Abstract
Objective
To test the hypothesis that a combination of PP13, PAPP-A and first-trimester uterine artery Doppler would improve the prediction of preeclampsia.
Methods
This is a prospective cohort study of pregnant women followed from the first-trimester to delivery. PP13 and PAPPA were determined by immunoassay of maternal serum at 11 – 14 weeks’, when uterine artery Doppler measurements were assessed. Cases identified with any form of preeclampsiawere compared with a control group without preeclampsia. The sensitivity of each marker or their combinations in predicting preeclampsia for different fixed false positive rates was calculated from the ROC curves.
Results
Forty two women were diagnosed with preeclampsia and 410 women with pregnancies not complicated by preeclampsia were used as controls. For a fixed false positive rate (FPR) of 20%, PP13, PAPP-A and mean uterine artery pulsatility index identified 49%, 58% and 62% respectively, of women who developed any form of preeclampsia. PP13 was best in predicting early onset preeclampsia with a sensitivity of 79% at a 20% FPR.Combinations of the three first trimester assessments did not improve the prediction of preeclampsia in later pregnancy.
Conclusion
First-trimester PP13, PAPP-A and uterine artery PI are reasonable, individual predictors of women at risk to develop preeclampsia. Combinations of these assessments do not further improve the prediction of preeclampsia
Keywords: PAPP-A, PP13, Uterine artery, Preeclampsia, Prediction
Introduction
Preeclampsia affects about 6% of pregnancies and is a significant contributor to maternal mortality and morbidity (1). Abnormal placenta development in the first-trimester leading to placental dysfunction is known to be a major cause of preeclampsia (2).Recent reports suggest the potential role of placental proteins including placental protein 13 (PP13) and pregnancy-associated plasma protein A (PAPP-A) in identifying pregnancies at risk for later development of preeclampsia. (3-8).
PPP13 is a member of the galectin family produced by the syncytiotrophoblast (5). It binds to proteins on the extracellular matrix between the placenta and the endometrium and is thought to be involved in placental implantation and vascular remodeling (9).Reduced levels of PP13 messenger RNA levels have been reported in the placentas of women who developed preterm preeclampsia (10)
A few case-control studies have examined the prediction of preeclampsia using either PP13 alone or in combination with either PAPP-A or uterine artery Doppler findings from the second trimester (4, 6-8, 11-13). Some of the studies have yielded conflicting results. For example, Stamatopoulou et al investigated the role of PP13 and PAPP-A as markers for hypertensive disorders and small for gestational age. The authors did not find PP13 levels to be different between the control group and affected pregnancies and concluded that PP13 needs to be further studied (13). In addition, little is known on how first-trimester uterine artery Doppler performs when combined with these serum markers.Our objective is to test the hypothesis that a combination of PP13, PAPP-A and first-trimester uterine artery Doppler would improve the prediction of preeclampsia.
Materials and Methods
This is a prospective cohort study of pregnant women followed from the first-trimester to delivery as part of a first-trimester screening study for adverse pregnancy outcomes.
Approval for the study was obtained from the institutional review board of Washington University School of Medicine in St Louis,MO and all women gave written, informed consent. Women with singleton pregnancies between 11 – 14 weeks’ gestation attending for first-trimester aneuploidy screening were approached to participate in the study from December 2009 to March 2011. Gestational age was calculated from the last menstrual period and confirmed by crown-rump length measurement.
After the first 477 women were recruited for the cohort study, the particular PP13 assay kit used became unavailable from the supplier. This study was therefore based on all women in whom PP13 evaluated. . Cases were defined as patients who developed pre-eclampsia at any gestational age. These were compared with a control group with pregnancies not complicated by preeclampsia followed over the same period.
Maternal serum analytes
Each patient provided approximately 10 cc of maternal blood which was drawn by venipuncture into non-heparinized tubes. The blood samples were allowed to clot,and centrifuged at 1,500g for 15 minutes. The serum was then removed, and aliquots were stored at − 80°C until analyzed. A serum sample of 25 μL from each was used to measure PP13 concentration by a time-resolved fluorescent immunoassay, where analyte concentration was directly proportional to the fluorescence measured on time-resolved fluorometer at 615 nm (DELFIA/AutoDELFIA research kit, PerkinElmer Life and Analytical Sciences, Turku, Finland). Duplicate measurements of each sample are obtained and the test of repeatability performed for the assay revealed a mean intra-assay coefficient of variation of 6.8% and an inter-assay of 8.1%.Concentrations of PP13 were expressed as multiples of the median (MoM) for gestational age and adjusted for maternal BMI. The samples were analyzed by an examiner blinded to the clinical outcomes.
