Abstract
Environmental contamination with hexavalent chromium (CrVI) has been increasing in the drinking water of the USA and developing countries. CrVI causes various health problems including menstrual disorders and infertility. Recently, we reported that CrVI causes granulosa cell apoptosis through the intrinsic apoptotic pathway. Our previous studies showed that postnatal exposure to CrVI arrests follicle development. In order to explore the underlying mechanism, primary and immortalized granulosa cells from rats were treated with 10 μM potassium dichromate and analyses of the cell cycle, and cell cycle regulatory proteins were performed. CrVI decreased cell proliferation as a result of cell cycle arrest and down-regulated cyclin-dependent kinases (CDK), cyclins, and PCNA while up-regulating CDK-inhibitors and down-regulating FSH receptor and ERβ. Vitamin C mitigated the effects of CrVI. This study shows that CrVI causes cell cycle arrest in granulosa cells by altering cell cycle regulatory proteins with potential intervention by vitamin C.
Keywords: Hexavalent chromium, vitamin C, ovary, granulosa cells, cell cycle, cyclins, cyclin-dependent kinases, CDK inhibitors, FSH receptor, ERβ
1. Introduction
Hexavalent chromium (CrVI) is widely used in various industries such as chrome plating, welding, wood processing and tanneries [1]. The extensive toxicokinetic and genotoxic literature associated with oral exposures to CrVI in animals and humans indicate that CrVI causes various health hazards including cancers, dermatititis, damage to the liver and kidneys, infertility in both males and females, defects in embryo/fetal development, and developmental problems in young children [2–9]. Chromium usage is increasing exponentially worldwide; and Cr pollution is a continuous, ongoing problem [6–7, 9–10]. CrVI gets into water supplies after being discharged from steel and pulp mills as well as metal plating and leather-tanning facilities [10]. It can also pollute water through erosion of soil and rock [10]. According to the Blacksmith’s Institute, various drinking water sources such as lakes and rivers, and wells in developing countries are highly polluted with CrVI, where its concentration far exceeds the USEPA approved safer limits in drinking water of 0.05 mg/L [9]. Recent report from the Environmental Working Group (EWG) indicates increasing CrVI levels in 35 cities in the United States [10]. At least 74 million people in nearly 7,000 communities drink tap water polluted with total chromium; and 1.7 million people from 42 communities from New Jersey drink tap water polluted with total Cr which includes hexavalent and other forms of the metal in the USA [10].
Unlike most other divalent metal ions, CrVI is unique and specific and behaves like anion due to its structural similarity with anions (such as CrO 42− and HCrO 4 −, SO42−) [11]. Due to its anionic nature CrVI is rapidly transported into cells through anion transporters and reduced to CrIII by various antioxidants such as ascorbate (vitamin C), glutathione (GSH), cysteine and antioxidant enzymes [12–13]. CrIII has been considered as less toxic than CrVI. However, recent studies show that CrIII causes more damage to DNA in mice and yeast than the CrVI once sequestered in cells [14]. The nutrition supplement, CrIII picolinate, was reported to cause damage to liver and kidney damage [15–16]. Cr is predominantly excreted through the urine and feces. Cr can cross the placenta and is transported in the mother’s milk. This facilitates damage to developing fetuses (through placental transfer) and neonates (through mother’s milk) [17].
Vitamin C accounts for ~80% of CrVI reduction in target tissues such as lung, liver and kidney, being the fastest reducer of CrVI [6–7]. In general, the intracellular concentration of vitamin C is very high, and maximum levels of vitamin C are present in the ovary, brain and placenta (1–5 mM). Vitamin C is concentrated in the granulosa and theca cells of the follicle, luteal cells of the corpus luteum, and cytoplasm of the oocyte [18–19]. Vitamin C uptake by granulosa cells in rats is hormonally regulated. Vitamin C plays a vital role in follicular development, implantation and maintenance of healthy pregnancy and vitamin C deficiency in guinea pigs resulted in anovulation with marked follicular degeneration [20]. These animals also had increased rates of implantation failures, and increased spontaneous abortions; vitamin C has been considered as the fertility factor in primates as it prevents apoptosis in cultured ovarian follicles and inhibits follicular atresia [21]. Vitamin C acts as a potent water-soluble antioxidant in biological fluids by scavenging physiologically relevant reactive oxygen species and reactive nitrogen species [22]. These include free radicals such as hydroxyl radicals, aqueous peroxyl radicals, superoxide anion, and nitrogen dioxide, as well as non-radical species such as hypochlorous acid, ozone, singlet oxygen, nitrosating species, nitroxide, and peroxynitrite. Vitamin C interacts with free radicals scavenging ROS and reactive nitrogen species; it can also regenerate other antioxidants, such as β-tocopherol, glutathione (GSH), and β-carotene, from their respective radical species [23–25]. Therefore, vitamin C is not only a reducing agent of CrVI, it is also an essential antioxidant for female reproductive functions.
Our recent studies reported that CrVI down-regulated mRNA levels of steroidogenic enzymes, FSH and estrogen receptors [26], and induced apoptosis of granulosa cells through the intrinsic apoptotic pathway, and increased sub-cellular translocation of p53 and MAPKs ERK1/2 and JNK in granulosa cells in vitro [27]. Vitamin C exhibited a selective and time-dependent molecular intervention of CrVI effects in several signaling pathways that lead to granulosa cell apoptosis. In brief, vitamin C prevented, or at least mitigated CrVI-induced decrease in expression or activity of Bcl-2, Bcl-XL, and AKT proteins; activation and mitochondrial translocation of pro-apoptotic BAD, BAX; phosphorylation of ERK1/2 and its sub-cellular translocation into nucleus and mitochondria; and phosphorylation of p53 at multiple serine sites that lead to apoptosis of granulosa cells [27]. Therefore, vitamin C could be a potential intervention to prevent or reduce the toxic effects of CrVI on the ovary to preserve the fertility. Our previous study showed that lactational exposure to CrVI caused pubertal delay in F1 females, decreased ovarian steroidogenesis, reduced follicle number, and arrested follicular development at the secondary follicular stage [26, 28]. However, the underlying mechanism behind this delay in the development of follicles remains unknown. Therefore, we hypothesized that CrVI induces cell cycle arrest in granulosa cells by altering cell cycle regulatory proteins and vitamin C mitigates the toxic effects of CrVI, and the current study was designed to test this hypothesis.
In primordial follicles, the oocyte is surrounded by a single layer of non-dividing granulosa cells arrested in G0 phase of the cell cycle [29]. Primordial follicles leave this quiescent state and initiate a phase of slow growth in which the granulosa cells enter the cell cycle at an exceedingly slow rate. Interestingly, as these slowly dividing granulosa cells acquire responsiveness to FSH and LH and begin producing estradiol (E2), cell cycle progression is accelerated leading to granulosa cell proliferation that results in the formation of large pre-ovulatory follicles [29]. Injections of E2 followed by FSH to hypophysectomized rats stimulate granulosa cell proliferation and follicle growth to the pre-ovulatory stage, indicating the predominant role of FSH and E2 in granulosa cell proliferation [29]. ERβ is the predominant ER form expressed in granulosa cells of growing and mature follicles of the rodent ovary; and ERβ-null mice exhibit partial arrest of folliculogenesis with ovulatory dysfunction [30]. Thus, any impairment in the FSH/E2 synthesis and/or their signaling pathways should hinder cell cycle regulation, and ultimately failure of follicle development. Therefore, the first objective of the present study was to understand the effect of CrVI on granulosa cell proliferation and cell cycle progression.
Cell cycle progression and cell proliferation are controlled by cyclin dependent kinases (CDK), cyclins, and CDK inhibitors (CDKIs) [31]. Cyclin D2 binds with CDK-4/-6 and thereby activates cell cycle progression through the G1 phase of the cell cycle. Cyclin E binds with CDK-2 and regulates the G1-S-phase transition. Progression through S phase is regulated by cyclin A-CDK-1 association followed by the initiation of mitosis (M) by cyclin B-CDK-1 association. In contrast, CDKIs, p15, p16 and p27 block cell cycle progression by inactivating CDK cascades resulting in cell cycle arrest [31]. In cyclin D2-null mice, granulosa cell proliferation is impaired; the follicles remain small, with the failure of ovulation [29]. In p27-null mice, primordial-to-primary follicle transition is accelerated resulting in the premature depletion of ovarian follicles and infertility [32]. Therefore, the second objective of the current study was to better understand the mechanism behind CrVI-toxicity on granulosa cell cycle progression and cell proliferation by analyzing the expression of cell cycle regulatory proteins cyclins, CDKs and CDKIs.
