Abstract
Renal strong ion compensation to chronic respiratory acidosis has been established but the nature of the response to acute respiratory acidosis is not well defined. We hypothesized that the response to acute respiratory acidosis in sheep is a rapid increase in the difference in renal fractional excretions of chloride and sodium (FeCl-FeNa). Inspired CO2 concentrations were increased for one hour to alter significantly PaCO2 and pHa from 32 ± 1 mm Hg and 7.52 ± 0.02 to 74 ± 2 mm Hg and 7.22 ± 0.02, respectively. FeCl-FeNa increased significantly from 0.372 ± 0.206 to 1.240 ± 0.217 % and returned to baseline at two hours when PaCO2 and pHa were 37 ± 0.6 mm Hg and 7.49 ± 0.01, respectively. Arterial pH and FeCl-FeNa were significantly correlated. We conclude that the kidney responds rapidly to acute respiratory acidosis, within 30 mins of onset, by differential reabsorption of sodium and chloride.
INTRODUCTION
Disturbances of acid-base balance are common in patients admitted to intensive care units; causes include acute respiratory failure, diabetic ketoacidosis and septic shock (Fencl & Rossing, 1989; Kaplan & Frangos, 2005). Moreover, even therapeutic drugs like diuretics (Koch & Taylor, 1992) that are administered to patients in intensive care units may lead to electrolyte-associated acid-base imbalances (Fencl & Rossing, 1989; Gehlbach & Schmidt, 2004). Acid-base disturbances are created immediately by respiratory insufficiency as a result of elevations of arterial partial pressure of carbon dioxide. This disturbance in pH is opposed by the kidney, the next major controller of acid-base balance. The kidney alters the handling of sodium and chloride ions which are the key players in determining the strong ion difference (SID) (Alfaro et al., 1996).
The kidney responds to chronic hypercapnea by increasing the strong ion difference. In early rat studies, chronic respiratory acidosis resulted in hypochloremia with selective increases in the filtered urine chloride but not sodium excretion (Carter, Seldin, & Teng, 1959; Polak, Haynie, Hays, & Schwartz, 1961). In dogs, exposure to 8% CO2 for 24 hours resulted in chloruresis and negative balance of chloride while sodium balance was unchanged (Levitin, Branscome, & Epstein, 1958). Yet, another study in humans demonstrated acute respiratory acidosis to have little variation in urinary sodium and chloride concentrations (Barker, Singer, Elkinton, & Clark, 1957). In a more recent study by Alfaro and coworkers (1996), it was demonstrated that hypercapnea and hypoxia in human patients with chronic obstructive pulmonary disease were associated with a decrease in plasma chloride without a significant change in plasma sodium, showing that these changes in SID were not due to hydration issues, but rather acid-base disorders. However, it is not known if the renal response can compensate for acute respiratory acidosis. One study demonstrated that chronic but not acute respiratory acidosis altered the function of renal proton ATPases (Eiam-ong, Laski, Kurtzman, & Sabatini, 1994). However, others have noted that acute respiratory acidosis for 4 hours resulted in morphological alterations in the tubulo-vesicular membrane compartment in specific nephron segments (Madsen & Tisher, 1983). It has also been demonstrated that the kidney adapts to acute respiratory acidosis through changes in the handling of amino acids (Gougoux, Vinay, Cardoso, Duplain, & Lemieux, 1982). Despite these reports on the ability of the kidney to handle acute respiratory acidosis, the principal method utilized by the kidney to compensate is unknown. Stewart hypothesized that the kidneys balance net chloride excretion against net sodium excretion to regulate plasma strong ion difference (Kellum & Elbers, 2009). In this study, we tested the hypothesis that the kidney would respond to acute respiratory acidosis within minutes by increasing the fractional excretion of chloride with respect to sodium. We utilized Stewart’s quantitative physicochemical analysis to explain the renal response to acute respiratory acidosis (Stewart, 1983).
MATERIALS AND METHODS
Subjects
The experimental procedures were approved by the Institutional Animal Care and Use Committee at Texas A&M University. Suffolk ewes, aged 2–6 years of age were maintained in an environmentally regulated facility (22°C and a 12:12 light/dark cycle) for the duration of the experiments. Animals were fed 2 kg/day of a “complete” ration (Sheep and Goat Pellet, Producers Cooperative, Bryan, TX). All animals consumed all of the feed offered.
