Abstract
Cell-to-cell spread of tobacco mosaic virus (TMV) through plant intercellular connections, the plasmodesmata, is mediated by a specialized viral movement protein (MP). In vivo studies using transgenic tobacco plants showed that MP is phosphorylated at its C-terminus at amino acid residues Ser258, Thr261 and Ser265. When MP phosphorylation was mimicked by negatively charged amino acid substitutions, MP lost its ability to gate plasmodesmata. This effect on MP–plasmodesmata interactions was specific because other activities of MP, such as RNA binding and interaction with pectin methylesterases, were not affected. Furthermore, TMV encoding the MP mutant mimicking phosphorylation was unable to spread from cell to cell in inoculated tobacco plants. The regulatory effect of MP phosphorylation on plasmodesmal permeability was host dependent, occurring in tobacco but not in a more promiscuous Nicotiana benthamiana host. Thus, phosphorylation may represent a regulatory mechanism for controlling the TMV MP–plasmodesmata interactions in a host-dependent fashion.
Keywords: cell-to-cell movement/cell wall associated kinase/phosphorylation/plasmodesmata/tobacco mosaic virus
Introduction
Katherine Esau first postulated that viruses moved throughout the plant via plant intercellular connections: the plasmodesmata (Esau, 1948). Plasmodesmata are structurally complex plasma membrane-lined pores that, in most cases, limit molecular transport between plant cells to small molecules and metabolites with a molecular mass of up to 1 kDa (reviewed by Lucas et al., 1993). This plasmodesmal size exclusion limit can be dynamically increased to allow intercellular movement of large endogenous proteins (Lucas et al., 1995) or invading viruses (Wolf et al., 1989). In the case of plant viruses, an increase in plasmodesmal permeability and the subsequent viral spread between host cells is mediated by virus-encoded cell-to-cell movement proteins (MPs) (reviewed by Lucas and Gilbertson, 1994; Carrington et al., 1996; Lazarowitz and Beachy, 1999; Rhee et al., 2000; Tzfira et al., 2000).
The best characterized MP is that of tobacco mosaic virus (TMV). Several biological activities have been attributed to TMV MP: interaction with plasmodesmata to increase their permeability (Wolf et al., 1989; Ding et al., 1992; Waigmann et al., 1994), binding to single-stranded RNA (Citovsky et al., 1990, 1992), phosphorylation by a cell wall-associated protein kinase (Citovsky et al., 1993) and interaction with cytoskeletal elements (Heinlein et al., 1995; McLean et al., 1995) and plant cell wall pectin methylesterases (PMEs) (Dorokhov et al., 1999; Chen et al., 2000). Based on these observations, MP was proposed to form complexes with the transported genomic TMV RNA, move these complexes throughout the cell using the cytoskeletal network, associate with the cell wall PMEs, increase plasmodesmal permeability and transverse the enlarged plasmodesmal channels (Ghoshroy et al., 1997; Rhee et al., 2000; Tzfira et al., 2000).
Potentially, MP activity within infected cells may be negatively regulated to prevent its continuous interference with the host plant intercellular communication. The molecular mechanism by which such regulation may occur is unknown. Here, we address this question by demonstrating that MP is phosphorylated in vivo at its C-terminus. Mimicking MP phosphorylation by negatively charged amino acid substitutions severely disturbs MP’s ability to interact with plasmodesmata and to promote viral cell-to-cell movement, suggesting that phosphorylation acts as a negative regulator of plasmodesmal transport. Interestingly, this regulatory effect on plasmodesmal permeability is host dependent and may contribute to the differential susceptibility of various host plants to TMV.
Results
MP phosphorylation in vivo and in vitro
Previously, we have shown that MP is phosphorylated in vitro by a cell wall-associated protein kinase at its C-terminal serine and threonine residues (Citovsky et al., 1993). Because in vitro modifications may not reflect the actual biological state of the protein, it was necessary to examine whether or not MP is phosphorylated in vivo in plant tissue. To this end, we generated transgenic tobacco (Nicotiana tabacum) plants that express either wild-type MP or one of its mutants with amino acid substitutions within the phosphorylation site identified previously in vitro (Citovsky et al., 1993). These plants were grown in the presence of radioactive phosphate, and cell walls, known to contain the majority of the accumulated MP (Deom et al., 1990; Citovsky et al., 1993), were purified from their leaves. MP contained in these cell wall fractions was immunoprecipitated and analyzed by autoradiography.
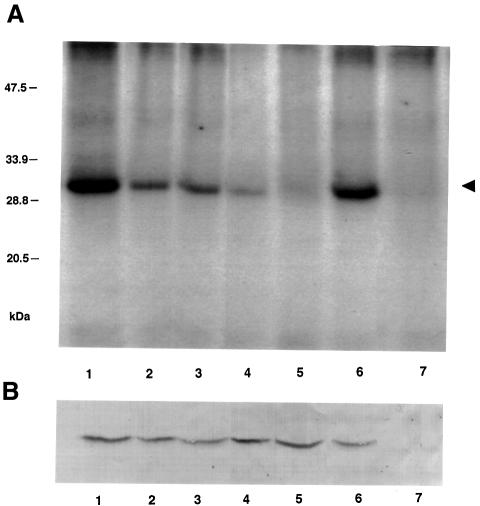
Wild-type MP was indeed phosphorylated within the cell walls of the transgenic tobacco plants (Figure 1A, lane 1). It was then important to determine whether the in vitro phosphorylation site of MP, comprising Ser258, Thr261 and Ser265 (Citovsky et al., 1993), is faithfully utilized in vivo. Thus, we analyzed transgenic plants that express MP mutants in which one phosphorylation site residue was left intact while the other two were substituted with non-phosphorylatable alanine residues. Specifically, mutants sb-10, sb-11 and sb-12 retained Ser258, Thr261 and Ser265 residues, respectively. Figure 1A shows that each of these MP mutants was phosphorylated in vivo. The phosphorylation intensity of sb-10, sb-11 and sb-12 (Figure 1, lanes 2, 3 and 4, respectively), however, was lower than that of the wild-type MP in which all three residues are presumably phosphorylated (Figure 1A, lane 1). Substitution of all three residues, sb-13 (Figure 1A, lane 5), abolished MP phosphorylation. These observations indicate that MP associated with cell walls in tobacco leaves is phosphorylated at amino acid residues Ser258, Thr261 and Ser265.
Fig. 1. MP phosphorylation in vivo. (A) PhosphorImage of MP immunopurified from plant cell walls and analyzed by SDS–PAGE as described in Materials and methods. Transgenic plants expressed the following MP derivatives: lane 1, wild-type MP; lane 2, sb-10; lane 3, sb-11; lane 4, sb-12; lane 5, sb-13. MP phosphorylation during TMV infection: lane 6, cell walls of TMV-infected plants; lane 7, cell walls of uninfected plants. The numbers on the left indicate molecular mass standards in thousands of daltons. An arrowhead indicates the position of TMV MP. (B) Western blot analysis of MP amounts used in the experiment described in (A).
To confirm further that MP is indeed phosphorylated within host cell walls during actual viral infection, we inoculated wild-type tobacco plants with TMV and grew them in the presence of radioactive phosphate followed by MP immunopurification and polyacrylamide gel electrophoretic analysis. Figure 1A shows that MP accumulated in cell walls of TMV-infected plants became phosphorylated (lane 6) whereas no such radioactively labeled protein species was detected in cell walls from uninfected tobacco plants (lane 7). Figure 1B demonstrates that all transgenic plants indeed contained similar quantities of immunopurified MP (lanes 1–5). The amount of MP accumulated within cell walls of TMV-infected plants was slightly lower (Figure 1B, lane 6) and, as expected, no MP was detected in uninfected plants (Figure 1B, lane 7).
