Abstract
Background
Despite recent success in pharmacologic treatment of depression, the inability to predict individual treatment response remains a liability. This study replicates and extends findings relating pretreatment EEG alpha to treatment outcomes for serotonergic medications.
Methods
Resting EEG (eyes-open and eyes-closed) was recorded from a 67-electrode montage in 41 unmedicated depressed patients and 41 healthy controls. Patients were tested prior to receiving antidepressants including a serotonergic mode of action (SSRI, SNRI, or SSRI plus NDRI). EEG was quantified by frequency principal components analysis (fPCA) of spectra derived from reference-free current source density (CSD) waveforms, which sharpens and simplifies EEG topographies, disentangles them from artifact, and yields measures that more closely represent underlying neuronal current generators.
Results
Patients who did not respond to treatment had significantly less alpha current source density compared with responders or healthy control subjects, localizable to well-defined posterior generators. The alpha difference between responders and nonresponders was greater for eyes-closed than eyes-open conditions and was present across alpha subbands. A classification criterion based on the median alpha for healthy controls showed good positive predictive value (93.3) and specificity (92.3). There was no evidence of differential value for predicting response to an SSRI alone or dual treatment targeting serotonergic plus other monoamine neurotransmitters.
Conclusions
Findings confirm the value of EEG alpha amplitude as a viable predictor of antidepressant response, and suggest that personalized treatments for depression may be identified using simple electrophysiologic CSD measures.
Keywords: depression, antidepressant treatment response, alpha rhythm, quantitative EEG (qEEG), current source density (CSD), principal components analysis (PCA)
Introduction
Pharmacologic treatments for major depressive disorder (MDD) have long focused on monoamine mechanisms, with the early success of tricyclic and MAOI antidepressants, and marked by the advent of selective serotonin reuptake inhibitors (SSRIs). Despite these advances, the failure rate for any specific treatment imposes formidable delays in relief from depression in patients for whom hopelessness and discouragement are already a concern. Without objective tests indicating the likelihood of an individual’s response to treatment, the risks to the patient grow with each failure. A reliable, objective, and readily available measure capable of differentiating between those who may or may not respond to specific treatments would find a much-needed place in clinical practice.
The alpha rhythm of the electroencephalogram (EEG) is a non-invasive and cost-effective index of the tonic state of the brain. The classical view of resting EEG alpha is of a posterior1, 8–13 Hz idling rhythm characteristic of a relaxed, wakeful state, which is blocked (desynchronized) when the individual is alert, or when visual processes are engaged by opening the eyes. An inverse association has been reported between scalp-recorded EEG alpha and local PET perfusion (3). Feige et al. (4) also reported an inverse association between posterior alpha (quantified by independent components analysis) and the fMRI BOLD response in cortical visual regions, but not in subcortical visual or reticular thalamic nuclei, which have also been implicated in the generation and synchronization of alpha (5–8). Alpha is also generated within the ventral visual stream, although its laminar organization differs considerably across cortical regions (9).
EEG alpha has found extensive use as an index of relative cortical deactivation (i.e., greater alpha, less activation) in studies of depressive disorders. In early studies, patients having a depressive disorder and elderly adults having a prior depressive disorder showed greater EEG alpha power than healthy controls (10–11). A number of additional studies have reported abnormal regional hemispheric asymmetries of alpha in individuals having a depressive disorder, with relatively less activity in left frontal (12–15) and right parietal regions (16–19).
There is evidence that EEG alpha may differentiate patients who clinically respond to pharmacological treatment from those who do not. Ulrich et al. (20) found increased posterior alpha in depressed patients who subsequently responded to amitriptyline. More recently, Bruder et al. (21) reported encouraging findings for predicting response to fluoxetine. Responders had greater alpha than nonresponders, with differences being topographically and functionally consistent with the classic alpha rhythm, i.e., evidence of reduced cortical activity in responders over posterior regions. They also found SSRI responders to differ from nonresponders in alpha symmetry, with responders showing relatively less cortical activity over right posterior regions. Neither alpha power nor asymmetry changed following treatment, which is consistent with alpha being a stable trait characteristic (22–24).
The present study was designed to confirm the predictive value of EEG alpha amplitude and asymmetry in a larger, independent sample of patients having a depressive disorder, and to extend the prior study in the following ways: First, we examined the predictive value not only for patients receiving an SSRI alone, but also those receiving dual treatment with an SSRI plus bupropion (i.e., an noradrenaline/dopamine reuptake inhibitor, NDRI) or an antidepressant with a dual mechanism of action (duloxetine or venlafaxine, both serontonin/noradrenaline reuptake inhibitors, SNRI). Second, to better identify and describe spectral topographic differences of interest, a dense recording montage (67-channels), and high-resolution EEG methods were used. Specifically, we used current source density (CSD) measures (25–26), which reduce volume conduction from distal sites, sharpen spatial resolution, and avoid problems associated with the recording reference (27–28). In contrast to rereferencing approaches to the ubiquitous reference problem for EEG recordings, CSD measures are unique (i.e, identical waveforms for any reference ), and are more closely related to neuronal activity, indicating the strength of underlying current generators as radial (transcranial) current flow (25–26, 29–30). Consequently CSD measures are less likely to be mislocalize activity than their reference-dependent counterparts. Third, CSD measures were quantified using frequency principal components analysis (fPCA) to obtain measures for empirically-derived frequency bands. Using this technique, Tenke and Kayser (29) identified factors with peaks corresponding to alpha subbands, including a high-frequency factor and a low-alpha/theta factor, that included activity typically classified as theta (i.e., between 4–8 Hz). Based on prior findings, we predicted that patients who respond to a serotonin reuptake inhibitor (SRI) would show greater global alpha than nonresponders, and also differ in their alpha asymmetry. Moreover, given evidence that pretreatment EEG theta also predicts antidepressant response (31–34), we examined whether the predicted differences between responders and nonresponders would be most prominent for the low-alpha/theta factor.
