in human PSGs and if it confers an additional function remains to be determined but we have no evidence of this occurring at this time. Understanding the function of PSGs is a complex but worthwhile undertaken as PSGs likely play an important role in angiogenesis and immune regulation, which are essential for successful pregnancy.
Acknowledgments
We would like to thank Anne Marie Dizon and Bandana Ajay Vishwakarma for technical assistance and Karen Wolcott for performing the cell sorting. We are very grateful to Dr. Guido David (University of Leuven, Belgium) for the Namalwa transfectants and the antibodies to syndecans and glypican-1. This study was supported by a grant from the National Institutes of Health, NICHD (R01HD035832), and a USUHS intramural grant to Dr. G.S. Dveksler.
Abbreviations
- PSGs
pregnancy specific glycoproteins
- GAG
glycosaminoglycan
- VEGF
vascular endothelial growth factor
- TGF
transforming growth factor
- HS
heparan sulfate
- CS
chondroitin sulfate
- HSPG
heparan sulfate proteoglycans
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Horne CH, Towler CM, Pugh-Humphreys RG, Thomson AW, Bohn H. Pregnancy specific beta1-glycoprotein--a product of the syncytiotrophoblast. Experientia. 1976;32(9):1197. doi: 10.1007/BF01927624. [DOI] [PubMed] [Google Scholar]
- 2.Kammerer R, Zimmermann W. Coevolution of activating and inhibitory receptors within mammalian carcinoembryonic antigen families. BMC Biol. 2010;8:12. doi: 10.1186/1741-7007-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kromer B, Finkenzeller D, Wessels J, Dveksler G, Thompson J, Zimmermann W. Coordinate expression of splice variants of the murine pregnancy-specific glycoprotein (PSG) gene family during placental development. Eur J Biochem. 1996;242(2):280–7. doi: 10.1111/j.1432-1033.1996.0280r.x. [DOI] [PubMed] [Google Scholar]
- 4.McLellan AS, Fischer B, Dveksler G, Hori T, Wynne F, Ball M, et al. Structure and evolution of the mouse pregnancy-specific glycoprotein (Psg) gene locus. BMC Genomics. 2005;6(1):4. doi: 10.1186/1471-2164-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camolotto S, Racca A, Rena V, Nores R, Patrito LC, Genti-Raimondi S, et al. Expression and transcriptional regulation of individual pregnancy-specific glycoprotein genes in differentiating trophoblast cells. Placenta. 2010;31(4):312–9. doi: 10.1016/j.placenta.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Johnson MR, Riddle AF, Grudzinskas JG, Sharma V, Collins WP, Nicolaides KH. Reduced circulating placental protein concentrations during the first trimester are associated with preterm labour and low birth weight. Hum Reprod. 1993;8(11):1942–7. doi: 10.1093/oxfordjournals.humrep.a137965. [DOI] [PubMed] [Google Scholar]
- 7.Pihl K, Larsen T, Laursen I, Krebs L, Christiansen M. First trimester maternal serum pregnancy-specific beta-1-glycoprotein (SP1) as a marker of adverse pregnancy outcome. Prenat Diagn. 2009;29(13):1256–61. doi: 10.1002/pd.2408. [DOI] [PubMed] [Google Scholar]
- 8.Towler CM, Horne CH, Jandial V, Campbell DM, MacGillivray I. Plasma levels of pregnancy-specific beta 1-glycoprotein in complicated pregnancies. Br J Obstet Gynaecol. 1977;84(4):258–63. doi: 10.1111/j.1471-0528.1977.tb12573.x. [DOI] [PubMed] [Google Scholar]
- 9.Grudzinskas JG, Gordon YB, Menabawey M, Lee JN, Wadsworth J, Chard T. Identification of high-risk pregnancy by the routine measurement of pregnancy-specific beta 1-glycoprotein. Am J Obstet Gynecol. 1983;147(1):10–2. doi: 10.1016/0002-9378(83)90075-3. [DOI] [PubMed] [Google Scholar]
- 10.Motran CC, Diaz FL, Montes CL, Bocco JL, Gruppi A. In vivo expression of recombinant pregnancy specific glycoprotein 1a induces alternative activation of monocytes and enhances Th2-type immune response. Eur J Immunol. 2003;33(11):3007–16. doi: 10.1002/eji.200323993. [DOI] [PubMed] [Google Scholar]
- 11.Snyder SK, Wessner DH, Wessells JL, Waterhouse RM, Wahl LM, Zimmermann W, et al. Pregnancy specific glycoproteins function as immunomodulators by inducing secretion of IL-10, IL-6 and TGF-beta1 by human monocytes. Am J Reprod Immunol. 2001;45(4):205–16. doi: 10.1111/j.8755-8920.2001.450403.x. [DOI] [PubMed] [Google Scholar]
