Abstract
Gene silencing via orally delivered small interfering RNAs (siRNAs) represents a promising treatment strategy for numerous gastrointestinal (GI) diseases associated with chronic intestinal inflammation; however, the oral delivery of siRNA to inflamed isntestinal tissues remains a major challenge. Here, we present a delivery vehicle for siRNA, termed thioketal nanoparticles (TKNs), that can localize orally deliver siRNA to sites of intestinal inflammation, and thus inhibit gene expression in diseased intestinal tissue. TKNs are formulated from a new polymer, poly-(1,4-phenyleneacetone dimethylene thioketal) (PPADT), that degrades selectively in response to reactive oxygen species (ROS). Therefore, when delivered orally, TKNs release siRNA in response to the abnormally high levels of ROS specific to sites of intestinal inflammation1–3. Using a murine model of ulcerative colitis (UC), we demonstrate that orally administered TKNs loaded with siRNA against the proinflammatory cytokine tumor necrosis factor-alpha (TNFα) diminish TNFα messenger RNA (mRNA) levels in the colon and protect mice from UC.
Small interfering RNAs directed against proinflammatory cytokines have the potential to treat diseases associated with intestinal inflammation4; however, the systemic depletion of proinflammatory cytokines can result in an elevated risk of infection5, lymphoma6, and cardiac dysfunction7. For this reason, there is great interest in targeting the delivery of immune suppressive siRNAs to sites of intestinal inflammation. Oral delivery vehicles have direct access to the intestinal mucosa, and thus represent an ideal platform for delivering siRNA to diseased intestinal tissues. Furthermore, by eliminating needles, oral delivery increases patient compliance and eliminates the risk of disease transmission by contaminated needles. Various delivery vehicles have been developed to orally deliver therapeutics to intestinal tissue8–10, yet none of these strategies have demonstrated the ability to protect siRNA from the harsh environment of the GI tract, localize its delivery to diseased intestinal tissue, and silence gene expression in intestinal tissues.
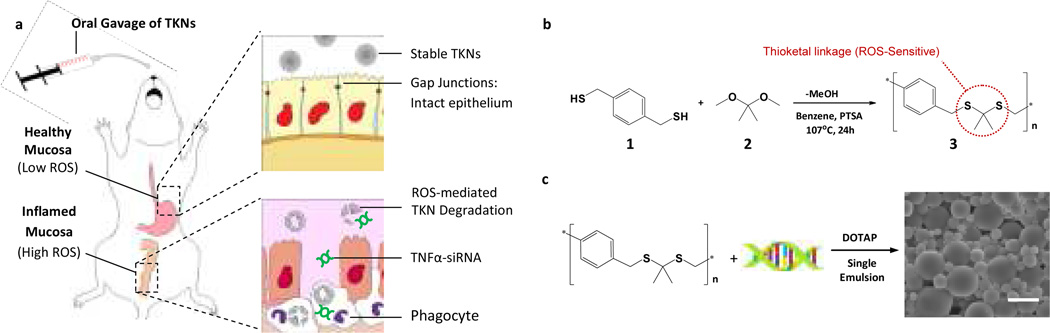
To localize the delivery of siRNA to diseased intestinal tissue, we identified the abnormally high levels of ROS produced at sites of intestinal inflammation as a disease specific triggering mechanism for siRNA release2. For example, biopsies taken from patients suffering from UC1,3,11, colon cancer12, and h. pylori infections2 have a ten- to hundred-fold increase in mucosal ROS concentrations that are confined to sites of disease development and correlate with disease progression. To orally deliver therapeutics to sites of intestinal inflammation we developed TKNs, which release encapsulated agents in response to ROS. TKNs are formulated from PPADT, a new polymer composed of ROS-sensitive thioketal linkages13 that are stable to acid-, base-, and protease-catalyzed degradation13–14 (Fig. 1a). Therefore, orally delivered TNFα-TKNs remain stable in the GI-tract, thereby protecting siRNA and preventing its release to non-inflamed tissues (Fig. 1b, upper panel). However, at sites of intestinal inflammation the elevated ROS levels trigger the degradation of the TNFα-TKNs, thus localizing the release of siRNA to inflamed intestinal tissues (Fig. 1b, lower panel).
Figure 1. Thioketal nanoparticles are formulated from a new ROS-sensitive polymer and release orally delivered siRNA at sites of intestinal inflammation.