Surgery
A week before the start of the experiment, ewes underwent surgery to chronically implant femoral arterial and venous catheters (0.050” inner diameter, 0.090” outer diameter polyvinyl chloride). Details of the surgery protocol have been described earlier (Cudd, Chen, Parnell, & West, 2001). In brief, anesthesia was induced by administering diazepam (0.2 mg/kg; Abbott Laboratories, North Chicago, IL) and ketamine (4 mg/kg intravenously, Ketaset®; Fort Dodge, IA). The ewes were intubated and a surgical plane of anesthesia was maintained using isoflurane (0.5 to 2.5%, IsoFlo®; Abbott Laboratories) and oxygen. Arterial and venous catheters were advanced into the aorta and vena cava via the femoral artery and vein, respectively. At the end of surgery, the ewes received an injection of flunixin meglumine (1.1 mg/kg intramuscularly, Banamine®; Scherring-Plough, Union, NJ), a prostaglandin synthase inhibitor, to reduce postoperative pain. Ewes also received postoperative antibiotics ampicillin trihydrate, polyflex® (25 mg/kg administered subcutaneously for 5 days); Aveco, Fort Dodge, IA and gentamicin sulfate, Gentavet® (2 mg/kg administered intramuscularly twice daily for 5 days); Velco, St Louis.
Experiment Protocol
Manipulation of inspired gases
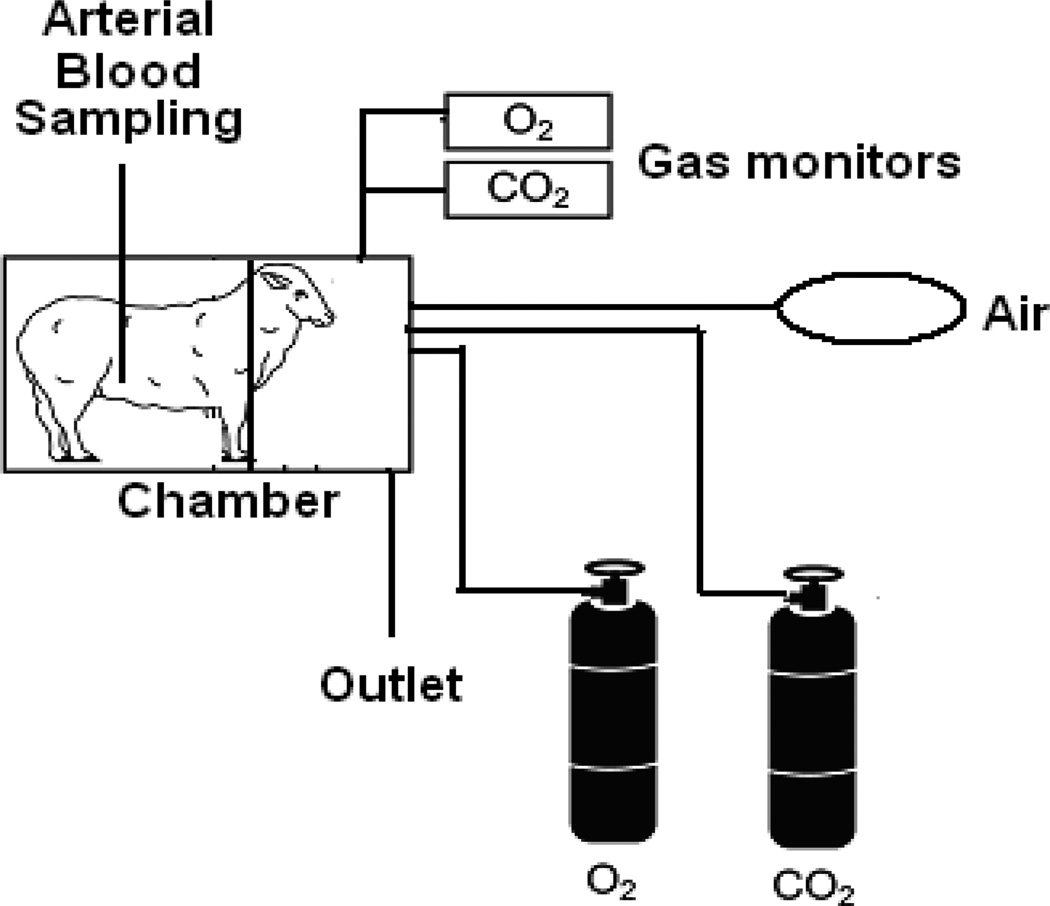
Data were collected on eight different experiment days from a total four ewes. On the day of an experiment, ewes were placed in a modified metabolism cart so that the animal’s head was inside a plexiglass chamber (Figure 1). A vinyl diaphragm attached to the open side of the chamber was drawn around the animal’s neck to isolate the atmosphere in the chamber from ambient air. Blood (1 ml) was drawn from the femoral artery catheter at 0, 30, 60, 90, and 120 minutes for blood gas analysis on each experiment day. Samples were collected in heparinized 3 ml syringes, capped and immediately analyzed using a blood gas analyzer (ABL 5; Radiometer, Westlake, OH). Subjects were made increasingly hypercapnic, peaking at 60 min (PaCO2 of ~ 72 mmHg), by increasing inspired fractional concentrations of carbon dioxide, values comparable to those occurring in cases of acute exacerbation of COPD (Brochard et al., 1995). The rates at which CO2 and O2 were introduced into the chamber were adjusted based on the subject’s pHa measured periodically utilizing a blood gas machine (ABL 5, Radiometer, Cleveland, OH). Gas concentrations in the chamber were measured continuously by gas analyzer (oxygen, model S-3A; carbon-dioxide, model CD-3A, Applied Technologies, Pittsburgh, PA). Gas concentrations in the chamber between blood gas measurements were held constant by continuous adjustment of gas flow into the chamber. The inspired oxygen was maintained at 20.94%, sufficient to maintain arterial partial pressure of oxygen of 99.5 ± 1.72 mm Hg at 60 minutes). At the end of 60 minutes, the subjects were allowed to breathe room air.