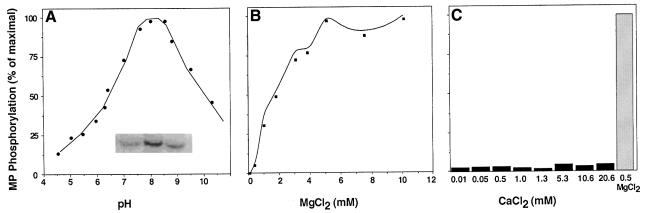
Nicotiana tabacum cell wall fraction was then used to characterize its protein kinase activity in vitro using MP as substrate. Figure 2 shows that this cell wall-associated enzyme functioned most efficiently at a slightly basic pH (Figure 2A) and required the presence of Mg2+ cations (Figure 2B). The optimal Mg2+ concentration was 5 mM. In contrast, the activity in the presence of 0.01–20.6 mM Ca2+ was negligible (Figure 2C), suggesting that MP-phosphorylating kinase is not responsible for the Ca2+-dependent general protein kinase activity of plant cell walls (Epel, 1994; Yahalom et al., 1998). Mn2+ and Zn2+ cations were also ineffective (data not shown).
Fig. 2. Effects of pH, Mg2+ and Ca2+ on MP phosphorylation. (A) Effect of pH. The cell wall preparation was dialyzed against 0.1 M citrate buffer pH 4.0–5.5, HEPES pH 6.0–7.5, Tris–HCl pH 8.0–9.0 or borate pH 9.5–10.5 supplemented in each case with 5 mM MgCl2. Each dialyzed sample was then assayed for its ability to phosphorylate MP. Inset (from left to right): MP phosphorylated at pH 5.0, 8.0 and 11.0, respectively. (B) Effect of MgCl2. MP phosphorylation was assayed in the pH 8.0 reaction buffer supplemented with the indicated concentrations of MgCl2. (C) Effect of CaCl2. MP phosphorylation was assayed in the pH 8.0 reaction buffer supplemented with the indicated concentrations of CaCl2. For a positive control, 5 mM MgCl2 was used. The degree of MP phosphorylation was quantified based on analysis of the gel area corresponding to the phosphorylated protein band, using a Molecular Dynamics phosphorimager.
Mimicking of phosphorylation by negatively charged substitutions blocks MP-induced increase in plasmodesmal permeability in a host-dependent fashion
One of the major biological functions of MP is to elevate the plasmodesmal size exclusion limit from 1 to 10 kDa (Wolf et al., 1989) or even 20 kDa (Waigmann et al., 1994). The effect of phosphorylation on MP’s ability to increase plasmodesmal permeability was directly tested by microinjection into two different Nicotiana hosts, N.tabacum (tobacco) and Nicotiana benthamiana. Nicotiana tabacum is a relatively restrictive host whereas N.benthamiana is well known for its promiscuity to viral infection (Gibbs et al., 1977; Dawson and Hilf, 1992).
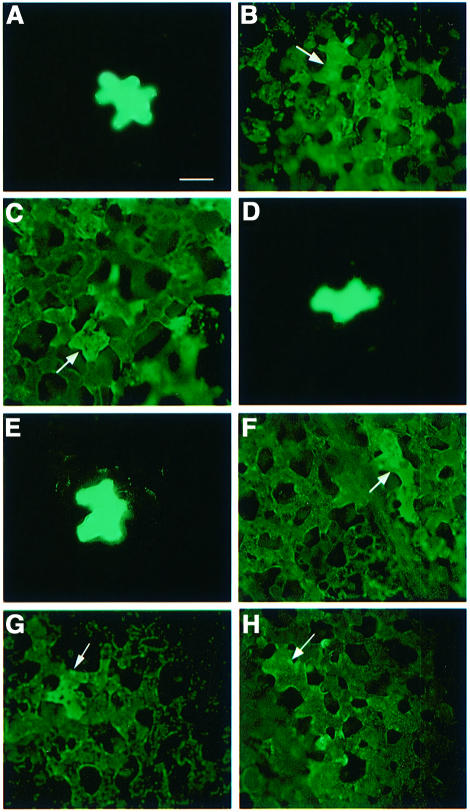
Purified MP and its derivatives that either cannot be phosphorylated or mimic phosphorylation were co- injected with 10 kDa fluorescently labeled dextran into spongy mesophyll cells of Nicotiana leaves (Waigmann et al., 1994; Kragler et al., 1998). Movement of 10 kDa dextran from the injected cell to other, non-injected cells was determined by fluorescence microscopy. Figure 3 and Table I show that 10 kDa dextran microinjected alone generally remained confined to the injected cell in N.tabacum as well as in N.benthamiana (Figure 3A and E, respectively; Table I), consistent with the known plasmodesmal size exclusion limit of this tissue (Wolf et al., 1989; Kragler et al., 1998). In contrast, microinjection of the wild-type MP promoted an extensive cell-to-cell movement of the dextran in both plant species in most microinjections (Figure 3B and F, respectively; Table I), indicating size exclusion limit-enhancing activity of MP. In addition, movement of fluorescent 10 kDa dextrans into the cells not directly connected to the injected cell indicates, as previously suggested (Waigmann et al., 1994), that MP itself moves into these distant cells to affect their plasmodesmal permeability.
Fig. 3. Host-dependent effect of mimicking MP phosphorylation on plasmodesmal permeability. (A and E) Fluorescently labeled dextran (10 kDa) microinjected alone into N.tabacum or N.benthamiana, respectively. (B and F) Wild-type MP mixed with 10 kDa fluorescently labeled dextran and microinjected into N.tabacum or N.benthamiana, respectively. (C and G) del 7 mixed with 10 kDa fluorescently labeled dextran and microinjected into N.tabacum or N.benthamiana, respectively. (D and H) sb-9 mixed with 10 kDa fluorescently labeled dextran and microinjected into N.tabacum or N.benthamiana, respectively. Intercellular distribution of the signal was visualized 25–45 min after injection. Arrows indicate injected cells. Magnification: 120×. Note that black spaces between cells represent intercellular air pockets characteristic for tobacco leaf mesophyll tissues (Waigmann et al., 1994).
Table I. Summary of microinjection experiments.
| Host plant and injected material | Microinjections |
|
|---|---|---|
| n (positive)/n (total)a | Movement competence | |
| Nicotiana tabacum | ||
| 10 kDa F-dextran alone | 1/12 | – |
| MP + 10 kDa F-dextran | 7/9 | + |
| del 7 + 10 kDa F-dextran | 13/14 | + |
| sb-9 + 10 kDa F-dextran | 1/23 | – |
| Nicotiana benthamiana | ||
| 10 kDa F-dextran alone | 1/10 | – |
| MP + 10 kDa F-dextran | 5/6 | + |
| del 7 + 10 kDa F-dextran | 6/8 | + |
| sb-9 + 10 kDa F-dextran | 16/20 | + |
an (positive), number of positive injections that are defined by movement of the fluorescent dextran from the injected cell into multiple surrounding cells; n (total), number of total injections.
To assess MP activity in the absence of phosphorylation, we used del 7, an MP derivative lacking amino acids 225–268 and thus the entire C-terminal phosphorylation site (Citovsky et al., 1990, 1992). Both in N.tabacum (see also Waigmann et al., 1994) and in N.benthamiana, del 7 promoted efficient intercellular movement of 10 kDa dextran molecules from the injected cell (arrows) into numerous surrounding cells in most injections (Figure 3C and G; Table I), indicating the active role of del 7 in the increase of plasmodesmal permeability observed in these experiments.