(a) PPADT is a new polymer composed of ROS-sensitive thioketal linkages (circled in red). TNFα-TKNs were prepared by first precomplexing TNFα-siRNA with the cationic lipid DOTAP. Next, these siRNA-DOTAP complexes were added to an organic solution containing PPADT. A single-emulsion procedure was then used to produce particles with mean diameters of ~600 nm. SEM image shows TNFα-TKNs (scale bar represents 1.5 µm). (b) When delivered orally, TNFα-TKNs remain stable in the harsh environment of the GI tract, protecting TNFα-siRNA and preventing its release to non-inflamed mucosal tissues. However, at sights of intestinal inflammation, where infiltrating phagocytes produce unusually high levels of ROS, the TKNs degrade, thus releasing TNFα-siRNA to the site of inflammation. (c) PPADT 3 was synthesized using the acetal exchange reaction.
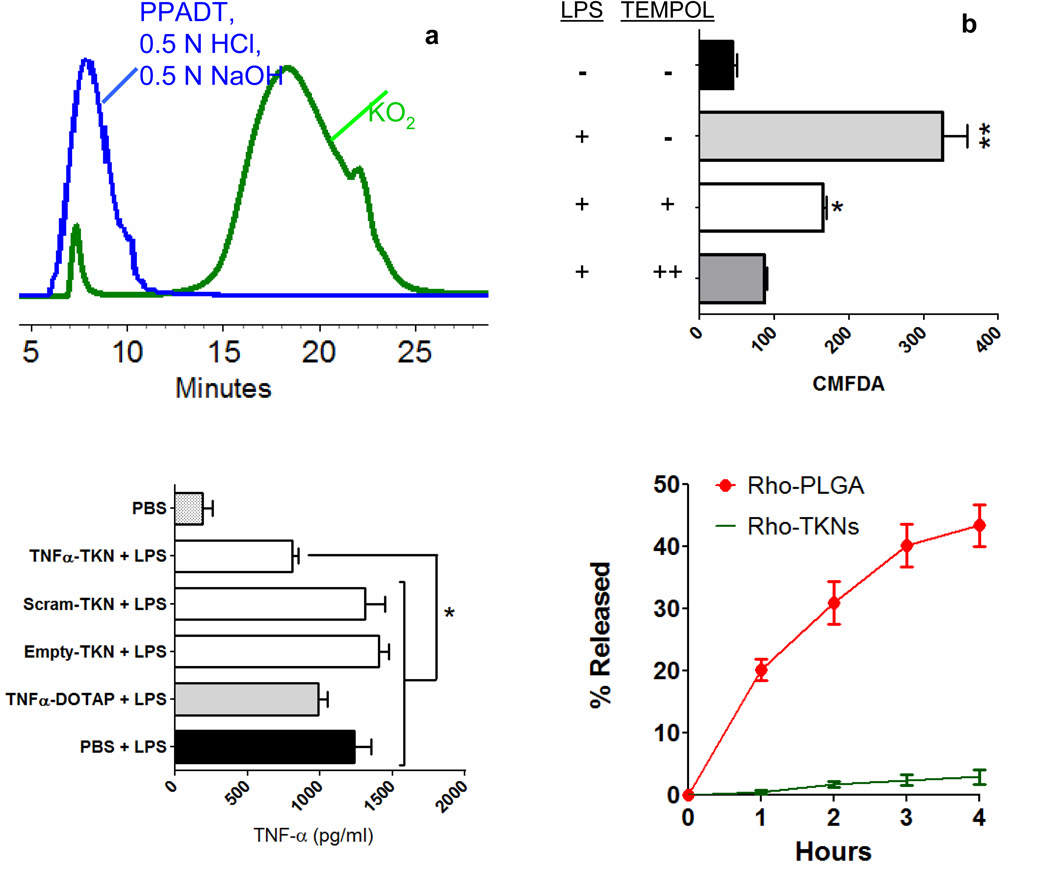
PPADT was synthesized from 1,4-benzenedimethanethiol 1 and 2,2-dimethoxypropane 2 via a step-growth polymerization that produces polymers with molecular weights (MN) of approximately 9,000 Da (Fig. 1c). To investigate the specificity of PPADT for ROS, we incubated PPADT with either a superoxide solution, 0.5 N HCl solution, or a 0.5 N NaOH solution and then analyzed the resulting product’s molecular weight via gel permeation chromatography (GPC). The GPC traces shown in Figure 2a demonstrate that exposing PPADT to superoxide decreases its molecular weight from almost 9,000 Da to approximately 810 Da in 8 h, whereas incubating PPADT in either an acidic or basic environment had no effect on its molecular weight.
Figure 2. PPADT is an ROS-sensitive polymer and nanoparticles formulated from it release their payloads in response to ROS produced by activated macrophages.