Figure 1.
On the day of an experiment, ewes were placed in a modified metabolism cart so that the animal’s head was inside a plexiglass chamber. A vinyl diaphragm attached to the open side of the chamber was drawn around the animal’s neck to isolate the atmosphere in the chamber from ambient air. Subjects were exposed to increased inspired fractional concentrations of carbon dioxide for 60 minutes. The percentage of oxygen and carbon dioxide in the chamber was measured using a gas analyzer.
Measurement of renal fractional clearance of electrolytes
Plasma and urine electrolyte concentrations were measured at 0, 30, 60, 90, and 120 minutes on each experiment day. In brief, urinary catheters (16 Fr) were aseptically inserted in the bladder of ewes before the beginning of the experiment. At 5 minutes before the sample collection time, the bladder was emptied by opening the catheter. Immediately following urine sample collection, arterial blood (1 ml) was collected and immediately centrifuged for 10 minutes at 3000 × g and 4°C, and the plasma was immediately frozen for electrolyte and creatinine analysis. Plasma and urine creatinine, sodium, and chloride concentrations were measured using a commercial analyzer (Vitros-250, Ortho Clinical Diagnostics, Rochester, NY) by the clinical chemistry laboratory of the College of Veterinary Medicine at the Texas A&M University.
The fractional excretion of chloride (FECl) and sodium (FENa) were calculated as follows:
| (1) |
| (2) |
where UCl−, UNa+, UCr are the urine concentrations of chloride, sodium and creatinine, respectively; and PCl−, PNa+, PCr are the plasma concentrations of chloride, sodium and creatinine, respectively.
Chloride/sodium excretion difference was calculated as follows:
| (3) |
Data analysis
FENa is a measure of the percentage of sodium filtered by the kidney that is excreted in the urine. Similarly, FECl represents the fraction of filtered chloride excreted. Our hypothesis suggested that, in response to respiratory acidosis, the body would increase the renal excretion of chloride relative to that of sodium and, therefore, the difference in fractional excretions of chloride and sodium (FECl - FENa) would increase.
FECl, FENa, FeCl-FeNa at the 0, 30, 60, 90 and 120 min time points were analyzed using one-way repeated measures ANOVA with time as the independent factor. Post-hoc pairwise comparisons were conducted using the Holm-Sidak method. In addition, the relationship between arterial pH and fractional excretion difference was determined by linear regression for each experiment as well as for the pooled data. The α level was established a priori at p < 0.05 for all analyses. The data are reported as mean ± SEM.