Next, MP phosphorylation was mimicked by substituting Ser258, Thr261 and Ser265 with negatively charged aspartate residues (sb-9 mutant), known to reproduce the electrostatic effects of phosphorylation (Dean and Koshland, 1990). Unlike del 7, sb-9 was unable to mediate cell-to-cell movement of dextran molecules in N.tabacum in nearly all injections (Figure 3D; Table I), indicating that the interaction between sb-9 and plasmodesmata was severely impaired, suggesting that phosphorylation is involved in down-regulation of the MP activity in N.tabacum.
In contrast to N.tabacum, sb-9 was fully functional in N.benthamiana, mediating efficient movement of fluorescently labeled 10 kDa dextran from the injected cell into neighboring non-injected cells in most injections (Figure 3H; Table I). This movement was comparable to that induced in the same host by the wild-type MP or its non-phosphorylatable derivative, del 7 (compare Figure 3F, G and H; Table I). These results suggest that plasmodesmal transport of MP in N.benthamiana is not regulated by MP phosphorylation.
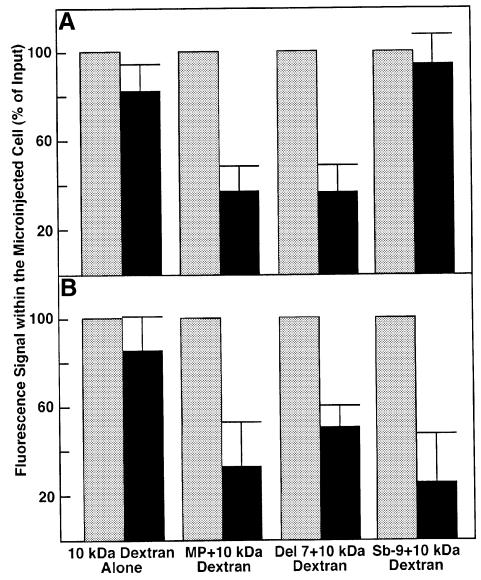
To characterize quantitatively the effects of microinjected MP and its derivatives on plasmodesmal permeability, the extent of dextran movement was determined on a per cell basis. Figure 4 shows that, at the end of the observation time, 81 or 83% of dextran microinjected alone was retained in N.tabacum and N.benthamiana, respectively. The small loss of signal observed in these experiments was most likely due to slow photobleaching of the fluorescent dye. In contrast, 30 min after co-injection with the wild-type MP or its del 7 derivative, the fluorescence signal had decreased by 62 or 63% in N.tabacum (Figure 4A) and 66 or 51% in N. benthamiana (Figure 4B), respectively. These observations indicate that MP and del 7 elevated plasmodesmal permeability of the injected cell to allow efflux of a significant portion of the microinjected dye. Microinjection of sb-9, however, resulted in the loss of only 6% of the injected dextran in N.tabacum (Figure 4A) but induced an efflux of 73% of the fluorescent signal in N.benthamiana (Figure 4B). Statistical evaluation of these results by the unpaired two-tailed Student’s t-test confirmed that, in N.tabacum, signal values after co-injection of dextran with wild-type MP or del 7 were significantly different from those obtained after microinjection of dextrans alone, indicating that MP and del 7 mediated cell-to-cell movement of the dye. In contrast, sb-9 did not promote a statistically significant dextran movement in N.tabacum. Micro injection of the wild-type MP or its del 7 and sb-9 derivatives in N.benthamiana resulted in a statistically sig nificant increase in plasmodesmal permeability compared with that observed after microinjections of 10 kDa dextran alone. The results of this analysis further support that sb-9 enhances plasmodesmal permeability in N.benthamiana but not in N.tabacum. Collectively, our analyses of the extent of plasmodesmal permeability changes induced by sb-9 suggest that intercellular transport machineries of N.tabacum and N.benthamiana responded differently to mimicking of MP phosphorylation.
Fig. 4. Quantification of MP-induced intercellular movement of fluorescent dextrans. Fluorescently labeled dextran (10 kDa) was microinjected either alone or as a mixture with the indicated MP derivatives into N.tabacum (A) or N.benthamiana (B). Fluorescent signal within the injected cell was quantified 5 min (shaded bars) and 30 min (black bars) after injection as described in Materials and methods. The measurements and the indicated standard deviations represent the results of a series of independent microinjections summarized in Table I.
Nicotiana benthamiana contains a protein kinase activity that phosphorylates MP
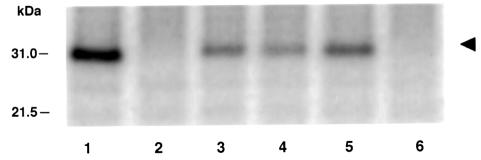
Since plasmodesmal transport in N.benthamiana was not sensitive to mimicking of MP phosphorylation, it was necessary to determine whether such phosphorylation occurs in this plant. Thus, we examined the ability of N.benthamiana cell walls to phosphorylate TMV MP at its C-terminal Ser258, Thr261 and Ser265 residues. Figure 5 shows that wild-type MP is efficiently phosphorylated by a protein kinase activity within N.benthamiana cell walls (lane 1). As expected, del 7, which lacks the entire phosphorylated region, was not phosphorylated (Figure 5, lane 2). Each of the sb-10, sb-11 and sb-12 mutants of MP was phosphorylated but to a markedly lesser degree than the wild-type protein (Figure 5, compare lanes 3, 4 and 5, respectively, with lane 1), suggesting that the substituted amino acid residues reside within the MP phosphorylation site. Finally, sb-9 completely failed to phosphorylate (Figure 5, lane 6). Thus, N.benthamiana is capable of phosphorylating TMV MP at the same amino acid residues as those utilized in N.tabacum. Taken together with the microinjection data, these results suggest that although MP is likely to be phosphorylated in N.benthamiana, the plasmodesmal transport machinery of this host does not respond to this regulatory mechanism of MP activity.
Fig. 5. MP phosphorylation in N.benthamiana. Cell walls purified from N.benthamiana leaves were used to phosphorylate MP or its derivatives as described in Materials and methods. Lane 1, wild-type MP; lane 2, del 7; lane 3, sb-10; lane 4, sb-11; lane 5, sb-12; lane 6, sb-9. The numbers on the left indicate molecular mass standards in thousands of daltons. The arrowhead indicates the position of TMV MP.
Mimicking phosphorylation does not impair MP binding to RNA or PME
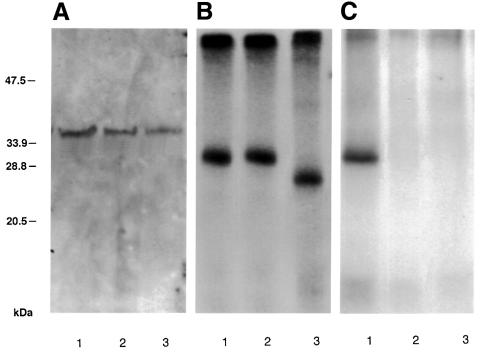
If mimicking phosphorylation specifically affects MP interaction with plasmodesmata, it should not interfere with biological activities of MP that are not directly related to plasmodesmal gating. Indeed, Figure 6A shows that sb-9 (lane 2) interacted in vitro with the 38 kDa tobacco PME thought to represent a potential cell wall receptor for TMV MP (Dorokhov et al., 1999; Chen et al., 2000). Importantly, the degree of sb-9–PME interaction was comparable to that observed with wild-type MP (Figure 6A, lane 1) or del 7 (lane 3). Furthermore, sb-9 binding to RNA, one of the functional hallmarks of TMV MP activity (Citovsky et al., 1990), was also indistinguishable from that of wild-type MP or del 7 (Figure 6B). These results suggest that the major protein structural characteristics were preserved in both sb-9 and del 7 derivatives of TMV MP. On the other hand, unlike wild-type MP, neither sb-9 nor del 7 was phosphorylated by the tobacco cell wall-associated protein kinase (Figure 6C), demonstrating that these mutations indeed targeted the C-terminal phosphorylation site of the protein. Taken together, our results suggest that mimicking MP phosphorylation did not compromise most biological activities of MP. Thus, the severely reduced plasmodesmata-gating activity of sb-9 observed in N.tabacum may indeed be due to a specific regulatory effect of phosphorylation on MP–plasmodesmata interactions, suggesting that it may represent a plant endogenous means to control TMV MP activity and that this regulatory mechanism is host dependent.