PAPP-A levels were measured by PerkinElmer laboratory as part of routine first-trimester aneuploidy screening and also converted to MoM. The PAPP-A levels were adjusted for maternal weight, ethnicity, smoking status, and for pregnancies conceived using assisted reproductive technologies.
Uterine artery Doppler evaluation
Doppler examination of the uterine arteries was performed using transabdominal ultrasound with color flow mapping. A mid-sagittal view of the uterus was obtained and the cervical canal identified. The transducer was then rotated until the paracervical vessels were visualized. The uterine artery was isolated and the pulsatility index (PI) measured at this point. The mean PI of the measurementsin each of the two uterine arteries was calculated and converted to MoM for the gestational age. All participating sonographers are certified by the Fetal Medicine Foundation for first trimester screening and Doppler measurements.
Clinical diagnoses
Normal or control patients were those without any medical or obstetrical complication during pregnancy or delivery. Preeclampsia was defined using guidelines of the American College of Obstetricians and Gynecology (14) and other hypertensive disorders by the criteria proposed by the National High Blood Pressure Education Program Working Group Report in Pregnancy (15). Early preeclampsia was defined as those requiring delivery prior to 34 weeks gestation (7).
Statistical analysis
Categorical and continuous variables were compared using chi-squared and student t test as appropriate. The distribution of the data was tested for normality using the Kolmogorov-Smirnov test. Logistic regression analysis was used to model the prediction of preeclampsia using PP13, PAPP-A and the mean uterine artery PI MoM individually or in combination. Backward stepwise approach was used to identify the final prediction model adjusting for confounding variables identified in the univariate analysis. The models were used to generate prediction scores for each marker and to produce receiver-operating characteristic curves (ROC). The performance of the variables in a screening paradigm was determined using receiver operator characteristic (ROC) curves, with the area under the curve (AUC) being of primary interest. The goodness of fit of each model was evaluated (16). The sensitivity of each marker or their combinations in predicting preeclampsia using variable assignments for the false positive rate (5%, 10%, and 20%) was calculated from the ROC curves. The same analyses were performed for cases of early onset preeclampsia.
All statistical analyses were performed using STATA version 10.0 (Stata Corp., College Station, TX). Tests with p values < 0.05 were considered significant.
Results
Among, 477 women in whom both PP13, PAPP-A and uterine artery Doppler were obtained, 452 met the inclusion criteria. Twenty five were excluded due to either spontaneous miscarriage prior to 20 weeks, loss to follow-up or fetal anomalies diagnosed in the second trimester.
Preeclampsia was diagnosed in 42 patients and the remaining 410 patients with pregnancies not complicated by preeclampsia serving as controls. There were 12 cases with early onset preeclampsia.
The demographic characteristics of the study population are shown in Table 1. Patients with preeclampsia were more likely to be of black race, have a higher BMI, and have higher rates of both hypertensive disorders as well as pre-gestational diabetes compared to control patients. The group with preeclampsia was also delivered at earlier gestational ages, and had lower mean birth weights compared with the control group.
Table 1.
Demographic characteristics of the study population.