Results
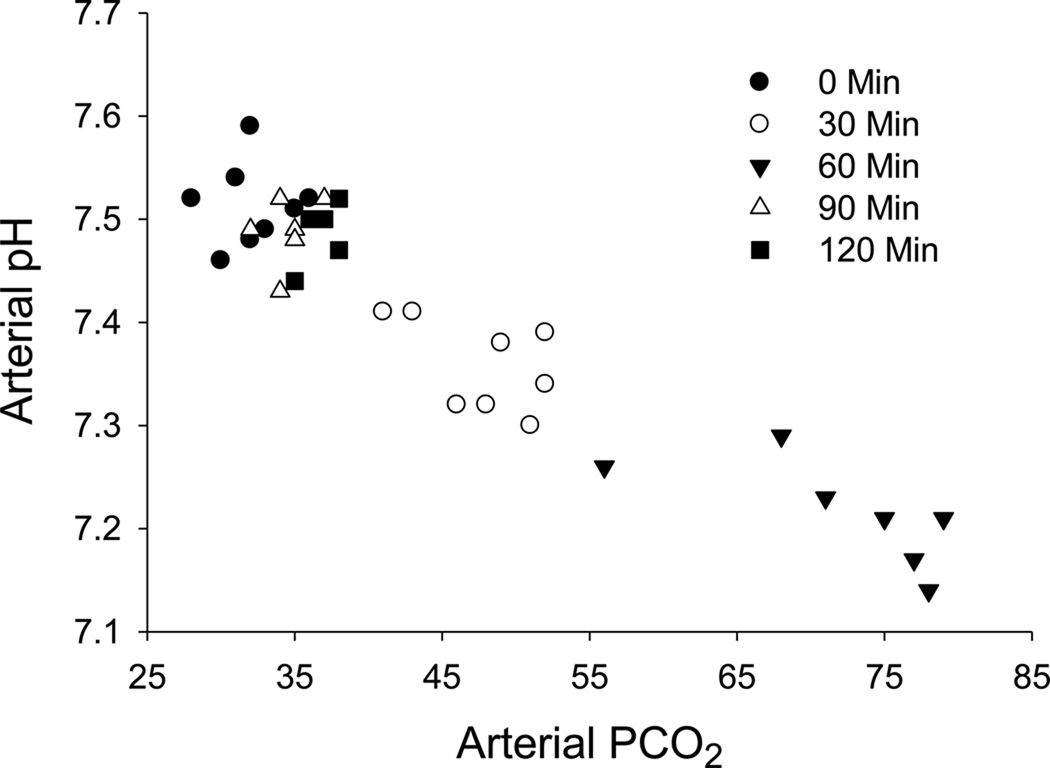
Subjects were exposed to increased inspired fractional concentrations of carbon dioxide for 60 minutes to produce acute respiratory acidosis. The arterial partial pressure of carbon dioxide (PaCO2) increased significantly from 32.13 ± 0.925 at 0 min to 47.75 ± 1.46 at 30 and 71.56 ± 2.69 at 60 min (Figure 2). This increase in PaCO2 resulted in a significant decrease in the pHa from 7.51 ± 0.01 at 0 min to 7.36 ±0.02 at 30 and 7.23 ± 0.02 at 60 min (Figure 2). By 90 and 120 minutes, the pHa and the PaCO2 values were not different from baseline (time 0).
Figure 2.
Arterial PCO2 and arterial pH values for each individual sampling from 4 subjects during 8 experiments at the 0, 30, 60, 90 and 120 min time points. The mean ± SEM arterial partial pressure of carbon dioxide (PaCO2) increased significantly from 32.13 ± 0.925 at 0 min to 47.75 ± 1.46 at 30 min and 71.56 ± 2.69 at 60 min while the mean ± SEM arterial pH decreased from 7.51 ± 0.01 at 0 min to 7.36 ± 0.02 at 30 min and 7.23 ± 0.02 at 60 min. By 90 and 120 minutes, pHa and Paco2 were not different from baseline (time 0).
