Abstract
The RNA polymerase II carboxyl-terminal domain (CTD) consists of tandem Y1S2P3T4S5P6S7 repeats. Dynamic remodeling of the CTD, especially its serine phosphorylation pattern, conveys informational cues about the transcription apparatus to a large ensemble of CTD-binding proteins. Our genetic dissection of fission yeast CTD function provides insights to the “CTD code”. Two concepts stand out. First, the Ser2 requirement for transcription during sexual differentiation is bypassed by subtracting Ser7, signifying that imbalance in the phosphorylation array, not absence of a phospho-CTD cue, underlies a CTD-associated pathology. Second, the essentiality of Ser5 for vegetative growth is circumvented by covalently tethering mRNA capping enzymes to the CTD, thus proving that capping enzyme recruitment is a chief function of the Ser5-PO4 mark. This illustrates that a key “letter” in the CTD code can be neutralized by delivering its essential cognate receptor to the transcription complex via an alternative route.
Keywords: carboxyl-terminal domain, serine phosphorylation, sexual differentiation, CTD receptors, mRNA capping enzymes
INTRODUCTION
The carboxyl-terminal domain (CTD) of the Rpb1 subunit of RNA polymerase II (Pol II) consists of tandem heptapeptide repeats of consensus sequence Y1S2P3T4S5P6S7. The CTD functions as a landing pad for cellular proteins that regulate the initiation, elongation and termination steps of Pol II transcription, modify chromatin structure, and catalyze or regulate RNA capping, splicing, and polyadenylation (Phatnani and Greenleaf, 2006; Buratowski, 2009). The inherently plastic CTD structure is sculpted by dynamic phosphorylation and dephosphorylation of the heptad serine residues. The combinatorial complexity of the CTD serine-2,5,7 phosphorylation array is enormous, comprising 8n distinct primary structures, where n is the number of heptad repeats. In addition, cis-trans isomerization at CTD residues Pro3 and Pro6 imparts another 4n conformational complexity. Thus, the total number of potential CTD structures is 32n! It is attractive to think that the instantaneous structure of the CTD provides a set of informational cues about the state of the transcription machinery (a CTD code) that is “read” by CTD receptors (Egloff and Murphy, 2008).
Clues to how protein receptors read the CTD code derive largely from biochemical and structural studies of particular CTD-binding proteins, especially the mRNA capping enzymes and CTD phosphatases (Ho and Shuman, 1999; Pei et al. 2001a; Fabrega et al. 2003; Hausmann et al. 2005; Zhang et al., 2006; Xiang et al., 2010; Werner-Allen et al., 2011). The following important points have emerged: (i) the CTD can assume markedly different conformations that are templated by the receptor proteins; (ii) the amino acid sequence of the CTD heptad and the CTD phosphorylation state are independent determinants of receptor binding and activity; (iii) CTD receptors are selective for trans or cis proline conformations; and (iv) CTD information can be assembled from multiple noncontiguous repeats of the heptad array binding simultaneously to the receptor.
These insights notwithstanding, the informational rules that govern the CTD code on a cellular or organismal level are incomplete. The linear information in the CTD code comprises the seven letters (amino acids) of the heptad, positionally numbered by convention (Tyr1, Ser2, etc.) and repeated tandemly. It has long been appreciated that the number of heptad repeats varies among taxa, with unicellular microsporidia (n=15) budding yeast (n=26) and fission yeast (n=29) being on the low end and mammals at the high end (n=52 in mouse and humans). It is also apparent that cell growth in each taxon is contingent on a minimum CTD length (which is less than the native CTD heptad number) and that the minimum heptad number increases with evolutionary complexity. Furthermore, it is established that not all letters of the heptad are equally important. For example, mutating all the Thr4 or Ser7 residues of the budding yeast Rpb1 CTD to alanine had little or no impact on cell growth (suggesting that Thr4 and Ser7 are place-keepers rather than informational), whereas converting all of the Tyr1, Ser2 or Ser5 residues to alanine was lethal (West and Corden, 1995; Stiller et al., 2000). More challenging questions pertain to the “grammar” of the CTD code and to elucidating which essential coding information is read by which essential receptors.
Here we address these issues genetically by manipulating the composition and structure of the Rpb1 CTD in the fission yeast Schizosaccharomyces pombe. The S. pombe CTD consists of 29 heptads. The most proximal 4 repeats deviate in size or sequence from the consensus heptad, and we refer to this junctional segment to the body of Rpb1 as the CTD “rump” (Fig. 1). Distal to the rump is an array of 25 heptads that adhere perfectly to the YSPTSPS consensus (with the single exception of an alanine in lieu of Pro3 in the fifth heptad downstream of the rump). We previously assessed the length requirements for fission yeast growth by serially truncating the heptads from the C-terminus of Rpb1. We found that: 8 repeats (comprising the rump plus 4 consensus heptads) was lethal; 10–13 repeats (rump plus 6 to 9 consensus heptads) resulted in slow growth and cold-sensitive phenotypes; 16 or more repeats (rump plus 12 or more consensus heptads) sufficed for normal growth (Schneider et al., 2010).