| Control N=410 |
Preeclampsia N=42 |
p-value | |
|---|---|---|---|
| Mean age (± SD) | 31.6 (5.6) | 30.2 (6.4) | 0.18 |
|
| |||
| Race | |||
| White (%) | 237 (57.8) | 17 (40.4) | |
| Black (%) | 113 (27.6) | 21 (50.0) | 0.03 |
| Hispanic (%) | 10 (2.4) | 0 (0) | |
| Asian (%) | 37 (9.0) | 2 (4.8) | |
| Others (%) | 13 (3.2) | 2 (4.8) | |
|
| |||
| Smoking (%) | 32 (7.8) | 7 (16.7) | 0.05 |
|
| |||
| Mean BMI (±SD) | 28.0 (7.2) | 34.0 (8.9) | <0.001 |
|
| |||
| Nulliparous (%) | 163 (39.8) | 20 (47.6) | 0.32 |
|
| |||
| Chronic hypertension (%) |
27 (6.6) | 15 (35.7) | <0.001 |
|
| |||
| Pre-gestational diabetes (%) |
25 (6.1) | 10 (23.8) | <0.001 |
|
| |||
| Mean gestational age (weeks) at ultrasound (± SD) |
12.1 (0.6) | 12.1 (0.7 | 0.89 |
|
| |||
| Mean gestational age(weeks) at delivery (± SD) |
38.2 (3.8) | 35.4 (4.1) | <0.001 |
|
| |||
| Mean birth weight (grams, ± SD) |
3265.7 (692.6) | 2581.6 (867.3) | <0.001 |
SD-= standard deviation.
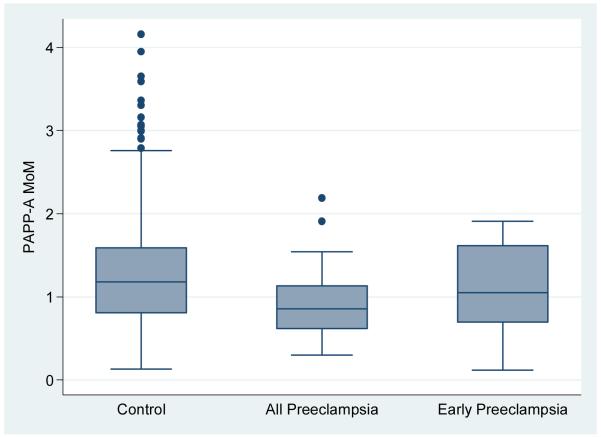
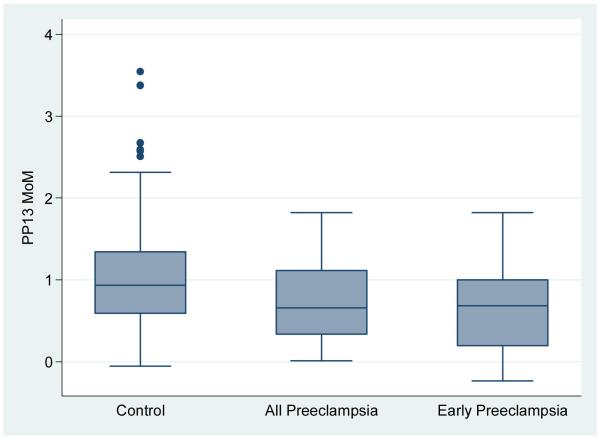
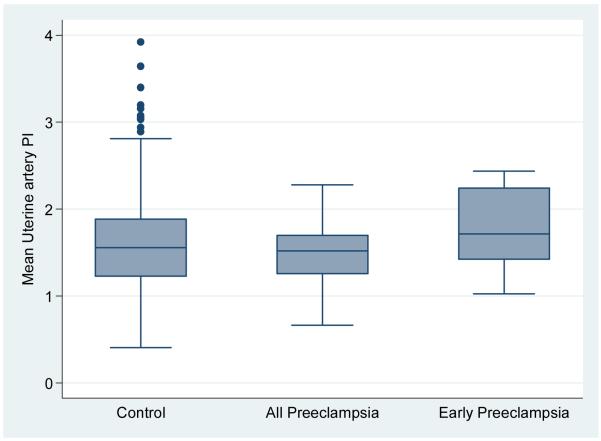
The PP13, PAPP-A and mean uterine artery levels were found to be normally distributed. The MoM for PP13, mean uterine artery PI and PAPP-A categorized by all preeclampsia, early preeclampsia versus the control groups is shown in Table 2 and depicted in Figures 1A, B and C. The median PP13 MoM was statistically significantly lower in the two groups with preeclampsia compared with the control group. Similarly, the PAPP-A MoM were lower in the groups with preeclampsia compared with the control group, but only statistically significant when all cases with Preeclampsia were combined. The median uterine artery MoM was higher in the group with early preeclampsia compared with any of the other groups but this was not statistically significant.
Table 2.