Methods
Participants
Outpatients (n = 41; 17 male) from the Depression Evaluation Service at the New York State Psychiatric Institute and healthy controls (n = 41; 17 male) with no history of psychopathology were recruited from the New York metropolitan area. Participants were right-handed as indicated by their Laterality Quotient (LQ > 0) on the Edinburgh Inventory (35). Participants were excluded for any of the following reasons: serious suicide risk, current substance use disorders (including alcohol abuse), psychotic disorders, seizure disorder, a history of head trauma, or other neurological disorder. Control participants were screened using the Structured Clinical Interview for DSM-IV, nonpatient edition (36) to exclude those with current or past psychopathology. The diagnostic assessment and treatment of patients were carried out by research psychiatrists. Patients met DSM-IV criteria for major depressive disorder (MDD; n = 22), dysthymia (n = 7), both disorders (n = 10), or depression not otherwise specified (n = 2). Five patients had a comorbid anxiety disorder. Beck Depression Inventory (37) scores of patients ranged from 13–55 (mean = 24.0 ±8.3; n = 39). All participants were paid $15 per hour. The study was approved by the institutional review board, and all participants signed an informed consent form.
Patients were tested after being unmedicated for a minimum of 7 days (6 weeks for two patients receiving fluoxetine), but most patients were drug-free for considerably longer or had not previously been treated with an antidepressant. Patients then received one of six treatments listed in Table 1, all of which included a SRI. A total of 16 patients received an SSRI alone, 15 received an SSRI plus an NDRI, and 10 received an SNRI. After 8–12 weeks of treatment, clinical response was assessed using with the Clinical Global Impression Improvement scale (CGI-I; 38) by a rater who was blind to the EEG data. Patients who had a CGI-I rating of “much improved” or “very much improved” were considered to be responders (n = 28) and all other patients were considered as nonresponders (n = 13). The final dosage levels for each antidepressant were comparable in responders and nonresponders (Table 1), who also did not differ from each other or from healthy controls in gender, age, education or handedness (Table 2). There was no significant difference between responders and nonresponders in severity of depression on Beck Depression Inventory or Hamilton Depression scale (HAM-D; 39) before treatment, but responders had significantly lower HAM-D scores than nonresponders post-treatment.
Table 1.
Antidepressant Treatments
| Responders | Nonresponders | |||
|---|---|---|---|---|
| n | Dosagea | n | Dosagea | |
| Monotherapy: | ||||
| Escitalopram (SSRI) | 7 | 20 (10–40) | 6 | 25 (20–40) |
| Fluoxetine (SSRI) | 0 | - | 2 | 60 |
| Sertraline (SSRI) | 1 | 50 | 0 | - |
| Dual Therapy: | ||||
| Escitalopram (SSRI) | 12 | 40 (7.5–40) | 3 | 40 (20–40) |
| plus Bupropion (NDRI) | 350 (150–450) | 450 (400–450) | ||
| Duloxetine (SNRI) | 5 | 60 (30–120) | 1 | 90 |
| Venlafaxine (SNRI) | 3 | 375 (125–375) | 1 | 375 |
Median Dosage in mg (range)
Notes: SSRI (selective serotonin reuptake inhibitor); NDRI (norepinephrine and dopamine reuptake inhibitor); SNRI (serotonin and norepinephrine reuptake inhibitor)
Table 2.
Characteristics of Treatment Responders, Nonresponders and Controls
| Responders | Nonresponders | Controls | |
|---|---|---|---|
| Gender (F/M) | 16/12 | 8/5 | 24/17 |
| Age (years)a | 34.9 ± 10.8 | 36.4 ± 9.8 | 33.1 ± 11.6 |
| Education (years) a | 16.4 ± 1.7 | 15.9 ± 4.8 | 15.9 ± 2.7 |
| Handedness (Laterality Quotient) a | 76.8 ± 20.2 | 79.9 ± 21.7 | 86.0 ± 18.4 |
| Beck Depression Inventorya,b | 23.0 ± 9.0 | 26.0 ± 6.3 | 2.1 ± 2.9 |
| Pre-Treatment HAM-Da | 14.8 ± 4.1 | 17.4 ± 5.4 | |
| Post-Treatment HAM-Da,c | 4.0 ± 2.6 | 14.8 ± 5.7 |
mean ± SD
Controls (n = 38), had significantly lower BDI than responders (n = 27), and nonresponders (n = 12) [F2,74 = 120.6, p<.001], but the patient groups did not differ from each other.
Responders (n = 22) had significantly lower HAM-D scores than nonresponders (n = 13) post-treatment [t 33 = 6.49, p <.001].
EEG Recordings
The EEG was recorded from 67 expanded 10–20 system locations (40) with a Lycra stretch electrode cap (ActiveTwo EEG system; 41) using an active reference at sites PO1 (common mode sense) and PO2 (driven right leg). Along with 11 midline sites, the montage consisted of 28 homologous pairs over the left and right hemisphere, extending laterally to include the inferior temporal lobes (cf. 42). Electrode placement was optimized by direct measurement of landmarks (nasion, inion, auditory meatus, vertex). The scalp placements were prepared using a conventional water-soluble electrolyte gel, and the interface verified (ActiView; 41). Additional electrodes above and below the right eye, and at the left and right outer canthi provided bipolar electrooculogram recordings for identification and rejection of blinks and eye movements.
EEG Acquisition and Artifact Procedures
Resting EEG was recorded while subjects sat quietly in a sound attenuated booth during four 2-minute resting EEG periods (eyes-closed [C] and eyes-open [O] counterbalanced across blocks: COOC or OCCO; order alternated across subjects in each group). Subjects were instructed to remain still and inhibit blinks or eye movements during each recording period. During the eyes-open condition, subjects fixated on a central cross. Continuous EEG was acquired at 256 samples/s using the 24-bit Biosemi system and nose-referenced data were exported into 16-bit Neuroscan format using Polyrex (43) to remove offsets, optimize scaling, and rereference the EEG.
