Introduction
Ginger, Zingiber officinale Roscoe (Zingiberaceae), is used worldwide not only as a spice in food, but also in various traditional systems of medicine against different ailments such as arthritis, rheumatism, infectious diseases, and vomiting (Afzal et al., 2001; Ali et al., 2008). The chemistry of ginger has been extensively investigated with over 100 compounds identified in both fresh and dried samples (Jolad et al., 2004; Jolad et al., 2005). Ginger's numerous biological activities have been attributed mainly to the gingerols and shogaols, the major pungent principles found in both fresh and dried ginger rhizomes (Funk et al., 2009; Lantz et al., 2004).
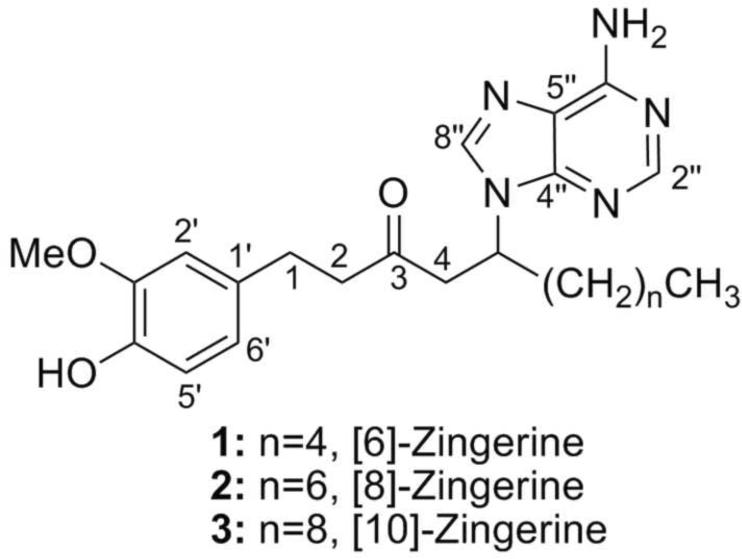
In the present article, we describe the isolation and structural determination of three novel purine- containing compounds (1–3) from dry powdered ginger rhizomes using a new phase-trafficking based method (Araya et al., 2010). The methanolic rhizome extract was subjected to our separation protocol using ion-exchange resins allowing for fast and selective resolution of the basic, acidic, and neutral components. Solid-phase reagents (SSR) are often used to facilitate and speed-up purification procedures in organic synthesis, especially in combinatorial chemistry (Flynn, 1999; Solinas and Taddei, 2007). However, application of SSR to natural products research has been limited (Alexandratos, 2009; Vanrensen and Veit, 1995; Zhao et al., 2008). Using this approach, we were able to isolate and identify three new nitrogenous compounds from ginger that were named [6]-, [8]-, and [10]-zingerines, as they are 5-(6-amino-9H-purin-9-yl) analogs of [6]-, [8]-, and [10]-gingerols respectively. Although present in selected microbial and marine products, secondary metabolites that contain a purine ring attached to a non-carbohydrate carbon skeleton are very rare in higher plants (Rosemeyer, 2004; Wang et al., 2008).
1. Results and Discussion
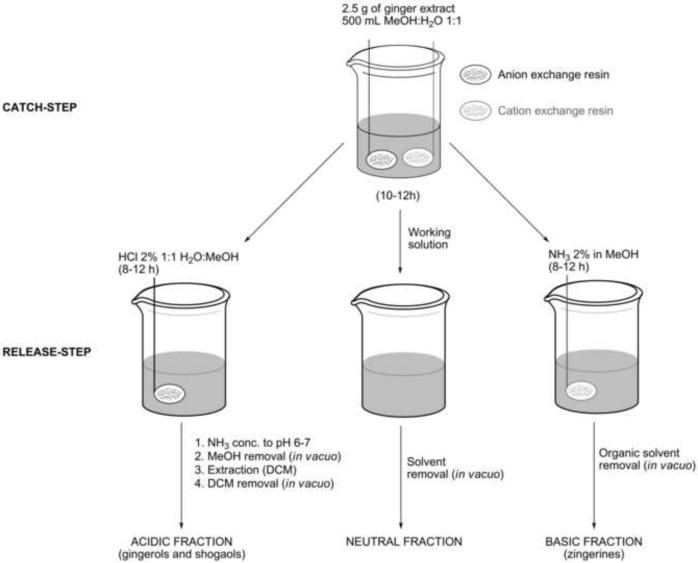
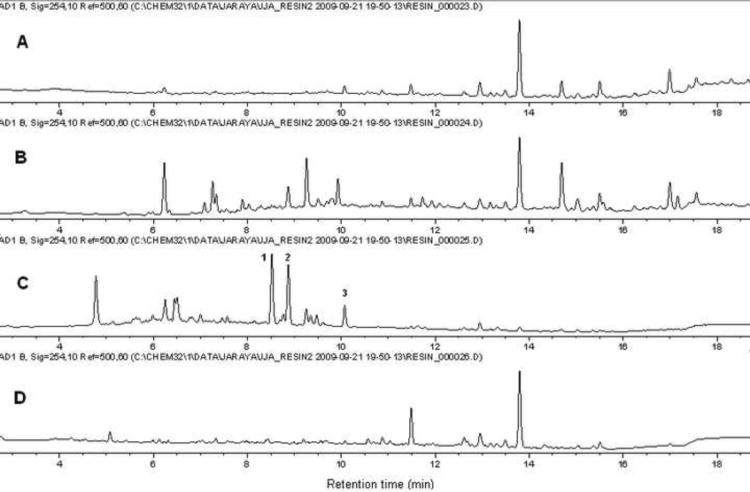
A methanolic extract of ginger rhizomes was submitted to the separation scheme illustrated in Fig. 2 in order to explore the scope of catch-and-release methodology for natural products resolution. This allows for quick and solvent sparing simultaneous separation of basic, acidic, and neutral components from a mixture taking advantage of ionic-exchange resins physically confined into spatially separated tea bags (Araya et al., 2010). As expected, the