In the ovary, E2 and FSH are essential signals for the growth of preovulatory follicles [32–33]. Each hormone acts via specific receptors and intracellular signaling pathways. Estrogens are known to be potent mitogens and increase the activity of CDK-2 and CDK-4, and expression of D-type cyclins of G1-S phase as well as decrease the levels of CDKIs [34]. E2 increases the levels of cyclins D1, D3 and E in the uterus [35], and expression of cyclins D2 and E in granulosa cells [36], with a reduction in levels of p27 [37]. Therefore, the third objective is to determine the effect of CrVI on FSH-receptor (FSHR) and ERβ in granulosa cells. We used primary cultures of rat granulosa cells (GC) and GC’s responses to CrVI toxicity was compared with a spontaneously immortalized rat granulosa cell line (SIGC) [38] to evaluate the suitability of SIGC as a model system to study the effect of heavy metal toxicity on granulosa cells.
2. Materials and methods
2.1. Chemicals
The reagents used in this study were purchased from the following suppliers: Antibiotic-antimycotic, Trypsin–EDTA (Invitrogen Life Technologies Inc., Carlsbad, CA); fetal bovine serum (Hyclone, Logan, UT); and tissue culture dishes and plates (Corning Inc., Corning, NY); potassium dichromate, ascorbate, Dulbecco’s Modified Eagle’s Medium (DMEM)-F12 (Sigma-Aldrish, Saint Louis, MO); QuantiTect reverse-transcription kit (Cat# 205311) and QuantiTect SYBR Green PCR Kit (Cat # 204141, Qiagen, Valencia, CA). Other chemicals used were molecular biology grade available from Fisher Scientific (Pittsburgh, PA) or Sigma-Aldrich (St. Louis, MO). Antibodies were from Cell Signaling Technology (Danvers, MA).
2.2. Animals
Animal use protocols were approved by the Animal Care and Use Committee of Texas A&M University and were in accordance with the standards established by Guiding Principles in the Use of Animals in Toxicology and Guidelines for the Care and Use of Experimental Animals by National Institutes of Health. Immature female Sprague-Dawley rats (23–25 days old) were euthanized by CO2 asphyxiation followed by cervical dislocation and ovaries were collected in DMEM-F12 media.
2.3. Cell Culture
2.3.1. Granulosa cell isolation from the ovary and primary culture
Granulosa cells (GC) from 50 ovaries collected from 25 rats were harvested as previously described [27, 39]. Briefly, ovaries were cleared from the surrounding fat under a stereo dissection microscope and punctured with 25-gauge needles. Cells were collected in phenol red-free DMEM-F12 containing 0.2% BSA, 10 mM HEPES, and 6.8 mM EGTA, incubated for 15 min at 37 C, and centrifuged for 5 min at 250 × g. The pellets were suspended in a solution containing 0.5 M sucrose, 0.2% BSA, and 1.8 mM EGTA in DMEM-F12 and incubated for 5 min. After incubation, the suspension was diluted with 3 vol DMEM-F12, centrifuged at 250 × g for 5 min and treated sequentially with trypsin (20 μg/ml) for 1 min, 300 μg/ml soybean trypsin inhibitor for 5 min, and DNase I (100 μg/ml) for 5 min at 37º C. The cells were washed with media and suspended in DMEM-F12. Cells were cultured in DMEM-F12 supplemented with 10% Dextran-Charcoal coated (steroid-stripped) Fetal Bovine Serum (DC-FBS), 20 mM HEPES (pH 7.4), 4 mM glutamine, 100 IU penicillin/ml, 100 μg/ml streptomycin and amphotericin-B (2.5 μg/ml) in a humidified atmosphere with 95% air, 5% CO2 at 37°C. To perform the vitro experiments, dishes were then divided into six groups with three dishes per group. Each group represents treatment as described below.
2.3.2. Culture of spontaneously immortalized rat granulosa cells
A spontaneously immortalized rat granulosa cell line (SIGC) [38] were cultured in DMEM-F12 (Sigma, Saint Louis, MO) containing 15 mM HEPES and supplemented with 5% DC-FBS (Hyclone, Logan, UT), penicillin 100 U/ml, streptomycin (100 μg/ml) and amphotericin-B (2.5 μg/ml) in a humidified atmosphere with 95% air, 5% CO2 at 37°C.
2.4. In vitro experimental design for CrVI treatment and vitamin C intervention
Chromium levels in worst polluted places in developing countries have been recorded as high as 31 mg Cr/L water, which is 640 times more than the USEPA approved safety levels of Cr in drinking water (0.05 mg/Liter water) [26, 40–41]. We have used 10 μM dose in the current study, ie., 2.94 mg/L base media, which is approximately 10.5 times lesser than the chromium levels in drinking water from the worst polluted area. As stated in the introduction, vitamin C is one of the most potent antioxidants, and reduces >86% of CrVI into CrIII. Therefore, we chose vitamin C to inhibit/prevent toxic effects of CrVI. Dose – response experiments with different doses of CrVI from 1 – 200 μM were performed and IC50 was calculated [42]. The chosen dose 10 μM is the IC50 value for CrVI response in granulosa cells. GC or SIGC were pre-treated with different doses of vitamin C from 0.1 mM – 5 mM for 24 h, followed by treated with CrVI (data not shown). Dose of 1 mM was chosen as the half-maximal effective dose (EC50) to mitigate toxic effect of CrVI on cell proliferation within a 24 h period. Also, vitamin C concentration 1mM is the average physiological dose in mammalian cells.
At 70% confluency, GC and SIGC were incubated in DMEM-F12 supplemented with 2% DC-FBS (base medium) in the presence or absence of vitamin C and divided into six treatment groups: (1) Control: cells were treated with base medium; (2) CrVI-12 h: cells were treated with 10 μM potassium dichromate for 12h in base medium; (3) CrVI -24 h: cells were treated with 10 μM potassium dichromate for 24 h in base medium; (4) Vitamin C: cells were treated with 1 mM vitamin C for 24 h in base medium; (5) vitamin C + CrVI – 12 h: cells were pre-treated with 1 mM vitamin C for 24 h and treated with 10 μM potassium dichromate for 12 h in base medium; (6) vitamin C + CrVI – 24 h: cells were pre-treated with 1 mM vitamin C for 24 h and treated with 10 μM potassium dichromate for 24 h in base medium. After the treatment, cells were harvested using 0.1% trypsin-EDTA and total protein and RNA were isolated [17–18]. All treatments were performed in triplicates on the same day and each experiment was repeated three times on different days.
2.5. Cell proliferation
GC and SIGC were cultured in 6-well tissue culture plates (Corning), and treated with CrVI with or without vitamin C pre-treatment as indicated in sections 2.3.1, 2.3.2, and 2.4. At 60–70% confluency, cells were incubated in DMEM/F12 supplemented with 2% DC-FBS with or without 1 mM vitamin C for 24 h; and treated with CrVI (potassium dichromate, (10 μM) for 48 h. After 48 h of CrVI treatment, cells were harvested using 0.1% trypsin-EDTA and cell numbers were determined using a Coulter counter [26].
2.6. Cell cycle analysis
GC and SIGC cells were cultured in T-75 flasks as indicated in sections 2.3.1, and 2.3.2 and treated with CrVI with or without vitamin C pre-treatment as described in section 2.4. Cells were fixed in 1% buffered paraformaldehyde saline for 15 minutes on ice, and then fixed in ice cold 70% ethanol and kept at −20°C for 30 minutes. Cells were rehydrated in phosphate-buffered saline (PBS) for 15 minutes, treated with DNase-free RNase (100 μg/mL), and stained with propidium iodide (25 μg/mL) in staining buffer (100 mM Tris, pH 7.4, 150 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2, 0.1% Nonidet P-40) for 30 minutes at room temperature. The number of cells distributed in G1, S, and G2–M phases of the cell cycle was determined by fluorescence-activated cell sorter (FACS) analysis of propidium-stained cells distribution using a flow cytometer (FACS Caliber; Becton Dickinson, San Jose, CA) and ModFit LT program (Verity Software House). Data are expressed as mean ± SEM of three independent experiments.
2.7. Protein extraction and immunoblotting
After the CrVI treatment with or without vitamin C pre-treatment, total protein from granulosa cells was isolated and immunoblotting/western blotting was performed as described previously [27]. Briefly, the cells were harvested using 1% Trypsin-EDTA and pelleted. The cell lysates were sonicated in sonication buffer which consisted of 20 mM Tris-Hcl, 0.5 mM EDTA, 100 μM DEDTC, 1% Tween, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail tablets: complete EDTA-free (1 tablet/50 ml) and PhosStop (1 tablet/10 ml). Sonication was performed using a Microson ultrasonic cell disruptor (Microsonix Incorporated, Farmingdale, NY). Protein concentration was determined using the Bradford method and a Bio-Rad Protein Assay kit. Protein samples (75 μg) were resolved using 7.5%, 10% or 12.5% SDS-PAGE. Chemiluminescent substrate was applied according to the manufacturer’s instructions (Pierce Biotechnology, Rockford, IL). The blots were exposed to Blue X-Ray film and densitometry of autoradiograms was performed using an Alpha Imager (Alpha Innotech Corporation, San Leandro, CA).