- 12.Wessells J, Wessner D, Parsells R, White K, Finkenzeller D, Zimmermann W, et al. Pregnancy specific glycoprotein 18 induces IL-10 expression in murine macrophages. Eur J Immunol. 2000;30(7):1830–40. doi: 10.1002/1521-4141(200007)30:7<1830::AID-IMMU1830>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 13.Bebo BF, Jr, Dveksler GS. Evidence that Pregnancy Specific Glycoproteins Regulate T-Cell Function and Inflammatory Autoimmune Disease During Pregnancy. Curr Drug Targets Inflamm Allergy. 2005;4(2):231–7. doi: 10.2174/1568010053586255. [DOI] [PubMed] [Google Scholar]
- 14.Ha CT, Wu JA, Irmak S, Lisboa FA, Dizon AM, Warren JW, et al. Human Pregnancy Specific Beta-1-Glycoprotein 1 (PSG1) Has a Potential Role in Placental Vascular Morphogenesis. Biol Reprod. 2010;83(1):27–35. doi: 10.1095/biolreprod.109.082412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu JA, Johnson BL, Chen Y, Ha CT, Dveksler GS. Murine pregnancy-specific glycoprotein 23 induces the proangiogenic factors transforming-growth factor beta 1 and vascular endothelial growth factor a in cell types involved in vascular remodeling in pregnancy. Biol Reprod. 2008;79(6):1054–61. doi: 10.1095/biolreprod.108.070268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lisboa FA, Warren J, Sulkowski G, Aparicio M, David G, Zudaire E, et al. Pregnancy-specific glycoprotein 1 induces endothelial tubulogenesis through interaction with cell surface proteoglycans. J Biol Chem. 2011;286 (9):7577–86. doi: 10.1074/jbc.M110.161810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waterhouse R, Ha C, Dveksler GS. Murine CD9 is the receptor for pregnancy-specific glycoprotein 17. J Exp Med. 2002;195(2):277–82. doi: 10.1084/jem.20011741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha CT, Waterhouse R, Warren J, Zimmermann W, Dveksler GS. N-glycosylation is required for binding of murine pregnancy-specific glycoproteins 17 and 19 to the receptor CD9. Am J Reprod Immunol. 2008;59(3):251–8. doi: 10.1111/j.1600-0897.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 19.McLellan AS, Zimmermann W, Moore T. Conservation of pregnancy-specific glycoprotein (PSG) N domains following independent expansions of the gene families in rodents and primates. BMC Evol Biol. 2005;5:39. doi: 10.1186/1471-2148-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Coomans C, David G. Membrane heparan sulfate proteoglycan-supported FGF2-FGFR1 signaling: evidence in support of the “cooperative end structures” model. J Biol Chem. 2001;276(45):41921–9. doi: 10.1074/jbc.M106608200. [DOI] [PubMed] [Google Scholar]
- 21.Ha CT, Waterhouse R, Wessells J, Wu JA, Dveksler GS. Binding of pregnancy-specific glycoprotein 17 to CD9 on macrophages induces secretion of IL-10, IL-6, PGE2, and TGF-beta1. J Leukoc Biol. 2005;77(6):948–57. doi: 10.1189/jlb.0804453. [DOI] [PubMed] [Google Scholar]
- 22.Uyama T, Ishida M, Izumikawa T, Trybala E, Tufaro F, Bergstrom T, et al. Chondroitin 4-O sulfotransferase-1 regulates E disaccharide expression of chondroitin sulfate required for herpes simplex virus infectivity. J Biol Chem. 2006;281(50):38668–74. doi: 10.1074/jbc.M609320200. [DOI] [PubMed] [Google Scholar]
- 23.Esko JD, Stewart TE, Taylor WH. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci U S A. 1985;82(10):3197–201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellerman DA, Ha C, Primakoff P, Myles DG, Dveksler GS. Direct Binding of the Ligand PSG17 to CD9 Requires a CD9 Site Essential for Sperm-Egg Fusion. Mol Biol Cell. 2003;14:5098–103. doi: 10.1091/mbc.E03-04-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osborne JC, Jr, Rosen SW, Nilsson B, Calvert I, Bohn H. Physicochemical studies of pregnancy specific beta 1-glycoprotein: unusual ultracentrifugal and circular dichroic properties. Biochemistry. 1982;21(22):5523–8. doi: 10.1021/bi00265a022. [DOI] [PubMed] [Google Scholar]
- 26.Sutherland AE, Sanderson RD, Mayes M, Seibert M, Calarco PG, Bernfield M, et al. Expression of syndecan, a putative low affinity fibroblast growth factor receptor, in the early mouse embryo. Development. 1991;113(1):339–51. doi: 10.1242/dev.113.1.339. [DOI] [PubMed] [Google Scholar]
- 27.Wynne F, Ball M, McLellan AS, Dockery P, Zimmermann W, Moore T. Mouse pregnancy-specific glycoproteins: tissue-specific expression and evidence of association with maternal vasculature. Reproduction. 2006;131(4):721–32. doi: 10.1530/rep.1.00869. [DOI] [PubMed] [Google Scholar]