phenolic compounds, including the main gingerols and shogaols, were trapped by the basic resin (Fig. 3). Interestingly, the LCMS traces of the basic fraction revealed the presence of several compounds with odd molecular weight values, which prompted us to investigate further the nature of such components because, to our knowledge, no nitrogen-containing basic metabolites have been reported from ginger to date.
Fig. 2.
General catch-and-release protocol scheme
Fig. 3.
HPLC trace (UV, 254 nm) of (A) methanolic extract, (B) acidic fraction, (C) basic fraction, and (D) neutral fraction.
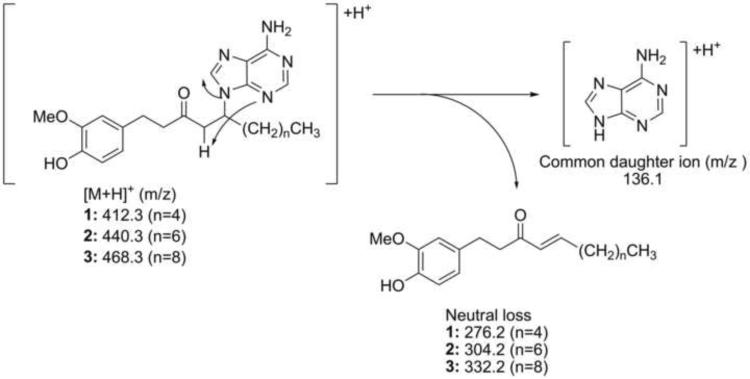
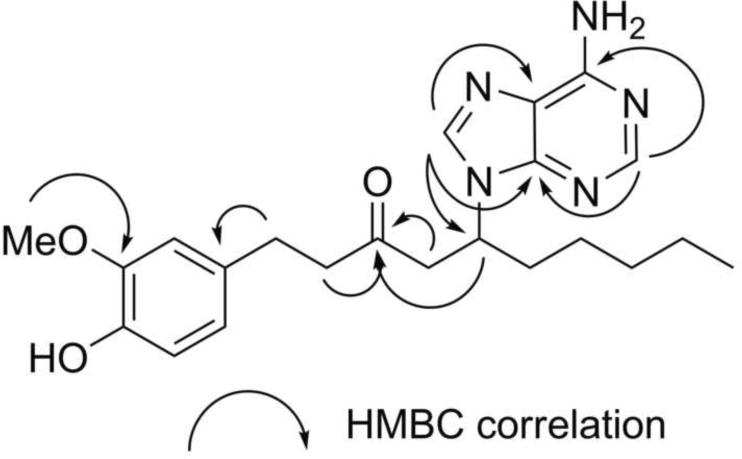
Initially, our small-scale separation protocol allowed for the isolation of main compound 1 from the basic fraction after two additional purification steps: column chromatography on Sephadex LH-20 followed by semi-preparative reverse phase HPLC. The HRMS m/z 412.2362 [M+H]+ (calc. for C22H30N5O2 412.2349) was consistent with the presence of five nitrogen atoms in the molecular formula. The ESIMS/MS showed the neutral loss of m/z 276.2, leaving the stable charged purine fragment of m/z 136.1 (Fig. 4). Furthermore, the structure of 1 was deduced based on analyses of the 1H, 13C NMR, 1H-1H COSY, HSQC and HMBC spectra (Supplementary Fig. 1–4) and supported by IR and UV spectra. First, the splitting pattern and coupling constants of the aromatic protons in the 1H NMR δH 6.63 (d, J=2.0 Hz, H-2'), 6.59 (d, J=8.0 Hz, H-5') and 6.44 (dd, J=8.0, 2.2 Hz, H-6') were consistent with a 1,2,4-trisubstituted benzene ring system and the corresponding aromatic carbons δC 133.7 (C-1'), 113.0 (C-2'), 148.9 (C-3'), 145.8 (C-4'), 116.2 (C-5') and 121.6 (C-6') were readily assigned with the assistance of correlations observed in the HSQC and HMBC spectra (Fig. 5). Second, the attachments of the methoxy group δH 3.76 (s, 3'-OCH3) to C-3' and methylene group δH 2.67 (m, H-1) to C-1' were established based on the correlations observed in the HMBC spectrum (Fig. 5). Third, the chemical shift of aromatic carbon C-4' (s, δC 145.8) suggested that this position was oxygenated. Analogous to [6]-gingerol, two spin systems corresponding to a decanyl chain were identified in compound 1 based on 1H-1H COSY, HSQC and HMBC spectra, showing two distinctive structural features: a ketone group δC 209.6 (s, C-3) and a methine proton δH 4.91 (m, H-5). Finally, with only two proton signals left δH 8.16 (brs, H-4”) and 8.12 (brs, H-8”), five