2.8. Real-Time Reverse Transcription (RT)-Polymerase Chain Reaction (PCR)
Total RNA was isolated using TRIZOL reagent according to manufacturer’s instructions. The ratio of absorbance was measured at 260 nm and 280 nm (NanoDrop™ 8000 Spectrophotometer), and samples with a ratio of 1.8 – 2.0 were considered pure and included for the RT-PCR analysis. The first strand cDNA was synthesized using 1 μg total RNA by QuantiTect® RT kit according to manufacturer’s instructions. Two μl cDNA was mixed with 10μl master mix (2X reaction buffer, dNTP mix, Hot Gold Star® DNA polymerase, MgCl2 and Sybr®Green dye), sense and anti-sense oligonucleotide primers for FSHR, ERβ and the internal control β-actin in a total reaction volume of 20 μl with RNase free water. The following primer sequences were used: FSHR: 5′-AGAGAGGCATCCTGACCCTCA AGTACC-3′ (forward), 3′–CACGTAGCAGAGCTTCTCCTTGATGTC–5′ (reverse); ERβ: 5′–CCA GCCCTGTTACTAGTCCAAACG–3′ (forward), 3′–GCTTTCTAAGAGCCGGACTTGGTCC–5′ (reverse), β-Actin: 5′-AGAGAGGCATCCTGACCCTCAAGTACC-3′ (forward), 3′–CACGTAGCA GAGCTTCTCCTTGATGTC–5′ (reverse). The reaction cycles were as follows: PCR enzyme initial activation at 95°C for 15 min.; initial denaturation at 94°C for 15 sec, annealing at 56°C for 30 sec and elongation at 72°C for 30 sec. All reactions were run in triplicate. The reaction was carried out with a Staratagene MX3000P real-time PCR machine. The fold differences were calculated by normalizing the relative expression of gene of interest with β-actin and the results expressed as fold changes.
Statistical analyses
All numerical data were subjected to one-way ANOVA to detect the effects of treatment and time interactions. Tukey-Kramer HSD test was used to adjust for multiple pair-wise comparisons of means. Least squares regression analysis was used to determine effects of treatment (Control, CrVI, Vitamin C, CrVI + Vitamin C); time (CrVI 12 h, CrVI 24 h) and treatment × time interactions. Each value is the mean ± SEM from 3 different plates per treatment, cultured using 50 ovaries collected from 25 immature rats. Similar results were obtained in three different experiments performed on three different days/time. P < 0.05 was considered to be significant. Statistical analyses were performed using general linear models of Statistical Analysis System (SAS, Cary, NC).
3. Results
3.1. CrVI decreased granulosa cell proliferation
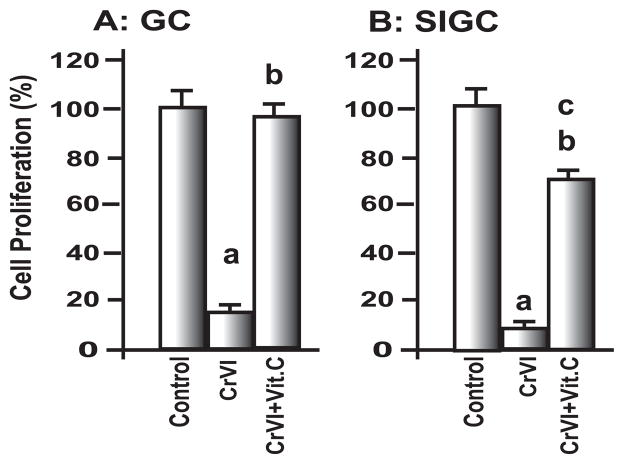
CrVI significantly (P<0.05) decreased proliferation of both primary cultures of rat granulosa cells, GC and the immortalized cell line, SIGC. Vitamin C prevented the effect of CrVI on GC cell proliferation and mitigated the adverse effect of CrVI on cell proliferation in SIGC (Fig. 1A & 1B).
Fig. 1. Effect of CrVI on granulosa cell proliferation.
Cell proliferation was assessed as described in Materials and Methods. Granulosa cells A, GC and B, SIGC were pre-treated with or without vitamin C (1 mM) and treated with CrVI (10μM) for 36 h, harvested and cell number was determined. Each value is the mean ± SEM from 3 different plates per treatment. P<0.05. a: Control vs CrVI; b: CrVI vs CrVI + Vitamin C; c: Control vs CrVI + Vitamin C.
3.2. CrVI induced cell cycle arrest in GC and SIGC
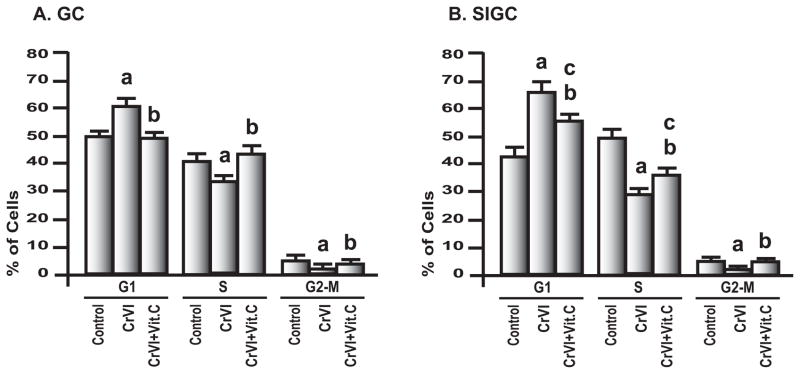
In order to understand CrVI-induced decrease in cell proliferation, effects of CrVI on cell cycle were determined. Results indicated that treatment with CrVI induced (i) cell cycle arrest in G1-phase progression; (ii) decreased cell population in S-phase; and (iii) decreased cell population in G2-M check-point in both GC and SIGC. However, there were variable effects of vitamin C between these two cells. In GC, vitamin C prevented CrVI effect in G1, S and G2-M phase check-points while vitamin C significantly (P<0.05) mitigated CrVI effects on G1 and S-phase check-points and prevented CrVI effect in G2-M check-point on SIGC (Fig. 2A & 2B). In order to obtain additional insight into CrVI effect on cell cycle regulation, we investigated the effects of CrVI on cell cycle regulatory proteins.
Fig. 2. Effect of CrVI on cell cycle progression.
Effects of CrVI on A, GC and B, SIGC cell cycle progression were analyzed as described in Materials and Methods. GC and SIGC were cultured; pre-treated with basal media or vitamin C (1 mM) for 24 h; and treated with CrVI (10 μM) for 24 h. Cell distributions in G1, S and G2-M phases of cell cycle are expressed as the mean ± SEM of three independent experiments, P<0.05. a: Control vs CrVI; b: CrVI vs CrVI + Vitamin C; c: Control vs CrVI + Vitamin C.