- 28.Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Nakamura E, Ito M, et al. Syndecan-4 deficiency impairs the fetal vessels in the placental labyrinth. Dev Dyn. 2000;219(4):539–44. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1081>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 29.Alam SM, Konno T, Sahgal N, Lu L, Soares MJ. Decidual cells produce a heparin-binding prolactin family cytokine with putative intrauterine regulatory actions. J Biol Chem. 2008;283(27):18957–68. doi: 10.1074/jbc.M801826200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott SP, Ranheim EA, Leatherberry VS, Khwaja SS, Klos KS, Alexander CM. Juvenile syndecan-1 null mice are protected from carcinogen-induced tumor development. Oncogene. 2007;26(10):1407–16. doi: 10.1038/sj.onc.1209930. [DOI] [PubMed] [Google Scholar]
- 31.Ball M, Carmody M, Wynne F, Dockery P, Aigner A, Cameron I, et al. Placenta. 2009;30 (7):649–53. doi: 10.1016/j.placenta.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Tai XG, Toyooka K, Yashiro Y, Abe R, Park CS, Hamaoka T, et al. CD9-mediated costimulation of TCR triggered naive T cells leads to activation followed by apoptosis. J Immunol. 1997;159(8):3799–807. [PubMed] [Google Scholar]
- 33.Klein-Soyer C, Azorsa DO, Cazenave JP, Lanza F. CD9 participates in endothelial cell migration during in vitro wound repair. Arterioscler Thromb Vasc Biol. 2000;20(2):360–9. doi: 10.1161/01.atv.20.2.360. [DOI] [PubMed] [Google Scholar]
- 34.Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC. Syndecan-1 regulates alphavbeta3 and alphavbeta5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med. 2009;206(3):691–705. doi: 10.1084/jem.20081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung JS, Bonkobara M, Tomihari M, Cruz PD, Jr, Ariizumi K. The DC-HIL/syndecan-4 pathway inhibits human allogeneic T-cell responses. Eur J Immunol. 2009;39(4):965–74. doi: 10.1002/eji.200838990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurzeder C, Koppold B, Sauer G, Pabst S, Kreienberg R, Deissler H. CD9 promotes adeno-associated virus type 2 infection of mammary carcinoma cells with low cell surface expression of heparan sulphate proteoglycans. Int J Mol Med. 2007;19(2):325–33. [PubMed] [Google Scholar]
- 37.Berditchevski F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sc. 2001;114(Pt 23):4143–51. doi: 10.1242/jcs.114.23.4143. [DOI] [PubMed] [Google Scholar]
- 38.Charrin S, le Naour F, Silvie O, Milhiet PE, Boucheix C, Rubinstein E. Lateral organization of membrane proteins: tetraspanins spin their web. Biochem J. 2009;420(2):133–54. doi: 10.1042/BJ20082422. [DOI] [PubMed] [Google Scholar]
- 39.Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397–422. doi: 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- 40.McQuade KJ, Beauvais DM, Burbach BJ, Rapraeger AC. Syndecan-1 regulates alphavbeta5 integrin activity in B82L fibroblasts. J Cell Sci. 2006;119(Pt 12):2445–56. doi: 10.1242/jcs.02970. [DOI] [PubMed] [Google Scholar]
- 41.Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8(12):957–69. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan K, Zelus BD, Meijers R, Liu JH, Bergelson JM, Duke N, et al. Crystal structure of murine sCEACAM1a[1,4]: a coronavirus receptor in the CEA family. Embo J. 2002;21(9):2076–86. doi: 10.1093/emboj/21.9.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virji M, Evans D, Hadfield A, Grunert F, Teixeira AM, Watt SM. Critical determinants of host receptor targeting by Neisseria meningitidis and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol Microbiol. 1999;34(3):538–51. doi: 10.1046/j.1365-2958.1999.01620.x. [DOI] [PubMed] [Google Scholar]
- 44.Wessner DR, Shick PC, Lu JH, Cardellichio CB, Gagneten SE, Beauchemin N, et al. Mutational analysis of the virus and monoclonal antibody binding sites in MHVR, the cellular receptor of the murine coronavirus mouse hepatitis virus strain A59. J Virol. 1998;72(3):1941–8. doi: 10.1128/jvi.72.3.1941-1948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immuno. 2006;6(6):433–46. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- 46.Hershkovitz O, Jarahian M, Zilka A, Bar-Ilan A, Landau G, Jivov S, et al. Altered glycosylation of recombinant NKp30 hampers binding to heparan sulfate: a lesson for the use of recombinant immunoreceptors as an immunological tool. Glycobiology. 2008;18(1):28–41. doi: 10.1093/glycob/cwm125. [DOI] [PubMed] [Google Scholar]