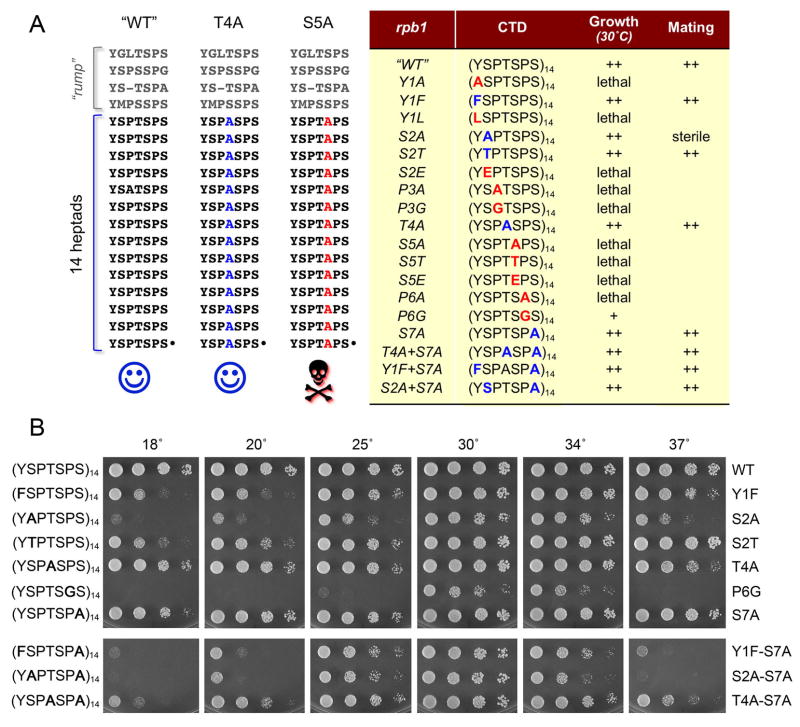
Figure 1. Effects of CTD mutations on fission yeast viability, vegetative growth, and mating proficiency.
(A) The amino acid sequences the CTDs encoded by rpb1 alleles “WT”, T4A and S5A are displayed at left with the heptad repeats aligned vertically. The alleles are named according to the amino acid substitutions introduced into all 14 consensus heptads appended to the “rump” that connects the CTD to the body of Pol II. A summary of the mutational effects on growth and mating is compiled in the Table at right. (B) Viable S. pombe strains with the indicated rpb1-CTD alleles were grown in liquid medium until A600 reached 0.6 to 0.9. The cultures were adjusted to A600 of 0.1 and aliquots (3 μl) of serial 5-fold dilutions were spotted on YES agar plates. The plates were photographed after incubation for 8 d at 18°C, 6 d at 20°C, 3 d at 25° or 2.5 d at 30°, 34° and 37°C.
In the present study, we introduced amino acid substitutions into a functional “wild-type” Rpb1 CTD composed of the rump plus 14 consensus heptads (Fig. 1). We thereby defined the essential letters (Tyr1, Pro3, Ser5, Pro6), their relevant side chain properties, and the epistasis relationships of compound intra-heptad mutations, especially with respect to the requirement for Ser2 for the fission yeast mating program. Our findings reveal significant differences in the CTD structure-activity relations of fission yeast versus budding yeast, while underscoring the apparently universal requirement for Ser5 as a coding letter in at least some of the heptad repeats (albeit without strong bias as to their locus within the CTD array: proximal, distal, or alternating). Finally, we provide evidence that capping enzymes are the critical receptors of the Ser5-PO4 coding letter, insofar as the lethality of an S5A CTD variant could be bypassed by tethering capping enzyme to the mutated CTD and thereby providing an alternative route to the transcription complex.
RESULTS and DISCUSSION
What amino acids of the CTD heptad are important for function in fission yeast?
We probed the function of all individual amino acids of the Y1S2P3T4S5P6S7 repeat by introducing alanine in lieu of Tyr1, Ser2, Pro3, Thr4, Ser5, Pro6 and Ser7 of each heptad of the S. pombe Rpb1 CTD array (see Fig. 1A for the full amino acid sequences of the “WT” CTD and exemplary T4A and S5A mutants). The rpb1-WT and rpb1-Ala CTD cassettes (marked with natMX 3′ of the ORF) were exchanged by homologous recombination for the CTD of one of the chromosomal rpb1+ alleles in a diploid strain of S. pombe. The diploids were sporulated at 30°C and large numbers (≥1000) of viable haploid progeny were scored for the presence of the natMX gene linked to the rpb1-Ala locus. Failure to recover any viable nourseothricin-resistant haploids signified that the pertinent CTD mutation was lethal. Where viable haploids were obtained, we sequenced the rpb1 locus to confirm that the entire mutated CTD was encoded in-frame with the body of Rpb1 and that no unwanted changes or partial allelic exchanges (e.g. by crossing over within the CTD ORF) had occurred. The results are summarized in Fig. 1A. The salient findings were that Tyr1, Pro3, Ser5, and Pro6 are essential for viability of fission yeast, whereas Ser2, Thr4, and Ser7 are not.
The viable CTD-Ala mutants were tested for growth on rich medium at 18°, 20°, 25°, 30°, 34°, and 37° C (Fig. 1B). T4A and S7A cells grew at all temperatures. These results show that: (i) Ser7 phosphorylation is not essential in fission yeast (in agreement with results for budding yeast); (ii) Thr4 phosphorylation, which occurs in vivo on the fission yeast CTD (Sakurai and Ishihama, 2002) is not essential. As noted previously (Schneider et al., 2010), our fission yeast rpb1-S2A mutant grew well at 30° C (Fig. 1B), signifying that Ser2 phosphorylation is not essential. This contrasts sharply with the situation in budding yeast, where the analogous rpb1-S2A change was lethal (West and Corden, 1995). Yet the S. pombe S2A mutation was not benign, i.e., S2A cells did not grow at 18° C and grew poorly at 37° C (Fig. 1B), implying that the hydroxyl moiety and/or Ser2 phosphorylation are important for CTD effector functions at extreme temperatures.
Structure-activity relations at Tyr1, Ser2 and Ser5
We went on to probe structure-activity relations (SARs) at the essential Tyr1 and Ser5 positions by introducing conservative or phosphomimetic changes. The results were instructive and, where noted below, different from analogous SARs in budding yeast. The S. pombe Y1F mutant was viable, though cold-sensitive (Fig. 1B), signifying that the Tyr1 hydroxyl, and hence any tyrosine phosphorylation [which does occur on the fission yeast CTD in vivo (Sakurai and Ishihama, 2002)], is not needed for the growth of fission yeast. Note that the analogous Y1F CTD mutation was lethal in S. cerevisiae (West and Corden, 1995). Here we found that the phenyl ring of Tyr1 is essential in S. pombe, insofar as the partially isosteric leucine in the Y1L mutant allele cannot sustain cell growth (Fig. 1A).