(a) GPC traces of PPADT before (blue) and after exposure to KO2 (green). Incubating PPADT in acidic and basic environments (0.5 N HCl & 0.5 N NaOH) had no effect on the molecular weight of PPADT (coincident traces represented with blue line). (b) Intracellular fluorescence of macrophages treated with CMFDA-loaded TKNs. Results expressed as mean fluorescence ± standard error of the mean (s.e.m.) for n = 3 per group. Statistical significance was determined by a one-way ANOVA using Bonferroni’s post hoc test (*p ≤ 0.05, **p ≤ 0.001). (c) Extracellular TNFα mRNA levels as determined by ELISA. Macrophages treated with 23.0 µg TNFα-siRNA/ml via TNFα-TKNs and activated with LPS expressed significantly less TNFα as compared to LPS-activated macrophages treated with PBS. Results depicted as mean pg of TNFα per ml of media ± s.e.m. for n = 3 per treatment group.
The unusually high concentrations of ROS localized to sites of intestinal inflammation are generated by activated phagocytes15. To determine the capability of the TKNs to release agents in response to a physiologically relevant source of ROS, we compared the amount of intracellular dye released from dye-loaded TKNs in activated (ROS overproducing) versus non-activated phagocytes. Macrophages were treated with TKNs loaded with 5-chloromethylfluorescein diacetate (CMFDA), washed of excess particles, and then activated with lipopolysaccharide (LPS). CMFDA is activated by intracellular proteases; therefore, the cellular fluorescence of cells treated with CMFDA-loaded TKNs (CMFDA-TKNs) will be proportional to the amount of CMFDA released from phagocytosed TKNs. Figure 2b shows that CMFDA-TKNs released CMFDA at an accelerated rate in response to ROS produced by activated macrophages. For example, LPS-activated macrophages showed a greater than seven-fold increase in cellular fluorescence compared to non-activated macrophages. This dramatic increase in cellular fluorescence was mitigated by treating LPS-activated macrophages with the ROS-scavenger TEMPOL (Fig. 2b), indicating that the amount of CMFDA released from CMFDA-TKNs is a function of ROS.
Due to the essential role played by TNFα in the onset and persistence of intestinal inflammation16, we chose to treat mice suffering from DSS-induced colitis with TNFα-siRNA17. To formulate siRNA-loaded particles for in vitro and in vivo studies, we first complexed siRNA with the cationic lipid 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), and then loaded these complexes into nanoparticles composed of PPADT. DOTAP has several properties that can increase the effectiveness of TNFα-TKNs. Complexing siRNA with cationic species, such as DOTAP, enhances siRNA trasfection by increasing siRNA stability18, cellular internalization19, mucosal transport20, and endosomal escape21–22. Thus, at sites of intestinal inflammation, we anticipate that the co-encapsulated DOTAP will improve the efficacy of siRNA released in response to ROS in both the intracellular and extracellular environments. Furthermore, incorporating DOTAP endows nanoparticles with a positive surface charge (Fig. S3), which can increase particle uptake by phagocytes23 and adhesion to the negatively charged intestinal mucosa24. Finally, to optimize the particles for oral delivery, we engineered siRNA-loaded TKNs to have diameters of ~600 nm. Nanoparticles of this size limit nonspecific uptake by enterocytes25, yet bind to inflamed colonic mucosa26 and are efficiently taken up phagocytes21,23, which are the major producers of TNFα at sites of intestinal inflammation27.
To demonstrate the ability of TNFα-TKNs to silence TNFα expression by immune cells, we treated LPS-activated macrophages with TNFα-TKNs or appropriate controls. Figure 2c shows that treating LPS-activated macrophages with TNFα-TKNs resulted in a statistically significant reduction in TNFα production as compared to cells treated with either PBS or TKNs loaded with a scrambled siRNA sequence (Sc-TKNs) (p ≤ 0.05). In addition, we discovered that TNFα-TKNs have a cytotoxicity profile that is similar to nanoparticles formulated from the FDA approved material poly(lactic-co-glycolic acid) (PLGA) (Fig. S4). These results show that TNFα-TKNs can protect siRNA from serum, deliver TNFα-siRNA in its active form, and decrease the expression of TNFα in activated phagocytes.