Median distribution of PP13, PAPP-A and uterine artery PI for the study population.
| Control (n=410) | All Preeclampsia (n=42) |
Early Preeclampsia (n=12) |
|
|---|---|---|---|
| PP13 (median, IQR) |
0.98 (0.59-1.34) | 0.66 (0.34- 1.11)* |
0.68 (0.18- 1.00)* |
| PAPP-A (median, IQR) |
1.18 (0.81-1.59) | 0.85 (0.62- 1.13)* |
1.05 (0.69-1.61) |
| Uterine artery PI (median, IQR) |
1.55 (1.23-1.88) | 1.52 (1.25-1.70) | 1.72 (1.42-2.24) |
IQR= interquartile range.
p<0.05 when compared to the control group.
Figure 1A.
box plot of PAPP-A in control group compared with all cases with preeclampsia and early preeclampsia.
Figure 1B.
box plot of PP13 in control group compared with all cases with preeclampsia and early preeclampsia.
Figure 1C.
box plot of mean uterine artery PI in control group compared with all cases with preeclampsia and early preeclampsia.
The logistic regression models adjusted for confounders identified from Table 1 with the final model including only the significant maternal characteristics. The ROC curves, AUC and sensitivity for the prediction of all cases of preeclampsia as well as cases of early preeclampsia are shown in Tables 3 and 4. We chose an assignment of 20% false positive rate (FPR) for our subsequent model performance description. PP13 could identify 49% of all preeclampsia and 79% of early onset preeclampsia. When combined with uterine artery Doppler, the detection rate for all or early preeclampsia did not significantly change (50% and 78%, respectively).
Table 3.
Screening performance of PP13, PAPP-A and uterine artery Doppler for all cases of preeclampsia.
| Marker | AUC (95% CI) |
p-value | Sensitivity for fixed false positive rates (FPR) | ||
|---|---|---|---|---|---|
| 5% | 10% | 20% | |||
| PP13 | 0.74 (0.66- 0.83) |
<0.001 | 0.30 | 0.45 | 0.49 |
| PAPP-A | 0.77 (0.63- 0.81) |
<0.001 | 0.21 | 0.50 | 0.58 |
| Mean uterine artery PI |
0.74 (0.61- 0.81) |
<0.001 | 0.21 | 0.51 | 0.62 |
| PP13 + Mean uterine artery PI |
0.75 (0.62- 0.82) |
<0.001 | 0.30 | 0.45 | 0.50 |
| PAPP-A + Mean uterine artery PI |
0.77 (0.63- 0.81) |
<0.001 | 0.25 | 0.45 | 0.64 |
| PP13 + PAPP-A |
0.76 (0.62- 0.82) |
<0.001 | 0.30 | 0.42 | 0.50 |
| PP13 + PAPP-A + Mean uterine artery PI |
0.77 (0.63- 0.81) |
<0.001 | 0.35 | 0.48 | 0.60 |
Table 4.
Screening performance of PP13, PAPP-A and uterine artery Doppler for all cases of preeclampsia delivered <34 weeks.
| Marker | AUC (95% CI) |
p-value | Sensitivity for fixed false positive rates (FPR) | ||
|---|---|---|---|---|---|
| 5% | 10% | 20% | |||
| PP13 | 0.85 (0.69- 1.00) |
<0.001 | 0.56 | 0.68 | 0.79 |
| PAPP-A | 0.80 (0.66- 0.99) |
<0.001 | 0.50 | 0.59 | 0.68 |
| Mean uterine artery PI |
0.77 (0.68- 0.98) |
<0.001 | 0.59 | 0.59 | 0.68 |
| PP13 + Mean uterine artery PI |
0.86 (0.69- 1.00) |
<0.001 | 0.55 | 0.68 | 0.78 |
| PAPP-A + Mean uterine artery PI |
0.80 (0.66- 0.98) |
<0.001 | 0.50 | 0.58 | 0.68 |
| PP13 + PAPP-A |
0.85 (0.69- 1.00) |
<0.001 | 0.68 | 0.68 | 0.79 |
| PP13 + PAPP-A + Mean uterine artery PI |
0.85 (0.69- 1.00) |
<0.001 | 0.68 | 0.68 | 0.79 |
The sensitivity for predicting all or early preeclampsia using PAPP-A alone was 58% and 68%, respectively. When PAPP-A is combined with uterine artery PI, the sensitivity for all preeclampsia improved to 64%, but was unchanged for early onset preeclampsia, at 68%. When PP13 and PAPP-A were combined, the sensitivity was 50% and 79% for all and early preeclampsia, respectively. The sensitivity when all markers are combined was 60% and 79%, for all and early preeclampsia, respectively. The sensitivity at lower false positive rates is also shown in both Tables 3 and 4.