Replacing Ser5 with threonine was lethal, as was the phosphomimetic change to glutamate (Fig. 1A). The S5A and S5E lethality agree with the budding yeast data (West and Corden, 1995) and indicate that a state mimicking constitutive Ser5 phosphorylation is deleterious across taxa. The inability of threonine to replace Ser5 in fission yeast implies that the extra methyl group of threonine is directly deleterious – either via steric hindrance with CTD binding proteins, interference with Ser5 phosphorylation or dephosphorylation, etc.
It is not simply the case that any threonine for serine change in the CTD is bad, insofar as an S2T CTD variant in S. pombe was viable and clearly grew better than the S2A mutant at low and high temperatures (Fig. 1B). Indeed S2T cells grew as well as WT cells at 37°, though they were impaired at 18° compared to WT (Fig. 1B). By contrast, an S2E mutation was lethal in fission yeast (Fig. 1A), suggesting that a constitutive phosphomimetic state at Ser2 is harmful.
Distinct coding cues at Pro3 and Pro6
The lethality of the P3A or P6A changes has implications for cis-trans isomerization of proline as part of the CTD code. In principle, substituting alanine for proline could have multiple deleterious effects. First, because a Ser-Pro dipeptide is a common target for CTD kinases, the Pro-to-Ala mutations might alter CTD phosphorylation patterns. We suspect this scenario does not account for the lethality of P3A, because Ser2 phosphorylation is not required for viability. However, the lethality of P6A could reflect aberrant Ser5 phosphorylation. Second, the Pro-to-Ala change subtracts two methylene groups from the proline ring that, in several available cocrystal structures, make van der Waals contacts at the interface of the CTD with its receptor proteins (Verdecia et al., 2000; Fabrega et al. 2003; Zhang et al. 2006; Xiang et al. 2010). Third, Ala in lieu of Pro alters the conformational options for the CTD main chain. Proline can adopt trans or cis conformations and switch from one to the other aided by peptidyl prolyl isomerases. By contrast, alanine adopts a trans conformation only. Thus, the P3A and P6A CTD variants might conceivably satisfy receptors that recognize the trans proline conformations of the CTD, but not those that require a cis proline conformation.
Here we tested the effects of replacing every Pro3 or Pro6 residue with glycine. The achiral glycine has the greatest conformational flexibility and is able to adopt cis-like or trans-like main chain conformations. We found that the P3G mutation was lethal (Fig. 1A), suggesting that the conformational flexibility might not be the sole essential property of the Pro3 residue and that the contacts of Pro3 ring carbons with CTD receptors are probably important per se. By contrast, the P6G mutant was viable at 30° C and 34° C, albeit slower growing that the wild-type control (Fig. 1B). The P6G strain was also cs and ts (Fig. 1B). This result underscores the requirement for conformational freedom at Pro6, and its sufficiency for a narrow window of cellular viability as sustained by P6G in the absence of the proline ring and its putative contacts. In this context, it is noteworthy that: (i) the Ser5-specific CTD phosphatase Ssu72 requires a cis conformation at Pro6 to bind the CTD; and (ii) Ssu72 also makes two van der Waals contacts to the Pro6 ring atoms (Xiang et al. 2010).
Effects of CTD double-mutations
To address possible functional redundancy of nonessential CTD residues Thr4 and Ser7, we generated a T4A+S7A double mutant, which grew normally at 30° C, but was both cs and ts compared to the T4A and S7A single mutants (Fig. 1B). (An analogous S. cerevisiae T4A+S7A mutant was also viable and moderately cs [Stiller et al. 2000]).
An S. pombe S7A+Y1F double mutant grew well at 30° C, but had a ts phenotype at 37° C that was not evident in either single mutant (Fig. 1B). The mutational synergy between the adjacent CTD residues Ser7-Tyr1 suggests an additive impact on CTD conformation, and hence CTD interactions with its receptors, at high temperature.
The effects of combining S2A and S7A were noteworthy, insofar as they eliminated two of the three potential serine phosphorylation sites in each CTD heptad. The S. pombe S2A+S7A mutant grew well at 30° C (Fig. 1B), signifying that Ser5 suffices for vegetative growth as the sole serine phosphorylation option for the Pol II CTD code. Like the S2A single mutant, the S2A+S7A cells were cs and ts (Fig. 1B).
A serine or threonine at CTD heptad position 2 is essential for mating in fission yeast
During our initial characterization of haploid S2A strains (Schneider et al. 2010), we noted that they were unable to mate with heterothallic wild-type S. pombe of either mating type, i.e., S2A was sterile. We found no such mating defects for haploid Y1F, T4A, S7A, T4A+S7A, or Y1F+S7A strains, thereby attesting to the specificity of the requirement for Ser2 (Fig. 1A). To examine the basis for the mating defect, we constructed homothallic h90 strains wherein rpb1+ was replaced with either the “WT” or S2A alleles. The mating program was induced by transfer to malt extract medium and mating efficiency was gauged after 24 h by light microscopy (Fig. 2A). The mating efficiency of the WT strain was 73%. By contrast, the S2A mating efficiency was only 8%.
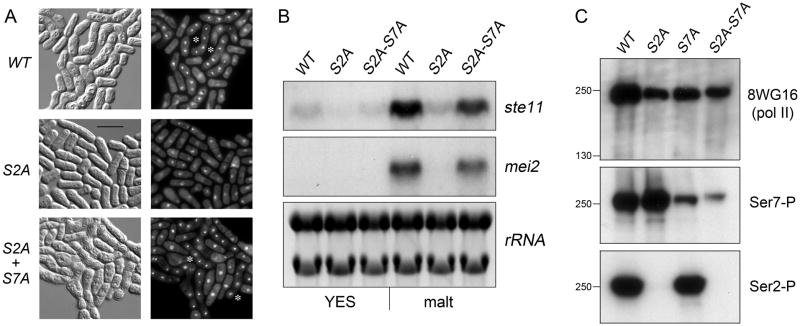
Figure 2. S7A suppresses the requirement for Ser2 for fission yeast mating and ste11 induction.