Continuous data were segmented into 1-s epochs every .5-s (50% overlap). After rejection of epochs contaminated by blink or eye-movement ( ±100 μV threshold, followed by interactive rejection), a reference-free approach identified isolated EEG channels containing amplifier drift, residual eye activity, muscle or movement-related artifacts (44), which were then replaced by spherical spline interpolations (45) from artifact-free channels whenever possible (i.e., fewer than 25% affected channels). Artifact detection and electrode interpolation was verified interactively, and accepted epochs screened for electrolyte bridges (46).
Current Source Density (CSD) Amplitude Spectra
Artifacted EEG epochs for each subject were transformed into reference-free current source density (CSD) estimates (μV/cm2 units; 10 cm head radius; 50 iterations; m = 4; λ = 10−5; 29) using a spherical spline surface Laplacian (26; 45; Matlab-based CSD toolbox and tutorial 47). The mean offset of each epoch was eliminated, a 50% Hanning taper window applied, and zeros padded to the beginning and end of each epoch to yield power spectra with a resolution of .25 Hz (i.e., 4 s at 1024 points/epoch). Averaged power spectra were then computed (48) for each condition (closed/open), and converted to amplitude spectra by square root transformation (i.e., RMS). After eliminating the first (DC) point, the next 299 points were used in subsequent analyses (i.e., .25 – 74.75 Hz).
Frequency Principal Components Analysis (fPCA)
Averaged CSD amplitude spectra (299 frequency points = variables) were submitted to unrestricted, covariance-based fPCA (29), with 67 electrodes, 2 conditions, and 82 participants (i.e., 10988 cases), followed by Varimax rotation of the covariance loadings (49; also see 50, 51). CSD-fPCA yields meaningful, physiologically identifiable spectral components that conform to the underlying data, while isolating and removing artifact (e.g., electromyogram and electrooculogram activity extracted as distinct components), thereby reducing noise and eliminating reference-related errors. Six factors accounted for 95% of the variance of amplitude spectra (cf. Section S1 in the Supplement), three of which (48% total variance) had clear peaks in alpha (low-alpha/theta, high-alpha and residual alpha; cf. 29), and had posterior maxima for eyes-closed.2
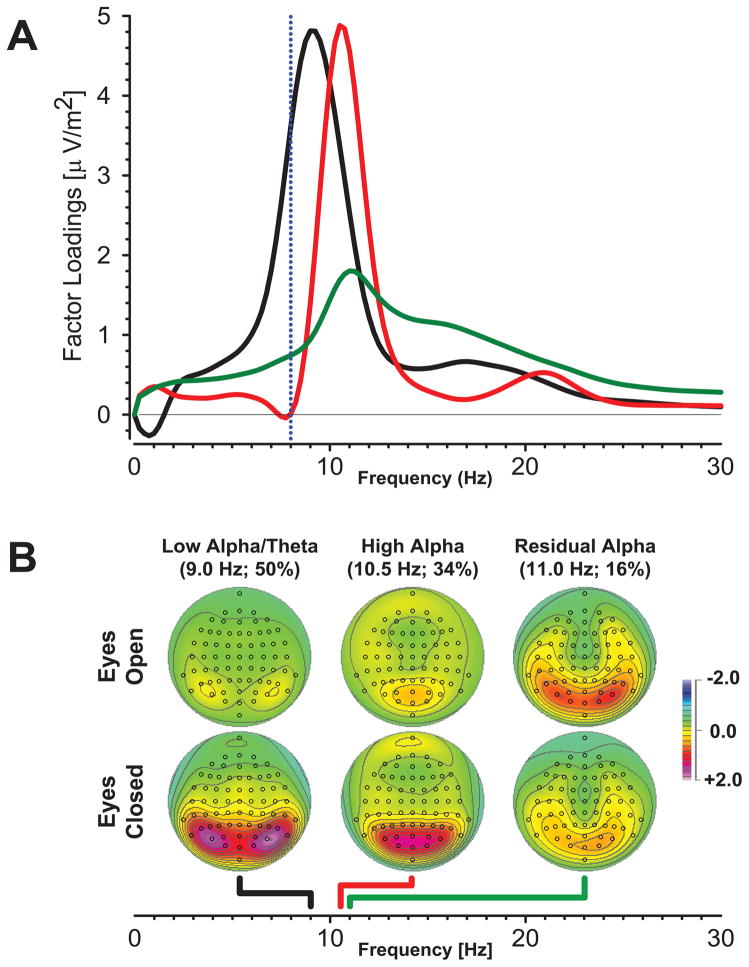
Although preliminary results for these three alpha factors revealed significant group differences (52), a comparison of corresponding factor loadings waveforms extracted separately for controls and patients indicated variability in the residual alpha factor loadings for patients that was eliminated by using these three factors as a spectral filter (Section S2 in the Supplement). This approach exploits the capacity of fPCA to isolate and remove overlapping broadband activity (cf. Figure S1 in the Supplement). A Varimax-fPCA of these data led to highly consistent alpha factors. As shown in Fig. 1A and B, these included low-alpha/theta (black lines, 9 Hz peak, 50% variance of filtered waveforms; waveform rise below 8 Hz) and high-alpha (red lines, 10.5 Hz, 34% variance) factors, with characteristic lateral and midline posterior maxima (respectively) for eyes-closed. The residual alpha factor (green lines, 11 Hz, 16% variance) will not be further considered, because it included a substantial low-beta contribution (12–20 Hz) and a maximum for eyes-open (i.e., a reversed condition effect), neither of which are characteristic of alpha activity.
Figure 1.