3.3. CrVI down-regulated CDKs in GC and SIGC
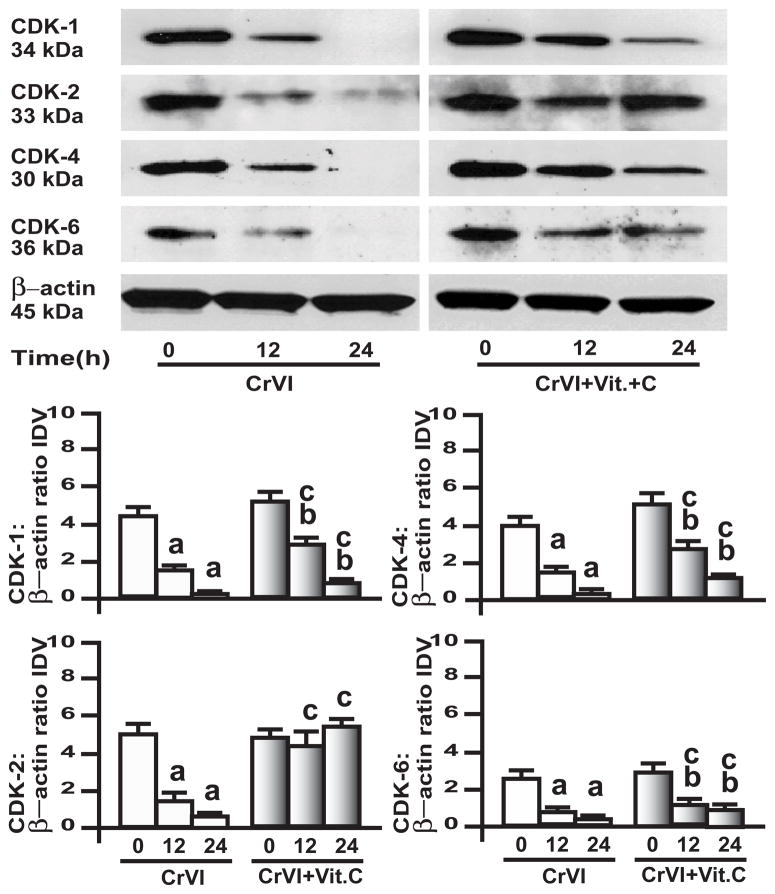
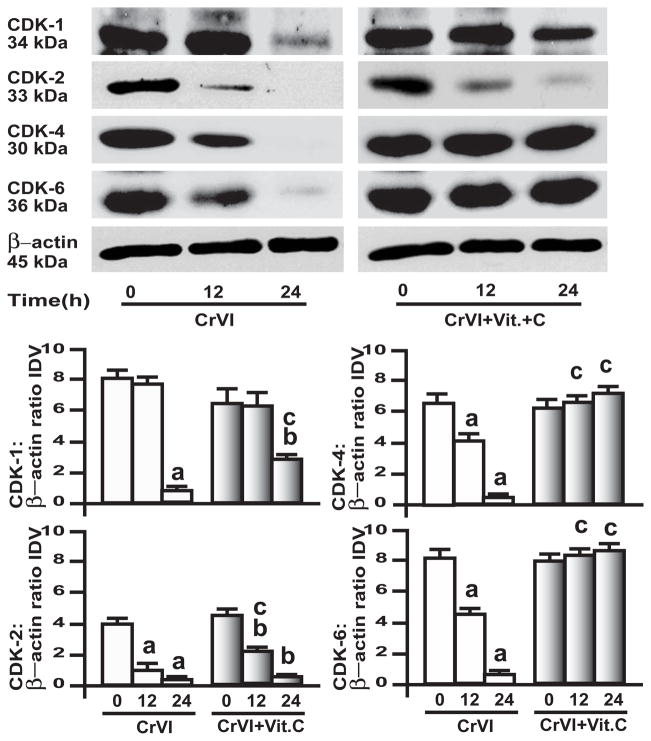
Association between cyclin D1-3/CDK-4 and -6; cyclin E/CDK-2; and cyclins A and B/CDK-1 is important in G1 phase, G1-S phase transition, and G2-M phase, respectively [31]. Results showed that CrVI down-regulated CDK-1, -2, -4 and -6 in both GC (Fig. 3) and SIGC (Fig. 4) in a time-dependent manner. In GC, vitamin C mitigated CrVI effect on CDK-1, CDK-4 and CDK-6, and prevented CrVI effect on CDK-2 (Fig. 3). In SIGC, vitamin C mitigated CrVI effect on CDK-1, prevented CrVI effect on CDK-4 and CDK-6, but did not change CrVI effect on CDK-2 (Fig. 4).
Fig. 3. Effect of CrVI on cyclin dependent kinases (CDK) in primary cultures of granulosa cells, GC.
GC were pre-treated with or without vitamin C (1 mM) for 24 h and treated with CrVI (10 μM) for 0 h, 12 h and 24 h period. Western blots were performed with 75 μg of protein, as described in Materials and Methods section. A, Representative western blots of CDK-1, CDK-2, CDK-4 and CDK-6 and β-actin. B, Histograms of Integrated Density Value (IDV) for each protein, normalized toβ-actin Each value is the mean ± SEM from 3 different plates per treatment, P<0.05; a: CrVI-treatment, 0 h vs 12 h or 24 h; b: Vitamin C vs CrVI+Vitamin C-treatment, 0 h vs 12 h or 24 h; c: CrVI (12 h or 24 h) vs CrVI+Vitamin C (12 h or 24 h).
Fig. 4. Effect of CrVI on cyclin dependent kinases (CDK) in immortalized granulosa cells, SIGC.
SIGC were pre-treated with or without vitamin C (1 mM) for 24 h and treated with CrVI (10 μM) for 0 h, 12 h and 24 h period. Western blots were performed with 75 μg of protein, as described in Materials and Methods section. A, Representative western blots of CDK-1, CDK-2, CDK-4 and CDK-6 and βactin. B, Histograms of Integrated Density Value (IDV) for each protein, normalized toβ-actin. Each value is the mean ± SEM from 3 different plates per treatment, P<0.05; a: CrVI-treatment, 0 h vs 12 h or 24 h; b: Vitamin C vs CrVI+Vitamin C-treatment, 0 h vs 12 h or 24 h; c: CrVI (12 h or 24 h) vs CrVI+Vitamin C (12 h or 24 h).
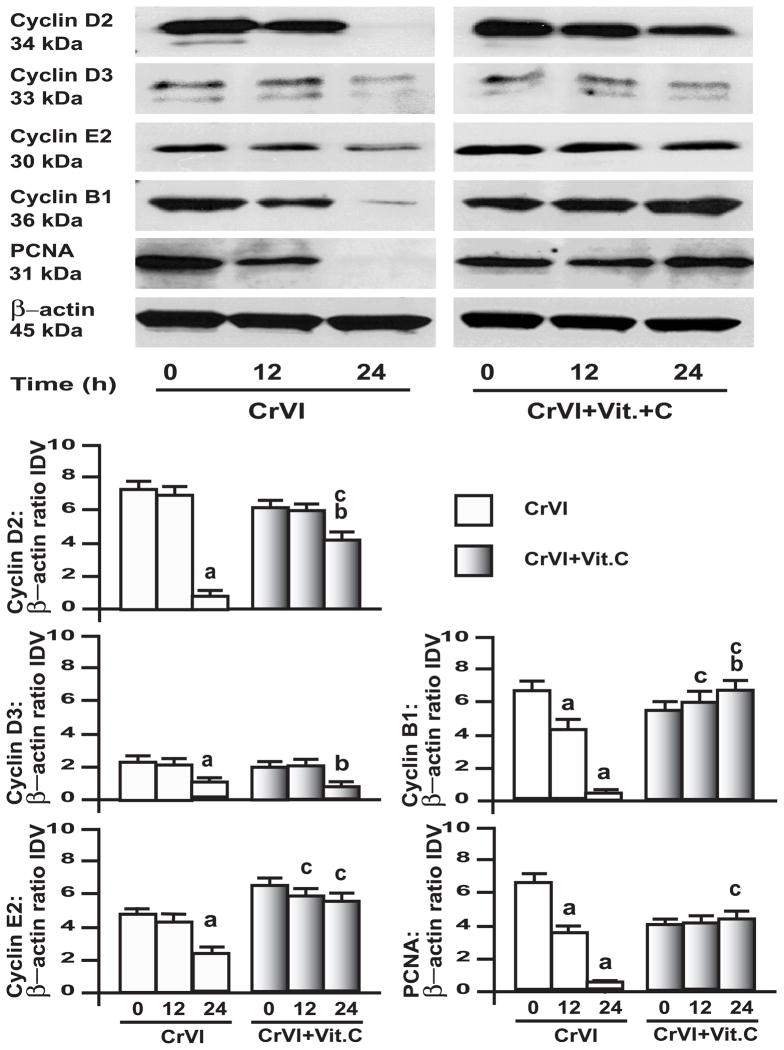
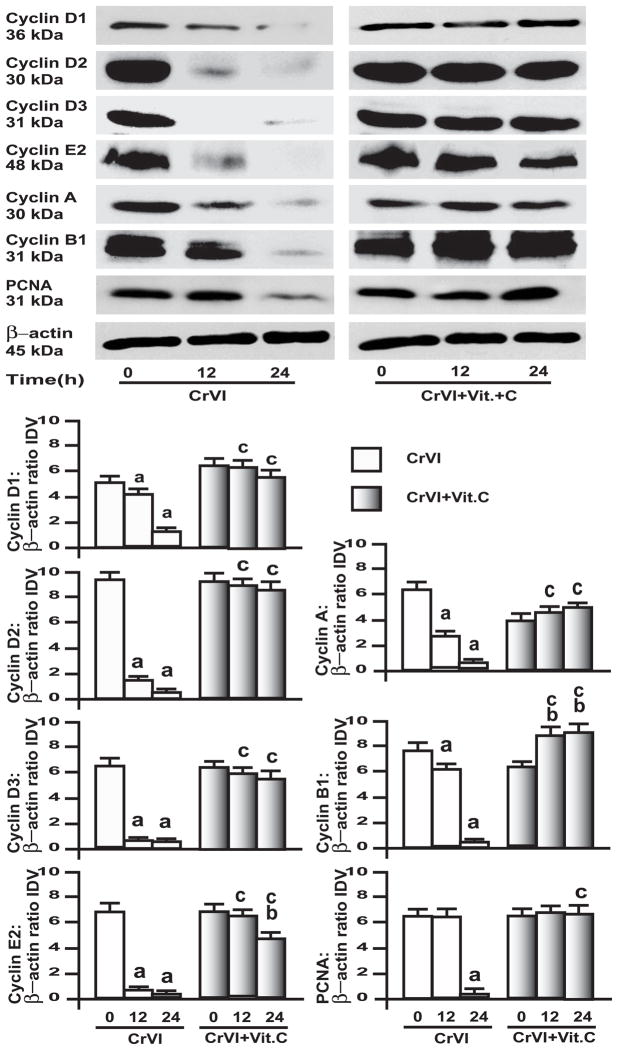
3.4. CrVI down-regulated cyclins and proliferating cell nuclear antigen (PCNA) in GC and SIGC
The effects of CrVI on cyclins and PCNA in GC and SIGC were examined. In GC, CrVI down-regulated cyclins D2, D3, E2 and B1 and PCNA, as well as PCNA; whereas, cyclins D1 and A were undetectable in GC. Vitamin C mitigated the effect of CrVI on cyclin D2 and PCNA; prevented CrVI effect on cyclins E2 and B1; and did not change CrVI effect on D3 (Fig. 5). In SIGC, CrVI down-regulated cyclins D1, D2, D3, E2, A, and B1; and vitamin C prevented CrVI-induced down-regulation of cyclins D1, D2, D3, B1, and PCNA; and mitigated CrVI effect on cyclins E2 and A (Fig. 6).