(A) Representative photomicrographs of homothallic WT, S2A and S2A+S7A cells after 24 h in mating/sporulation medium (malt). Cells were treated with DAPI and then visualized by differential interference contrast (left panels) and fluorescence (right panels) microscopy. The stars indicate examples of asci. (B) Northern blot analysis of ste11+ and mei2+ mRNA levels in exponentially growing homothallic WT, S2A and S2A+S7A cells before (YES), or 5 hr after transfer to mating medium (malt). As a control for equal loading of RNA in each lane, ribosomal RNAs (28S and 18S) stained with ethidium bromide (negative image) are shown in the bottom panel. (C) Western blot analysis of Rpb1 CTD phosphorylation status in exponentially growing heterothallic WT, S2A, S7A and S2A+S7A cells using antibody to bulk Rpb1 (8WG16, top panel) and phospho-specific antibodies against Ser7-P (middle panel) and Ser2-P (bottom panel). The positions and sizes (kDa) of marker proteins are indicated at left.
The fission yeast meiotic sexual differentiation pathway is triggered by transcriptional induction of ste11+, which encodes the Ste11 transcription factor. In turn, Ste11 induces the expression of downstream genes in the meiotic program, including mei2+ (Yamamoto 1996). Northern blot analysis of RNA isolated from the homothallic “WT” strain before and 5 h after transfer to mating/sporulation medium (malt) revealed upregulation of the ste11 and mei2 mRNAs (Fig. 2B). This transcriptional response was effaced in the homothallic S2A strain (Fig. 2B), thereby explaining the S2A mating defect. Our finding that haploid heterothallic S2T strains mated readily with heterothallic strains of one or the other mating type indicated that serine or threonine sufficed for execution of the mating program, whereas alanine did not. A tentative inference from these data is that Ser2 phosphorylation is required for mating. While our studies were in progress, Coudreuse et al. (2010) reported that deletion of the gene encoding the S. pombe CTD Ser2-specific kinase Lsk1 inhibited mating and ste11 expression after nitrogen deprivation. They elicited a stronger mating phenotype by replacing CTD Ser2 with alanine and concluded that Ser2-PO4 per se plays a specific regulatory role in cell differentiation. In experiments described below, we instate a more nuanced view of the impact of erasing Ser2 phosphorylation from the CTD code.
S7A suppresses the mating defect of S2A
Combining the S2A and S7A mutations exerted a profound effect on the S2A mating phenotype, i.e., the S. pombe heterothallic S2A+S7A mutant was competent to mate with heterothallic rpb1+ cells (Fig. 1A). A homothallic S2A+S7A strain was also proficient at mating, as gauged by microscopy (Fig. 2A). The mating efficiency of h90 S2A+S7A cells was 47%. Concomitantly, the S2A+S7A allele restored the induction of the ste11 and mei2 mRNAs (Fig. 2B). Thus, eliminating the Ser7 hydroxyl effectively bypassed the requirement for the CTD Ser2 hydroxyl to execute the mating program. We found that S7A also alleviated the mating defect caused by deletion of the Ser2-specific kinase Lsk1. Our homothallic lsk1Δ strain had a mating efficiency of 22%, compared to 42% efficiency for an lsk1Δ rpb1-S7A double-mutant. A homothallic rpb1-S7A single-mutant mated with 74% efficiency, equivalent to a WT strain.
To gauge the impact of the S2A and S7A mutations on the CTD phosphorylation pattern, we prepared trichloroacetic acid extracts of total protein from “WT”, S2A, S7A and S2A+S7A strains and analyzed them by SDS-PAGE and western blotting. The monoclonal antibody 8WG16 recognized total S. pombe Rpb1 in each sample as a discrete ~250 kDa species (Fig. 2C, top panel). A Ser2-specific phospho-CTD antibody reacted with Rpb1 in the WT and S7A samples, but there were no Ser2-P signals detected in the S2A and S2A+S7A extracts (Fig. 2C, bottom panel). These results attest to the specificity of the Ser2-P antibody and the genetic ablation of the Ser2 “letter” of the CTD code.
The instructive findings were that a Ser7-specific monoclonal phospho-CTD antibody reacted strongly with WT Rpb1 and Rpb1-S2A (Fig. 2C, middle panel). Indeed, the Ser7-P signal was higher in the S2A sample than in WT, even though the total Rpb1 signal was higher in WT than S2A (compare top and middle panels). This was a consistent finding in several iterations of the experiment, whether the samples were immunoblotted with one antibody per membrane or immunoblotted sequentially with the set of three antibodies by probing, stripping and re-probing a single membrane (not shown). Thus, it appears that replacing Ser2 with alanine increased the steady-state level of Ser7 phosphorylation. We found that the Ser7-P signal was suppressed, but not abolished, in the S7A and S2A+S7A samples (Fig. 2C, middle panel). We suspect that the residual Ser7-P signal originates from the two potential Ser7 phosphorylation sites in the rump segment of Rpb1 to which the S7A and S2A+S7A CTD heptad arrays were fused (Fig. 1A).
The S2A+S7A mating rescue phenotype, in conjunction with the analysis of Rpb1 phosphorylation, has potentially deep implications for the CTD code: to wit, that the absence of Ser2 (and Ser2-PO4) allows for increased phosphorylation of Ser7, and that unopposed Ser7 phosphorylation is deleterious for proper mating. Therefore, the sterility of S2A can be reversed by S7A, which blocks Ser7 phosphorylation. The key point is that an imbalance in the CTD phosphorylation array, not absence of a particular phospho-CTD cue, underlies a CTD-associated pathology.