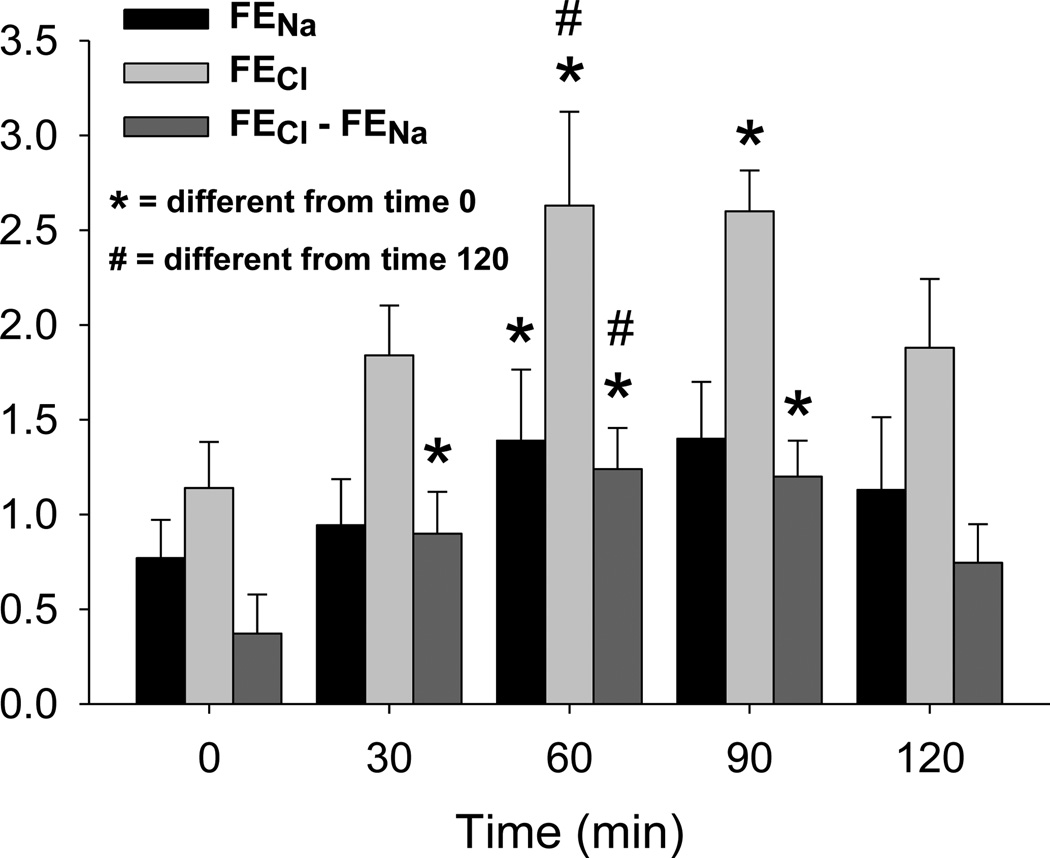
The difference in fractional excretions of chloride and sodium was significantly greater at 30, 60 and 90 minutes than baseline (Figure 3). Both fractional excretion of chloride and that of sodium increased in response to respiratory acidosis; however, the increase in fractional excretion of chloride exceeded that of the fractional excretion of sodium. In addition, the change in differential fractional excretion of chloride and sodium in response to acute respiratory acidosis preceded the changes in fraction excretion of chloride and fractional excretion of sodium and was proportionately greater, 233%, 131% and 81% respectively. No significant changes were noted in plasma concentration of chloride or urine concentrations of sodium or chloride. Plasma sodium concentration was significantly greater at 30 minutes than baseline, 149.0 ± 0.9 and 147.3 ± 0.9 mEq/L respectively; however no differences could be demonstrated at any other time point.
Figure 3.
Fractional excretion of chloride (FECl), fractional excretion of sodium (FENa) and difference in fractional excretions of chloride and sodium (FECl-FENa). While FENa, FECl and FECl-FENa all increased significantly in response to acute respiratory acidosis, 81, 131 and 233% respectively, FECl-FENa increased the soonest after onset of acidosis and exhibited the greatest proportionate change. This data was collected from 4 subject on 8 experimental days. Data are presented as means ± SEM, * = significantly different from time 0, # = significantly different from time 120.