Fig. 5. Effect of CrVI on cyclins and PCNA in primary cultures of granulosa cells, GC.
GC were pre-treated with or without vitamin C (1 mM) for 24 h and treated with CrVI (10 μM) for 0 h, 12 h and 24 h period. Western blots were performed with 75 μg of protein, as described in Materials and Methods section. A, Representative western blots of cyclins D2, D3, E2, B1, PCNA andβ-actin. B, Histograms of Integrated Density Value (IDV) for each protein, normalized toβ-actin. Each value is the mean ± SEM from 3 different plates per treatment, P<0.05; a: CrVI-treatment, 0 h vs 12 h or 24 h; b: Vitamin C vs CrVI+Vitamin C-treatment, 0 h vs 12 h or 24 h; c: CrVI (12 h or 24 h) vs CrVI+Vitamin C (12 h or 24 h).
Fig. 6. Effect of CrVI on cyclins and PCNA in immortalized granulosa cells, SIGC.
SIGC were pre-treated with or without vitamin C (1 mM) for 24 h and treated with CrVI (10 μM) for 0 h, 12 h and 24 h period. Western blots were performed with 75 μg of protein, as described in Materials and Methods section. A, Representative western blots of cyclins D1, D2, D3, E2, A, B1, PCNA and b-actin. B, Histograms of Integrated Density Value (IDV) for each protein, normalized toβ-actin. Each value is the mean ± SEM from 3 different plates per treatment, P<0.05; a: CrVI-treatment, 0 h vs 12 h or 24 h; b: Vitamin C vs CrVI+Vitamin C-treatment, 0 h vs 12 h or 24 h; c: CrVI (12 h or 24 h) vs CrVI+Vitamin C (12 h or 24 h).
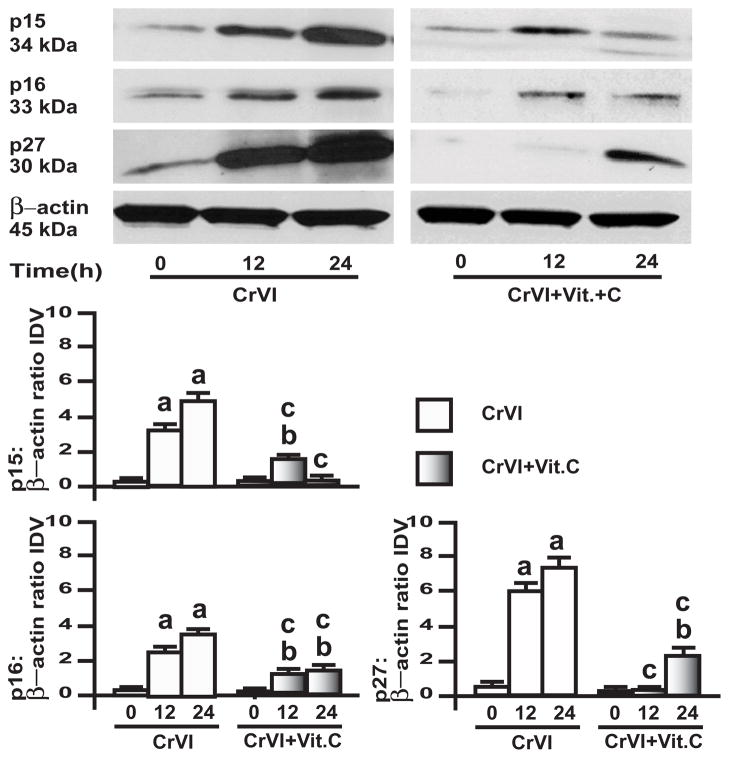
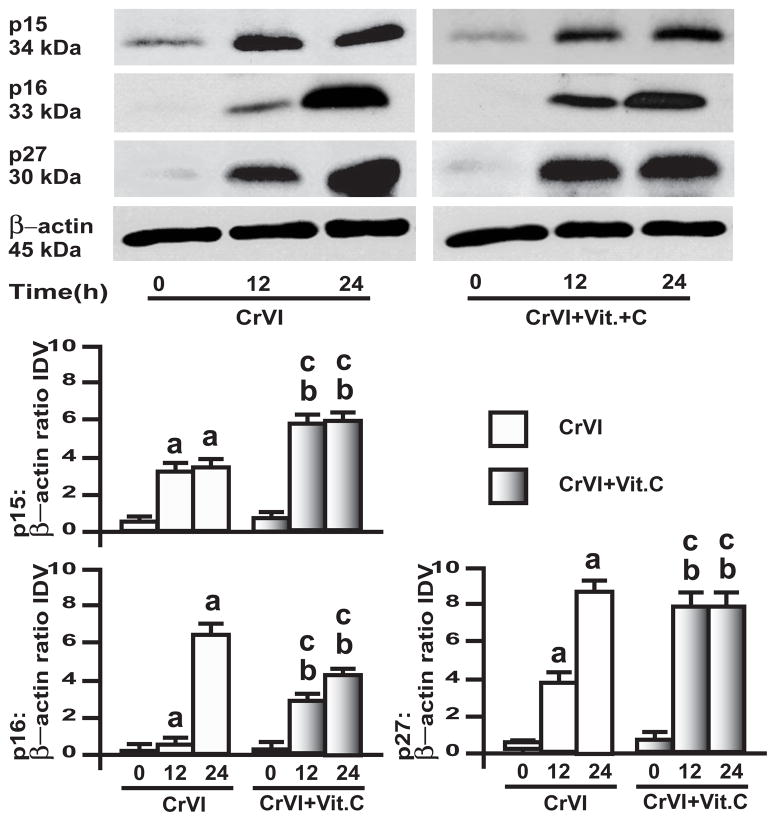
3.5. CrVI up-regulated cyclin dependent kinases p15, p16 and p27 in GC and SIGC
CDK inhibitors are one of the major determining regulators in primordial to primary follicle transition [32]. Expression of p16 and p27 is negatively correlated with the expression of cyclin D2 in rodent ovary [43]. Moreover, CDKIs regulate CDKs during cell cycle progression [31]. Therefore, CrVI effects on CDKIs in GC and SIGC were investigated. In GC, CrVI up-regulated CDKIs p15, p16 and p27 in a time-dependent manner, and vitamin C mitigated the effect of CrVI (Fig. 7). In SIGC, CrVI up-regulated p15, p16 and p27 in a time-dependent manner; and vitamin C mitigated CrVI effect only on p16 at 24 h time point, but did not alter the CrVI effect on p15, and p27 (Fig. 8).
Fig. 7. Effects of CrVI on cyclin dependent kinase inhibitors (CDKIs) in primary cultures of granulosa cells, GC.
GC were pre-treated with or without vitamin C (1 mM) for 24 h and treated with CrVI (10 μM) for 0 h, 12 h and 24 h period. Western blots were performed with 75 μg of protein, as described in Materials and Methods section. A, Representative western blots of CDKIs p15, p16 and p27 and b-actin. B, Histograms of Integrated Density Value (IDV) for each protein, normalized to βactin. Each value is the mean ± SEM from 3 different plates per treatment, P<0.05;; a: CrVI-treatment, 0 h vs 12 h or 24 h; b: Vitamin C vs CrVI+Vitamin C-treatment, 0 h vs 12 h or 24 h; c: CrVI (12 h or 24 h) vs CrVI+Vitamin C (12 h or 24 h).
Fig. 8. Effects of CrVI on cyclin dependent kinase inhibitors (CDKIs) in immortalized granulosa cells, SIGC.