The lethality of S5A is bypassed by fusion of capping enzyme to the CTD
No less daunting than the combinatorial complexity of the CTD structure and code are the prospects that; (i) cellular receptor proteins recognize many different “words” encoded in the CTD; and (ii) any particular CTD word or combination of words is recognized by more that one essential receptor protein. If this is so, then it becomes quite challenging to assign an essential CTD “byte” or single letter to a specific CTD-receptor pair in the sea of available receptors. Arguably, the strongest genetic evidence for a uniquely essential function of a letter in the CTD code would be to bypass the requirement for that letter; for example, by delivering the cognate receptor protein(s) to the Pol II transcription complex via other means. Here we attempted to override the universal requirement for Ser5 (and Ser5 phosphorylation) by targeting an essential cellular Ser5-PO4 receptor (the mRNA capping enzymes RNA triphosphatase and RNA guanylyltransferase) to the Pol II elongation complex by fusing the receptor in cis to the carboxyl terminus of the otherwise nonfunctional Rpb1-CTD-S5A protein.
The fission yeast capping enzymes RNA triphosphatase (Pct1) and RNA guanylyltransferase (Pce1) are separately encoded essential proteins that catalyze the first two steps in mRNA cap formation: (i) the conversion of a 5′ triphosphate RNA end to a 5′ diphosphate; and (ii) transfer of GMP from GTP to the RNA diphosphate end to form a GpppRNA cap (Shuman et al. 1994; Pei et al. 2001b, c). Pct1 and Pce1 do not interact with each other, but bind independently to the Ser5-phosphorylated Rpb1 CTD heptad array (Pei et al. 2001a). By contrast, the mammalian capping enzyme Mce1 is a single modular 68-kDa polypeptide composed of N-terminal triphosphatase and C-terminal guanylyltransferase domains. The Mce1 guanylyltransferase domain binds to the Ser5-phosphorylated Pol II CTD and thereby ferries the covalently tethered triphosphatase module, which does not bind to Pol II CTD on its own, to the mammalian Pol II elongation complex (Ho and Shuman, 1999). Expression of Mce1 in S. cerevisiae complements lethal deletions of the budding yeast triphosphatase and guanylyltransferase enzymes (Sawaya and Shuman, 2003). Here we find that a plasmid-borne MCE1 gene driven by a fission yeast promoter complemented the otherwise lethal S. pombe pct1Δ and pce1Δ mutations (Fig. S1A), thus verifying that Mce1 is active for capping in S. pombe. We had shown previously that high gene dosage of MCE1 (on a multicopy plasmid) could suppress the conditional phenotypes caused by deletions of the C-terminal domain of S. pombe Spt5, an essential transcription elongation factor implicated (along with the Pol II CTD) in recruiting the capping enzymes to the transcription elongation complex (Schneider et al. 2010). However, we found here that increased MCE1 dosage did not rescue the lethality of rpb1-S5A (not shown), consistent with the stringent requirement for Ser5-PO4 for binding of Mce1 to the Rpb1 CTD in trans (Ho and Shuman, 1999).
We proceeded to fuse the MCE1 ORF in-frame to the “WT” (Ser5-containing) and mutant S5A CTD cassettes (Fig. 3) and exchanged them by homologous recombination for the CTD of one of the chromosomal rpb1+ alleles in a diploid strain of S. pombe. Viable rpb1-S5-MCE1 and rpb1-S5A-MCE1 haploids (Fig. 3A) were obtained after sporulating diploids at 30° C and scoring random haploid progeny for natMX marker linked to the MCE1-fused rpb1 locus. We sequenced the rpb1 gene in the haploids to confirm that the entire CTD-MCE1 cassette was encoded in-frame with the body of Rpb1 and that no unwanted changes or partial allelic exchanges had occurred. This remarkable result showed that the stringent requirement for Ser5-PO4 as a letter in the CTD code could be elided when the capping enzymes were delivered by an alternate route. Note that the rpb1-S5A-MCE1 haploids were proficient for mating with heterothallic strains, thereby proving that Ser5 phosphorylation per se is not required for sexual differentiation.
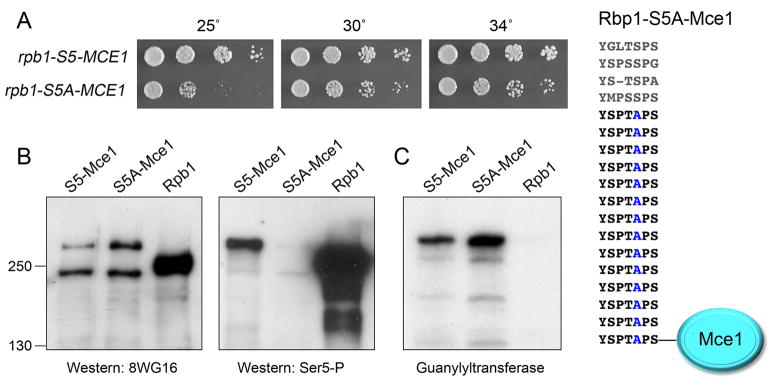
Figure 3. The lethality of S5A is bypassed by fusion of capping enzyme to the CTD.
(A) The S5A-Mce1 fusion is depicted at right. rpb1-S5-MCE1 and rpb1-S5A-MCE1 cells were grown in liquid culture at 30°C and serial dilutions were spotted to YES agar. The plates were photographed after 4 d at 25°C, or 3 d at 30 and 34°C. (B) Pol II immunoblots of extracts of rpb1+, S5-MCE1, and S5A-MCE1 cells using 8WG16 (left panel) or anti-Ser5-P (right panel) antibodies. The positions and sizes (kDa) of marker proteins are indicated at left. (C) Guanylyltransferase activity was gauged by label transfer from [α32P]GTP to the active capping enzymes in the extract to form covalent enzyme-[32P]GMP adducts detectable by SDS-PAGE and autoradiography. The positions of marker proteins are as in panel B. (See also Fig. S1.)