Notably, the best AUC for prediction of all types of preeclampsia were seen for the models containing PAPP-A, while those for early onset preeclampsia were seen in the models containing PP13. The overlapping 95% confidence intervals for the models show that these AUC were not significantly different in their discriminating ability. The regression equations for the final models predicting preeclampsia are as below:
Using only PAPP-A: Y=−3.389 −0.716 X PAPP-A MoM (SE, 0.324) + 0.05 X BMI (SE, 0.021) + 0.319 if race is black (SE, 0.378) + 1.57 if history of chronic hypertension (SE, 0.431); R2=0.149, p<0.0001
Using only PP13: Y = −2.607 – 0.502 X PP13 MoM (SE, 0.387) + 0.759 if pre-gestational diabetic (0.556) + 0.777 if race is black (SE, 0.395) + 1.268 if history of chronic hypertension (SE, 0.468); R2= 0.111, p<0.0001
Using only Mean uterine artery PI: Y = −3.895 – 0.593 X Mean uterine artery PI (SE, 0.541) + 0.944 if pre-gestational diabetic (SE, 0.480) + 0.059 X BMI (SE, 0.021) + 1.532 if history of chronic hypertension (SE, 0.432); R2 =0.149, p=<0.0001
Using all three markers: Y= −1.308 −0.574 X PP13 MoM (SE, 0.427) −0.502 X PAPP-A MoM (SE, 0.344) – 0.643 X Mean uterine artery PI (SE, 0.598) + 0.799 if pre-gestational diabetic (SE, 0.564) + 0.664 if race is black (SE, 0.410) + 1.340 if history of chronic hypertension (SE, 0.483); R2= 0.131, p<0.0001
Final model for early onset preeclampsia: Y = −4.678 −0.443 X PP13 MoM (SE, 0.762) −0.009 X PAPP-A MoM (SE, 0.555) + 0.347 Mean uterine artery PI (SE, 0.908) + 3.059 if history of chronic hypertension (SE, 0.768); R2=0.236, p=0.0005.
Where SE = standard error.
Discussion
The data show that PP13 and PAPP-A as first trimester serum markers of placental mal-development identify pregnancies at an increased risk for development of preeclampsia in the second half of pregnancy, compared to a control population who subsequently exhibit an uncomplicated pregnancy outcome. Moreover, increased uterine artery resistance in the first-trimester, reflected by a high mean PI, also predicts an increased risk for later development of preeclampsia. Each of the three markers identify women whose pregnancies are at higher than normal risk for delivery prior to 34 weeks because of the diagnosis of early onset preeclampsia. Importantly, combinations of two or more of the first trimester assessments failed to yield an improvement in risk identification for the prediction of preeclampsia.
The strengths of our study include the prospect design and the use of a robust statistical approach to design a clinical prediction model. Although our sample size forearly onset preeclampsia was limited, our study buttresses others with larger numbers,especially of early onset preeclampsia, tobuild support for the premise that first trimester screening can identify populations at substantial increased risk for development of preeclampsia, enriching the population that might then be enrolled inrandomized clinical trials to test therapies for prophylaxis of this condition.
Our findings that the PI from the first trimester uterine Doppler evaluation predicts women at increased risk for preeclampsia are similar to results reported by Spencer et al. (7) who used second trimester uterine artery Doppler assessments with similar sensitivity and area under the curve to predict 44 cases of early onset preeclampsia,compared with 446 controls. Khalil et al also reported similar findings to ours in their nested case-control study comparing 42 women who developed preeclampsia to 210 controls (9). The later study however also included pulse wave analysis in their combined model and had a higher sensitivity of 85.7% for a 10% FPR. Our findings are different from those of Nicolaides et al (17) who studied only 10 cases with early preeclampsia and found an improvement in sensitivity when PP13 was combined with first-trimester uterine artery Doppler assessments. On the other hand, Akolekar et al found PP13 levels to be significantly lower only in cases that developed early onset preeclampsia (10).The sensitivity of PAPP-A and PP13 reported in this study for predicting preeclampsia at FPR of 5% and 10% (Tables 3 and 4) are higher than those reported in a recent study which evaluated early onset preeclampsia in 88 cases compared with 480 controls (4). The later study used a traditional parsimonious approach to model building that has been used in Down syndrome and neural tube defect screening unlike the present study which used a logistic regression approach with the advantage of being able to adjust for confounding in the population. It is still uncertain which approach is best.