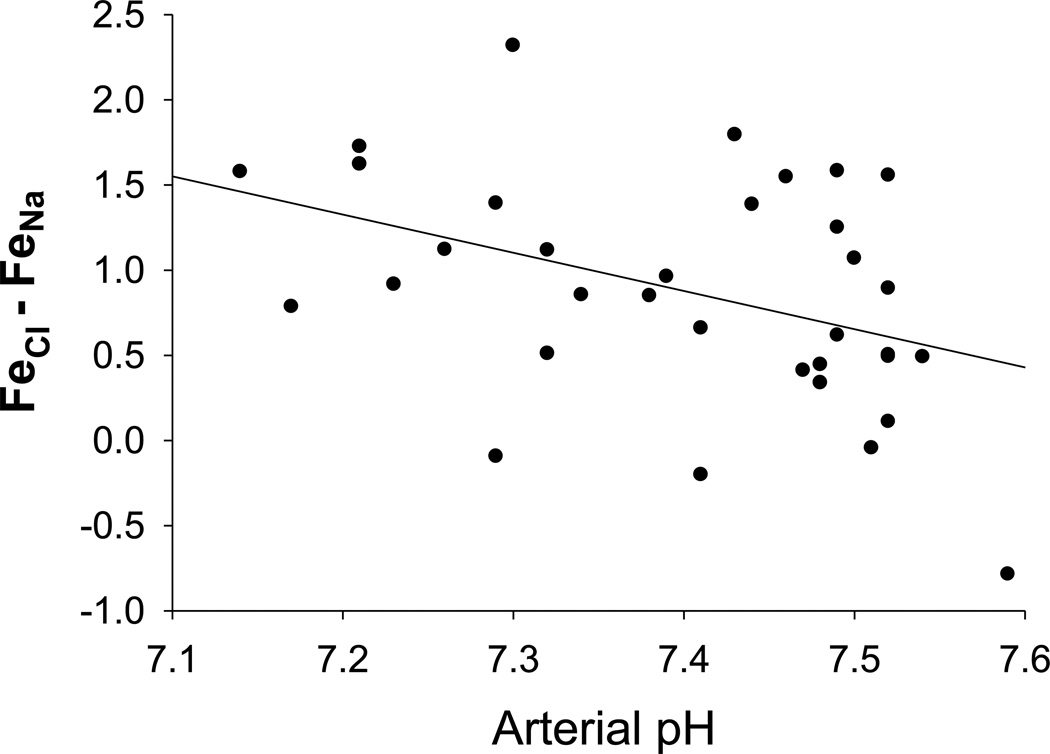
Linear regression demonstrated a significant negative correlation between pHa and FECl and FENa difference (Figure 4). In addition, negative correlations between the same parameters were demonstrated by analysis of data from each individual experiment.
Figure 4.
Data points represent arterial pH and FECl-FENa for each individual sampling from 4 subjects on 8 experimental days at all time points. There was a statistically significant negative correlation between arterial pH and FECl-FENa (p = 0.016).
Discussion
This study is the first to document increased renal chloride excretion with respect to sodium within 30 minutes of the onset of acute hypercapnea. Our hypothesis does not predict that clearance of sodium or chloride will increase or decrease, but that the relationship between them will change in response to alterations in acid-base status. The acute hypercapnea produced in this study was not accompanied by hypoxemia. The arterial partial pressure of oxygen was controlled by manipulating the inspired fractional concentration of gases. In support of our findings, Alfaro and coworkers demonstrated an increase in the ratio of plasma [Na+]:[Cl−] due to a decrease in plasma [Cl−] in human patients with chronic obstructive pulmonary disease (blood Pco2 = 54 mm Hg). However, that study did not examine if the decrease in plasma Cl− concentration is mediated by a differential regulation of the two ions by the kidney (Alfaro, Torras, Ibanez, & Palacios, 1996).
The kidney and the GI tract are the two main organ systems that have been proposed to compensate for primary respiratory acidosis (Stewart, 1983). It has been demonstrated that acute respiratory acidosis (pH = 7.21 ± 0.01) increases sodium and chloride absorption by 98% and 32% respectively in anesthetized rabbit ileum (Charney, Arnold, & Johnstone, 1983). Similarly, in anesthetized rats, both ileum and the colon compensate for respiratory acidosis (pH = 7.26 ± 0.01) by increasing absorption of sodium and chloride but sodium to a greater extent (Feldman & Charney, 1982). Therefore, it appears that the gastrointestinal system increases the absorption of sodium with respect to chloride whereas the renal system increases the chloride clearance with respect to sodium in response to acute respiratory acidosis. Though mechanisms underlying renal compensation for chronic metabolic acidosis has been reported (Wang, Egbert, Aronson, & Giebisch, 1998), the specific cellular pathways underlying altered fractional clearance of chloride as against sodium in response to respiratory acidosis have not been well studied.
We conclude that the renal response to acute respiratory acidosis is a rapid (within 30 min of onset) increase in the differential fractional excretion of chloride over sodium. While both the fractional excretion of sodium and the fractional clearance of chloride were increased by acute respiratory acidosis, 81% and 131% respectively, the earliest and greatest proportionate change was the differential fractional excretion of chloride over sodium, by 233%, which is consistent with strong ion acid base theory and that sodium reabsorption during respiratory acidosis is not independent of renal regulation of extracellular fluid volume. Therefore, net chloride excretion relative to sodium is the primary mechanism for renal regulation of acid-base balance.
