Abstract
Sister chromatid cohesion and separation are two fundamental chromosome dynamics that are essential to equal chromosome segregation during cell proliferation. In this review, I will discuss the major steps that regulate these dynamics during mitosis, with an emphasis on vertebrate cells. The implications of these machineries outside of sister chromatid cohesion and separation are also discussed.
Introduction
Cellular organisms proliferate by cell division following cell growth. During these processes, cellular contents are accumulated and redistributed, symmetrically during stem cell renewal or asymmetrically during differentiation. The genetic materials, on the other hand, are always duplicated, packaged in the form of chromosome, and divided equally. The equal segregation of chromosomes is critical for maintaining genome stability during both cell proliferation and differentiation.
To execute equal chromosome segregation, elaborate and redundant mechanisms are deployed to ensure precise chromosome dynamics that are conserved across the eukaryotic domain. Among them, sister chromatid cohesion plays multiple roles during various stages of the cell division cycle. First, it provides a simple mechanism to identify pairs of sister chromatids. Second, it balances the pulling force from the opposing spindle poles. This is essential for the function of the mitotic spindle checkpoint. Third, it facilitates DNA repair in S and G2 via homologous recombination. All these events are critical for genome stability during cell divisions.
The pairing of sister chromatids was first observed by German cytologist Walter Flemming in 1885. Despite of obvious importance, it was until about a century later that mechanistic studies began to emerge. The most significant discovery is perhaps the identification of cohesin as the glue that bond sister chromatids together [1, 2]. Genetic and biochemical studies thereafter have revealed a four-step process that executes sister chromatid cohesion during the mitotic cell cycle. Cohesins are first loaded onto unreplicated chromosomes as early as telophase of the previous cell cycle. Sister chromatid cohesion is next established between duplicated chromosomes during S phase. In vertebrate cells, sister chromatid cohesion is further remodeled in early mitosis. Finally, sister chromatid cohesion is severed in anaphase to allow sister chromatid separation. Each step will be reviewed in this article, with more emphasis on recent developments in vertebrate cells.
Cohesin is a ring-shaped protein complex
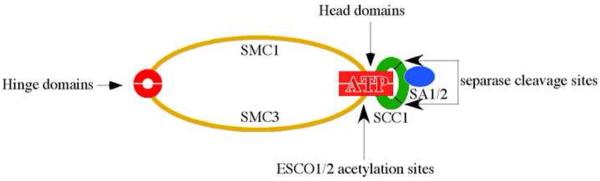
Sister chromatids are physically held together by cohesin, which is a protein complex consisting of four core subunits (Fig. 1). Two of the subunits are SMC1 and SMC3, both of which have a 50 nm long coiled coil structure connecting a head domain and a hinge domain. In the SMC1/3 heterodimers, the two hinge domains interact with each other while the two head domains are separated, forming a V-shaped structure [3]. It is generally accepted that with the addition of the SCC1 (aka MCD1 and RAD21) and SCC3 (aka SA1/2 and STAG in vertebrates) subunits, the gaps between the head domains are sealed and the final cohesin complex forms a ring-shaped structure.
Fig. 1.
Cohesin structures and key posttranslational modifications. The red circle and box are the hinge domains and head domains of SMC1/3, respectively. Acetylations of SMC3 by ESCO1/2 and cleavages of SCC1 by separase are also indicated.
Short of direct visualization by electron microscopy or crystallography, the evidence for the ring-shaped cohesin is very strong. First, the head domains of SMC1 and 3 interact with the carboxyl and amino termini of SCC1, respectively [3, 4]. By doing so, SCC1 closes the opening span of the V-shaped SMC1/3 heterodimer. Second, the head domains of SMCs are ABC-like ATPases, which are capable of heterodimerizing in the presence of ATP [5, 6]. Such interaction also brings the head domains together and seals the opening. Third, the association of cohesin with a circular DNA molecule is disrupted either by a proteolytic cleavage at the coil-coiled motif of SMC1 or by a restrictive cleavage of the DNA [7, 8], indicating a topological concatenation between two closed circles. Together, these pieces of evidences strongly argue that the cohesin complex is a closed protein circle that wraps around a DNA molecule.
It is still under debate whether a pair of sister chromatids is entrapped by many of individual cohesin ring or handcuffed by many of two interlinked cohesin rings. Most of biochemical characterizations suggest that the cohesin rings do not stably interact with each other, consistent with the entrapment model. On the other hand, one study where cohesin was tagged differently showed that cohesins forms oligomers [9], a result that lends support to the handcuff model. Additional scrutiny is apparently necessary to reconcile these differences.
Loading of cohesin onto chromosomes
Just like all ring-shaped protein complexes that function by topologically wrapping around DNA or chromosomes, the loading of cohesin is more complicated than simple affinity binding (Fig. 2). The complex may assemble around chromosomes to form a topological concatenate. Alternatively, preassembled complex may be transiently opened up to allow the entry of chromosomes. The fact that cohesin can be biochemically purified in the absence of chromosome indicates that the complex can assemble in the absence of DNA, favoring the second possibility.
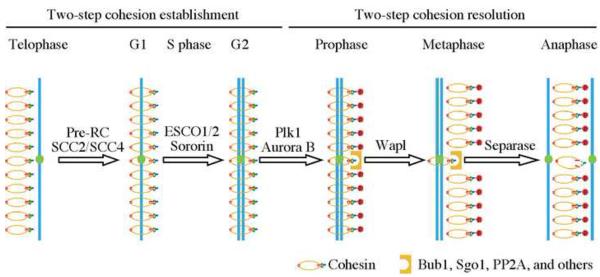
Fig. 2.
Regulations of sister chromatid cohesion and separation in various stages of the cell cycle. Key regulators are indicated at each transition.
The cohesin ring could be transiently open up at two places. First, cohesin may open at the head domains (Fig. 1). Genetic studies demonstrated that ATP hydrolyses catalyzed by the head domains are required for sister chromatid cohesion [5, 6]. Similar to other members of this ATPase family, the head domains dimmerize when both bound to ATP, where as hydrolysis of the ATP destabilizes the interaction. The attractive feature of this model is that this interaction can be regulated by ATP binding and hydrolysis. Activation of the ATPase activity may open the complex to allow the entry of sister chromatid during cohesion establishment, while as inhibition of the ATPase activity may secure the closure of the complex for cohesion maintenance.