The lack of improvement above single marker sensitivity when multiple markers are combined likely reflects that the parameters studied are involved in the same pathway of maternal decidual modification in early pregnancy; thus, the markers identify pregnancies at risk for a selected phenotype within the spectrum of the preeclamptic syndrome, one involving the dysregulated change in maternal vascular blood flow to the chorioallantoic placenta. For example, PAPP-A cleaves insulin-like growth factor binding proteins4 and 5 (18). This protease activity modulates the activity of insulin-like growth factors1 and 2 to alter the development of the implantation site in the maternal decidua where vessels are normally modified. PP13 is a member of the galectin family of proteins and contributes to immune tolerance at the maternal-fetal interface (9). The origin of preeclampsia is now considered multifaceted as reported by the NIH Consensus report (15) and the end organ similarities commonly evolve only when the clinical phenotype manifests hypertension and proteinuria, these findings suggest the need for further research with the goal of identifying markers that predict other pathways to preeclampsia. Studies on placental pathology and morphology as they relate to other analytes reflective of placenta dysfunction will shed light on novel markers (19,20).
Our study is not without limitations, including the sample size was small, especially with regards to cases with early preeclampsia mentioned above. We opted not to use a different method for analyzing PP13 after the difficulty obtaining further supplies of the same kit in order to avoid the possibility of inconsistent results (3). We were therefore unable to adjust include many variables in the regression models to avoid over-fitting. Despite this limitation, our findings are comparable to others with larger numbers with early preeclampsia.
In conclusion, our study confirm the potential role of PP13, PAPP-A and uterine artery PI as predictors of preeclampsia. However, more studies devoted to identifying robust makers with potential additive effects when combined are needed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lyell DJ, Lambert-Messerlian GM, Giudice LC. Prenatal screening, epidemiology, diagnosis, and management of preeclampsia. Clin Lab Med. 2003;23:413–42. doi: 10.1016/s0272-2712(03)00027-1. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM, Cooper DW. Pathogenesis and genetics of preeclampsia. Lancet. 2001;357:53–6. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 3.Cowans NJ, Stamatopoulou A, Khalil A, Spencer K. PP13 as a marker of pre-eclampsia: A two platform comparison study. Placenta. 2011 Feb;32(Suppl):S37–41. doi: 10.1016/j.placenta.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Wortelboer EJ, Koster MP, Cuckle HS, Stoutenbeek PH, Schielen PC, Visser GH. First-trimester placental protein 13 and placental growth factor: markers for identification of women destined to develop early-onset pre-eclampsia. BJOG. 2010 Oct;117(11):1384–9. doi: 10.1111/j.1471-0528.2010.02690.x. [DOI] [PubMed] [Google Scholar]
- 5.ROMERO R, KUSANOVIC JP, THAN NG, EREZ O, GOTSCH F, ESPINOZA J, EDWIN S, CHEFETZ I, GOMEZ R, NIEN JK, SAMMAR M, PINELES B, HASSAN SS, MEIRI H, TAL Y, KUHNREICH I, PAPP Z, CUCKLE HS. First-trimester maternal serum PP13 in the risk assessment for preeclampsia. Am J Obstet Gynecol. 2008;199:122 e1–122 e11. doi: 10.1016/j.ajog.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CHAFETZ I, KUHNREICH I, SAMMAR M, TAL Y, GIBOR Y, MEIRI H, CUCKLE H, WOLF M. First-trimester placental protein 13 screening for preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2007;197:35 e1–7. doi: 10.1016/j.ajog.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 7.SPENCER K, COWANS NJ, CHEFETZ I, TAL J, MEIRI H. First-trimester maternal serum PP-13, PAPP-A and second-trimester uterine artery Doppler pulsatility index as markers of pre-eclampsia. Ultrasound Obstet Gynecol. 2007;29:128–34. doi: 10.1002/uog.3876. (2007) [DOI] [PubMed] [Google Scholar]