Fig. 6. Effect of mimicking MP phosphorylation on MP’s ability to bind PME (A) or RNA (B), and undergo phosphorylation (C). Lane 1, wild-type MP; lane 2, sb-9; lane 3, del 7. All conditions are described in Materials and methods. The numbers on the left indicate molecular mass standards in thousands of daltons.
Mimicking MP phosphorylation by negatively charged substitutions blocks cell-to-cell movement of TMV
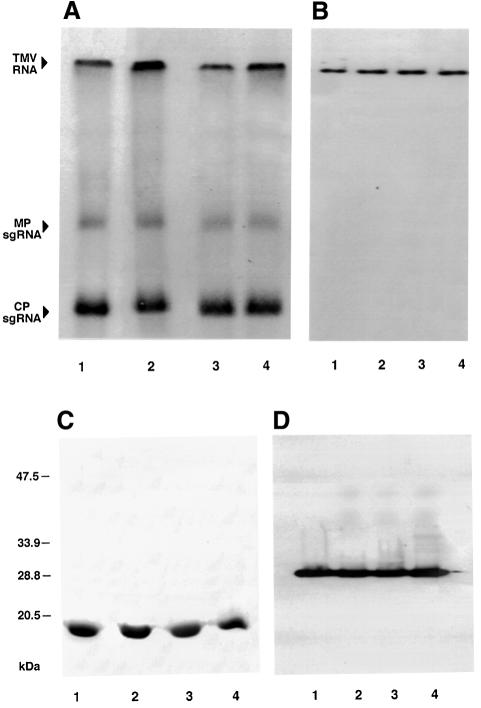
If phosphorylation indeed functions as a negative regulator of MP function, sb-9, which mimics the phosphorylated state of MP, must be unable to promote viral cell-to-cell spread in vivo. To test this hypothesis, sb-9 was introduced into the infectious cDNA clone of TMV in place of the wild-type MP. First, the wild-type and sb-9-encoding TMV cDNAs were transcribed in vitro and the resulting viral RNAs tested for their ability to replicate and accumulate genomic and subgenomic (sg) TMV RNA within inoculated protoplasts derived from N.tabacum and N.benthamiana hosts. Figure 7 shows that, in both hosts, the sb-9 mutation did not affect viral replication as judged from accumulation of positive- and negative-stranded TMV RNA in the infected protoplasts (Figure 7A and B, respectively). In addition, the appearance of the replication intermediate, negative-stranded TMV RNA, indicates that viral RNA accumulation was due to replication rather than input transcripts that persisted in the inoculated protoplasts.
Fig. 7. Replication and translation of TMV RNA encoding MP or sb-9 in protoplasts. (A and B) Northern analyses of positive-stranded and negative-stranded viral RNA, respectively. (C and D) Western blot analyses of CP and MP accumulation, respectively. Bands corresponding to TMV RNA and MP and CP sgRNAs are indicated. Lanes 1 and 2, N.tabacum protoplasts infected with TMV RNA encoding MP or sb-9, respectively; lanes 3 and 4, N.benthamiana protoplasts infected with TMV RNA encoding MP or sb-9, respectively. The numbers on the left indicate molecular mass standards in thousands of daltons.
Figure 7A also shows accumulation of 3′ co-terminal sgRNAs transcribed during the replication cycle and encoding the viral MP and coat protein (CP) gene products (Ishikawa et al., 1991). Similarly to the genomic TMV RNA, no significant differences in accumulation of MP or CP sgRNAs were found between transcripts encoding MP or sb-9 in either of the host species. The CP and MP sgRNAs produced were biologically functional, generating their respective protein products both in N.tabacum and N.benthamiana protoplasts (Figure 7C and D). Furthermore, these results indicate that the sb-9 mutation did not affect the stability and overall accumulation levels of MP in the infected protoplasts of both host plants (Figure 7D).
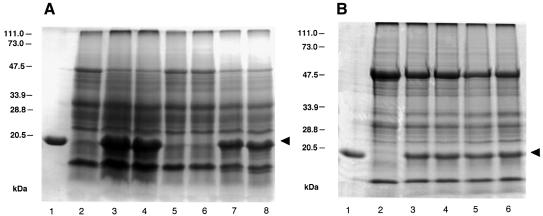
Next, the effect of mimicking MP phosphorylation on local and systemic TMV movement was examined following inoculation of N.tabacum plants with TMV RNA encoding either wild-type MP or its sb-9 derivative. The spread of the virus was assessed from the appearance of CP, a known hallmark of viral infection (Lartey et al., 1997; Ghoshroy et al., 1998). Figure 8A shows that inoculation of N.tabacum with TMV RNA containing the intact MP gene resulted in efficient viral infection and accumulation of CP (lanes 3 and 4). Furthermore, CP was found both in the inoculated (Figure 8A, lane 3) and uninoculated leaves (lane 4), indicating cell-to-cell (within the inoculated leaf) as well as systemic (from the inoculated leaf into uninoculated leaves) viral movement. In contrast, neither cell-to-cell movement nor systemic spread of the virus occurred when plants were inoculated with TMV RNA encoding the sb-9 derivative of MP (Figure 8A, lanes 5 and 6; compare with uninfected plant, lane 2). However, sb-9 TMV RNA became infectious both locally (Figure 8A, lane 7) and systemically (lane 8) on transgenic N.tabacum plants expressing the wild-type MP, indicating that its failure to infect wild-type N.tabacum is the result of non-functional MP. These results suggest that mimicking MP phosphorylation blocks viral cell-to-cell and systemic spread in wild-type N.tabacum but does not interfere with replication of viral genomes or their ability to spread when the functional MP is provided in trans in MP-transgenic plants.
Fig. 8. Effect of mimicking MP phosphorylation on viral cell-to-cell and systemic movement. Ten days after infection, leaves were harvested from plants inoculated with TMV RNA encoding wild-type MP or sb-9 and analyzed for the presence of viral CP as described in Materials and methods. (A) Nicotiana tabacum plants. Lane 1, TMV CP; lane 2, uninfected plant; lanes 3 and 4, inoculated and uninoculated leaves, respectively, of wild-type plants infected with TMV RNA encoding wild-type MP; lanes 5 and 6, inoculated and uninoculated leaves, respectively, of wild-type plants infected with TMV RNA encoding sb-9; lanes 7 and 8, inoculated and uninoculated leaves, respectively, of MP transgenic plants infected with TMV RNA encoding sb-9. (B) Nicotiana benthamiana plants. Lane 1, TMV CP; lane 2, uninfected plant; lanes 3 and 4, inoculated and uninoculated leaves, respectively, of plants infected with TMV RNA encoding sb-9; lanes 5 and 6, inoculated and uninoculated leaves, respectively, of plants infected with TMV RNA encoding wild-type MP. The arrowhead indicates the position of TMV CP. Protein molecular mass standards are indicated on the left in thousands of daltons.