SIGC were pre-treated with or without vitamin C (1 mM) for 24 h and treated with CrVI (10 μM) for 0 h, 12 h and 24 h period. Western blots were performed with 75 μg of protein, as described in Materials and Methods section. A, Representative western blots of CDKIs p15, p16 and p27 and β-actin. B, Histograms of Integrated Density Value (IDV) for each protein, normalized toβ-actin. Each value is the mean ± SEM from 3 different plates per treatment, P<0.05; a: CrVI-treatment, 0 h vs 12 h or 24 h; b: Vitamin C vs CrVI+Vitamin C-treatment, 0 h vs 12 h or 24 h; c: CrVI (12 h or 24 h) vs CrVI+Vitamin C (12 h or 24 h).
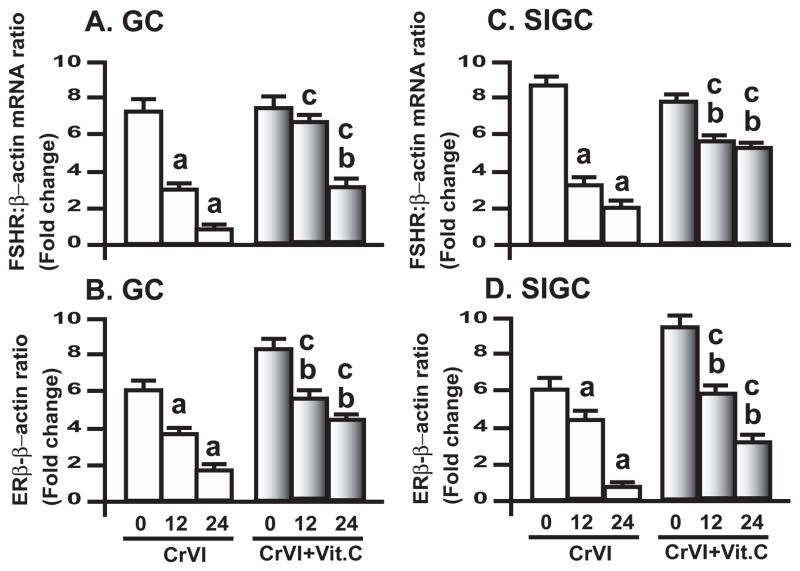
3.6. CrVI down-regulated FSHR mRNA and ERβ mRNA in GC and SIGC
FSH and E2 not only regulate granulosa cell proliferation but they also inhibit granulosa cell apoptosis [44]. Therefore, in order to understand the mechanism underlying CrVI-induced delay/arrest in cell cycle check-points, and deregulation of cell cycle regulatory proteins, mRNA levels of FSH-receptor (FSHR), and E2-receptor (ERβ) in GC and SIGC were examined. CrVI down-regulated FSHR mRNA and ERβ mRNA in GC and SIGC; and vitamin C mitigated CrVI effect in both GC and SIGC (Fig. 9A–D).
Fig. 9. Effect of CrVI on FSHR and ERβ in GC and SIGC.
GC and SIGC were pre-treated with or without vitamin C (1 mM) for 24 h and treated with CrVI (10 μM) for 0 h, 12 h and 24 h period. Total RNA was isolated and real-time qPCR was performed as described in Materials and Methods section. Histograms showing A, FSHR mRNA levels in GC; B, ERβ mRNA levels in GC; C, FSHR mRNA levels in SIGC; D, ERβ mRNA levels in SIGC. The fold differences were calculated by normalizing the relative expression of gene of interest with β-actin and the results were expressed as fold changes. Each value is the mean ± SEM from 3 different plates per treatment, P<0.05; a: CrVI-treatment, 0 h vs 12 h or 24 h; b: Vitamin C vs CrVI+Vitamin C-treatment, 0 h vs 12 h or 24 h; c: CrVI (12 h or 24 h) vs CrVI+Vitamin C (12 h or 24 h).
4. Discussion
Our previous study demonstrated that lactational exposure to CrVI delayed puberty and decreased steroidogenesis in F1 female rats [26]. CrVI induced a decrease in follicle number and delay/arrest in follicle development at the secondary stage, accompanied by a decrease in E2 levels. In general, antral follicles develop in rats between PND-9 and PND-20 [45]. However, in CrVI-exposed females of the F1 generation, follicle development was arrested at the preantral stage, without further development into antral follicles [26]. Withdrawal of CrVI exposure after PND-21 resulted in the normal development of antral follicles by PND-45 [26]. Moreover, it is evident from our previous study that CrVI down-regulated FSHR, 17β-HSD-1, -2, SF-1, ERβ and ERβ in the ovarian granulosa cells in vitro, and vitamin C mitigated these effects [26]. In order to understand the mechanism behind CrVI-induced delay in follicle development/differentiation, the effect of CrVI on cell proliferation, cell cycle regulation, and levels of cyclins, CDKs, and CDKIs, as well as FSHR and ERβ were monitored in the present study. Data indicate that CrVI decreased proliferation and induced cell cycle arrest at the G1 phase, decreased cell population in S phase and G2-M phase in both GC and SIGC cells. Interestingly, as given in Table-1, cellular response to CrVI-induced toxicity is very similar in both GC and SIGC, only the mitigative effects of vitamin C varied some what between GC and SIGC, in terms of cell proliferation, cell cycle regulation at G1-S phase check-point, as well as regulation of CDKIs p15 and p27. While vitamin C prevented the CrVI-induced decrease in cell proliferation, cell cycle arrest at the G1-S phase and decrease in cyclins and CDKs, and increase in CDKIs p15, p16 and p27 in GC, it mitigated (partial intervention) CrVI-induced effects in SIGC. Interestingly, vitamin C did not change CrVI-induced inhibition of p15 and p27 in SIGC.
Table 1.
Comparison between rat granulosa cell primary cultures (GC) and immortalized rat granulosa cell line (SIGC) on the effect of CrVI and intervention of vitamin C.
| End points | Effect of CrVI | Intervention of vitamin C on CrVI effect | ||
|---|---|---|---|---|
| GC | SIGC | GC | SIGC | |
| Cell proliferation | ↓ | ↓ | + + | + |
| G1-S phase arrest | ↑ | ↑ | + + | + |
| Cell population in S-phase | ↓ | ↓ | + + | + |
| Cell population in G2-M phase | ↓ | ↓ | + + | + + |
|
| ||||
| Cyclin-Dependent Kinases (CDKs): | ||||
| CDK-4 | ↓ | ↓ | + | + + |
| CDK-6 | ↓ | ↓ | + | + + |
| CDK-2 | ↓ | ↓ | + + | — |
| CDK-1 | ↓ | ↓ | + | + |
|
| ||||
| Cyclins: | ||||
| Cyclin D1 | ND | ↓ | ND | + + |
| Cyclin D2 | ↓ | ↓ | + | + + |
| Cyclin D3 | ↓ | ↓ | — | + + |
| Cyclin E2 | ↓ | ↓ | + + | + |
| Cyclin A | ND | ↓ | ND | + |
| Cyclin B1 | ↓ | ↓ | + + | + + |
| PCNA | ↓ | ↓ | + | + + |
|
| ||||
| CDK inhibitors: | ||||
| p15 | ↑ | ↑ | + + | — |
| p16 | ↑ | ↑ | + + | + |
| p27 | ↑ | ↑ | + | — |
|
| ||||
| FSH-Receptor | ↓ | ↓ | + | + |
| Estrogen Receptor-β | ↓ | ↓ | + | + |
[↓] - Down-regulated; [↑] - Up-regulated; [+]- Mitigated; [+ +] - Prevented/Inhibited; [—] No change; [ND]-Not detected or not expressed.
CrVI induced cell cycle arrest in GC and SIGC at the G1 phase [31]. As noted previously, the G1 phase check-point is regulated by association between CDK-4/-6, and cyclins D1, D2 and D3 [31]. In the ovary, cyclin D2 is the predominant cyclin in granulosa cells, whereas D1 and D3 are the major candidates in theca cells [29]. In GC, CrVI decreased protein levels of G1-S phase regulators CDK-4, -6 and cyclins D2 and D3, and vitamin C mitigated CrVI effects on CDK-4, -6 and cyclin D2. Similar to results in GC, CrVI significantly decreased CDK-4, -6 and cyclins D1, D2 and D3 in SIGC, and vitamin C mitigated CrVI effects on CDK-1, prevented CrVI effects on CDK-4 and CDK-6; and also prevented CrVI-induced down-regulation of cyclins D1, D2, D3 and PCNA. These data suggest that accumulation of GC and SIGC at the G1-phase is due to down-regulation of CDK-4,-6/D-type cyclins. Also, efficacy of vitamin C to mitigate or prevent CrVI-induced toxicity on G1-phase CDKs and cyclins is similar in GC and SIGC.