The rpb1-S5-MCE1 and rpb1-S5A-MCE1 strains grew on agar medium at 30° and 34° C, with the latter forming slightly smaller colonies (Fig. 3A). Their growth disparity was exacerbated at 25° C (Fig. 3A). Neither strain grew at 18° (not shown), indicating that fusing Mce1 to an otherwise functional S5 CTD was deleterious at cold temperatures. The rpb1-S5-MCE1 cells grew at 37° C, but rpb1-S5A-MCE1 cells did not (not shown). Extracts prepared under nondenaturing (“native”) conditions from S5-MCE1 and S5A-MCE1 cells grown in liquid culture at 30° C were probed by western blotting with antibodies directed against Pol II, as were native extracts from an rpb1+ strain that has the full-length native CTD composed of 29 repeats. The monoclonal antibody 8WG16 recognized full-length S. pombe Rpb1, which migrated as an ~250 kDa polypeptide during SDS-PAGE (Fig. 3B, left panel). Two immunoreactive polypeptides were detected in this size range in the S5-MCE1 and S5A-MCE1 extracts: (i) a species larger than Rpb1 that corresponds to the Rpb1-S5/S5A-Mce1 fusion proteins; and (ii) a species slightly smaller than Rpb1 that we presume corresponds to Rpb1 subunit from which Mce1 (and perhaps some CTD heptads) had been removed by proteolysis. Parallel samples were immunoblotted with a polyclonal antibody that recognizes the Ser5-phosphorylated CTD (Fig. 3B, right panel). Full-length Rpb1 reacted with this antibody, as expected. The pertinent findings were that the larger Rpb1-S5-Mce1 fusion protein also reacted with anti-Ser5-P, whereas the Rpb1-S5A-Mce1 fusion protein did not (Fig. 3B, right panel). The putative “clipped” shorter form of Rpb1-S5 (and Rpb1-S5A) that reacted 8WG16 did not react with anti-Ser5-P (Fig. 3B). We detected uniquely in the S5-MCE1 extract a ~100 kDa anti-Ser5-P-reactive polypeptide (not shown) that we presume corresponds to a CTD(S5P)-Mce1 fragment formed by proteolytic incision of Rpb1-S5-Mce1 within the CTD or near the Rpb1 body-CTD junction.
The native extracts were also assayed for guanylyltransferase activity by label transfer from [α32P]GTP to the active capping enzyme in the extract to form a covalent enzyme-[32P]GMP adduct detectable by SDS-PAGE and autoradiography (Fig. 3C). The S5-MCE1 and S5A-MCE1 extracts transferred the radiolabeled GMP to the ~300 kDa polypeptides corresponding to the Rpb1-S5/S5A-Mce1 fusions. By contrast, no polypeptides in this size range were labeled in the Rpb1 extract (Fig. 3C). The endogenous 50 kDa S. pombe guanylyltransferase Pce1 was labeled in all three extracts (not shown). We detected labeling of two fragments derived from Rpb1-S5/S5A-Mce1 in the S5-MCE1 and S5A-MCE1 extracts: an ~70 kDa species that co-migrated with Mce1-GMP (which we attribute to cleavage of the fusion protein near the CTD/Mce1 junction) and a ~100 kDa species that likely comprised a CTD-Mce1 fragment (not shown).
The point of this analysis is to emphasize that the Rpb1-S5A-Mce1 fusion protein that sustained viability of fission yeast at 30–34° C was present and catalytically active, albeit susceptible to proteolysis, as was its Rpb1-S5-Mce1 counterpart. Our immunoblotting data suggest that clipping of the fusion constructs resulted in a lower cellular pool of intact Pol II large subunits (compared to rpb1+) that might account for why the fusion strains had conditional growth defects. This complexity does not detract from the key finding that the essentiality of the Ser5-PO4 mark reflects its singular requirement for capping enzyme recruitment, which can be bypassed by fusing the triphosphatase and guanylyltransferase enzymes to the S5A mutant CTD.
Two further lines of evidence confirmed that the rescue of lethality of rpb1-S5A by Mce1 fusion reflected provision of capping activity in cis. First, we showed that the rpb1-S5A-MCE1 strain and the control rpb1-S5-MCE1 strain were both viable after deletion of the otherwise essential S. pombe pct1+ gene (Fig. S1B). Thus, the Rpb1-S5A-Mce1 fusion suffices as the sole source of RNA triphosphatase activity in the absence of a Ser5-PO4 recruitment mark. Second, we tested the activity of variant CTD fusions – S5-MCE1-(D438A) and S5A-MCE1-(D438A) – containing a guanylyltransferase-inactivating mutation. Asp438 is a constituent of the guanylyltransferase active site (in motif IV, implicated in metal-binding and catalysis); the D438A change abolishes Mce1 capping activity in vivo (Sawaya and Shuman, 2003). We exchanged the S5 and S5A MCE1-(D438A) CTD fusions by homologous recombination for the CTD of one of the chromosomal rpb1+ alleles in a diploid strain. Viable rpb1-S5-MCE1-(D438A) haploids were obtained after sporulation, because the endogenous yeast Pce1 guanylyltransferase could recognize the Ser5-containing CTD. By contrast, we were unable to recover any viable rpb1-S5A-MCE1-(D438A) haploids, because the S5A CTD is unable to recruit Pce1. These results signify that the fusion of active wild-type Mce1 to Rpb1-S5A is providing the essential guanylyltransferase activity when the Ser5 letter is missing in the rpb1-S5A-MCE1 strain.