nitrogen atoms, and five carbon signals δC 153.4 (C-2”), 150.6 (C-4”), 120.4 (C-5”), 157.3 (C-6”), and 142.6 (C-8”); the presence of an adenine ring was proposed and supported by ESIMS/MS fragmentation and HMBC spectra (Fig. 5). Furthermore, adenine substitution at the C-5 position explained satisfactorily the shift to lower filed of H-5 (m, δH 4.91) when compared with the corresponding signal of [6]-gingerol (Kim et al., 2008). This assignment was confirmed by synthesis (vide infra). After scaling-up the separation protocol, compounds 2 and 3 were isolated showing similar fragmentation patterns in the ESIMS/MS, almost identical low field signals in 1H- and 13C-NMR spectra (Table 1), and differing from compound 1 only in the number of methylene groups in the aliphatic region. Accordingly, their structures 2 and 3 were assigned relative to that previously described for compound 1.
Fig. 4.
Proposed fragmentation of compounds 1–3
Fig. 5.
Selected HMBC correlations of observed for compound 1
Table 1.
1H-NMR Spectroscopic data (500 MHz, CD3OD) for zingerines (1–3)a
| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
|
|
||||||
| δC (mult) | δH (J in Hz) | δ C | δH (J in Hz) | δC (mult) | δH (J in Hz) | |
| 1 | 30.4 t | 2.67 m | 30.4 t | 2.68 m | 30.4 t | 2.67 m |
| 2 | 45.7 t | 2.67 m | 45.7 t | 2.68 m | 45.7 t | 2.67 m |
| 3 | 209.6 s | - | 209.6 s | - | 209.6 s | - |
| 4a | 47.7 t | 3.39 dd (17.6, 8.8) | 47.8 t | 3.40 dd (17.8, 8.9) | 47.8 t | 3.39 dd (17.9, 8.7) |
| 4b | 3.05 dd (17.6, 4.7) | 3.05 dd (17.8, 4.8) | 3.05 dd (17.9, 4.9) | |||
| 5 | 54.0 d | 4.91 m | 54.0 d | 4.92 m | 54.0 d | 4.92 m |
| 6a | 35.1 t | 1.82 m | 35.1 t | 1.80 m | 35.1 t | 1.80 m |
| 6b | 2.08 m | 2.08 m | 2.08 m | |||
| 7a | 32.3 t | 0.98 m | 32.9 t | 1.24 m | 33.2 t | 1.24 m |
| 7b | 1.20 m | |||||
| 8 | 26.8 t | 1.20 m | 30.2 t | 1.24 m | 30.6 t | 1.24 m |
| 9 | 23.5 t | 1.20 m | 30.1 t | 1.24 m | 30.5 t | 1.24 m |
| 10 | 14.3 q | 0.81 t (6.9) | 27.1 t | 1.24 m | 30.5 t | 1.24 m |
| 11 | - | - | 23.7 t | 1.24 m | 30.0 t | 1.24 m |
| 12 | - | - | 14.5 q | 0.84 t (7.0) | 27.1 t | 1.24 m |
| 13 | - | - | - | - | 23.8 t | 1.24 m |
| 14 | - | - | - | - | 14.6 q | 0.87 t (7.0) |
| 1' | 133.7 s | - | 133.7 s | - | 133.7 s | - |
| 2' | 113.0 d | 6.63 d (2.0) | 113.0 d | 6.63 d (1.8) | 113.0 d | 6.63 d (1.9) |
| 3' | 148.9 s | - | 148.9 s | - | 148.9 s | - |
| 4' | 145.8 s | - | 145.9 s | - | 145.9 s | - |
| 5' | 116.2 d | 6.59 d (8.0) | 116.2 d | 6.59 d (7.9) | 116.2 d | 6.59 d (7.9) |