Acknowledgments
Supported by NIAAA Grant AA10940 and by the NIH Pediatrics Initiatives (TAC).
References
- Alfaro V, Torras R, Ibanez J, Palacios L. A physical-chemical analysis of the acid-base response to chronic obstructive pulmonary disease. Can J Physio Pharmacol. 1996;74(11):1229–1235. [PubMed] [Google Scholar]
- Barker ES, Singer RB, Elkinton JR, Clark JK. The renal response in man to acute experimental respiratory alkalosis and acidosis. J Clin Invest. 1957;36(4):515–529. doi: 10.1172/JCI103449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333(13):817–822. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- Carter NW, Seldin DW, Teng HC. Tissue and renal response to chronic respiratory acidosis. J Clin Invest. 1959;38(6):949–960. doi: 10.1172/JCI103878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudd TA, Chen WJ, Parnell SE, West JR. Third trimester binge ethanol exposure results in fetal hypercapnea and acidemia but not hypoxemia in pregnant sheep. Alcohol Clin Exp Res. 2001;25(2):269–276. [PubMed] [Google Scholar]
- Eiam-ong S, Laski ME, Kurtzman NA, Sabatini S. Effect of respiratory acidosis and respiratory alkalosis on renal transport enzymes. Am J Physiol. 1994;267(3 Pt 2):F390–F399. doi: 10.1152/ajprenal.1994.267.3.F390. [DOI] [PubMed] [Google Scholar]
- Feldman GM, Charney AN. Effect of acute respiratory alkalosis and acidosis on intestinal ion transport in vivo. Am J Physiol. 1982;242(5):G486–G492. doi: 10.1152/ajpgi.1982.242.5.G486. [DOI] [PubMed] [Google Scholar]
- Fencl V, Rossing TH. Acid-base disorders in critical care medicine. Annu Rev Med. 1989;40:17–29. doi: 10.1146/annurev.me.40.020189.000313. [DOI] [PubMed] [Google Scholar]
- Gehlbach BK, Schmidt GA. Bench-to-bedside review: treating acid-base abnormalities in the intensive care unit - the role of buffers. Crit Care. 2004;8(4):259–265. doi: 10.1186/cc2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougoux A, Vinay P, Cardoso M, Duplain M, Lemieux G. Immediate adaptation of the dog kidney to acute hypercapnia. Am J Physiol. 1982;243(3):F227–F234. doi: 10.1152/ajprenal.1982.243.3.F227. [DOI] [PubMed] [Google Scholar]
- Kaplan LJ, Frangos S. Clinical review: Acid-base abnormalities in the intensive care unit -- part II. Crit Care. 2005;9(2):198–203. doi: 10.1186/cc2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum JA, Elbers PW. Stewart's Textbook of Acid-Base. Netherlands: Lulu; 2009. [Google Scholar]
- Koch SM, Taylor RW. Chloride ion in intensive care medicine. Crit Care Med. 1992;20(2):227–240. doi: 10.1097/00003246-199202000-00012. [DOI] [PubMed] [Google Scholar]
- Levitin H, Branscome W, Epstein FH. The pathogenesis of hypochloremia in respiratory acidosis. J Clin Invest. 1958;37(12):1667–1675. doi: 10.1172/JCI103758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KM, Tisher CC. Cellular response to acute respiratory acidosis in rat medullary collecting duct. Am J Physiol. 1983;245(6):F670–F679. doi: 10.1152/ajprenal.1983.245.6.F670. [DOI] [PubMed] [Google Scholar]
- Polak A, Haynie GD, Hays RM, Schwartz WB. Effects of chronic hypercapnia on electrolyte and acid-base equilibrium. I. Adaptation. J Clin Invest. 1961;40:1223–1237. doi: 10.1172/JCI104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PA. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983;61(12):1444–1461. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- Wang T, Egbert AL, Jr., Aronson PS, Giebisch G. Effect of metabolic acidosis on NaCl transport in the proximal tubule. Am J Physiol. 1998;274(6 Pt 2):F1015–F1019. doi: 10.1152/ajprenal.1998.274.6.F1015. [DOI] [PubMed] [Google Scholar]