A. Rotated CSD-fPCA factor loadings waveforms for filtered spectra consisted of low-alpha/theta (black), high-alpha (red), and residual-alpha (green). The rising slope of the low-alpha/theta waveform includes frequencies below 8 Hz (conventional theta band, left of the dotted blue line), while the residual-alpha component includes low-beta. B. Corresponding factor score topographies show that all three alpha factors were greatest at posterior sites, as previously described (Tenke & Kayser, 2005). Low-alpha/theta and high-alpha were greater for eyes-closed, but residual alpha was not. Dots indicate the spherical positions of electrodes (nose at top). Colored lines below maps point to the peak frequencies of corresponding factor loadings on the common abscissa (colors as shown in A).
Statistical Analyses
By virtue of the spatial sharpening and removal of ambiguity with CSD, low-alpha/theta and high-alpha may be concisely quantified as means across sites spanning these well-defined maxima: P9/10, P7/8, P5/6, PO7/8, PO3/4, O1/2 for low-alpha/theta; PO7/8, PO3/4, O1/2, POz, Oz for high-alpha. Neither the overall topographies of posterior condition-dependent alpha, nor repeated measures ANOVA including Hemisphere (Left, Right) as a within-subject factor, suggested differential alpha asymmetries between groups. Consequently, regional alpha means were subjected to a simpler repeated measures ANOVA (GLM procedure; 53) for Alpha Frequency (low-alpha/theta, high-alpha) and Condition (eyes-closed, eyes-open), with Group (control, responder, nonresponder; or responder, nonresponder for patients only) and Gender (male, female) as between-subjects factors and Age as a covariate. An exploratory analysis was also conducted using Treatment (monotherapy, dual therapy) and Response Group (responder, nonresponder) as between-subjects factors, excluding Gender because of insufficient cell sizes.
Based on our prior study (21), we predicted that patients with greater alpha than expected for controls would respond well to SRI treatment, whereas those with less alpha would not. This prediction was tested by using the median of condition-dependent alpha for healthy controls (eyes-closed minus eyes-open, averaged across low- and high-alpha factors) to classify patients as having greater alpha (predicted to be responders) or less alpha (predicted nonresponders) than healthy controls. The capacity of condition-dependent alpha to predict treatment outcome was also explored using logistic regression. The association between condition-dependent alpha and measures related to depression (e.g., BDI and HAM-D) were examined using product-moment correlations.
Results
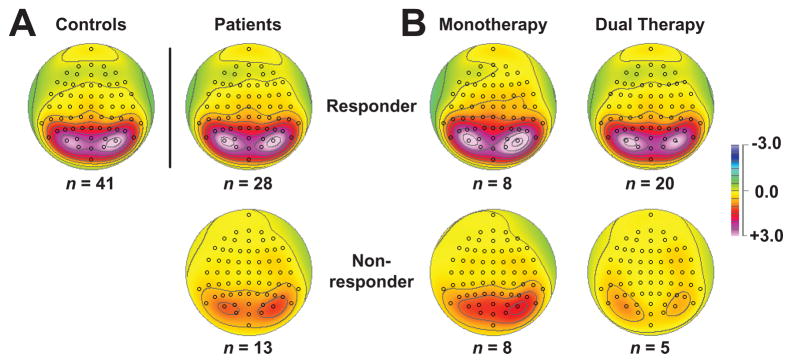
Alpha topographies were consistent across the three groups, but nonresponders showed substantially less alpha than responders and controls, particularly in the eyes-closed condition. The smaller condition-dependent alpha in nonresponders is illustrated in Figure 2A, and this group difference was supported by a significant Condition × Group interaction, F[2, 75] = 3.56, p = .033. No additional Group effects attained significance, and there were no significant Gender interactions.3 An Age (covariate) effect (F[1,75] = 12.0, p = .001; corresponding Pearson r = −.41, p < .001 for grand mean) indicating alpha decreases with age, and a Condition × Age interaction (F[1, 75] = 10.9, p = .001; corresponding Pearson r = −.38, p < .001 for mean closed-minus-open difference) were also observed, but they did not interact with Group. Aside from the defining Condition effect (F[1, 75] = 36.4, p < .001 cf. Fig. 1), there were no additional significant effects.
Figure 2.
A. Condition-dependent (eyes-closed minus eyes-open) difference topographies, averaged across high- and low-alpha/theta factors for controls, responders and nonresponders. Alpha reduction was pronounced for nonresponders. B. Corresponding condition-dependent topographies for patients treated with a monotherapy or dual therapy. Both nonresponder groups showed a marked alpha reduction compared to responders.
When the ANOVA model was restricted to patients (i.e., responders vs. nonresponders), the Condition × Response Group effect was prominent (F[1, 36] = 7.86, p = .008 ), and a Response Group effect attained significance (F[1, 36] = 4.71, p = .037), but the Age effects did not. Response Group did not interact with Alpha Frequency (low vs. high), and no significant Condition × Response Group effect was observed for separate ANOVA models for low- and high-alpha factors, suggesting that the alpha reduction in nonresponders is a shared property that is maximal for eyes-closed and cuts broadly across alpha subbands (Section S3 in the Supplement).
The marked difference in alpha between nonresponders and responders contrasts with the lack of differentiation between mono- and dual-therapies, evident in Fig. 2B. The exploratory ANOVA in patients (i.e., Response Group and Treatment as between group factors; Age covariate) replicated the Condition × Response Group effect, F[1,36] = 7.23; p = .011, but revealed no significant effects or interactions involving Treatment.