Alternatively, cohesin may open at the hinge domain (Fig. 1). Evidence for this pathway came from a single elegant study where, by artificially locking the hinge-hinge interaction, the resulting cohesin is defective at mediating sister chromatid cohesion. On the other hands, locking the head-head interaction with the same strategy has no effect [10]. The simplest explanation is that the interaction between the hinge domains, but not the head domains, needs to be disrupted to allow chromosome entry. The hinge domain exhibits DNA binding activity [11, 12], which may be utilized to initiate the loading process. It has been proposed that the hinge domains may initially contact with chromosome DNA, which in turn induces a conformational change and leads to the entrapment of sister chromatids [11]. These two possibilities are not mutually exclusive. For example, the hinge and head domains may influence each other through the 50-nm-long coiled coil motif. Alternatively, the two domains may contact each other spatially. Definitive answer will arrive when the structure of the whole complex is solved, preferably in the presence of DNA.
The opening of cohesin may be further regulated by cohesin loaders, such as the SCC2/SCC4 complex [13–16]. This loader is conserved among eukaryotes, despite of significant sequence diversity among the SCC4 subunits [17]. In vertebrates, the pre-replication complex (Pre-RC) facilitates the recruitment of the loader to chromosomes [13, 14]. However, the loader is also recruited in G2 when the pre-RC is no longer on chromosome, suggestive of alternative mechanism [18–21]. Regardless the mode of recruitment, the SCC2/4 complex is required in both G1 and G2 [21]. The mechanism of the loading process is poorly understood.
Recent evidence also suggests that the opening of cohesin may require the removal of a pair of SMC3 acetylations, which are added during sister chromatid cohesion establishment in S phase [22–24]. The strongest evidence comes from a study in budding yeast, where the putative acetylation-mimicking mutant cohesin failed to be loaded on chromosomes. If the mutation faithfully mimics the acetylated form, this observation indicates that the acetylation of cohesin negatively regulates the loading process [22]. It is possible that the SCC2/4 complex may not be able to open the acetylated cohesins.
Establishment of sister chromatid cohesion
The loading of cohesin onto chromosomes is required but not sufficient for sister chromatid cohesion [25]. What else is required after the loading is currently under intense study. The best understood event is the aforementioned cohesin acetylation catalyzed by a conserved acetyltransferase [22–24]. It was first identified as Eco1/Ctf7 in budding yeast [25, 26] and homologues were later identified in higher eukaryotes [27–29]. They acetylate cohesin at a pair of lysines on the SMC3 subunit [30, 31] and converts cohesin from a non-cohesive state into a cohesive state (Fig. 1).
The exact natures of these cohesin states are poorly understood. In addition to the acetylation on SMC3, the Wapl-Pds5 complex appears to play a central role in determining the non-cohesive states, perhaps by destabilizing the cohesin-chromosome interaction [32, 33]. The cohesin that is associated with Wapl-Pds5 is in the non-cohesive state where as the cohesin that is not associated with Wapl-Pds5 is in the cohesive state. It has been suggested that cohesin in the non-cohesive state may be an unlocked ring that can be transiently opened to embrace chromosomes during loading and/or establishment. Conversely, the cohesive cohesin may be a locked ring that securely keeps the sister chromatids bonded together after establishment. Proper switch of the cohesin status at appropriate stages of sister chromatid cohesion cycle is therefore critical during cohesin loading, sister chromatid cohesion establishment and maintenance.
During and/or after the establishment of sister chromatid cohesion, the destabilizing activity of Wapl is prevented by the cohesin acetylation. The mechanism remains unclear and appears to be different between yeast and metazoans. In yeast, the acetylation alone may be sufficient. The acetylated lysines are in or near the ATPase head domain of SMC3 and the modification may inhibit the ATPase activity and stabilize the head-head interaction. In metazoans, the acetylation recruits sororin to help antagonize Wapl [34, 35]. No sequence homologues of sororin have been identified in yeast, raising the possibility that the requirement of sororin may be specific to metazoans. The difference is also apparent in the function of Wapl, which is required for the loading and establishment of sister chromatid cohesin in yeast but dispensable for sister chromatid cohesion in HeLa cells [32, 33, 45]. Instead, Wapl is required for the prophase pathway to remove cohesins from chromosome arms in vertebrates (see below)
Finally, the establishment of sister chromatid cohesion occurs in S phase and is likely coupled with DNA replication machinery. The strongest evidence came from studies in budding yeast where Eco1/Ctf7 is recruited by replication factor PCNA [36]. In human cells, the homologues of Eco1/Ctf7 are ESCO1 and ESCO2 [27]. Although a putative PCNA-interacting box exists on both of them, its functionality and significance remain to be demonstrated. Nonetheless, the acetylation is also dependent on replication factors in cultured human cells [37]. Furthermore, the metazoan-specific sororin is recruited to chromosomes in a replication-dependent manner [34, 35]. These pieces of evidence suggest that the establishment is also coupled with DNA replication in human. Consistent with this notion, a number of replication factors are implicated in sister chromatid cohesion in cultured human cells [38–41].
Maintenance of sister chromatid cohesion at the centromeres
Sister chromatid cohesion is maintained from its establishment in S phase until its final separation in anaphase. In vertebrates, the maintenance is regulated spatially along chromosomes. Specifically, the cohesion at chromosome arms is dissolved in prophase while as the cohesion at the centromeres and some heterochromatin regions are maintained until anaphase (Fig. 2). The prophase pathway depends on the kinase activity of Plk1 and Aurora B [42, 43], a series of phosphorylations on the SCC3 subunit of the cohesin complex [44] and the cohesin-destabilization factor Wapl [32, 33, 45]. The connections between these events are poorly understood and additional researches are necessary to fill the gaps. The biological significance of this spatial regulation may be multifold. It is possible that removing a significant portion of cohesin from chromosomes may speed up the process of sister chromatid separation in anaphase because fewer cohesins need to be removed [42]. Supporting this idea, cells depleted of Wapl maintains sister chromatid cohesion along chromosome arms and separate sister chromatids with a detectable delay [32]. Alternatively, cohesin has been implicated in transcription regulation in interphase cells. It is therefore possible that the prophase removal may preserve a pool of cohesin from separase cleavage so that they can be immediately used in the following G1 [46]. This hypothesis has yet been tested directly.