Unlike N.tabacum, inoculation of N.benthamiana with TMV RNA encoding the sb-9 derivative of MP still produced efficient accumulation of viral CP both in the inoculated and systemic leaves (Figure 8B, lanes 3 and 4) as compared with N.benthamiana plants inoculated with the wild-type TMV RNA (lanes 5 and 6). As expected, no TMV CP band was observed in uninfected N.benthamiana plants (Figure 8B, lane 2). Thus, the regulatory effect of mimicking MP phosphorylation on TMV movement is host dependent, occurring in N.tabacum but not in N.benthamiana.
Discussion
Recent evidence suggests that plasmodesmata function to transport large molecules such as viral MPs and MP–nucleic acid complexes (reviewed by Carrington et al., 1996; Lazarowitz and Beachy, 1999; Rhee et al., 2000; Tzfira et al., 2000), transcription factors (Lucas et al., 1995) and gene silencing signals (Palauqui et al., 1997; Vaucheret et al., 1998). Plasmodesmal transport of these biologically active factors should be tightly regulated. No such regulatory mechanism, however, has been described to date. Here, we utilized one of the best characterized proteins known to traffic from cell to cell, TMV MP, to show that its interaction with plasmodesmata is controlled by phosphorylation.
Although MP phosphorylation has been demonstrated in plant protoplasts (Watanabe et al., 1992; Haley et al., 1995), these cells lack cell walls and their cognate plasmodesmata, which represent the biological sites of action for MP (Tomenius et al., 1987; Atkins et al., 1991; Ding et al., 1992). Thus, it was still unknown whether MP phosphorylation takes place in vivo, within plant tissues, where MP is biologically active. Our present results show that phosphorylation of MP contained in the cell walls, presumably within plasmodesmata (Ding et al., 1992), occurs in vivo in transgenic plants. The MP phosphorylation site utilized in vivo, i.e. C-terminal amino acid residues Ser258, Thr261 and Ser265, is identical to that determined in vitro using purified cell wall fractions (Citovsky et al., 1993). So far, this MP phosphorylation has been detected only in N.tabacum; here, we show that a similar MP kinase activity is also present in N.benthamiana. Furthermore, we provide evidence that MP produced during the wild-type TMV infection of tobacco plants is also phosphorylated. C-terminal phosphorylation of MP is carried out by a serine/threonine-specific protein kinase associated with the cell wall of the host cell. This enzyme activity requires Mg2+ but not Ca2+ cations. Independence from Ca2+ distinguishes it from several known protein kinases that associate with plant cell walls (He et al., 1996; Yahalom et al., 1998).
The biological function of MP C-terminal phosphorylation was examined using negatively charged amino acid substitutions within the phosphorylation site. Substitutions with aspartate or glutamate are known to reveal the electrostatic effects of phosphorylation (Dean and Koshland, 1990). For example, replacement of serine by aspartate in the HPr protein has been shown to cause shifts in two-dimensional NMR spectra similar to those elicited by phosphorylation (Wittekind et al., 1989). Also, inactivation by phosphorylation of Ser113 in isocitrate dehydrogenase is mimicked when aspartate is substituted at this site (Thorsness and Koshland, 1987). Our studies show that substituting Ser258, Thr261 and Ser265 with aspartate residues (sb-9 derivative of MP) inactivated the MP–plasmodesmata interactions leading to gating of these channels. Specific interference of MP phosphorylation with plasmodesmal transport is supported by the observation that mimicking this protein modification did not affect several other known activities of MP in vitro nor did it compromise MP stability in vivo. Interestingly, the negative regulation of MP interactions with plasmodesmata was host dependent. In N.tabacum, mimicking phosphorylation blocked MP’s ability to interact efficiently with plasmodesmata, whereas in N.benthamiana the same MP derivative remained fully active as compared with non-phosphorylated MP. This result, taken together with the observation that both N.tabacum and N.benthamiana are competent for MP phosphorylation, suggests that the plasmodesmal transport machineries of the two hosts differ. Specifically, the plasmodesmal transport machinery of N.benthamiana is likely to be able to mediate movement of phosphorylated and non-phosphorylated MP, whereas the plasmodesmal transport machinery of N.tabacum may support only movement of non-phosphorylated MP. Thus, the inactivation strategy by phosphorylation, highly successful in N.tabacum, is not effective in N.benthamiana, consistent with the known fact that N.benthamiana is one of the most susceptible hosts for plant viruses (Gibbs et al., 1977; Dawson and Hilf, 1992).
Besides TMV MP, few plant viral MPs have been tested for phosphorylation. However, recently reported phosphorylation of the 17 kDa MP of potato leafroll virus has also been proposed to regulate negatively the function of this transport protein (Sokolova et al., 1997). Thus, it is tempting to speculate that the regulatory activity of MP phosphorylation or other strategies to inactivate this viral protein (Hughes et al., 1995) may underlie the well known but previously unexplained differences between various tobacco species in their susceptibility to viral infection (Gibbs et al., 1977; Dawson and Hilf, 1992).
Because C-terminal phosphorylation of MP blocks its interaction with plasmodesmata, it should also inhibit the ability of the virus to spread from cell to cell in vivo. Indeed, TMV encoding the sb-9 derivative of MP within its genomic RNA was unable to move locally and systemically within N.tabacum. The mutant virus was fully capable of movement in transgenic plants expressing wild-type TMV MP, indicating that the mutation did not interfere with replication and translation of viral gene products. Indeed, the sb-9 mutation did not affect replication of viral RNA, production of MP and CP sgRNAs, and expression and stability of MP and CP in N.tabacum and N.benthamiana protoplasts. Thus, mimicking MP phosphorylation blocks TMV movement through plasmodesmata, probably by preventing the MP-induced increase in permeability of these intercellular channels. Similarly to microinjection experiments, the inhibitory effect of mimicking MP phosphorylation on TMV movement in vivo was host dependent, occurring in N.tabacum but not in a more promiscuous N.benthamiana host. These results were supported by direct evaluation of the cell-to-cell movement capacity of wild-type TMV MP, sb-9 and del 7, which were expressed as green fluorescent protein (GFP) fusion proteins following microbombardment of their encoding genes into epidermal cells of mature leaves from N.tabacum or N.benthamiana. del 7–GFP and wild-type TMV MP–GFP showed significant movement in both hosts. In contrast, cell-to-cell transport of sb-9–GFP was comparable to that of wild-type TMV MP in N.benthamiana, but was severely reduced in N.tabacum (K.Trutnyeva, R.Bachmaier and E.Waigmann, unpublished).
Negative regulation of MP effects on plasmodesmata makes biological sense. Unlike other TMV-coded proteins expressed throughout the course of viral infection, MP is synthesized only transiently (reviewed in Hull, 1989). However, the produced protein does not appear to have a rapid turnover; instead, it persists in the infected cells, accumulating within their plasmodesmata (Tomenius et al., 1987; Ding et al., 1992). The continuous presence of active MP may elevate plasmodesmal permeability and alter intercellular communication, an important biological process. Potentially, phosphorylation minimizes this MP interference with plasmodesmal transport during viral infection. Thus, viral cell-to-cell movement may involve plasmodesmal gating by the newly synthesized, unphosphorylated MP followed by phosphorylation of MP that has already performed its function within plasmodesmata, preventing it from further action. This model suggests that MP is most active in freshly infected cells where it is known to be produced vigorously (Dawson and Lehto, 1990) while MP contained within cells at later stages of the infection is expected to become permanently inactivated. Indeed, recent observations demonstrate that plasmodesmal gating is restricted to the outer edge of an expanding TMV infection site even though MP itself is also present in plasmodesmata of cells located in the center of this infected area (Oparka et al., 1997).
Because viruses often adapt existing cellular machinery for their own needs, TMV is likely to employ an endogenous pathway for cell-to-cell transport of proteins and nucleic acids. Thus, MP phosphorylation may represent a general mechanism by which plants regulate intercellular transport of macromolecules.