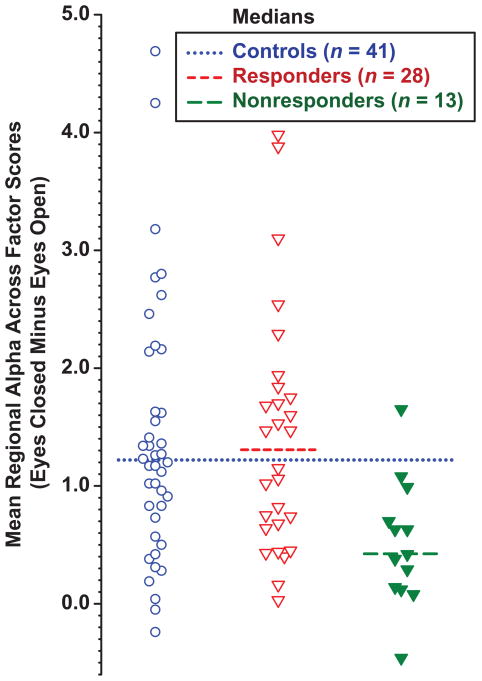
The value of these alpha scores for predicting treatment response was examined by classifying patients as to whether they had prominent alpha above the median for controls (dotted line in Fig. 3; predicted responders), or less alpha than the control median (predicted nonresponders). By this classification, 14 of the 15 patients with prominent alpha responded to treatment, whereas only 14 of 26 with less alpha were responders (Fisher’s exact test, 2-tail p = .014). This indicates that prominent alpha had a high positive predictive value of 93.3 (response rate for those predicted to be responders) and specificity of 92.3 (percentage of nonresponders predicted to be nonresponders). In contrast, sensitivity was only 50.0, and negative predictive value 46.1, with an overall predictive value of 63.4. Eyes-closed-minus-open alpha was also significantly correlated with post-treatment HAM-D (r = −.35, n = 35, p = .04), but not with pretreatment HAM-D (r = −.14, n = 41, ns) or BDI (r = −.12, n = 39, ns). Logistic regression also indicated that posterior condition-dependent alpha was predictive of treatment response (Wald test = 6.183, df = 1, p = .013).
Figure 3.
Scatterplot of mean condition-dependent (eyes-closed minus eyes-open) posterior alpha, averaged across factors for controls, responders and nonresponders. The median for controls (dotted line) was comparable to that for responders (short dashes), but differed markedly from that for nonresponders (long dashes).
Supplementary Analyses: Conventional EEG Measures and Low-Density CSD
Even though high-resolution CSD measures were crucial for the present study, we also submitted a reduced-electrode CSD montage to fPCA, resulting in significant findings (Section S4 in the Supplement). In addition, nose-referenced EEG power spectra were analyzed at standard 10–20 system sites to provide a bridge to the literature using conventional “low-resolution” EEG in alpha and theta bands. Although group differences in alpha showed trends consistent with those reported for CSD measures, they were not statistically significant and alpha asymmetry did not differ between groups (Section S5 in the Supplement). However, an ANOVA of EEG theta revealed one significant group effect: a Group × Condition × Electrode interaction in which the slope of the anterior-to-posterior increase in power for eyes-closed (i.e., also seen in maps at low-alpha/theta frequencies; Figure S5 in the Supplement) was flatter in nonresponders (Figure S6 in the Supplement).
Discussion
These findings replicate, extend and formalize the previous finding (21) that depressed patients with prominent (large amplitude) EEG alpha benefit from treatment with an SRI. Patients who fail to respond to these antidepressants had markedly less alpha than responders and healthy controls. The present study was based on independent and considerably larger samples of depressed patients and healthy adults. Additionally, the EEG was recorded at a higher spatial resolution, and further enhanced by CSD measures, yielding reference-independent topographies that clearly implicate posterior anatomical regions in the generation of these condition-dependent alpha effects. A simple classification scheme, based on median alpha for healthy controls, yielded predictions of treatment outcome with a surprisingly high positive predictive value and specificity. There was no evidence to support a differential value for predicting response to SSRI monotherapy as opposed to dual therapy targeting both serotonergic and nonserotonergic neurotransmitters. These results are consistent with the view of alpha as a marker for SRI responsivity. In contrast to our previous findings (21), treatment response was not related to hemispheric asymmetry of posterior alpha. Although the reason for the lack of replication of the alpha asymmetry findings is unclear, alpha asymmetry is known to be less reliable than alpha power (21–24), and may be influenced by a number of moderator or mediator variables (18).
Advantages, caveats and constraints
The findings on the value of alpha as a predictor of treatment outcome are quite encouraging, but additional study is required with diverse treatments (e.g., nonserotonergic antidepressants, cognitive behavioral therapy or placebo) to determine whether the prediction uniquely reflects a serotonergic response. Using alpha levels in healthy controls as the threshold cutoff (cf. 21) proved again to be successful for predicting treatment response. Positive predictive value and specificity were very high (>90%), the clinical implication being that depressed patients having prominent alpha can be predicted to be responsive with a high degree of confidence. However, sensitivity was low (50%); about half of the responders had alpha below the control median, and were therefore not predicted to be responders. It is not known how many responders may have been placebo responders, shown spontaneous remission, or otherwise not been true drug responders. Further studies should explore other alpha thresholds, as well as combining alpha with other electrophysiologic (32, 54) or neurocognitive (55) measures to improve prediction of treatment response. Also, despite evidence that EEG alpha has high reliability and heritability (21–24), few studies have directly examined whether acute or chronic administration of an SSRI alters alpha in depressed patients. A multisite study is now underway assessing neuroimaging, electrophysiologic, and neurocognitive measures before and after one week of SSRI or placebo so as to further examine their differential predictive value.
The observed differences in alpha were robust, and used methods that unambiguously localized the effect to posterior EEG generators. Another fundamental advantage of CSD-fPCA is that it improves the quality of the data by separating and removing sources of artifact. For example, electromyogram and residual electrooculogram, which are broadly distributed and vary with the recording reference, are isolated as separate CSD factors (cf. Fig. S1 in the Supplement). Although the generators of these factors may largely be outside the braincase and might otherwise obscure the effects reported here, they nevertheless represent physiological activity that could be separately explored by CSD-fPCA.4
Relationship to activity in the theta band
As previously observed (29), the spectral loadings of the low-alpha/theta factor extend below the conventional 8 Hz border into theta. Consequently, conventional qEEG measures based on standard frequency band cutoffs would unjustifiably split the factor in two, just below its peak (Fig. 1A; see also Figures S4 and S5 in the Supplement). For this reason, some differences reported here between treatment responders and nonresponders might have previously been attributed to theta activity.