It is critical that the centromeric cohesion is spared from the prophase pathway so that sister chromatids remain paired before the onset of anaphase. Counteracting the prophase pathway, the presence of a pair of proteins, Shugoshin and PP2A, at the centromeres is critical to the centromeric cohesion. In yeast, Shugoshin is critically involved in protecting centromeric cohesin from separase durig meiosis I [47–50]. Surprisingly, the vertebrate homologue Sgo1 plays a mitotic role by protecting the centromeric cohesin from the prophase pathway [49, 51, 52]. Mechanistically, studies in yeast strongly indicate that Shugoshin functions upstream by recruiting the PP2A phosphatase [53, 54]. Similar observation has been made in Xenopus extract [55]. However, this linear relationship appears less clear-cut in human cells. Depletion of PP2A in HeLa cells reduces or removes kinetochore Sgo1 [54, 56]. Furthermore, depletion of Sgo1 does not affect the PP2A localization, yet causes premature sister chromatid separation [56], suggesting that Sgo1 may also facilitate sister chromatid cohesion independent of PP2A in HeLa cells.
Upstream of the Shugoshin and PP2A, both yeast and human cells use a histone modification, catalyzed by the Bub1 kinase, to recruit Shugoshin to the centromeres [51, 57–60]. The phosphorylation of histone H2A at Thr120 in human cells is believed to a part of the histone code that is important for sister chromatid cohesion. Further support of such a histone code comes from the involvement of haspin in sister chromatid cohesion in human cells [61]. Haspin is a kinase that phosphorylates histone H3 at Ser3 [62]. Instead of regulating the recruitment of Sgo1, this histone modification regulates the localization of the chromosome passenger complex (CPC) [63–65], which is required for the mitotic spindle checkpoint. The misregulated checkpoint in the absence of haspin may cause apparent premature sister chromatid separation and defective chromosome segregation. It appears that histone modifications plays a central role in determine many aspects of chromosome dynamics in the mitotic phases of the cell cycle.
In addition to the Bub1-Shugoshin-PP2A network, another acetyltransferase called San is required for centromeric cohesion in Drosophila and human cells [28, 66]. This requirement appears to be metazoan specific as no such activity has been identified in yeast. Furthermore, the localization of Sgo1 is not affected in cells depleted of San, suggesting that it may function downstream or define a parallel pathway. Identification of the relevant substrate will provide much-needed insight into the mechanism.
The final separation of sister chromatid
Sister chromatid separation occurs in anaphase, during which separase becomes active. Separase is an endopeptidase that specifically cleaves cohesin subunit SCC1 [67–70]. The cleavage irreversibly opens the cohesin ring and allows the final separation of sister chromatids (Fig. 2). To ensure a timely execution of sister chromatid separation, the activity of separase is regulated both temporally and spatially.
In human cells, separase only cleaves about 5% of cohesin in anaphase [42]. This is due to the spatial regulation of separase, in which separase specifically cleaves the chromosome-associated cohesins [42, 46]. Mechanistically, separase cleaves cohesin in a DNA-dependent manner [46]. Similar selective cohesin cleavage was also described in budding yeast, although mediated by a different mechanism [71].
The temporal regulations restrict separase activity in anaphase. In metazoans, separase activity is inhibited by at least two mechanisms. Securin is a chaperone inhibitor that physically associates with separase [70, 72–75]. Although lacking any sequence similarity, this function is conserved from yeast to human [69, 73–78]. Surprisingly, this inhibitor is not essential as deletion mutants exhibit subtle phenotypes in yeast and animal cells [79–83]. The lethality observed in fission yeast [70] and Drosophila [84] may be related to the chaperone function of securin. In these organisms, the correct folding of separase critically depends on securin and deletion of securin leads to the absence of separase activity, which is the underlying cause of the lethality.
In animal cells, separase is also inhibited by a specific phosphorylation [85]. When combined with securin deletion, cells that are heterozygous of the phosphorylation site mutation exhibit premature sister chromatid separation in the presence of microtubule poison [86]. In addition, the phosphorylation is also required for oocyte maturation, during which the levels of securin are much lower than that in other somatic cells [87, 88]. The biochemical mechanism of this inhibition remains to be worked out. The current model is that the CDK1/cyclin B complex catalyzes the phosphorylation, binds to separase with higher affinity, and inhibits separase activity [89, 90]. This was nicely demonstrated in vitro. However, what remain under debate are the cyclin B concentrations that are needed to achieve this inhibition. In some studies physiological level of cyclin B is sufficient [91, 92], while in other studies overexpression of cyclin B is required [93]. Regardless of the mechanism, it is clear that the phosphorylation inhibition of separase is required for preventing premature sister chromatid separation in the absence of securin.
To activate separase in anaphase, both inhibitory mechanisms need to be removed. The mechanism has been largely worked out. In fact, both securin and cyclin B1 are substrates of the anaphase promoting complex/cyclosome (APC/C) [72, 73, 76, 78]. Consequently, both are degraded in anaphase to allow the activation of separase and sister chromatid separation. In vertebrate cells, separase also undergoes auto-cleavage [94–96]. But the biochemical significance of this event is unclear.
Less clear is the regulation of separase in interphase. As mentioned above, the protein levels of both securin and cyclin B are controlled by APC/C, which is activated at the metaphase-anaphase transition and inactivated until the G1-S transition. This begging the question how separase is inhibited in G1 cells? Furthermore, what inhibit separase from G1 to G2 in cells deleted of securin? It is possible that yet another inhibitor regulates separase in interphase. Alternatively, the nuclear exclusion of separase can prevent cohesin cleavage during these periods [97]. Different from budding yeast, separase in human cells are excluded from the nuclei by active nuclear export. Such mechanism separates separase from the nuclear cohesins and prevents premature cohesin cleavage. Remarkably, in human breast and bone cancer tissues, separase were found mislocalized into the nuclei [98]. Because a complete loss of sister chromatid cohesion has been shown to cause lethality, it is likely that separase is regulated by more than one mechanism in interphase and the mislocalization of separase may compromise the integrity of sister chromatid cohesion and contributes to tumorigenesis. More research is necessary in this area to address these important questions.