Materials and methods
Plant lines
Nicotiana tabacum cv. Turk and N.benthamiana were used as hosts. Transgenic N.tabacum plants expressing MP, sb-10, sb-11, sb-12 and sb-13 were generated as described (Citovsky et al., 1992).
Mutagenesis and expression of MP
Substitution mutants sb-9, sb-10, sb-11, sb-12 and sb-13 of MP were constructed using oligonucleotide-directed mutagenesis (McClary et al., 1989) and confirmed by dideoxynucleotide sequencing (Kraft et al., 1988). Generation of deletion mutant del 7 of MP was described previously (Citovsky et al., 1990, 1992). MP and its del 7, sb-9, sb-10, sb-11 and sb-12 derivatives were expressed in Escherichia coli using the T7 RNA polymerase system (Studier et al., 1990), purified to near homogeneity (95–98% pure as determined by silver-stained SDS–polyacrylamide gels), and verified by western blot analysis as described (Citovsky et al., 1990, 1992, 1993; Waigmann et al., 1994).
TMV cDNA clone encoding MP sb-9 was produced directly from a full-length infectious cDNA of TMV under the control of SP6 promoter (pTMV304, kindly provided by Dr W.Dawson, University of Florida, Lake Alfred, FL) using the Transformer™ Site-Directed Mutagenesis Kit according to the manufacturer’s instructions (Clontech Laboratories, Inc.) followed by dideoxynucleotide sequencing (Kraft et al., 1988).
Infection of plants and detection of TMV in plant extracts
TMV cDNA clones encoding wild-type MP or sb-9 in the pTMV304 construct were linearized with KpnI. Infectious transcripts were produced from the SP6 promoter and capped with 7m-diguanosine triphosphate using the RiboMax large scale RNA Production System kit according to the manufacturer’s directions (Promega). Nicotiana tabacum or N.benthamiana plants were mechanically inoculated with 10 µl of the resulting 100 µg/ml RNA solution supplemented with 3 mg/ml celite. Four individual plants were inoculated with each viral transcript.
Ten days after inoculation, leaf samples (50 mg fresh weight) were removed and homogenized in 100 µl of extraction buffer (75 mM Tris–HCl pH 6.8, 9 M urea, 4.3% SDS and 7.5% β-mercaptoethanol). The homogenate was heated at 100°C for 5 min, centrifuged (10 000 g for 10 min) to remove all insoluble material, and 10 µl of the supernatant were analyzed by SDS–PAGE (Laemmli, 1970) on 12.5% gels. Following electrophoresis, gels were stained with Coomassie Blue and photographed using a Fotodyne gel documentation system. The position of TMV CP was verified by western blot analysis (data not shown). No significant differences between individual plants inoculated with the same viral construct were observed.
Plant cell wall fractions
Cell wall fractions were prepared as described (Citovsky et al., 1993). Briefly, fresh plant tissue derived from mature leaves of wild-type or transgenic N.tabacum plants was ground to a fine powder and homogenized at 4°C in 1 vol. of buffer H (0.1 M HEPES pH 7.4, 100 mM NaCl, 10 mM EDTA, 5 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 mM leupeptin, 1 mM pepstatin). These homogenates, which represented the total plant cell extract, were centrifuged (1000 g for 5 min at 4°C) to give a crude cell wall pellet and supernatant. Cell walls were further purified by homogenization at 4°C in 10 vols of buffer H with 2% Triton X-100 and centrifugation. This procedure was repeated twice, followed by six washes (1000 g for 5 min at 4°C) in buffer H with 2% Triton X-100 and two washes in buffer H without the detergent. The resulting white insoluble material was resuspended in 0.5 vols of buffer H, quick-frozen in liquid nitrogen and stored at –70°C until use.
MP phosphorylation in vivo
Transgenic plants expressing MP, sb-10, sb-11, sb-12 and sb-13 were grown from seeds in sealed Magenta jars on 0.7% agar in a low phosphate MS medium formulated in-house by replacing potassium phosphate from the MS recipe (Murashige and Skoog, 1962) with potassium chloride and supplementing it with 10 mCi (1 Ci = 37 GBq) of 32Pi. When the plants reached 6 cm in height, one of their leaves was harvested and used for isolation of cell walls as described above except that buffer H was supplemented with 10 µM okadaic acid, 50 mM sodium fluoride and 0.2 mM sodium vanadate to inhibit cellular phosphatases. The purified cell wall fraction was extracted in 1 vol. of 75 mM Tris–HCl pH 6.8, 9 M urea, 7.5% β-mercaptoethanol, centrifuged (15 000 g for 15 min at 4°C), and the supernatant dialyzed for 16 h at 4°C against buffer H. MP was then immunoprecipitated with affinity-purified rabbit anti-MP antibodies (McLean et al., 1995) using standard procedures (Ausubel et al., 1987) and analyzed on 12.5% SDS–polyacrylamide gels, followed by autoradiography. Equal loading of all lanes was confirmed by western blot analysis using mouse anti-MP antibodies followed by alkaline phosphatase detection.
MP phosphorylation in vitro
Cell wall fractions (10 µg of protein in 25 µl of buffer H) were mixed with 5 µl of 10× kinase buffer (0.5 M Tris–HCl pH 8.0, 50 mM MgCl2) and 20 µl of buffer H containing 2 µg of MP or its derivatives. The reaction was started by addition of 0.5 µl of [γ-32P]ATP (equal to 5 µCi, 3000 Ci/mmol), continued for 15 min at room temperature, and stopped with 50 µl of loading buffer (75 mM Tris–HCl pH 6.8, 9 M urea, 4.3% SDS, 7.5% β-mercaptoethanol). The phosphorylated protein was resolved on a 12.5% SDS–polyacrylamide gel and detected by autoradiography.
MP binding to RNA
The 300 bp fragment of the spinach chloroplast plastidic electron transport D gene was used as template for T7 RNA polymerase to generate a radioactively labeled RNA probe with a specific activity of 107 c.p.m./µg (Stern and Gruissem, 1987). A gel-purified RNA probe (1.0 ng) was incubated for 15 min at 4°C in 20 µl of buffer H with 0.1 µg of purified MP or its derivatives. RNA–protein complexes were cross-linked by UV-light irradiation and analyzed on 12.5% SDS–polyacrylamide gels as described (Citovsky et al., 1990, 1992).
MP binding to PME
A renatured blot overlay assay (Chen et al., 2000) was used to examine binding of MP or its derivatives to PME. Briefly, PME (1 µg) purified from tobacco leaves (Chen et al., 2000) was electrophoresed on a 12.5% SDS–polyacrylamide gel, electroblotted onto a PVDF membrane, depleted of SDS by guanidine hydrochloride extraction, renatured, blocked with 1% bovine serum albumin, and incubated with 10 µg/ml purified MP, del 7 or sb-9 as described (Chen et al., 2000). Following incubation, the membrane was washed and protein binding was detected using anti-MP antibody and the ECL western blotting kit (Amersham) (Chen et al., 2000).
Replication and translation of TMV RNA encoding MP or sb-9 in N.tabacum and N.benthamiana protoplasts
Protoplasts were prepared from mature leaves of N.tabacum and N.benthamiana plants as described (McLean et al., 1995), electroporated with 10 µl of the infectious transcripts of TMV cDNA clones encoding wild-type MP or sb-9 (see above), and incubated for 20 h at 25°C as described (Lewandowski and Dawson, 1998). For analysis of viral RNA, total RNA from 106 protoplasts was extracted, resolved on 0.9% agarose gels and analyzed by northern blotting as described (Lewandowski and Dawson, 1998). Strand-specific digoxigenin-labeled riboprobes were used to detect TMV-derived RNAs. Positive-stranded full-length and sg TMV RNAs were detected using a probe complementary to TMV nucleotides 5545–5845 and negative-stranded RNA was detected with a probe identical to the same TMV sequence. Because negative-strand TMV RNA is produced at much lower levels than positive-strand RNA (Ishikawa et al., 1991), 20-fold larger RNA samples were used for its detection. For protein analysis, 106 protoplasts were resuspended and boiled in the gel loading buffer (Laemmli, 1970), and the resulting total cell protein extracts resolved on 12.5% SDS–polyacrylamide gels followed by western blot analysis using affinity-purified anti-CP or anti-MP antibodies.