| 6' | 121.6 d | 6.44 dd (8.0, 2.0) | 121.6 d | 6.45 dd (7.9, 1.8) | 121.7 d | 6.45 dd (7.9, 1.9) |
| 2” | 153.4 s | 153.5 s | - | 153.5 s | - | |
| 4” | 150.6 d | 8.16 br s | 150.7 d | 8.16 br s | 150.7 d | 8.16 br s |
| 5” | 120.4 s | - | 120.4 s | - | 120.5 s | - |
| 6” | 157.3 s | - | 157.4 s | - | 157.4 s | - |
| 8” | 142.6 d | 8.126 br s | 142.7 d | 8.12 br s | 142.6 d | 8.12 br s |
| 3'-OMe | 56.4 q | 3.76 s | 56.4 q | 3.76 s | 56.4 q | 3.76 s |
br = broad; s = singlet; d = doublet; dd = doublet of doublets; q = quartet; m = multiplet.
Although purine is the most widely distributed N-heterocyclic ring in nature, purine-containing plant secondary metabolites are not common (Rosemeyer, 2004). Simple purine alkaloids, like caffeine or theanine, have a limited taxonomic distribution in the plant kingdom and a purine ring attached to a non-carbohydrate carbon skeleton, as in the case of compounds 1–3, is an unusual structural feature. Among the limited reports of this class of natural products are the cucurbitane triterpenoids isolated from Cucurbita pepo cv dayangua (Wang et al., 2008), alachalasine F–G from the fungus Podospora vesticola (Zhang et al., 2008, 2009), and a limited number of examples from marine sponges (Appenzeller et al., 2008; Wright et al., 2009; Yosief et al., 2000).
To the best of our knowledge, compounds 1–3 have not been previously reported in the literature and they represent the first basic nitrogen-containing secondary metabolites in ginger. We named this family of compounds zingerines, as they are 5-(6-amino-9H-purin-9-yl) analogs of gingerols. Compounds 1–3 were detected in the original extract prior any purification step using targeted LCMS analysis as well as in two extracts prepared from additional commercial samples of ginger rizhomes (Supplementary Fig. 9–12). The results showed a strong evidence that the three new compounds were not likely to be artifacts of our new isolation process. Interestingly, we unsuccessfully attempted to obtain zingerines using classic liquid/liquid partition protocols (data not shown) presumably due to the acidic phenolic group they contain that makes these compounds amphoteric and thus soluble in the aqueous phase under basic conditions. We believe that the amphoteric behavior of the zingerines coupled with their strong adsorption on silica gel are the two main reasons that have prevented identification and isolation of these compounds in previous phytochemical studies of ginger.