Beyond sister chromatid dynamics
The machineries that regulate sister chromatid dynamics are also used to regulate other important biological processes. Notably, both cohesin and its loading and establishing factors are implicated in regulating transcription [99–103]. It appears that cohesin is a component of chromosome insulator and is able to mediate higher order chromatin structures by generating intra-chromosomal loops. Defectives in this process have been suggested to be the underlying mechanisms of a couple of human diseases [104–106]. In addition, cohesin [107] and separase [108] are required for DNA repair. Separase may remove the cohesin sitting on the damage sites so that the DNA repair machinery can gain access. On the other hand, cohesin is thought to keep duplicated chromosomes in close proximity so that the sequence information on the intact sister chromatid may be used to repair double-strand breaks on the other sister using homologous recombination. Finally, separase is also involved in centrosome duplication [109, 110] and vesicle trafficking [111, 112]. The mechanisms of separase in these events remain unknown. The studies of sister chromatid dynamics will help us to understand these important biological processes.
Acknowledgements
H. Zou is supported by a grant (R01GM081466) from NIH and a grant (I-1594) from the Welch Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Guacci V, Koshland D, Strunnikov A. A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell. 1997;91:47–57. doi: 10.1016/s0092-8674(01)80008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Michaelis C, Ciosk R, Nasmyth K. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell. 1997;91:35–45. doi: 10.1016/s0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- [3].Haering CH, Lowe J, Hochwagen A, Nasmyth K. Molecular architecture of SMC proteins and the yeast cohesin complex. Mol Cell. 2002;9:773–88. doi: 10.1016/s1097-2765(02)00515-4. [DOI] [PubMed] [Google Scholar]
- [4].Haering CH, Schoffnegger D, Nishino T, Helmhart W, Nasmyth K, Lowe J. Structure and stability of cohesin's Smc1-kleisin interaction. Mol Cell. 2004;15:951–64. doi: 10.1016/j.molcel.2004.08.030. [DOI] [PubMed] [Google Scholar]
- [5].Arumugam P, Gruber S, Tanaka K, Haering CH, Mechtler K, Nasmyth K. ATP hydrolysis is required for cohesin's association with chromosomes. Curr Biol. 2003;13:1941–53. doi: 10.1016/j.cub.2003.10.036. [DOI] [PubMed] [Google Scholar]
- [6].Weitzer S, Lehane C, Uhlmann F. A model for ATP hydrolysis-dependent binding of cohesin to DNA. Curr Biol. 2003;13:1930–40. doi: 10.1016/j.cub.2003.10.030. [DOI] [PubMed] [Google Scholar]
- [7].Ivanov D, Nasmyth K. A topological interaction between cohesin rings and a circular minichromosome. Cell. 2005;122:849–60. doi: 10.1016/j.cell.2005.07.018. [DOI] [PubMed] [Google Scholar]
- [8].Haering CH, Farcas AM, Arumugam P, Metson J, Nasmyth K. The cohesin ring concatenates sister DNA molecules. Nature. 2008;454:297–301. doi: 10.1038/nature07098. [DOI] [PubMed] [Google Scholar]
- [9].Zhang N, Kuznetsov SG, Sharan SK, Li K, Rao PH, Pati D. A handcuff model for the cohesin complex. J Cell Biol. 2008;183:1019–31. doi: 10.1083/jcb.200801157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gruber S, Arumugam P, Katou Y, Kuglitsch D, Helmhart W, Shirahige K, et al. Evidence that loading of cohesin onto chromosomes involves opening of its SMC hinge. Cell. 2006;127:523–37. doi: 10.1016/j.cell.2006.08.048. [DOI] [PubMed] [Google Scholar]
- [11].Hirano M, Hirano T. Opening Closed Arms: Long-Distance Activation of SMC ATPase by Hinge-DNA Interactions. Mol Cell. 2006;21:175–86. doi: 10.1016/j.molcel.2005.11.026. [DOI] [PubMed] [Google Scholar]
- [12].Chiu A, Revenkova E, Jessberger R. DNA interaction and dimerization of eukaryotic SMC hinge domains. J Biol Chem. 2004;279:26233–42. doi: 10.1074/jbc.M402439200. [DOI] [PubMed] [Google Scholar]
- [13].Takahashi TS, Yiu P, Chou MF, Gygi S, Walter JC. Recruitment of Xenopus Scc2 and cohesin to chromatin requires the pre-replication complex. Nat Cell Biol. 2004;6:991–6. doi: 10.1038/ncb1177. [DOI] [PubMed] [Google Scholar]
- [14].Gillespie PJ, Hirano T. Scc2 couples replication licensing to sister chromatid cohesion in Xenopus egg extracts. Curr Biol. 2004;14:1598–603. doi: 10.1016/j.cub.2004.07.053. [DOI] [PubMed] [Google Scholar]
- [15].Toyoda Y, Furuya K, Goshima G, Nagao K, Takahashi K, Yanagida M. Requirement of chromatid cohesion proteins rad21/scc1 and mis4/scc2 for normal spindle-kinetochore interaction in fission yeast. Curr Biol. 2002;12:347–58. doi: 10.1016/s0960-9822(02)00692-9. [DOI] [PubMed] [Google Scholar]
- [16].Ciosk R, Shirayama M, Shevchenko A, Tanaka T, Toth A, Nasmyth K. Cohesin's binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Mol Cell. 2000;5:243–54. doi: 10.1016/s1097-2765(00)80420-7. [DOI] [PubMed] [Google Scholar]
- [17].Watrin E, Schleiffer A, Tanaka K, Eisenhaber F, Nasmyth K, Peters JM. Human Scc4 is required for cohesin binding to chromatin, sister-chromatid cohesion, and mitotic progression. Curr Biol. 2006;16:863–74. doi: 10.1016/j.cub.2006.03.049. [DOI] [PubMed] [Google Scholar]
- [18].Unal E, Heidinger-Pauli JM, Koshland D. DNA double-strand breaks trigger genome-wide sister-chromatid cohesion through Eco1 (Ctf7) Science. 2007;317:245–8. doi: 10.1126/science.1140637. [DOI] [PubMed] [Google Scholar]