The linear geometry of Ser5 heptads is flexible
Candida mRNA guanylyltransferase Cgt1 has two distinct CTD docking sites that bind noncontiguous Ser5-PO4 heptad elements in different registers: one site binds TSPSYSP while the other engages SYSPTSP (Fabrega et al., 2003). In S. pombe, the Ser5 phospho-CTD array independently recruits the triphosphatase Pct1 and the guanylyltransferase Pce1 (Pei et al., 2001a), presumably at the same time, shortly after transcription initiation and deposition of the Ser5-PO4 marks. These scenarios raise the issue of whether the Ser5 phospho-CTD code for capping enzyme recruitment entails geometric constraints on the linear arrangement or Ser5-PO4 heptads. We addressed this question by generating CTD chimaeras in which the 14 repeats appended to the rump were composed of a mixture of S5 and S5A heptads, as follows: (i) the proximal 7 heptads were S5 and the distal 7 heptads were S5A; (ii) the proximal half were S5A and the distal half were S5; (iii) the S5 and S5A heptads alternated (with heptads 1, 3, 5, etc. being S5A and 2, 4, 6, etc. being S5). This approach should, in principle, distinguish the impact of the bulk content of Ser5-phophorylatable (and non-phosphorylatable) heptads versus their positions en bloc and individually within the linear CTD array.
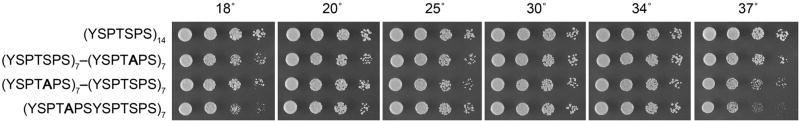
We found that the (YSPTSPS)7(YSPTAPS)7 and (YSPTAPS)7(YSPTSPS)7 chimaeras were both viable and grew about as well as the “WT” CTD strain at temperatures between 20° and 34° C (Fig. 4). At 37° C, the (YSPTAPS)7(YSPTSPS)7 chimaera formed smaller colonies. We conclude that that: (i) seven consensus Ser5-containing heptad repeats suffice for S. pombe growth when supplemented by an additional seven S5A mutant heptads; and (ii) there is no major proximal-distal distinction in the fission yeast CTD, at least with respect to Ser5 heptads and viability. [Similar conclusions were drawn for S. cerevisiae proximal-distal Ser5-S5A block chimeras (West and Corden, 1995).]
Figure 4. S5/S5A CTD chimaeras.
S. pombe strains with the indicated rpb1-CTD alleles were grown in liquid YES medium. The cultures were adjusted to A600 of 0.1 and aliquots (3 μl) of serial 5-fold dilutions were spotted on YES agar plates. The plates were photographed after 7 d at 18°C, 6 d at 20°C, 3 d at 25°C, or 2.5 d at 30°C, 34°C, and 37°C.
The fission yeast (YSPTAPSYSPTSPS)7 chimera, composed of alternating S5A and Ser5 heptads, grew normally at 20° to 34°, but more slowly than WT or the proximal-distal chimeras at 18° and 37°, as gauged by colony size (Fig. 4). An analogous S. cerevisiae mutant with a CTD composed of alternating S5A and Ser5 heptad repeats was viable, but also slow-growing at 15° C and 37° C (Liu et al. 2010). The cs and ts effects of the alternating S5A mutations suggest that consecutive Ser5-PO4 marks are recognized by one or more cellular receptors and that this recognition is relevant for yeast fitness at low and high temperatures.
EXPERIMENTAL PROCEDURES
To introduce mutations within each of 14 heptad repeats appended in-frame to amino acid 1577 of S. pombe Rpb1, synthetic 504-bp DNA segments (GenScript, Piscataway, NJ) were digested with BamHI and XbaI and inserted into a rpb1 CTD integration cassette marked with natMX (Schneider et al. 2010). The plasmids harboring the mutated integration cassettes were linearized and transformed into a diploid S. pombe strain. Nourseothricin-resistant transformants were selected and diagnostic PCR and/or Southern blotting was used to confirm correct integrations at one of the rpb1+ loci. A segment of the rpb1::natMX allele was amplified by PCR and sequenced to verify that the desired mutations were present in all 14 repeats and that the mutated CTD variants were in frame with the body of Rpb1. The heterozygous rpb1+/rpb1::natMX diploids were then sporulated and subjected to random spore analysis. Spores (~5,000) were plated to YES agar medium (to gauge the number of viable offspring) and to YES medium containing clonNAT. A finding that no viable nourseothricin-resistant haploids were recovered after 10 d at 30°C was deemed to indicate lethality of a given rpb1-CTD mutant allele.
To generate homothallic rpb1-CTD mutant cells, strain AJC-D16 (h90 ura4-D18 leu1-32) was transformed with linear “WT”, S2A, S7A, or S2A+S7A CTD integration cassettes. Nourseothricin-resistant transformants were selected and diagnostic Southern blotting and sequencing of the PCR products from the respective alleles confirmed allelic replacements.
The Rpb1 S5 and S5A CTDs were fused in-frame to mammalian capping enzyme Mce1 by two stage overlap extension PCR and then inserted into the CTD integration plasmid. The plasmids were sequenced to verify the fusion junctions and confirm that no unwanted mutations were introduced during PCR and cloning. Allelic replacements at the rpb1+ locus of diploid S. pombe cells, sporulations yielding S5-MCE1 and S5A-MCE1 haploids, and verification of the rpb1 S5-MCE1 and S5A-MCE1 alleles were performed as described above.
Other methods are described in detail in Supplemental Experimental Procedures.
Supplementary Material
Highlights.