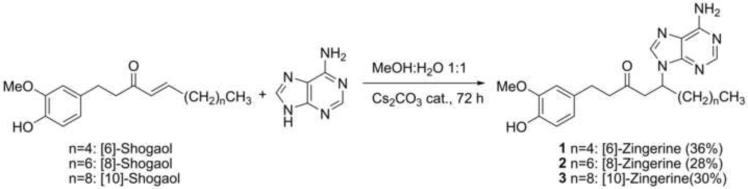
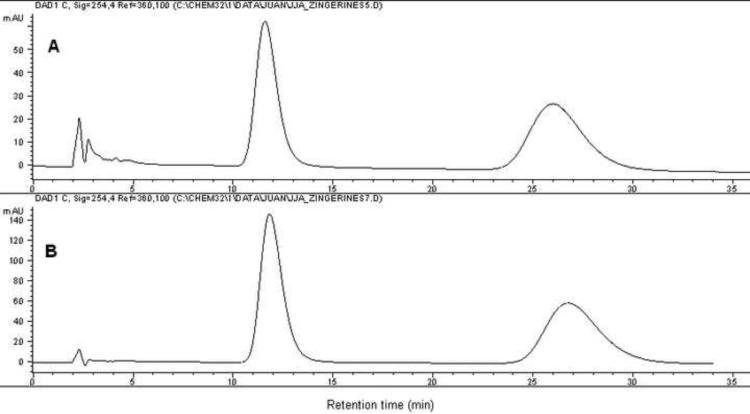
The presence of zingerines in the dried ginger rhizome extract could in theory arise from a Michael-type reaction between shogaols, dehydration products of gingerols during drying of ginger rizhome, and adenine. To explore the feasibility of such a reaction, we dissolved an equimolar mixture of [6]-shogaol and adenine in a mixture of 1:1 MeOH:H2O and stirred the reaction mixture during 72 h at room temperature. The formation of [6]-zingerine was only detected by means of LCMS analysis, but not using UV detection, suggesting that only minute quantities of the compound is produced under those conditions (Supplementary Fig. 13–14). As expected, basic and acidic catalysis increased the yield of the desired product. Using basic conditions, the reaction was scaled-up to 90 mg of [6]-shogaol and 45.2 mg (1.2 eq.) of adenine under agitation for 72 h at room temperature to obtain compound 1 in 36% isolated yield. Similarly, [8]-shogaol (50 mg) and [10]-shogaol (50 mg) were subjected to identical reaction conditions thereupon we obtained compounds 2 and 3 in 28% and 30% isolated yield, respectively (Fig. 6). The synthetically obtained products were identical to the ones isolated from ginger plant material by means of 1H- and 13C-NMR spectra (Supplementary Fig. 15–20) and HPLC traces (Supplementary Fig. 21–23). This confirmed the proposed structures of the zingerines. In addition, both natural and synthetic samples were optically inactive demonstrating that the compounds were racemic mixtures. This was confirmed by means of chiral HPLC resolution of both synthetic and natural [6]-zingerine, showing two peaks with 1:1 area under the curve (AUC) ratio (Fig. 7). Although natural [6]-zingerine was not optically active, an enzymatic origin cannot be ruled out definitively because of the possible operation of a retro Michael/Michael type reaction equilibrium leading to racemization under experimental conditions. Furthermore, identification of biosynthetic genes and their enzymatic products responsible for the origin of zingerines would definitely exclude an artificial genesis. Although gingerols are present in relatively high quantities in ginger rhizome, we were unable to detect free adenine in the initial extract or basic fraction using LCMS analysis (data not shown).
Fig. 6.
Synthesis of compounds 1–3
Fig. 7.
HPLC chiral resolution of [6]-zingerine (1) (A) isolated from ginger rhizome and (B) obtained by synthesis.
2. Conclusions
The use of our new catch-and-release methodology allowed us to isolate and identify three compounds new to the literature from extensively studied ginger rhizomes. The new compounds contain an adenine group attached to the gingerol-type carbon skeleton, an unusual structural feature in higher plants. Experiments showed that traditional solvent partition/chromatographic methods would have missed these alkaloids. We believe that catch-and-release methodology can be applied more widely to natural products resolution not only rapidly and conveniently to prepare high quality samples for high throughput screening campaigns, but also for isolation and structural determination of novel compounds present as very minor components as demonstrated in this report. Biological testing of the newly isolated compounds is currently underway and will be reported in due course.
3. Experimental section
3.1. General Procedures
Melting points were recorded with an OptiMelt automatic apparatus. Optical rotations were measured with a Rudolph RS Autopol IV automatic polarimeter. IR spectra were obtained with a Thermo Nicolet Avatar 380 FT-IR. UV-Vis measurements were conducted with a Varian Cary 50 UV-Vis spectrophotometer. 1H-NMR, 13C-NMR and two dimensional spectra were recorded with a Bruker Avance AV-III 500 with a dual carbon/proton cryoprobe. Agitation of samples was carried out with a New Brunswick Scientific Excella E1 platform shaker. Semi-preparative HPLC was conducted using an Agilent 1200 HPLC system with a Phenomenex Luna C18 column (5μm, 250×10 mm), flow rate of 4.5 mL/min (approx. 160 bar), injection volume of 50 μL (ca. 10 mg sample), and UV detection using diode array. Preparative HPLC separations were done using an Agilent 1100 HPLC system with a Varian Dynamax C8 (8 μm, 250×21.4 mm), flow rate of 15 mL/min (aprox. 35 bar), injection volume of 800 μL (ca. 100 mg sample), and UV detection using diode array.
Chiral HPLC analysis was performed using a Chiralcel OD-H column (No. ODHOCD-LH013) purchased from Chiral Technologies Inc. (West Chester, PA) in an Agilent 1100 system. The solvent system used was isocratic hexanes:isopropanol 70:30 (v/v), the flow rate was 0.9 mL/min, the injection volume was 25 μL, and UV detection at 254 nm.