- [19].Strom L, Sjogren C. DNA damage-induced cohesion. Cell Cycle. 2005;4:536–9. doi: 10.4161/cc.4.4.1613. [DOI] [PubMed] [Google Scholar]
- [20].Strom L, Lindroos HB, Shirahige K, Sjogren C. Postreplicative recruitment of cohesin to double-strand breaks is required for DNA repair. Mol Cell. 2004;16:1003–15. doi: 10.1016/j.molcel.2004.11.026. [DOI] [PubMed] [Google Scholar]
- [21].Unal E, Arbel-Eden A, Sattler U, Shroff R, Lichten M, Haber JE, et al. DNA damage response pathway uses histone modification to assemble a double-strand break-specific cohesin domain. Mol Cell. 2004;16:991–1002. doi: 10.1016/j.molcel.2004.11.027. [DOI] [PubMed] [Google Scholar]
- [22].Borges V, Lehane C, Lopez-Serra L, Flynn H, Skehel M, Rolef Ben-Shahar T, et al. Hos1 deacetylates Smc3 to close the cohesin acetylation cycle. Mol Cell. 2010;39:677–88. doi: 10.1016/j.molcel.2010.08.009. [DOI] [PubMed] [Google Scholar]
- [23].Beckouet F, Hu B, Roig MB, Sutani T, Komata M, Uluocak P, et al. An Smc3 acetylation cycle is essential for establishment of sister chromatid cohesion. Mol Cell. 2010;39:689–99. doi: 10.1016/j.molcel.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xiong B, Lu S, Gerton JL. Hos1 is a lysine deacetylase for the smc3 subunit of cohesin. Curr Biol. 2010;20:1660–5. doi: 10.1016/j.cub.2010.08.019. [DOI] [PubMed] [Google Scholar]
- [25].Toth A, Ciosk R, Uhlmann F, Galova M, Schleiffer A, Nasmyth K. Yeast cohesin complex requires a conserved protein, Eco1p(Ctf7), to establish cohesion between sister chromatids during DNA replication. Genes Dev. 1999;13:320–33. doi: 10.1101/gad.13.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Skibbens RV, Corson LB, Koshland D, Hieter P. Ctf7p is essential for sister chromatid cohesion and links mitotic chromosome structure to the DNA replication machinery. Genes Dev. 1999;13:307–19. doi: 10.1101/gad.13.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hou F, Zou H. Two human orthologues of Eco1/Ctf7 acetyltransferases are both required for proper sister-chromatid cohesion. Mol Biol Cell. 2005;16:3908–18. doi: 10.1091/mbc.E04-12-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Williams BC, Garrett-Engele CM, Li Z, Williams EV, Rosenman ED, Goldberg ML. Two putative acetyltransferases, san and deco, are required for establishing sister chromatid cohesion in Drosophila. Curr Biol. 2003;13:2025–36. doi: 10.1016/j.cub.2003.11.018. [DOI] [PubMed] [Google Scholar]
- [29].Bellows AM, Kenna MA, Cassimeris L, Skibbens RV. Human EFO1p exhibits acetyltransferase activity and is a unique combination of linker histone and Ctf7p/Eco1p chromatid cohesion establishment domains. Nucleic Acids Res. 2003;31:6334–43. doi: 10.1093/nar/gkg811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang J, Shi X, Li Y, Kim BJ, Jia J, Huang Z, et al. Acetylation of Smc3 by Eco1 is required for S phase sister chromatid cohesion in both human and yeast. Mol Cell. 2008;31:143–51. doi: 10.1016/j.molcel.2008.06.006. [DOI] [PubMed] [Google Scholar]
- [31].Rolef Ben-Shahar T, Heeger S, Lehane C, East P, Flynn H, Skehel M, et al. Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science. 2008;321:563–6. doi: 10.1126/science.1157774. [DOI] [PubMed] [Google Scholar]
- [32].Kueng S, Hegemann B, Peters BH, Lipp JJ, Schleiffer A, Mechtler K, et al. Wapl controls the dynamic association of cohesin with chromatin. Cell. 2006;127:955–67. doi: 10.1016/j.cell.2006.09.040. [DOI] [PubMed] [Google Scholar]
- [33].Gandhi R, Gillespie PJ, Hirano T. Human Wapl Is a Cohesin-Binding Protein that Promotes Sister-Chromatid Resolution in Mitotic Prophase. Curr Biol. 2006;16:2406–17. doi: 10.1016/j.cub.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nishiyama T, Ladurner R, Schmitz J, Kreidl E, Schleiffer A, Bhaskara V, et al. Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell. 2010;143:737–49. doi: 10.1016/j.cell.2010.10.031. [DOI] [PubMed] [Google Scholar]
- [35].Lafont AL, Song J, Rankin S. Sororin cooperates with the acetyltransferase Eco2 to ensure DNA replication-dependent sister chromatid cohesion. Proc Natl Acad Sci U S A. 2010;107:20364–9. doi: 10.1073/pnas.1011069107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moldovan GL, Pfander B, Jentsch S. PCNA controls establishment of sister chromatid cohesion during S phase. Mol Cell. 2006;23:723–32. doi: 10.1016/j.molcel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- [37].Terret ME, Sherwood R, Rahman S, Qin J, Jallepalli PV. Cohesin acetylation speeds the replication fork. Nature. 2009;462:231–4. doi: 10.1038/nature08550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Maradeo ME, Skibbens RV. Replication factor C complexes play unique pro- and anti-establishment roles in sister chromatid cohesion. PLoS ONE. 2010;5:e15381. doi: 10.1371/journal.pone.0015381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Maradeo ME, Garg A, Skibbens RV. Rfc5p regulates alternate RFC complex functions in sister chromatid pairing reactions in budding yeast. Cell Cycle. 2010;9:4370–8. doi: 10.4161/cc.9.21.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Skibbens RV. Chl1p, a DNA helicase-like protein in budding yeast, functions in sister-chromatid cohesion. Genetics. 2004;166:33–42. doi: 10.1534/genetics.166.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kenna MA, Skibbens RV. Mechanical link between cohesion establishment and DNA replication: Ctf7p/Eco1p, a cohesion establishment factor, associates with three different replication factor C complexes. Mol Cell Biol. 2003;23:2999–3007. doi: 10.1128/MCB.23.8.2999-3007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Waizenegger IC, Hauf S, Meinke A, Peters JM. Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell. 2000;103:399–410. doi: 10.1016/s0092-8674(00)00132-x. [DOI] [PubMed] [Google Scholar]