Using imipramine, Knott et al. (31) reported a trend for greater alpha, but significantly less theta, in treatment responders. For fluoxetine, Cook et al. (56) reported differences in theta band “cordance,” a measure related to the local Laplacian, but derived from a complex combination of relative and absolute power. Additional qEEG studies of treatment response have focused on midline frontal theta as identified by LORETA. Greater theta current density, localized by LORETA to the anterior cingulate cortex, has been reported for patients who respond to treatment with nortriptyline (33), citalopram or reboxetine (32). Korb et al. (34) also reported greater theta current density for responders than noresponders treated with fluoxetine or venlafaxine, but not between placebo responders and nonresponders. Given the secondary midline topography of our low-alpha/theta factor, midline findings might be anticipated,5 but differences between responders and nonresponders were not supported in analyses (Section S3 in the Supplement). Moreover, both low-alpha/theta and high-alpha were required to reliably differentiate between responders and nonresponders. Conventional EEG analysis did show a reduction in anterior-to-posterior gradient of condition-dependent theta power in nonresponders (Section S5 in the Supplement). We conclude that the observed group differences in the low-alpha/theta factor do not reflect theta, or midline frontal theta in particular, but rather are specific to classic posterior alpha.
The identified alpha subbands, and an absence of a distinct theta, are consistent with the findings of Shackman et al (57), based on a spectral factor analysis of common-average EEG. These investigators suggested that alpha subbands provide no advantage over the broader alpha band in describing differences related to temperament or task demands. Although the two CSD-fPCA factors likewise do not differentially predict treatment response, there is no a priori rationale for generalizing to other clinical or physiological classifications. It should also be noted that these findings discount the possibility that a simple shift between two distinct spectral components is involved (e.g., alpha-slowing).
Alpha and treatment response
Previous work suggests that resting alpha is a stable trait characteristic (22–24) and alpha differences between depressed patients and controls (10) or between treatment response groups (21) persist following treatment. Condition-dependent posterior alpha was also found to be greatest in individuals with a strong familial risk for depression (i.e., both parents having MDD; 58). Consequently, prominent alpha in patients may be a trait marker for a form of depression that is responsive to serotonergic agents. The correspondence between serotonergic activity and behavioral arousal (59) and the inverse relationship between posterior alpha and physiologic or emotional arousal (60) may both have implications for depression and SRI response. Further study is, however, needed to identify their relevance for different forms of depression.
The present study exploited the tonic nature of posterior EEG alpha, and the capacity of high-resolution CSD-fPCA to identify and quantify neuronal generator patterns, to produce a promising predictor of antidepressant response. The factor score topographies suggest that a considerably smaller array of electrodes may provide a sufficient predictor, which is supported by exploratory findings (supplement S4). It is also hoped that the judicious choice of additional, phasic electrophysiologic measures (e.g., loudness dependency of auditory evoked potential; 32, 54) may provide a complementary source of information on serotonergic function to further improve outcome predictions for a range of treatments.
Supplementary Material
Acknowledgments
Supported by grant MH36295 (GEB) from the National Institute of Mental Health (NIMH). We would like to thank Charles L. Brown, III, for providing waveform plotting software, and Malarie Mitchell for secretarial help.
Footnotes
Additional “alpha” rhythms include sensorimotor mu, a temporal third ‘rhythm’(1), and sleep-spindles (2).
We analyzed only alpha components, noting that their orthogonality allows them to be analyzed separately or in combination, without concern about the shared variance of nonorthogonal methods.
However, the Condition × Group interaction was present for females (n=48; F[2, 44] = 4.27, p = .02), but not for males (n=34; F[2, 60] = .95, n.s.).
Preliminary analysis of the remaining components identified in Fig. S1 suggested no additional differences related to the treatment response.