CrVI decreased the S-phase cell populations both in GC and SIGC. Similar to the results from G1-phase, vitamin C prevented CrVI effects on S phase check point in GC and mitigated CrVI effects in SIGC. The association of cyclin E with CDK2 is active at the G1/S transition and directs entry into S phase. During the G1-S phase transition, cyclin E binds to CDK-2, and thus hyperphosphorylates Rb, resulting in the activation of transcription factors and S-phase proteins such as thymidylate synthase and dihydrofolate reductase. S-phase progression is directed by the cyclin A/CDK2 complex [31]. In order to understand the mechanism behind the CrVI-induced decrease in cell population in the S-phase in GC and SIGC, cylins E2, A, and their counterpart CDK-2 levels were estimated. As noted in the G1 phase check-point, CrVI down-regulated cyclin E2 and CDK-2 in both GC and SIGC in a similar pattern. Although cyclin A was undetectable in GC, it was abundant in SIGC, down-regulated by CrVI, and mitigated by vitamin C in CrVI-exposed cells. Vitamin C also mitigated CrVI effects on cyclin E2 and its counterpart CDK-2 in GC; and cyclin E2 in SIGC.
Further, we detected the effect of CrVI on CDK inhibitors p15, p16 and p27. p27 negatively regulates cyclin E/CDK2 and cyclin A/CDK2 complexes, and is a predominant CDK-inhibitor in the mammalian ovary which tightly regulates primordial to primary follicle transition. The p27-knock out mouse has an accelerated primordial to primary follicle transition that exhausts follicle reserve prematurely leading to infertility [46]. Interestingly, CrVI up-regulated p15, p16 and p27 in both GC and SIGC in a similar manner. Vitamin C mitigated CrVI effects on p15, p16 and p27 in GC, and p16 alone in SIGC. There are other CDKIs such as p18, p19, p57 and p21 that inhibit CDK-4/-6, and CDK-2, respectively. At present, we do not know whether vitamin C mitigates CrVI effects on p18, p19, p57 and/or p21. Collectively, these data suggest that failure of vitamin C to mitigate CrVI effect on CDK-2 and p27 could be the major reasons behind partial intervention of vitamin C against CrVI in SIGC compared to GC in the progression of cells in S-phase check point.
PCNA is required during DNA replication [47], and is expressed in the nuclear matrix of cells during all phases of the cell cycle, reaching a maximum in S and G2-phase [48]. Expression of PCNA in granulosa cells begins upon the formation of a primary follicle and its level of expression increases during the FSH-dependent stages of preovulatory follicular development [49]. CrVI decreased PCNA in both GC and SIGC, and vitamin C mitigated the CrVI-induced decrease in PCNA in both GC and SIGC, suggesting that the CrVI-induced decrease in follicle number, and delay/arrest in follicle development at the secondary follicular stage [26] might also be due to the decrease in PCNA levels, in addition to its effects on cell cycle machinery.
We next determined the effect of CrVI on the G2-M phase check point in GC and SIGC. The association of cyclin A with CDK1 (also known as cdc2) is important in G2 while CDK1/cyclin B is necessary for mitosis to occur [50]. CrVI decreased cell populations in the G2-M phase in both GC and SIGC, and vitamin C mitigated the effect of CrVI in both GC and SIGC. The current study shows that CrVI decreased cyclin B1/CDK-1 in GC and cyclins A, B1 and CDK-1 in SIGC which was mitigated by vitamin C. This shows that CrVI delayed cells from entering into, and progressing through the G2-M phase by decreasing the levels of cyclins A and B1, and CDK-1 in GC and SIGC, which could be prevented by vitamin C. Collectively, our data suggests that the response of both GC and SIGC to CrVI toxicity is very similar, while vitamin C intervention on CrVI is complete in GC and partial in SIGC. This is because of the immortalized nature of SIGC which possess defects in certain cell-cycle check-point mechanisms.
In hypophysectomized rats, cyclin D2 mRNA and protein were increased in granulosa cells by treatment with E2 or FSH. In serum-free cultures of rat granulosa cells, cyclin D2 mRNA was rapidly elevated in response to E2, FSH and forskolin [29]. Follicles of either cyclin D2-null mice or FSH-null mice [51] do not proceed beyond the secondary stage of follicle development. FSH promotes proliferation of granulosa cells and development of pre-ovulatory follicles by activating more than 100 different target genes including cyclin D2 and PCNA [33]. FSH dissociates FOXO1 (a transcriptional repressor) from cyclin D2-promoter and increases transcription of cyclin D2 in granulosa cells [33, 36]. A recent study suggests that FSH decreases p27 levels in primary cultures of rat GC [37], indicating the importance of FSH signaling on GC proliferation and follicle development. E2 affords protection against VCD-induced atresia/apoptosis of small preantral follicles [52]. These studies support the importance of FSH and E2 not only for granulosa cell proliferation, but also to protect them from apoptosis. CrVI down-regulated FSHR and ERβ in GC and SIGC, which was mitigated by vitamin C. Therefore, we suggest that down-regulation of FSHR and ERβ signaling could be a potential mechanism for delay/arrest in follicular development at the secondary follicular stage due to CrVI exposure [26]; and decrease in cell proliferation mediated through disruption in cell cycle regulation as indicated in the current study.
Future studies are needed to determine the relationships between CrVI-induced cell cycle arrest and apoptosis that lead to arrested follicle development. Studies currently being undertaken are targeting gene/protein expression profiles from 1–12 h following exposure to CrVI in order to determine whether the changes in cell cycle related gene expression are a cause or consequence of programmed cell death. Further, understanding the mechanisms through which various toxicants affect/disrupt ovarian steroidogenesis and folliculogenesis at the molecular and cellular level requires readily available cells for in vitro studies [53]. Due to several limitations in obtaining granulosa cells, particularly from human, the use of granulosa cell lines for in vitro model systems has become an attractive alternative. Current study and several ongoing experiments in our laboratory are expected to (i) provide a thorough understanding of the molecular and cellular aspects of SIGC in response to CrVI toxicity, (ii) correlate and validate SIGC as an alternative or substitute for granulosa cells cultured from the rat ovary; and (iii) determine SIGC as the most appropriate model for evaluating ovarian function. As a first step in achieving these goals, we evaluated molecular characteristics of SIGC in terms of cell cycle regulation, and we suggest that SIGC may be a good model system to study metal toxicity, and mitigative effects of nutrioxidants such as vitamin C.