- [43].Losada A, Hirano M, Hirano T. Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev. 2002;16:3004–16. doi: 10.1101/gad.249202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hauf S, Roitinger E, Koch B, Dittrich CM, Mechtler K, Peters JM. Dissociation of Cohesin from Chromosome Arms and Loss of Arm Cohesion during Early Mitosis Depends on Phosphorylation of SA2. PLoS Biol. 2005;3:1–14. doi: 10.1371/journal.pbio.0030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Verni F, Gandhi R, Goldberg ML, Gatti M. Genetic and molecular analysis of wings apart-like (wapl), a gene controlling heterochromatin organization in Drosophila melanogaster. Genetics. 2000;154:1693–710. doi: 10.1093/genetics/154.4.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sun Y, Kucej M, Fan HY, Yu H, Sun QY, Zou H. Separase is recruited to mitotic chromosomes to dissolve sister chromatid cohesion in a DNA-dependent manner. Cell. 2009;137:123–32. doi: 10.1016/j.cell.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rabitsch KP, Gregan J, Schleiffer A, Javerzat JP, Eisenhaber F, Nasmyth K. Two fission yeast homologs of Drosophila Mei-S332 are required for chromosome segregation during meiosis I and II. Curr Biol. 2004;14:287–301. doi: 10.1016/j.cub.2004.01.051. [DOI] [PubMed] [Google Scholar]
- [48].Marston AL, Tham WH, Shah H, Amon A. A genome-wide screen identifies genes required for centromeric cohesion. Science. 2004;303:1367–70. doi: 10.1126/science.1094220. [DOI] [PubMed] [Google Scholar]
- [49].Kitajima TS, Kawashima SA, Watanabe Y. The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature. 2004;427:510–7. doi: 10.1038/nature02312. [DOI] [PubMed] [Google Scholar]
- [50].Katis VL, Galova M, Rabitsch KP, Gregan J, Nasmyth K. Maintenance of cohesin at centromeres after meiosis I in budding yeast requires a kinetochore-associated protein related to MEI-S332. Curr Biol. 2004;14:560–72. doi: 10.1016/j.cub.2004.03.001. [DOI] [PubMed] [Google Scholar]
- [51].Tang Z, Sun Y, Harley SE, Zou H, Yu H. Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis. Proc Natl Acad Sci U S A. 2004;101:18012–7. doi: 10.1073/pnas.0408600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Salic A, Waters JC, Mitchison TJ. Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell. 2004;118:567–78. doi: 10.1016/j.cell.2004.08.016. [DOI] [PubMed] [Google Scholar]
- [53].Riedel CG, Katis VL, Katou Y, Mori S, Itoh T, Helmhart W, et al. Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature. 2006;441:53–61. doi: 10.1038/nature04664. [DOI] [PubMed] [Google Scholar]
- [54].Kitajima TS, Sakuno T, Ishiguro K, Iemura S, Natsume T, Kawashima SA, et al. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature. 2006;441:46–52. doi: 10.1038/nature04663. [DOI] [PubMed] [Google Scholar]
- [55].Rivera T, Losada A. Shugoshin regulates cohesion by driving relocalization of PP2A in Xenopus extracts. Chromosoma. 2009;118:223–33. doi: 10.1007/s00412-008-0190-4. [DOI] [PubMed] [Google Scholar]
- [56].Tang Z, Shu H, Qi W, Mahmood NA, Mumby MC, Yu H. PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev Cell. 2006;10:575–85. doi: 10.1016/j.devcel.2006.03.010. [DOI] [PubMed] [Google Scholar]
- [57].Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science. 2010;327:172–7. doi: 10.1126/science.1180189. [DOI] [PubMed] [Google Scholar]
- [58].Fernius J, Hardwick KG. Bub1 kinase targets Sgo1 to ensure efficient chromosome biorientation in budding yeast mitosis. PLoS genetics. 2007;3:e213. doi: 10.1371/journal.pgen.0030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Boyarchuk Y, Salic A, Dasso M, Arnaoutov A. Bub1 is essential for assembly of the functional inner centromere. J Cell Biol. 2007;176:919–28. doi: 10.1083/jcb.200609044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kitajima TS, Hauf S, Ohsugi M, Yamamoto T, Watanabe Y. Human Bub1 Defines the Persistent Cohesion Site along the Mitotic Chromosome by Affecting Shugoshin Localization. Curr Biol. 2005;15:353–9. doi: 10.1016/j.cub.2004.12.044. [DOI] [PubMed] [Google Scholar]
- [61].Dai J, Sullivan BA, Higgins JM. Regulation of mitotic chromosome cohesion by Haspin and Aurora B. Dev Cell. 2006;11:741–50. doi: 10.1016/j.devcel.2006.09.018. [DOI] [PubMed] [Google Scholar]
- [62].Dai J, Sultan S, Taylor SS, Higgins JM. The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev. 2005;19:472–88. doi: 10.1101/gad.1267105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yamagishi Y, Honda T, Tanno Y, Watanabe Y. Two histone marks establish the inner centromere and chromosome bi-orientation. Science. 2010;330:239–43. doi: 10.1126/science.1194498. [DOI] [PubMed] [Google Scholar]
- [64].Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, et al. Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science. 2010;330:231–5. doi: 10.1126/science.1189435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science. 2010;330:235–9. doi: 10.1126/science.1189505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hou F, Chu CW, Kong X, Yokomori K, Zou H. The acetyltransferase activity of San stabilizes the mitotic cohesin at the centromeres in a shugoshin-independent manner. J Cell Biol. 2007;177:587–97. doi: 10.1083/jcb.200701043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hauf S, Waizenegger IC, Peters JM. Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science. 2001;293:1320–3. doi: 10.1126/science.1061376. [DOI] [PubMed] [Google Scholar]