Compounds and resins were purchased from Sigma-Aldrich (St. Louis, MO): adenine 99%; cesium carbonate 99%, hydrochloric acid; Dowex™ Marathon™ WBA Anion-Exchange resin); Dowex™ MAC-3 ion exchange resin; Sephadex LH-20. RP-TLC plates (C-18) were obtained from Sorbent Technologies (Atlanta, GA). Aromatreu® Finum tea filters were purchased from www.cheftools.com
3.2. Plant Material and Extraction
Dry powdered ginger rhizomes (150 kg) were purchased from Naturex (South Hackensack, NJ) Lot. # E99/03/B8. Plant material (40 kg) was subjected to a first extraction using CH2Cl2 (130 L) twice during 48 h. Removal of the organic solvent under reduced pressure afforded 1.7 kg of CH2Cl2 extract (4.3 %). The remaining plant material was then subjected to a second extraction using MeOH (130 L) twice during 48h. After concentration under reduce pressure, 1.2 kg of MeOH extract residue was obtained (3.0 %). In the same way, two smaller samples (100 g) of ginger powder obtained from the same company (Lots # E99/01/B8 and #E94/01/B8) were extracted sequentially with CH2Cl2 (500 mL) and MeOH (500 mL) to afford CH2Cl2 and methanolic extract respectively.
3.3. General catch-and-release procedure
The procedure was carried out as described previously (Araya et al., 2010) using 5.0 g of MeOH extract. The resulting basic, acidic and neutral fractions were analyzed by LCMS and purified by semi-preparative HPLC.
3.4. Scale-up catch-and-release procedure
The methanolic rhizome extract (100 g) was suspended in 2 L of MeOH:H2O 1:1 mixture (v/v). A total of 200 g of prewashed Dowex MAC-3 resin was added to the mixture and left stirring overnight. The resin was then filtered off, washed several times with pure MeOH (until the filtrate was clear) and the resin was finally left overnight stirring in 2 L of NH3 2% in MeOH. The resin was filtered off, and filtrate concentrated under reduced pressure to afford 1.9 g of basic fraction residue (1.9 %).
3.5. Isolation of Zingerines
The basic fractions from the general catch-and-release protocol were then separated by Sephadex LH-20 column chromatography (100 g) using MeOH as eluent to give 80 fractions (10 mL each) that were combined into 7 fractions (A–G) according to RP-TLC. Fraction F (210 mg) was then purified using semipreparative HPLC with a linear gradient program of acetonitrile and water from 25:75 (v/v) (t=0 min) to 35:65 (t=5 min), then 55:45 (t=15 min), 100:0 (t=25 min), and finally recovery to 25:75 (t=35 min). Compound 1 (18.2 mg, tR =14.7 min), compound 2 (5.2 mg, tR = 19.1 min), and compound 3 (4.8 mg, tR = 22.5 min) were obtained after removal of the mobile phase under reduce pressure.
3.6. Large Scale Isolation of Shogaols
Pure [6]-, [8]-, and [10]-shogaol were isolated as described previously and the structures of the shogaols were confirmed by spectroscopic methods (Kim et al., 2008; Sang et al., 2009).
3.7. [6]-Zingerine (1)
5-(6-Amino-9H-purin-9-yl)-1-(4-hydroxy-3-methoxyphenyl)decan-3-one; pale yellow oil; [α]D25=−0.004 (c. 0.03, MeOH); UV (MeOH, c=0.5 mM) λmax nm: 208, 262; IR ν cm−1: 3326 (N-H), 3153 (N-H), 1711 (conj. >C=O), 1514 (C=C); 1H NMR(CD3OD, 500 MHz) and 13C NMR (CD3OD, 133 MHz) see Table 1; ESIMS m/z 412.3 [M+H]+; ESIMS/MS m/z 136.1 [M+H-276.2]; HRMS m/z 412.2362 [M+H]+ (calc. for C22H30N5O2 412.2349).
3.8. [8]-Zingerine (2)
5-(6-Amino-9H-purin-9-yl)-1-(4-hydroxy-3-methoxyphenyl)dodecan-3-one; pale yellow oil; [α]D25=−0.002 (c. 0.02, MeOH); UV (MeOH, c=0.5 mM) λmax nm: 208, 262; IR ν cm−1: 3325 (N-H), 3153 (N-H), 1713 (conj. >C=O), 1513 (C=C); 1H NMR(CD3OD, 500 MHz) and 13C NMR (CD3OD, 133 MHz) see Table 1; ESIMS m/z 440.3 [M+H]+; ESIMS/MS m/z 136.1 [M+H-304.2]; HRMS m/z 440.2695 [M+H]+ (calc. for C24H34N5O3 440.2662).