Of the seven letters in the CTD code (YSPTSPS), Y1, P3, S5 and P6 are essential
S2 is optional for vegetative growth, but essential for the yeast mating program
S7A suppresses the mating defect of S2A cells
The essentiality of S5 is bypassed by fusing mRNA capping enzymes to the CTD
Acknowledgments
We thank Ana Sanchez for technical assistance. This work was supported by NIH grant GM52470. SS is an American Cancer Society Research Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buratowski S. Progression through the RNA polymerase II cycle. Mol Cell. 2009;36:541–546. doi: 10.1016/j.molcel.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudreuse D, van Bakel H, Dewez M, Soutourina J, Parnell T, Vandenhaute J, Cairns B, Werner M, Hermand D. A gene-specific requirement of RNA polymerase II CTD phosphorylation for sexual differentiation in S. pombe. Curr Biol. 2010;20:1053–1064. doi: 10.1016/j.cub.2010.04.054. [DOI] [PubMed] [Google Scholar]
- Egloff S, Murphy S. Cracking the RNA polymerase II CTD code. Trends Genetics. 2008;24:280–288. doi: 10.1016/j.tig.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Fabrega C, Shen V, Shuman S, Lima CD. Structure of an mRNA capping enzyme bound to the phosphorylated carboxyl-terminal domain of RNA polymerase II. Mol Cell. 2003;11:1549–1561. doi: 10.1016/s1097-2765(03)00187-4. [DOI] [PubMed] [Google Scholar]
- Hausmann S, Koiwa H, Krishnamurthy S, Hampsey M, Shuman S. Different strategies for carboxyl-terminal domain (CTD) recognition by Serine5-specific CTD phosphatases. J Biol Chem. 2005;280:37681–37688. doi: 10.1074/jbc.M505292200. [DOI] [PubMed] [Google Scholar]
- Ho CK, Shuman S. Distinct roles for CTD Ser2 and Ser5 phosphorylation in the recruitment and allosteric activation of mammalian capping enzyme. Mol Cell. 1999;3:405–411. doi: 10.1016/s1097-2765(00)80468-2. [DOI] [PubMed] [Google Scholar]
- Liu P, Kenney JM, Stiller JW, Greenleaf AL. Genetic organization, length conservation, and evolution of the RNA polymerase II carboxyl-terminal domain. Mol Biol Evol. 2010;27:2628–2641. doi: 10.1093/molbev/msq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Hausmann S, Ho CK, Schwer B, Shuman S. The length, phosphorylation state, and primary structure of the RNA polymerase II carboxyl-terminal domain dictate interactions with mRNA capping enzymes. J Biol Chem. 2001a;276:28075–28082. doi: 10.1074/jbc.M102170200. [DOI] [PubMed] [Google Scholar]
- Pei Y, Schwer B, Hausmann S, Shuman S. Characterization of Schizosaccharomyces pombe RNA triphosphatase. Nucleic Acids Res. 2001b;29:387–396. doi: 10.1093/nar/29.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Schwer B, Saiz J, Fisher RP, Shuman S. RNA triphosphatase is essential in Schizosaccharomyces pombe and Candida albicans. BMC Microbiology. 2001c;1:29. doi: 10.1186/1471-2180-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phatnani HP, Greenleaf AL. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 2006;20:2922–2936. doi: 10.1101/gad.1477006. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Ishihama A. Level of the RNA polymerase II in the fission yeast stays constant but phosphorylation of its carboxyl terminal domain varies depending on the phase and rate of cell growth. Genes Cells. 2002;7:273–284. doi: 10.1046/j.1365-2443.2002.00522.x. [DOI] [PubMed] [Google Scholar]
- Sawaya R, Shuman S. Mutational analysis of the guanylyltransferase component of mammalian mRNA capping enzyme. Biochemistry. 2003;42:8240–8249. doi: 10.1021/bi034396d. [DOI] [PubMed] [Google Scholar]
- Schneider S, Pei Y, Shuman S, Schwer B. Separable functions of the fission yeast Spt5 CTD in capping enzyme binding and transcription elongation overlap with those of the RNA polymerase II CTD. Mol Cell Biol. 2010;30:2353–2364. doi: 10.1128/MCB.00116-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S, Liu Y, Schwer B. Covalent catalysis in nucleotidyl transfer reactions: essential motifs in Saccharomyces cerevisiae RNA capping enzyme are conserved in Schizosaccharomyces pombe and viral capping enzymes and among polynucleotide ligases. Proc Natl Acad Sci USA. 1994;91:12046–12050. doi: 10.1073/pnas.91.25.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller JW, McConaughy BL, Hall BD. Evolutionary complementation for polymerase II CTD function. Yeast. 2000;16:57–64. doi: 10.1002/(SICI)1097-0061(20000115)16:1<57::AID-YEA509>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Stiller JW, Cook MS. Functional unit of the RNA polymerase II C-terminal domain lies within heptapeptide pairs. Eukaryotic Cell. 2004;3:735–740. doi: 10.1128/EC.3.3.735-740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecia MA, Bowman ME, Lu KP, Hunter T, Noel JP. Structural basis for phosphoserine-proline recognition by group IV WW domains. Nat Struct Biol. 2000;7:639–643. doi: 10.1038/77929. [DOI] [PubMed] [Google Scholar]
- West ML, Corden JL. Construction and analysis of yeast RNA polymerase II CTD deletion and substitution mutations. Genetics. 1995;140:1223–1233. doi: 10.1093/genetics/140.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Allen JW, Lee CL, Liu P, Nicely NI, Wang S, Greenleaf AL, Zhou P. Cis proline-mediated pSer5-dephosphorylation by the RNA polymerase II CTD phosphatase Ssu72. J Biol Chem. 2011;286:5717–5726. doi: 10.1074/jbc.M110.197129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang K, Nagaike T, Xiang S, Kilic T, Beh MM, Manley JL, Tong L. Crystal structure of the human sympelkin-Ssu72-CTD phosphopeptide complex. Nature. 2010;467:729–733. doi: 10.1038/nature09391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M. Regulation of meiosis in fission yeast. Cell Struct Funct. 1996;21:431–436. doi: 10.1247/csf.21.431. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kim Y, Genoud N, Gao J, Kelly JW, Pfaff SL, Gill GN, Dixon JE, Noel JP. Determinants for dephosphorylation of the RNA polymerase II C-terminal domain by Scp1. Mol Cell. 2006;24:759–770. doi: 10.1016/j.molcel.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.