- [68].Uhlmann F, Lottspeich F, Nasmyth K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature. 1999;400:37–42. doi: 10.1038/21831. [DOI] [PubMed] [Google Scholar]
- [69].Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M, Nasmyth K. An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell. 1998;93:1067–76. doi: 10.1016/s0092-8674(00)81211-8. [DOI] [PubMed] [Google Scholar]
- [70].Funabiki H, Kumada K, Yanagida M. Fission yeast Cut1 and Cut2 are essential for sister chromatid separation, concentrate along the metaphase spindle and form large complexes. Embo J. 1996;15:6617–28. [PMC free article] [PubMed] [Google Scholar]
- [71].Hornig NC, Uhlmann F. Preferential cleavage of chromatin-bound cohesin after targeted phosphorylation by Polo-like kinase. Embo J. 2004;23:3144–53. doi: 10.1038/sj.emboj.7600303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Leismann O, Herzig A, Heidmann S, Lehner CF. Degradation of Drosophila PIM regulates sister chromatid separation during mitosis. Genes Dev. 2000;14:2192–205. doi: 10.1101/gad.176700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zou H, McGarry TJ, Bernal T, Kirschner MW. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999;285:418–22. doi: 10.1126/science.285.5426.418. [DOI] [PubMed] [Google Scholar]
- [74].Yamamoto A, Guacci V, Koshland D. Pds1p, an inhibitor of anaphase in budding yeast, plays a critical role in the APC and checkpoint pathway(s) J Cell Biol. 1996;133:99–110. doi: 10.1083/jcb.133.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yamamoto A, Guacci V, Koshland D. Pds1p is required for faithful execution of anaphase in the yeast, Saccharomyces cerevisiae. J Cell Biol. 1996;133:85–97. doi: 10.1083/jcb.133.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Funabiki H, Yamano H, Nagao K, Tanaka H, Yasuda H, Hunt T, et al. Fission yeast Cut2 required for anaphase has two destruction boxes. Embo J. 1997;16:5977–87. doi: 10.1093/emboj/16.19.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Funabiki H, Yamano H, Kumada K, Nagao K, Hunt T, Yanagida M. Cut2 proteolysis required for sister-chromatid seperation in fission yeast. Nature. 1996;381:438–41. doi: 10.1038/381438a0. [DOI] [PubMed] [Google Scholar]
- [78].Cohen-Fix O, Peters JM, Kirschner MW, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 1996;10:3081–93. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- [79].Pfleghaar K, Heubes S, Cox J, Stemmann O, Speicher MR. Securin is not required for chromosomal stability in human cells. PLoS Biol. 2005;3:e416. doi: 10.1371/journal.pbio.0030416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wang Z, Moro E, Kovacs K, Yu R, Melmed S. Pituitary tumor transforming gene-null male mice exhibit impaired pancreatic beta cell proliferation and diabetes. Proc Natl Acad Sci U S A. 2003;100:3428–32. doi: 10.1073/pnas.0638052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wang Z, Yu R, Melmed S. Mice lacking pituitary tumor transforming gene show testicular and splenic hypoplasia, thymic hyperplasia, thrombocytopenia, aberrant cell cycle progression, and premature centromere division. Mol Endocrinol. 2001;15:1870–9. doi: 10.1210/mend.15.11.0729. [DOI] [PubMed] [Google Scholar]
- [82].Mei J, Huang X, Zhang P. Securin is not required for cellular viability, but is required for normal growth of mouse embryonic fibroblasts. Curr Biol. 2001;11:1197–201. doi: 10.1016/s0960-9822(01)00325-6. [DOI] [PubMed] [Google Scholar]
- [83].Jallepalli PV, Waizenegger IC, Bunz F, Langer S, Speicher MR, Peters JM, et al. Securin is required for chromosomal stability in human cells. Cell. 2001;105:445–57. doi: 10.1016/s0092-8674(01)00340-3. [DOI] [PubMed] [Google Scholar]
- [84].Stratmann R, Lehner CF. Separation of sister chromatids in mitosis requires the Drosophila pimples product, a protein degraded after the metaphase/anaphase transition. Cell. 1996;84:25–35. doi: 10.1016/s0092-8674(00)80990-3. [DOI] [PubMed] [Google Scholar]
- [85].Stemmann O, Zou H, Gerber SA, Gygi SP, Kirschner MW. Dual inhibition of sister chromatid separation at metaphase. Cell. 2001;107:715–26. doi: 10.1016/s0092-8674(01)00603-1. [DOI] [PubMed] [Google Scholar]
- [86].Huang X, Hatcher R, York JP, Zhang P. Securin and Separase Phosphorylation Act Redundantly to Maintain Sister Chromatid Cohesion in Mammalian Cells. Mol Biol Cell. 2005;16:4725–32. doi: 10.1091/mbc.E05-03-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Huang X, Andreu-Vieyra CV, Wang M, Cooney AJ, Matzuk MM, Zhang P. Preimplantation mouse embryos depend on inhibitory phosphorylation of separase to prevent chromosome missegregation. Mol Cell Biol. 2009;29:1498–505. doi: 10.1128/MCB.01778-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Huang X, Andreu-Vieyra CV, York JP, Hatcher R, Lu T, Matzuk MM, et al. Inhibitory phosphorylation of separase is essential for genome stability and viability of murine embryonic germ cells. PLoS Biol. 2008;6:e15. doi: 10.1371/journal.pbio.0060015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Boos D, Kuffer C, Lenobel R, Korner R, Stemmann O. Phosphorylation-dependent binding of cyclin B1 to a Cdc6-like domain of human separase. J Biol Chem. 2008;283:816–23. doi: 10.1074/jbc.M706748200. [DOI] [PubMed] [Google Scholar]