Phase-locking of midline with posterior activity is also possible (cf. 29)
Financial disclosures
Drs. Tenke, Kayser, Manna and Bruder, Ms.Fekri, Ms. Schaller, Mr. Kroppmann and Mr. Alschuler reported no biomedical financial interests or potential conflicts of interest. Dr. Stewart reports during the past two years having served on an external drug monotoring committee for Pfizer Inc and on an advisory board for Alkermes. In addition, Dr. Stewart has received study medication from Forest Laboratories, Inc. Dr. McGrath has provided scientific consultation or served on advisory boards for Novartis Pharmaceuticals, and has received research grant support from Roche Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Niedermeyer E. Alpha rhythms as physiological and abnormal phenomena. Int J Psychophysiol. 1997;26:31–49. doi: 10.1016/s0167-8760(97)00754-x. [DOI] [PubMed] [Google Scholar]
- 2.Ishii R, Dziewas R, Chau W, Soros P, Okamoto H, Gunji A, Pantev C. Current source density distribution of sleep spindles in humans as found by synthetic aperture magnetometry. Neurosci Lett. 2003;340:25–8. doi: 10.1016/s0304-3940(03)00048-x. [DOI] [PubMed] [Google Scholar]
- 3.Cook IA, O’Hara R, Uijtdehaage SH, Mandelkern M, Leuchter AF. Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalogr Clin Neurophysiol. 1998;107:408–14. doi: 10.1016/s0013-4694(98)00092-3. [DOI] [PubMed] [Google Scholar]
- 4.Feige B, Scheffler K, Esposito F, Di Salle F, Hennig J, Seifritz E. Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. J Neurophysiol. 2005;93:2864–72. doi: 10.1152/jn.00721.2004. [DOI] [PubMed] [Google Scholar]
- 5.Buzsaki G. The thalamic clock: emergent network properties. Neuroscience. 1991;41:351–64. doi: 10.1016/0306-4522(91)90332-i. [DOI] [PubMed] [Google Scholar]
- 6.Lopes da Silva F. Neural mechanisms underlying brain waves: from neural membranes to networks. Electroencephalogr Clin Neurophysiol. 1991;79:81–93. doi: 10.1016/0013-4694(91)90044-5. [DOI] [PubMed] [Google Scholar]
- 7.Larson CL, Davidson RJ, Abercrombie HC, Ward RT, Schaefer SM, Jackson DC, Holden JE, Perlman SB. Relations between PET-derived measures of thalamic glucose metabolism and EEG alpha power. Psychophysiology. 1998;35:162–9. [PubMed] [Google Scholar]
- 8.Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101:243–76. doi: 10.1016/s0306-4522(00)00353-5. [DOI] [PubMed] [Google Scholar]
- 9.Bollimunta A, Chen Y, Schroeder CE, Ding M. Neuronal mechanisms of cortical alpha oscillations in awake-behaving macaques. J Neurosci. 2008;28:9976–88. doi: 10.1523/JNEUROSCI.2699-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollock VE, Schneider LS. Topographic electroencephalographic alpha in recovered depressed elderly. J Abnorm Psychol. 1989;98:268–73. doi: 10.1037//0021-843x.98.3.268. [DOI] [PubMed] [Google Scholar]
- 11.Pollock VE, Schneider LS. Topographic quantitative EEG in elderly subjects with major depression. Psychophysiology. 1990;27:438–444. doi: 10.1111/j.1469-8986.1990.tb02340.x. [DOI] [PubMed] [Google Scholar]
- 12.Gotlib IH, Ranganath C, Rosenfeld P. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cogn Emotion. 1998;12:449–478. [Google Scholar]
- 13.Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J Abnorm Psychol. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- 14.Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. J Abnorm Psychol. 2010;119:502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. J Abnorm Psychol. 2006;115:715–29. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- 16.Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, McGrath PJ, Quitkin FM. Regional brain asymmetries in major depression with or without an anxiety disorder: a quantitative electroencephalographic study. Biol Psychiatry. 1997;41:939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- 17.Kentgen LM, Tenke CE, Pine DS, Fong R, Klein RG, Bruder GE. Electroencephalographic asymmetries in adolescents with major depression: influence of cormorbidity with anxiety disorders. J Abnorm Psychol. 2000;109:797–802. doi: 10.1037//0021-843x.109.4.797. [DOI] [PubMed] [Google Scholar]
- 18.Reid SA, Duke LM, Allen JJ. Resting frontal electroencephalographic asymmetry in depression: inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35:389–404. [PubMed] [Google Scholar]
- 19.Stewart JL, Towers DN, Coan JA, Allen JJB. The oft-neglected role of parietal EEG asymmetry and risk for major depressive disorder. Psychophysiology. 2011;48:82–95. doi: 10.1111/j.1469-8986.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulrich G, Renfordt E, Frick K. The topographical distribution of alpha-activity in the resting eeg of endogenous-depressive inpatients with and without clinical-response to pharmacotherapy. Pharmacopsychiatry. 1986;19:272–273. [Google Scholar]
- 21.Bruder GE, Sedoruk JP, Stewart JW, McGrath PJ, Quitkin FM, Tenke CE. Electroencephalographic alpha measures predict therapeutic response to a selective serotonin reuptake inhibitor antidepressant: pre- and post-treatment findings. Biol Psychiatry. 2008;63:1171–1177. doi: 10.1016/j.biopsych.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagemann D, Hewig J, Seifert J, Naumann E, Bartussek D. The latent state-trait structure of resting EEG asymmetry: replication and extension. Psychophysiology. 2005;42:740–52. doi: 10.1111/j.1469-8986.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- 23.Smit DJ, Posthuma D, Boomsma DI, Geus EJ. Heritability of background EEG across the power spectrum. Psychophysiology. 2005;42:691–7. doi: 10.1111/j.1469-8986.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 24.Allen JJB, Urry HL, Hitt SK, Coan JA. Stability of Resting Frontal EEG Asymmetry Across Different Clinical States of Depression. Psychophysiology. 2004;41:269–280. doi: 10.1111/j.1469-8986.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 25.Nunez PL, Srinivasan R. Electric Fields of the Brain: The Neurophysics of EEG. 2. New York: Oxford University Press; 2006. [Google Scholar]