- 8.SPENCER K, COWANS NJ, NICOLAIDES KH. Low levels of maternal serum PAPP-A in the first trimester and the risk of pre-eclampsia. Prenat Diagn. 2008;28:7–10. doi: 10.1002/pd.1890. [DOI] [PubMed] [Google Scholar]
- 9.Than NG, Pick E, Bellyei S, Szigeti A, Burger O, Berente Z, Janaky T, Boronkai A, Kliman H, Meiri H, Bohn H, Than GN, Sumegi B. Functional analyses of placental protein 13/galectin-13. Eur J Biochem. 2004;271:1065–78. doi: 10.1111/j.1432-1033.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 10.SEKIZAWA A, PURWOSUNU Y, YOSHIMURA S, NAKAMURA M, SHIMIZU H, OKAI T, RIZZO N, FARINA A. PP13 mRNA expression in trophoblasts from preeclamptic placentas. Reproductive Sciences. 2009;16:408–13. doi: 10.1177/1933719108328615. [DOI] [PubMed] [Google Scholar]
- 11.Khalil A, Cowans NJ, Spencer K, Goichman S, Meiri H, Harrington K. First-trimester markers for the prediction of pre-eclampsia in women with a-priori high risk. Ultrasound Obstet Gynecol. 2010;35(6):671–9. doi: 10.1002/uog.7559. [DOI] [PubMed] [Google Scholar]
- 12.Akolekar R, Syngelaki A, Beta J, Kocylowski R, Nicolaides KH. Maternal serum placental protein 13 at 11-13 weeks of gestation in preeclampsia. Prenat Diagn. 2009;29(12):1103–8. doi: 10.1002/pd.2375. [DOI] [PubMed] [Google Scholar]
- 13.Stamatopoulou A, Cowans NJ, Matwejew E, von Kaisenberg C, Spencer K. Placental Protein-13 and Pregnancy-Associated Plasma Protein-A as First Trimester Screening Markers for Hypertensive Disorders and Small for Gestational Age Outcomes. Hypertens Pregnancy. 2010 Aug 11; doi: 10.3109/10641955.2010.484081. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.American College of Obstetricians and Gynecologists . Diagnosis and management of preeclampsia and eclampsia. The College; Washington, DC: 2002. ACOG practice bulletin no. 33. [Google Scholar]
- 15.Report of the National High Blood Pressure Education Program Working Group. Report on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 16.Mikolajczyk RT, DiSilvestro A, Zhang J. Evaluation of logistic regression reporting in current obstetrics and gynecology literature. Obstet Gynecol. 2008;111(2 Pt 1):413–9. doi: 10.1097/AOG.0b013e318160f38e. [DOI] [PubMed] [Google Scholar]
- 17.Nicolaides KH, Bindra R, Turzan OM, Cefetz I, Sammar M, Meiri H, Tal J, Cuckle HS. A novel approach to first trimester screening for early pre-eclampsia combining PP-13 and Doppler ultrasound. Ultrasound Obstet Gynecol. 2006;27:13–17. doi: 10.1002/uog.2686. [DOI] [PubMed] [Google Scholar]
- 18.Laursen LS, Overgaard MT, Søe R, Boldt HB, Sottrup-Jensen L, Giudice LC, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001 Aug 24;504(1-2):36–40. doi: 10.1016/s0014-5793(01)02760-0. [DOI] [PubMed] [Google Scholar]
- 19.Odibo AO, Zhong Y, Longtine M, Tuuli M, Odibo L, Cahill AG, Macones GA, Nelson DM. First-trimester serum analytes, biophysical tests and the association with pathological morphometry in the placenta of pregnancies with preeclampsia and fetal growth restriction. Placenta. 2011 Apr;32(4):333–8. doi: 10.1016/j.placenta.2011.01.016. Epub 2011 Feb 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayhew TM. Stereology and the placenta: where’s the point? -- a review. Placenta. 2006 Apr;27(Suppl A):S17–25. doi: 10.1016/j.placenta.2005.11.006. [DOI] [PubMed] [Google Scholar]