- [90].Gorr IH, Boos D, Stemmann O. Mutual inhibition of separase and Cdk1 by two-step complex formation. Mol Cell. 2005;19:135–41. doi: 10.1016/j.molcel.2005.05.022. [DOI] [PubMed] [Google Scholar]
- [91].Fan HY, Sun QY, Zou H. Regulation of Separase in Meiosis: Separase is Activated at the Metaphase I-II Transition in Xenopus Oocytes during Meiosis. Cell Cycle. 2006;5:198–204. doi: 10.4161/cc.5.2.2321. [DOI] [PubMed] [Google Scholar]
- [92].Chang DC, Xu N, Luo KQ. Degradation of cyclin B is required for the onset of anaphase in Mammalian cells. J Biol Chem. 2003;278:37865–73. doi: 10.1074/jbc.M306376200. [DOI] [PubMed] [Google Scholar]
- [93].Hagting A, Den Elzen N, Vodermaier HC, Waizenegger IC, Peters JM, Pines J. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J Cell Biol. 2002;157:1125–37. doi: 10.1083/jcb.200111001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zou H, Stemman O, Anderson JS, Mann M, Kirschner MW. Anaphase specific auto-cleavage of separase. FEBS Lett. 2002;528:246–50. doi: 10.1016/s0014-5793(02)03238-6. [DOI] [PubMed] [Google Scholar]
- [95].Waizenegger I, Gimenez-Abian JF, Wernic D, Peters JM. Regulation of human separase by securin binding and autocleavage. Curr Biol. 2002;12:1368–78. doi: 10.1016/s0960-9822(02)01073-4. [DOI] [PubMed] [Google Scholar]
- [96].Chestukhin A, Pfeffer C, Milligan S, DeCaprio JA, Pellman D. Processing, localization, and requirement of human separase for normal anaphase progression. Proc Natl Acad Sci U S A. 2003;100:4574–9. doi: 10.1073/pnas.0730733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sun Y, Yu H, Zou H. Nuclear Exclusion of Separase Prevents Cohesin Cleavage in Interphase Cells. Cell Cycle. 2006;5:2537–42. doi: 10.4161/cc.5.21.3407. [DOI] [PubMed] [Google Scholar]
- [98].Meyer R, Fofanov V, Panigrahi A, Merchant F, Zhang N, Pati D. Overexpression and mislocalization of the chromosomal segregation protein separase in multiple human cancers. Clin Cancer Res. 2009;15:2703–10. doi: 10.1158/1078-0432.CCR-08-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wendt KS, Yoshida K, Itoh T, Bando M, Koch B, Schirghuber E, et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature. 2008;451:796–801. doi: 10.1038/nature06634. [DOI] [PubMed] [Google Scholar]
- [100].Stedman W, Kang H, Lin S, Kissil JL, Bartolomei MS, Lieberman PM. Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/Igf2 insulators. Embo J. 2008;27:654–66. doi: 10.1038/emboj.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Rubio ED, Reiss DJ, Welcsh PL, Disteche CM, Filippova GN, Baliga NS, et al. CTCF physically links cohesin to chromatin. Proc Natl Acad Sci U S A. 2008;105:8309–14. doi: 10.1073/pnas.0801273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Parelho V, Hadjur S, Spivakov M, Leleu M, Sauer S, Gregson HC, et al. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell. 2008;132:422–33. doi: 10.1016/j.cell.2008.01.011. [DOI] [PubMed] [Google Scholar]
- [103].Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–5. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Gordillo M, Vega H, Trainer AH, Hou F, Sakai N, Luque R, et al. The molecular mechanism underlying Roberts syndrome involves loss of ESCO2 acetyltransferase activity. Human molecular genetics. 2008;17:2172–80. doi: 10.1093/hmg/ddn116. [DOI] [PubMed] [Google Scholar]
- [105].Deardorff MA, Kaur M, Yaeger D, Rampuria A, Korolev S, Pie J, et al. Mutations in cohesin complex members SMC3 and SMC1A cause a mild variant of cornelia de Lange syndrome with predominant mental retardation. Am J Hum Genet. 2007;80:485–94. doi: 10.1086/511888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Tonkin ET, Wang TJ, Lisgo S, Bamshad MJ, Strachan T. NIPBL, encoding a homolog of fungal Scc2-type sister chromatid cohesion proteins and fly Nipped-B, is mutated in Cornelia de Lange syndrome. Nat Genet. 2004;36:636–41. doi: 10.1038/ng1363. [DOI] [PubMed] [Google Scholar]
- [107].Sjogren C, Nasmyth K. Sister chromatid cohesion is required for postreplicative double-strand break repair in Saccharomyces cerevisiae. Curr Biol. 2001;11:991–5. doi: 10.1016/s0960-9822(01)00271-8. [DOI] [PubMed] [Google Scholar]
- [108].Nagao K, Adachi Y, Yanagida M. Separase-mediated cleavage of cohesin at interphase is required for DNA repair. Nature. 2004;430:1044–8. doi: 10.1038/nature02803. [DOI] [PubMed] [Google Scholar]
- [109].Tsou MF, Wang WJ, George KA, Uryu K, Stearns T, Jallepalli PV. Polo kinase and separase regulate the mitotic licensing of centriole duplication in human cells. Dev Cell. 2009;17:344–54. doi: 10.1016/j.devcel.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Tsou MF, Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006;442:947–51. doi: 10.1038/nature04985. [DOI] [PubMed] [Google Scholar]
- [111].Bacac M, Fusco C, Planche A, Santodomingo J, Demaurex N, Leemann-Zakaryan R, et al. Securin and separase modulate membrane traffic by affecting endosomal acidification. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01169.x. [DOI] [PubMed] [Google Scholar]
- [112].Bembenek JN, White JG, Zheng Y. A role for separase in the regulation of RAB-11-positive vesicles at the cleavage furrow and midbody. Curr Biol. 2010;20:259–64. doi: 10.1016/j.cub.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]