- 26.Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin Neurophysiol. 2006;117:348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 27.Kayser J, Tenke CE. In search of the Rosetta Stone for scalp EEG: converging on reference-free techniques. Clin Neurophysiol. 2010;121:1973–1975. doi: 10.1016/j.clinph.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin Y, Xu P, Yao D. A comparative study of different references for EEG default mode network: the use of the infinity reference. Clin Neurophysiol. 2010;121:1981–91. doi: 10.1016/j.clinph.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 29.Tenke CE, Kayser J. Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA) Clin Neurophysiol. 2005;116:2826–2846. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Nicholson C. Theoretical analysis of field potentials in anisotropic ensembles of neuronal elements. IEEE Trans Biomed Eng. 1973;20:278–88. doi: 10.1109/TBME.1973.324192. [DOI] [PubMed] [Google Scholar]
- 31.Knott VJ, Telner JI, Lapierre YD, Browne M, Horn ER. Quantitative EEG in the prediction of antidepressant response to imipramine. J Affect Disord. 1996;39:175–184. doi: 10.1016/0165-0327(96)00003-1. [DOI] [PubMed] [Google Scholar]
- 32.Mulert C, Juckel G, Brunnmeier M, Karch S, Leicht G, Mergl R, Moller HJ, Hegerl U, Pogarell O. Prediction of treatment response in major depression: integration of concepts. J Affect Disord. 2007;98:215–225. doi: 10.1016/j.jad.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 34.Korb AS, Hunter AM, Cook IA, Leuchter AF. Rostral anterior cingulate cortex theta current density and response to antidepressants and placebo in major depression. Clin Neurophysiol. 2009;120:1313–1319. doi: 10.1016/j.clinph.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Non patient Edition (SCID-NP) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 37.Beck AT, Ward CH, Mendelson M, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 38.Guy W. ECDEU Assesment Manual for Psychopharmocology: Publication ADM 76-338. Washington D.C: U.S. Department of Health, Education, and Welfare; 1976. pp. 534–537. [Google Scholar]
- 39.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–58. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- 41.BioSemi Inc. Amsterdam, NL: Author; 2001. ActiveTwo - Multichannel, DC amplifier, 24-bit resolution, biopotential measurement system with active electrodes. ( http://www.biosemi.com) [Google Scholar]
- 42.Tenke CE, Kayser J, Stewart JW, Bruder GE. Novelty P3 reductions in depression: characterization using principal components analysis (PCA) of current source density (CSD) waveforms. Psychophysiology. 2010;47:133–146. doi: 10.1111/j.1469-8986.2009.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kayser J. Polygraphic Recording Data Exchange - PolyRex. New York State Psychiatric Institute: Department of Biopsychology; 2003. ( http://psychophysiology.cpmc.columbia.edu/PolyRex.htm) [Google Scholar]
- 44.Kayser J, Tenke CE. Electrical distance as a reference-free measure for identifying artifacts in multichannel electroencephalogram (EEG) recordings. Psychophysiology. 2006;43:S51. [Google Scholar]
- 45.Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989;72:184–7. doi: 10.1016/0013-4694(89)90180-6. [(Corrigenda EEG 02274 (1990): Electroencephalogr Clin Neurophysiol 76:565] [DOI] [PubMed] [Google Scholar]
- 46.Tenke CE, Kayser J. A convenient method for detecting electrolyte bridges in multichannel electroencephalogram and event-related potential recordings. Clin Neurophysiol. 2001;112:545–550. doi: 10.1016/s1388-2457(00)00553-8. [DOI] [PubMed] [Google Scholar]
- 47.Kayser J. Current Source Density (CSD) Interpolation using Spherical Splines: CSD toolbox. New York State Psychiatric Institute: Division of Cognitive Neuroscience; 2009. ( http://psychophysiology.cpmc.columbia.edu/Software/CSDtoolbox) [Google Scholar]
- 48.NeuroScan Inc. SCAN 4.3 - Vol. II. EDIT 4.3 - Offline analysis of acquired data: Document number 2203, Revision D. El Paso, TX: Compumedics Neuroscan; 2003. [Google Scholar]
- 49.Kayser J, Tenke CE. Optimizing PCA methodology for ERP component identification and measurement: theoretical rationale and empirical evaluation. Clin Neurophysiol. 2003;114:2307–25. doi: 10.1016/s1388-2457(03)00241-4. [DOI] [PubMed] [Google Scholar]
- 50.Donchin E, Heffley EF. Multivariate analysis of event-related potential data: a tutorial review. In: Otto DA, editor. Multidisciplinary perspectives in event-related brain potential research; Proceedings of the fourth international congress on event-related slow potentials of the brain (EPIC IV); Hendersonville, NC. April 4–10, 1976; Washington, DC: The Office; 1976. pp. 555–72. 1978. [Google Scholar]
- 51.Glaser EM, Ruchkin DS. Principles of neurobiological signal analysis. New York: Academic Press; 1976. [Google Scholar]
- 52.Tenke CE, Kayser J, Gates NA, Alschuler DM, Kroppmann CJ, Fekri S, Stewart JW, McGrath PJ, Bruder GE. Auditory evoked potential (AEP) and EEG measures in depressed patients predict response to antidepressants. Biol Psychiatry. 2010;67:98S. [Google Scholar]
- 53.SPSS Inc. PASW Statistics 18 Command Syntax Reference. Chicago, Il: Author; 2010. [Google Scholar]
- 54.Hegerl U, Juckel G. Intensity dependence of auditory evoked potentials as an indicator of central serotonergic neurotransmission: a new hypothesis. Biol Psychiatry. 1993;33:173–187. doi: 10.1016/0006-3223(93)90137-3. [DOI] [PubMed] [Google Scholar]
- 55.Taylor BP, Bruder GE, Stewart JW, McGrath PJ, Halperin J, Ehrlichman H, Quitkin FM. Psychomotor slowing as a predictor of fluoxetine nonresponse in depressed patients. Am J Psychiatry. 2006;163:73–78. doi: 10.1176/appi.ajp.163.1.73. [DOI] [PubMed] [Google Scholar]
- 56.Cook IA, Leuchter AF, Witte E, Abrams M, Uijtdehaage SH, Stubbeman W, Rosenberg-Thompson S, Anderson-Hanley C. Neurophysiologic predictors of treatment response to fluoxetine in major depression. Psychiatry Res. 1999;85:263–73. doi: 10.1016/s0165-1781(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 57.Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Identifying robust and sensitive frequency bands for interrogating neural oscillations. Neuroimage. 2010;51:1319–33. doi: 10.1016/j.neuroimage.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruder GE, Tenke CE, Warner V, Nomura Y, Grillon C, Hille J, Leite P, Weissman MM. Electroencephalographic measures of regional hemispheric activity in offspring at risk for depressive disorders. Biol Psychiatry. 2005;57:328–335. doi: 10.1016/j.biopsych.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 59.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 60.Heller W, Etienne MA, Miller GA. Patterns of perceptual asymmetry in depression and anxiety: Implications for neuropsychological models of emotion and psychopathology. J Abnorm Psychol. 1995;104:327–333. doi: 10.1037//0021-843x.104.2.327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.