Microinjection, image analysis and fluorescence quantification
A mixture containing ∼0.4 µg/µl TMV MP, sb-9 or del 7 and 0.5 mM 10 kDa fluorescein isothiocyanate-labeled dextran (FITC–dextran; Sigma-Aldrich) was microinjected into N.tabacum cv. Turk or N.benthamiana leaf mesophyll cells essentially as described (Ding et al., 1992; Waigmann et al., 1994). Before use, FITC–10 kDa dextran was separated from free dye by centrifugation through a 10 kDa cut-off spin column (Ultrafree-MC; Millipore, MA). Intercellular movement of FITC–10 kDa dextran was observed and documented with a cooled slow scan, high resolution CCD camera (Quantix-G2; Photometrics, Tucson, AZ). Each experiment was repeated numerous times (Table I).
For quantification of dextran fluorescence on a per cell level, 5 and 30 min after injection the signal was recorded with the CCD camera and quantified using the ‘analyze’ function of the IPlab software program (Scanalytics, Virginia, VA). In this function, all signal values within a region of interest, i.e. the area of the injected cell (S), can be summed. Then, the system background (B) was obtained by combining the values in an area identical in size to that of the injected cell but in a region containing non-injected cells. Intracellular fluorescence values (F) for each time point were calculated as F = S – B. Finally, the movement of fluorescent dextrans out of the injected cell was expressed as a percentage of signal input calculated as the ratio of the signal retained within the injected cell 30 min after injection to that measured 5 min after injection, i.e. F30min/F5min × 100. The 5 min period was chosen as the first time point at which the image could be reliably recorded in all experiments whereas the 30 min time point represented a standard observation time during which MP-mediated dextran movement is known to occur (Waigmann et al., 1994). For statistical evaluation, mean values and standard deviations were calculated based on 5–8 independent microinjection experiments, i.e. number of measured cells n = 5–8. To determine whether any two sets of fluorescent signal measurements were statistically different from each other, data were subjected to the unpaired two-tailed Student’s t-test. For this test, a combined n = 10–15 (derived from the two sets of fluorescent signal measurements to be compared) was used. 2P values <0.01, corresponding to the statistical probability of >99%, were considered statistically significant.
Acknowledgments
Acknowledgements
We are indebted to Gail McLean for helpful discussions and to Pat Zambryski in whose laboratory the story of MP phosphorylation began. We also thank Heinrich Kowalski for valuable help with statistical analysis. This work was supported by grants from the National Institutes of Health (Grant No. R01-GM50224), the National Science Foundation Functional Genomic Initiative (Grant No. DBI 9975717), the US Department of Agriculture (Grant No. 94-02564) and the US–Israel Binational Research and Development Fund (BARD) (Grant No. US-2969-98) to V.C. and a grant from the Austrian Science Foundation (Grant No. P12614-MOB) to E.W. E.W. was supported by an APART fellowship (APART 441) from the Austrian Academy of Science.
References
- Atkins D., Hull,R., Wells,B., Roberts,K., Moore,P. and Beachy,R.N. (1991) The tobacco mosaic virus 30 K movement protein in transgenic tobacco plants is localized to plasmodesmata. J. Gen. Virol., 72, 209–211. [DOI] [PubMed] [Google Scholar]
- Ausubel F.M., Brent,R., Kingston,R.E., Moore,D.D., Smith,J.A., Seidman,J.G. and Struhl,K. (1987) Current Protocols in Molecular Biology. Greene Publishing–Wiley Interscience, New York, NY. [Google Scholar]
- Carrington J.C., Kassachau,K.D., Mahajan,S.K. and Schaad,M.C. (1996) Cell-to-cell and long-distance transport of viruses in plants. Plant Cell, 8, 1669–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.-H., Sheng,J., Hind,G., Handa,A. and Citovsky,V. (2000) Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell-to-cell movement. EMBO J., 19, 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V., Knorr,D., Schuster,G. and Zambryski,P. (1990) The P30 movement protein of tobacco mosaic virus is a single-strand nucleic acid binding protein. Cell, 60, 637–647. [DOI] [PubMed] [Google Scholar]
- Citovsky V., Wong,M.L., Shaw,A., Prasad,B.V.V. and Zambryski,P. (1992) Visualization and characterization of tobacco mosaic virus movement protein binding to single-stranded nucleic acids. Plant Cell, 4, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V., McLean,B.G., Zupan,J. and Zambryski,P. (1993) Phosphorylation of tobacco mosaic virus cell-to-cell movement protein by a developmentally-regulated plant cell wall-associated protein kinase. Genes Dev., 7, 904–910. [DOI] [PubMed] [Google Scholar]
- Dawson W.O. and Hilf,M.E. (1992) Host-range determinants of plant viruses. Annu. Rev. Plant Physiol. Plant Mol. Biol., 43, 527–555. [Google Scholar]
- Dawson W.O. and Lehto,K.M. (1990) Regulation of tobamovirus gene expression. Adv. Virus Res., 38, 307–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A.M. and Koshland,D.E. (1990) Electrostatic and steric contributions to regulation at the active site of isocitrate dehydrogenase. Science, 249, 1044–1046. [DOI] [PubMed] [Google Scholar]
- Deom C.M., Schubert,K.R., Wolf,S., Holt,C.A., Lucas,W.J. and Beachy,R.N. (1990) Molecular characterization and biological function of the movement protein of tobacco mosaic virus in transgenic plants. Proc. Natl Acad. Sci. USA, 87, 3284–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B., Haudenshield,J.S., Hull,R.J., Wolf,S., Beachy,R.N. and Lucas,W.J. (1992) Secondary plasmodesmata are specific sites of localization of the tobacco mosaic virus movement protein in transgenic tobacco plants. Plant Cell, 4, 915–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorokhov Y.L., Makinen,K., Frolova,O.Y., Merits,A., Saarinen,J., Kalkkinen,N., Atabekov,J.G. and Saarma,M. (1999) A novel function for a ubiquitous plant enzyme pectin methylesterase: the host-cell receptor for the tobacco mosaic virus movement protein. FEBS Lett., 461, 223–228. [DOI] [PubMed] [Google Scholar]
- Epel B.L. (1994) Plasmodesmata: composition, structure and trafficking. Plant Mol. Biol., 26, 1343–1356. [DOI] [PubMed] [Google Scholar]
- Esau K. (1948) Some anatomical aspects of plant virus disease problems. II. Bot. Rev., 14, 413–449. [Google Scholar]
- Ghoshroy S., Lartey,R., Sheng,J. and Citovsky,V. (1997) Transport of proteins and nucleic acids through plasmodesmata. Annu. Rev. Plant Physiol. Plant Mol. Biol., 48, 27–49. [DOI] [PubMed] [Google Scholar]
- Ghoshroy S., Freedman,K., Lartey,R. and Citovsky,V. (1998) Inhibition of plant viral systemic infection by non-toxic concentrations of cadmium. Plant J., 13, 591–602. [DOI] [PubMed] [Google Scholar]