3.9. [10]-Zingerine (3)
5-(6-Amino-9H-purin-9-yl)-1-(4-hydroxy-3-methoxyphenyl)tetradecan-3-one; pale yellow oil; [α]D25= −0.001 (c. 0.02 MeOH); UV (MeOH, c=0.5 mM) λmax nm: 208, 262; IR ν cm−1: 3326 (N-H), 3152 (N-H), 1710 (conj. >C=O), 1513 (C=C); 1H NMR(CD3OD, 500 MHz) and 13C NMR (CD3OD, 133 MHz) see Table 1; ESIMS m/z 468.3 [M+H]+; ESIMS/MS m/z 136.1 [M+H-332.2]; HRMS m/z 468.2921 [M+H]+ (calc. for C26H38N5O3 468.2975)
3.10. HPLC MSn analyses
The on-line HPLC/MSn analyses of extracts and fractions were performed using an Agilent 1200 Series liquid chromatography system coupled to a Agilent IonTrap LCMS 6310 mass spectrometer. The positive ion ESI-MS experimental conditions were as follows: HV capillary voltage, 3.5 kV; drying temperature, 350°C; drying gas, 12.0 L/min; nebulizer, 15 psi; and capillary exit voltage, 124.8V. The Frag Ampl was set to 1.0V and the smart fragmentation function was used (Smart Frag Ampl was 30–200%). Targeted analysis of zingerines was programmed as follows: (a) for [6]-zingerine, isolation and fragmentation of ions m/z 411–413, then isolation and detection of ion m/z 136.1; (b) for [8]-zingerine, isolation and fragmentation of ions m/z 439–441, then isolation and detection of ion m/z 136.1; (c) for [10]-zingerine, isolation and fragmentation of ions m/z 467–469, then isolation and detection of ion m/z 136.1. The HPLC separations were done using an Agilent Eclipse XDB-C18 column (5μm, 4.6×150 mm) and the flow rate was 1.0 mL/min (approx. 80 bar). The mobile phase was a linear gradient of acetonitrile and water from 25:75 (v/v) (t=0 min) to 35:65 (t=5 min), then 55:45 (t=15 min), 100:0 (t=25 min), and finally recovery to 25:75 (t=35 min).
3.11. General synthesis of zingerines
A mixture of shogaol (0.33 mmol) and adenine (0.40 mmol, 1.2 eq.) and Cs2CO3 (5 mg) in MeOH:H2O 1:1 (15 mL) was stirred at room temperature for 72 h. Then, organic solvent was removed under reduced pressure and filtration, the reaction mixtures were purified by semi preparative HPLC to afford the corresponding zingerine. Accordingly, [6]-zingerine (42.7 mg), [8]-zingerine (19.4 mg), and [10]-zingerine (19.4 mg) were obtained in 36%, 28%, and 30% yields respectively.
Supplementary Material
Fig. 1.
Structure of compounds 1–3
4. Acknowledgment
The chemistry work was supported by NCCAM/ODS grant 1R21AT004182-01A2 from the National Institutes of Health (NIH) and the University of Kansas Center for Research project 2506014-910/099 to B.N.T. J.A. thanks LASPAU-Fulbright program and the University of Costa Rica for financial support. J.A. also thanks Kimberly Lovell for assistance during chiral HPLC separations. The contents are solely the responsibilities of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afzal M, Al-Hadidi D, Menon M, Pesek J, Dhami MSI. Ginger: an Ethomedical, Chemical And Pharmacological Review. Drug Metabol. Drug Interact. 2001;18:159–191. doi: 10.1515/dmdi.2001.18.3-4.159. [DOI] [PubMed] [Google Scholar]
- Alexandratos SD. Ion-Exchange Resins: A Retrospective from Industrial and Engineering Chemistry Research. Ind. Eng. Chem. Res. 2009;48:388–398. [Google Scholar]
- Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Appenzeller J, Mihci G, Martin MT, Gallard JF, Menou JL, Boury-Esnalllt N, Hooper J, Petek S, Chevalley S, Valentin A, Zaparucha A, Al-Mourabit A, Debitus C. Agelasines J, K, and L from the Solomon Islands marine sponge Agelas cf. mauritiana. J. Nat. Prod. 2008;71:1451–1454. doi: 10.1021/np800212g. [DOI] [PubMed] [Google Scholar]