Acknowledgments
This work was supported by National Institute of Health (NIH)/National Institute of Environmental Health Sciences (NIEHS) grants ES016605-01A1 to S.K.B. We acknowledge Dr. Roger Smith, Dept of Veterinary Pathobiology, Texas A&M University for assistance with cell cycle analysis.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nriagu JO. Historical Perspectives. In: Nriagu JO, Nieboer E, editors. Chromium in the Natural and Human Environments. John Wiley and Sons; 1988. pp. 1–19. [Google Scholar]
- 2.Junaid M, Murthy RC, Saxena DK. Embryo- and fetotoxicity of chromium in pregestationally exposed mice. Bull Environ Contam Toxicol. 1996;57:327–34. doi: 10.1007/s001289900194. [DOI] [PubMed] [Google Scholar]
- 3.Kamath SM, Stoecker BJ, Davis-Whitenack ML, Smith MM, Adeleye BO, Sangiah S. Absorption, retention and urinary excretion of chromium-51 in rats pretreated with indomethacin and dosed with dimethylprostaglandin E2, misoprostol or prostacyclin. J Nutr. 1997;127:478–82. doi: 10.1093/jn/127.3.478. [DOI] [PubMed] [Google Scholar]
- 4.Kanojia RK, Junaid M, Murthy RC. Embryo and fetotoxicity of hexavalent chromium: a long-term study. Toxicol Lett. 1998;95:165–72. doi: 10.1016/s0378-4274(98)00034-4. [DOI] [PubMed] [Google Scholar]
- 5.Murthy RC, Junaid M, Saxena DK. Ovarian dysfunction in mice following chromium (VI) exposure. Toxicol Lett. 1996;89:147–54. doi: 10.1016/s0378-4274(96)03803-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhitkovich A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI) Chem Res Toxicol. 2005;18:3–11. doi: 10.1021/tx049774+. [DOI] [PubMed] [Google Scholar]
- 7.Zhitkovich A, Peterson-Roth E, Reynolds M. Killing of chromium-damaged cells by mismatch repair and its relevance to carcinogenesis. Cell Cycle. 2005;4:1050–2. [PubMed] [Google Scholar]
- 8.Makarov Y, Shimtova LA. Occupational conditions and gynecological illness in workers engaged in the production of chromium compounds. Environ Health Perspect. 1978;24:1–128. [Google Scholar]
- 9.BlacksmithInstitute. Top 10 worst polluted sites. New York: The Blacksmith Institute; 2007. [Google Scholar]
- 10.Sutton R. Chromium-6 in US Tap Water. Environmental Working Group; 2010. pp. 1–22. [Google Scholar]
- 11.Mitiche ASaL. Extraction and transport of chromium (VI) through a bulk liquid membrane containing triphenylphosphine. Annali di Chimica. 2004;94:1–10. doi: 10.1002/adic.200490115. [DOI] [PubMed] [Google Scholar]
- 12.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 13.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Kirpnick-Sobol Z, Reliene R, Schiestl RH. Carcinogenic Cr(VI) and the nutritional supplement Cr(III) induce DNA deletions in yeast and mice. Cancer Res. 2006;66:3480–4. doi: 10.1158/0008-5472.CAN-05-3944. [DOI] [PubMed] [Google Scholar]
- 15.Wani S, Weskamp C, Marple J, Spry L. Acute tubular necrosis associated with chromium picolinate-containing dietary supplement. Ann Pharmacother. 2006;40:563–6. doi: 10.1345/aph.1G469. [DOI] [PubMed] [Google Scholar]
- 16.Juturu V, Komorowski JR. Chromium compounds: cytotoxicity and carcinogenesis. Toxicology. 2003;186:171–3. doi: 10.1016/s0300-483x(02)00707-2. author reply 5–7. [DOI] [PubMed] [Google Scholar]
- 17.Barceloux DG. Chromium. J Toxicol Clin Toxicol. 1999;37:173–94. doi: 10.1081/clt-100102418. [DOI] [PubMed] [Google Scholar]
- 18.Hoch-Ligeti C, Bourne GH. Changes in the concentration and histological distribution of ascorbic acid in ovaries, adrenals and livers of rats during oestrus cycles. Br J Pathol. 1948;29:400–7. [PMC free article] [PubMed] [Google Scholar]
- 19.Deane HW. Histochemical observations on the ovary and oviduct of the albino rat during the estrous cycle. Am J Anat. 1952;91:363–93. doi: 10.1002/aja.1000910303. [DOI] [PubMed] [Google Scholar]
- 20.Kramer MM, Harman MT, Brill AK. Disturbances of reproduction and ovarian changes in the guinea-pig in relation to vitamin C deficiency. Am J Physiol. 1933;106:611–22. [Google Scholar]
- 21.Millar J. Vitamin C--the primate fertility factor? Med Hypotheses. 1992;38:292–5. doi: 10.1016/0306-9877(92)90019-9. [DOI] [PubMed] [Google Scholar]
- 22.Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999;13:1007–24. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- 23.Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 24.Padayatty SJ, Levine M. New insights into the physiology and pharmacology of vitamin C. CMAJ. 2001;164:353–5. [PMC free article] [PubMed] [Google Scholar]
- 25.Berger TM, Polidori MC, Dabbagh A, Evans PJ, Halliwell B, Morrow JD, et al. Antioxidant activity of vitamin C in iron-overloaded human plasma. J Biol Chem. 1997;272:15656–60. doi: 10.1074/jbc.272.25.15656. [DOI] [PubMed] [Google Scholar]
- 26.Banu SK, Samuel JB, Arosh JA, Burghardt RC, Aruldhas MM. Lactational exposure to hexavalent chromium delays puberty by impairing ovarian development, steroidogenesis and pituitary hormone synthesis in developing Wistar rats. Toxicol Appl Pharmacol. 2008;232:180–9. doi: 10.1016/j.taap.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Banu SK, Stanley JA, Lee J, Stephen SD, Arosh JA, Hoyer PB, et al. Hexavalent chromium-induced apoptosis of granulosa cells involves selective sub-cellular translocation of Bcl-2 members, ERK1/2 and p53. Toxicol Appl Pharmacol. 2011;251:253–66. doi: 10.1016/j.taap.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuel JB, Stanley JA, Roopha DP, Vengatesh G, Anbalagan J, Banu SK, et al. Lactational hexavalent chromium exposure-induced oxidative stress in rat uterus is associated with delayed puberty and impaired gonadotropin levels. Hum Exp Toxicol. 2011;30:91–101. doi: 10.1177/0960327110364638. [DOI] [PubMed] [Google Scholar]
- 29.Robker RL, Richards JS. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol. 1998;12:924–40. doi: 10.1210/mend.12.7.0138. [DOI] [PubMed] [Google Scholar]
- 30.Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15:725–51. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23:9408–21. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 32.Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30:438–64. doi: 10.1210/er.2008-0048. [DOI] [PubMed] [Google Scholar]
- 33.Hunzicker-Dunn M, Maizels ET. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal. 2006;18:1351–9. doi: 10.1016/j.cellsig.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prall OW, Sarcevic B, Musgrove EA, Watts CK, Sutherland RL. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J Biol Chem. 1997;272:10882–94. doi: 10.1074/jbc.272.16.10882. [DOI] [PubMed] [Google Scholar]
- 35.Geum D, Sun W, Paik SK, Lee CC, Kim K. Estrogen-induced cyclin D1 and D3 gene expressions during mouse uterine cell proliferation in vivo: differential induction mechanism of cyclin D1 and D3. Mol Reprod Dev. 1997;46:450–8. doi: 10.1002/(SICI)1098-2795(199704)46:4<450::AID-MRD2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 36.Park Y, Maizels ET, Feiger ZJ, Alam H, Peters CA, Woodruff TK, et al. Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. J Biol Chem. 2005;280:9135–48. doi: 10.1074/jbc.M409486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kayampilly PP, Menon KM. Follicle-stimulating hormone inhibits adenosine 5’-monophosphate-activated protein kinase activation and promotes cell proliferation of primary granulosa cells in culture through an Akt-dependent pathway. Endocrinology. 2009;150:929–35. doi: 10.1210/en.2008-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein LS, Stoica G, Tilley R, Burghardt RC. Rat ovarian granulosa cell culture: a model system for the study of cell-cell communication during multistep transformation. Cancer Res. 1991;51:696–706. [PubMed] [Google Scholar]
- 39.Kayampilly PP, Menon KM. Follicle-stimulating hormone increases tuberin phosphorylation and mammalian target of rapamycin signaling through an extracellular signal-regulated kinase-dependent pathway in rat granulosa cells. Endocrinology. 2007;148:3950–484. doi: 10.1210/en.2007-0202. [DOI] [PubMed] [Google Scholar]
- 40.ATSDR. US Public Health Service. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 1993. Update Toxicological Profile for Chromium; p. ATSDR/TP-92/08. [Google Scholar]
- 41.ATSDR. Case Studies in Environmental Medicine: Chromium Toxicity. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2001. [Google Scholar]
- 42.Litchfield JT, Jr, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 43.Bayrak A, Oktay K. The expression of cyclin-dependent kinase inhibitors p15, p16, p21, and p27 during ovarian follicle growth initiation in the mouse. Reprod Biol Endocrinol. 2003;1:41. doi: 10.1186/1477-7827-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin P, Rui R. Effects of follicular size and FSH on granulosa cell apoptosis and atresia in porcine antral follicles. Mol Reprod Dev. 2010;77:670–8. doi: 10.1002/mrd.21202. [DOI] [PubMed] [Google Scholar]
- 45.Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 46.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaskulski D, deRiel JK, Mercer WE, Calabretta B, Baserga R. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988;240:1544–6. doi: 10.1126/science.2897717. [DOI] [PubMed] [Google Scholar]
- 48.Casasco A, Calligaro A, Casasco E, Marchetti C, Poggi P, Casasco M. An immunocytochemical method for studying embryo cytokinetics. Basic Appl Histochem. 1988;32:293–6. [PubMed] [Google Scholar]
- 49.Oktay K, Schenken RS, Nelson JF. Proliferating cell nuclear antigen marks the initiation of follicular growth in the rat. Biol Reprod. 1995;53:295–301. doi: 10.1095/biolreprod53.2.295. [DOI] [PubMed] [Google Scholar]
- 50.Pomerening JR. Positive-feedback loops in cell cycle progression. FEBS Lett. 2009;583:3388–96. doi: 10.1016/j.febslet.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar TR. FSHbeta knockout mouse model: a decade ago and into the future. Endocrine. 2009;36:1–5. doi: 10.1007/s12020-009-9199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson KE, Sipes IG, Greenstein BD, Hoyer PB. 17beta-estradiol affords protection against 4-vinylcyclohexene diepoxide-induced ovarian follicle loss in Fischer-344 rats. Endocrinology. 2002;143:1058–65. doi: 10.1210/endo.143.3.8665. [DOI] [PubMed] [Google Scholar]
- 53.Havelock JC, Rainey WE, Carr BR. Ovarian granulosa cell lines. Mol Cell Endocrinol. 2004;228:67–78. doi: 10.1016/j.mce.2004.04.018. [DOI] [PubMed] [Google Scholar]