- Gibbs M.J., Armstrong,J., Weiller,G.F. and Gibbs,A.J. (1977) Virus evolution: the past, a window on the future? In Tepfer,M. and Balazs,E. (eds), Virus-Resistant Transgenic Plants: Potential Ecological Impact. Springer-Verlag, Berlin, Germany, pp. 1–17. [Google Scholar]
- Haley A., Hunter,T., Kiberstis,P. and Zimmern,D. (1995) Multiple serine phosphorylation sites on the 30 kDa TMV cell-to-cell movement protein synthesized in tobacco protoplasts. Plant J., 8, 715–724. [DOI] [PubMed] [Google Scholar]
- He Z.H., Fujiki,M. and Kohorn,B.D. (1996) A cell wall-associated, receptor-like protein kinase. J. Biol. Chem., 271, 19789–19793. [DOI] [PubMed] [Google Scholar]
- Heinlein M., Epel,B.L., Padgett,H.S. and Beachy,R.N. (1995) Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science, 270, 1983–1985. [DOI] [PubMed] [Google Scholar]
- Hughes R.K., Perbal,M.C., Maule,A.J. and Hull,R. (1995) Evidence for proteolytic processing of tobacco mosaic virus movement protein in Arabidopsis thaliana. Mol. Plant Microbe Interact., 8, 658–665. [DOI] [PubMed] [Google Scholar]
- Hull R. (1989) The movement of viruses in plants. Annu. Rev. Phytopathol., 27, 213–240. [Google Scholar]
- Ishikawa M., Meshi,T., Ohno,T. and Okada,Y. (1991) Specific cessation of minus-strand RNA accumulation at an early stage of tobacco mosaic virus infection. J. Virol., 65, 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft R., Tardiff,J., Kranter,K.S. and Leinwand,L.A. (1988) Using miniprep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques, 6, 544–547. [PubMed] [Google Scholar]
- Kragler F., Monzer,J., Shash,K., Xoconostle-Cazares,B. and Lucas,W.J. (1998) Cell-to-cell transport of proteins: requirement for unfolding and characterization of binding to a putative plasmodesmal receptor. Plant J., 15, 367–381. [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 277, 680–685. [DOI] [PubMed] [Google Scholar]
- Lartey R., Ghoshroy,S., Ho,J. and Citovsky,V. (1997) Movement and subcellular localization of a tobamovirus in Arabidopsis. Plant J., 12, 537–545. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S.G. and Beachy,R.N. (1999) Viral movement proteins as probes for intracellular and intercellular trafficking in plants. Plant Cell, 11, 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski D.J. and Dawson,W.O. (1998) Deletion of internal sequences results in tobacco mosaic virus defective RNAs that accumulate to high levels without interfering with replication of the helper virus. Virology, 251, 427–437. [DOI] [PubMed] [Google Scholar]
- Lucas W.J. and Gilbertson,R.L. (1994) Plasmodesmata in relation to viral movement within leaf tissues. Annu. Rev. Phytopathol., 32, 387–411. [Google Scholar]
- Lucas W.J., Ding,B. and van der Schoot,C. (1993) Plasmodesmata and the supracellular nature of plants. New Phytol., 125, 435–476. [DOI] [PubMed] [Google Scholar]
- Lucas W.J., Bouche-Pillon,S., Jackson,D.P., Nguyen,L., Baker,L., Ding,B. and Hake,S. (1995) Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science, 270, 1980–1983. [DOI] [PubMed] [Google Scholar]
- McClary J.A., Witney,F. and Geisselsoder,J. (1989) Efficient site-directed in vitro mutagenesis using phagemid vectors. Biotechniques, 7, 282–289. [PubMed] [Google Scholar]
- McLean B.G., Zupan,J. and Zambryski,P. (1995) Tobacco mosaic virus movement protein associates with the cytoskeleton in tobacco cells. Plant Cell, 7, 2101–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T. and Skoog,F. (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant., 15, 473–497. [Google Scholar]
- Oparka K.J., Prior,D.A.M., Santa-Cruz,S., Padgett,H.S. and Beachy,R.N. (1997) Gating of epidermal plasmodesmata is restricted to the leading edge of expanding infection sites of tobacco mosaic virus (TMV). Plant J., 12, 781–789. [DOI] [PubMed] [Google Scholar]
- Palauqui J.C., Elmayan,T., Pollien,J.M. and Vaucheret,H. (1997) Systemic acquired silencing: transgene-specific post-translational silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J., 16, 4738–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee Y., Tzfira,T., Chen,M.-H., Waigmann,E. and Citovsky,V. (2000) Cell-to-cell movement of tobacco mosaic virus: enigmas and explanations. Mol. Plant Pathol., 1, 33–39. [DOI] [PubMed] [Google Scholar]
- Sokolova M., Prufer,D., Tacke,E. and Rohde,W. (1997) The potato leafroll virus 17K movement protein is phosphorylated by a membrane-associated protein kinase from potato with biochemical features of protein kinase C. FEBS Lett., 400, 201–205. [DOI] [PubMed] [Google Scholar]
- Stern B.D. and Gruissem,W. (1987) Control of plastid gene expression: 3′ inverted repeats act as mRNA processing and stabilizing elements, but do not terminate transcription. Cell, 51, 1145–1157. [DOI] [PubMed] [Google Scholar]
- Studier F.W., Rosenberg,A.H., Dunn,J.J. and Dubendorff,J.W. (1990) Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol., 185, 60–89. [DOI] [PubMed] [Google Scholar]
- Thorsness P.E. and Koshland,D.E. (1987) Inactivation of isocitrate dehydrogenase by phosphorylation is mediated by the negative charge of the phosphate. J. Biol. Chem., 262, 10422–10425. [PubMed] [Google Scholar]
- Tomenius K., Clapham,D. and Meshi,T. (1987) Localization by immunogold cytochemistry of the virus coded 30 K protein in plasmodesmata of leaves infected with tobacco mosaic virus. Virology, 160, 363–371. [DOI] [PubMed] [Google Scholar]
- Tzfira T., Rhee,Y., Chen,M.-H. and Citovsky,V. (2000) Nucleic acid transport in plant–microbe interactions: the molecules that walk through the walls. Annu. Rev. Microbiol., 54, 187–219. [DOI] [PubMed] [Google Scholar]
- Vaucheret H., Beclin,C., Elmayan,T., Feuerbach,F., Godon,C., Morel,J.B., Mourrain,P., Palauqui,J.C. and Vernhettes,S. (1998) Transgene-induced gene silencing in plants. Plant J., 16, 651–659. [DOI] [PubMed] [Google Scholar]
- Waigmann E., Lucas,W., Citovsky,V. and Zambryski,P. (1994) Direct functional assay for tobacco mosaic virus cell-to-cell movement protein and identification of a domain involved in increasing plasmodesmal permeability. Proc. Natl Acad. Sci. USA, 91, 1433–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Ogawa,T. and Okada,Y. (1992) In vivo phosphorylation of the 30 kDa protein of tobacco mosaic virus. FEBS Lett., 313, 181–184. [DOI] [PubMed] [Google Scholar]
- Wittekind M., Reizer,J., Deutscher,J., Saier,M.H. and Klevit,R.E. (1989) Common structural changes accompany the functional inactivation of HPr by seryl phosphorylation or by serine to aspartate substitution. Biochemistry, 28, 9908–9912. [DOI] [PubMed] [Google Scholar]
- Wolf S., Deom,C.M., Beachy,R.N. and Lucas,W.J. (1989) Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science, 246, 377–379. [DOI] [PubMed] [Google Scholar]
- Yahalom A., Lando,R., Katz,A. and Epel,B. (1998) A calcium-dependent protein kinase is associated with maize mesocotyl plasmodesmata. J. Plant Physiol., 153, 354–362. [Google Scholar]