- Araya JJ, Montenegro G, Mitscher LA, Timmermann BN. Application of Phase-Trafficking Methods to Natural Products Research. J. Nat. Prod. 2010;73:1568–1572. doi: 10.1021/np100465h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn DL. Phase-trafficking reagents and phase-switching strategies for parallel synthesis. Med. Res. Rev. 1999;19:408–431. doi: 10.1002/(sici)1098-1128(199909)19:5<408::aid-med7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Funk JL, Frye JB, Oyarzo JN, Timmermann BN. Comparative Effects of Two Gingerol-Containing Zingiber officinale Extracts on Experimental Rheumatoid Arthritis. J. Nat. Prod. 2009;72:403–407. doi: 10.1021/np8006183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolad SD, Lantz RC, Solyom AM, Chen GJ, Bates RB, Timmermann BN. Fresh organically grown ginger (Zingiber officinale): composition and effects on LPS-induced PGE(2) production. Phytochemistry. 2004;65:1937–1954. doi: 10.1016/j.phytochem.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Jolad SD, Lantz RC, Chen GJ, Bates RB, Timmermann BN. Commercially processed dry ginger (Zingiber officinale): Composition and effects on LPS-stimulated PGE(2) production. Phytochemistry. 2005;66:1614–1635. doi: 10.1016/j.phytochem.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Kim JS, Lee SI, Park HW, Yang JH, Shin TY, Kim YC, Baek NI, Kim SH, Choi SU, Kwon BM, Leem KH, Jung MY, Kim DK. Cytotoxic components from the dried rhizomes of Zingiber officinale Roscoe. Arch. Pharmacal Res. 2008;31:415–418. doi: 10.1007/s12272-001-1172-y. [DOI] [PubMed] [Google Scholar]
- Lantz RC, Sarihan M, Chen GJ, Timmermann B. Sites of action of compounds isolated from ginger. FASEB J. 2004;18:A99–A99. [Google Scholar]
- Rosemeyer H. The chemodiversity of purine as a constituent of natural products. Chem. Biodivers. 2004;1:361–401. doi: 10.1002/cbdv.200490033. [DOI] [PubMed] [Google Scholar]
- Sang S, Hong J, Wu H, Liu J, Yang CS, Pan M-H, Badmaev V, Ho C-T. Increased Growth Inhibitory Effects on Human Cancer Cells and Anti-inflammatory Potency of Shogaols from Zingiber officinale Relative to Gingerols. J. Agric. Food. Chem. 2009;57:10645–10650. doi: 10.1021/jf9027443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas A, Taddei M. Solid-supported reagents and catch-and-release techniques in organic synthesis. Synthesis-Stuttgart. 2007;16:2409–2453. [Google Scholar]
- Vanrensen I, Veit M. Simultaneous Determination of Phenolics and Alkaloids Using Ion Exchange Chromatography for Sample Preparation. Phytochem. Anal. 1995;6:121–124. [Google Scholar]
- Wang DC, Xiang H, Li D, Gao HY, Cai H, Wu LJ, Deng XM. Purine-containing cucurbitane triterpenoids from Cucurbita pepo cv dayangua. Phytochemistry. 2008;69:1434–1438. doi: 10.1016/j.phytochem.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Wright AE, Roth GP, Hoffman JK, Divlianska DB, Pechter D, Sennett SH, Guzman EA, Linley P, McCarthy PJ, Pitts TP, Pomponi SA, Reed JK. Isolation, Synthesis, and Biological Activity of Aphrocallistin, an Adenine-Substituted Bromotyramine Metabolite from the Hexactinellida Sponge Aphrocallistes beatrix. J. Nat. Prod. 2009;72:1178–1183. doi: 10.1021/np900183v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosief T, Rudi A, Kashman Y. Asmarines A-F, novel cytotoxic compounds from the marine sponge Raspailia species. J. Nat. Prod. 2000;63:299–304. doi: 10.1021/np9902690. [DOI] [PubMed] [Google Scholar]
- Zhang YG, Tian RR, Liu SC, Chen XL, Liu XZ, Che YS. Alachalasins AG, new cytochalasins from the fungus Stachybotrys charatum. Biorg. Med. Chem. 2008;16:2627–2634. doi: 10.1016/j.bmc.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Zhang YG, Tian RR, Liu SC, Chen XL, Liu XZ, Che YS. Alachalasins AG, new cytochalasins from the fungus Stachybotrys charatum (vol 16, pg 2627, 2008) Biorg. Med. Chem. 2009;17:428–428. doi: 10.1016/j.bmc.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Zhao RY, Yan Y, Li MX, Yan HS. Selective adsorption of tea polyphenols from aqueous solution of the mixture with caffeine on macroporous crosslinked poly(N-vinyl-2-pyrrolidinone) Reac. Funct. Pol. 2008;68:768–774. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.