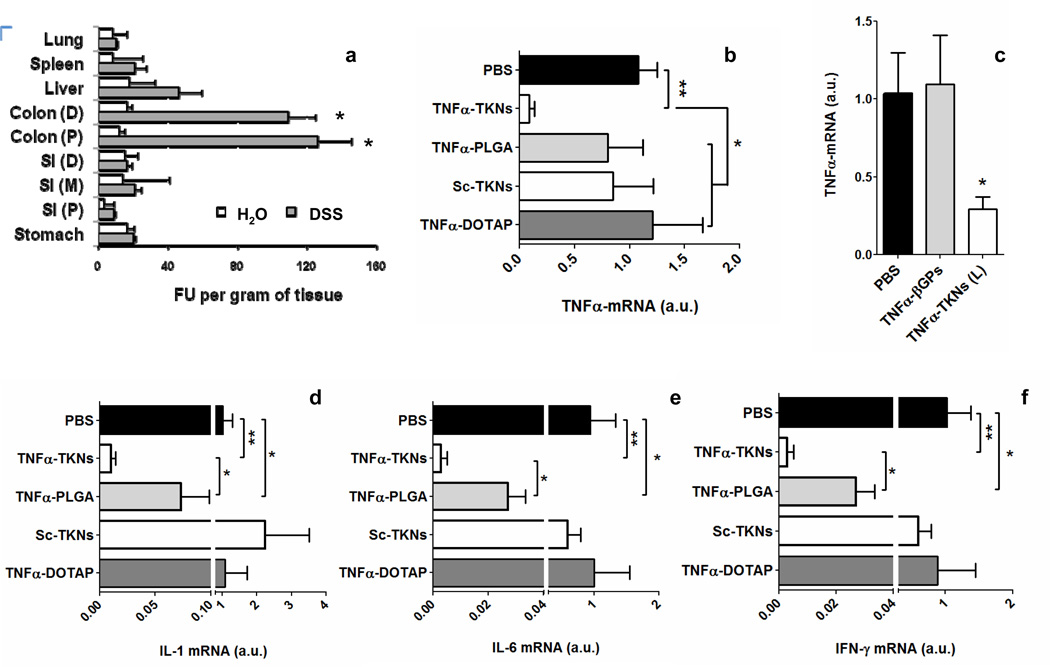
The stability of the TKNs to simulated GI fluids (Fig. S6) and their ability to release encapsulated agents, including siRNA (Fig S1), in response to ROS, motivated us to determine if orally delivered TKNs could target siRNA to inflamed intestinal tissues. Intestinal inflammation was induced in female C57BL/6 mice by replacing their drinking water on day zero with a 3% solution of DSS28. DSS supplementation induces an inflammatory response in the colon that mimics human UC and is characterized by: immune cell infiltration, a marked loss in body weight, and elevated colonic ROS and cytokine production27. Starting on day zero, mice receiving either DSS or normal drinking water were given a daily oral gavage of TKNs loaded with a Cy3-tagged scrambled siRNA (Cy3siRNA). On day seven, the biodistribution of the delivered siRNA was measured by fluorescence. Our results demonstrate that the TKNs can localize orally delivered siRNA to sites of intestinal inflammation (Fig. 3a). For example, Figure 3a shows a greater than 3-fold increase in the amount of Cy3siRNA delivered to the distal and proximal sections of the colons of mice receiving DSS and Cy3siRNA-TKNs as compared to mice receiving normal water and treated with Cy3siRNA-TKNs. Moreover, we observed relatively low levels of Cy3siRNA-TKNs delivered to the other organs of both treatment groups. Since the inflammation induced by DSS is confined to the colon28, these results confirm that the siRNA-loaded TKNs remain stable in non-inflamed regions of the GI-tract while targeting siRNA to inflamed intestinal tissues.
Figure 3. TKNs target orally delivered siRNA to inflamed intestinal tissues and when loaded with TNFα-siRNA reduce the colonic mRNA levels of proinflammatory cytokines in mice suffering from DSS-induced UC.
(a) Biodistribution of Cy3-tagged siRNA in the organs of mice treated with a daily gavage of Cy3siRNA-TKN s for 6 days (D: distal, M: medial, P: proximal, SI: small intestine). Fluorescent units (FU) per gram of tissues depicted as the mean ± s.d. for n = 10 mice per group. Mice received either a solution of DSS (DSS) or water (H2O). Asterisk represents statistical significance between the florescence in the same organs of DSS-treated mice and mice receiving water, and was determined using a two-sample student t-test (*p ≤ 0.05). (b) TNFα mRNA levels in mice receiving DSS and treated scrambled- or TNFα-siRNA (2.3 mg siRNA/kg/day) via either TNFα-TKNs (n = 6), TNFα-PLGA (n = 5), Sc-TKNs (n = 5), siRNA-DOTAP (n = 6). (c) TNFα mRNA levels in mice receiving DSS and treated with 0.23 mg TNFα-siRNA/kg/day via either TNFα-TKNs (n = 10) or TNFα-βGPs (n=10). (d) Colonic cytokine mRNA levels in mice receiving DSS and treated with either PBS or 0.23 mg TNFα-siRNA 0.23/kg/day via TNFα-TKNs (n = 10). Statistical differences in Figures a-d determined by a one-way ANOVA using Bonferroni’s post hoc test (*p ≤ 0.05, **p ≤ 0.001).
Based on these results, we hypothesized that orally administered TNFα-TKNs could silence TNFα expression in the colons of mice suffering from DSS-induced colitis. To test our hypothesis, mice receiving DSS were given TNFα-siRNA or scrambled siRNA (2.3 mg siRNA/kg) encapsulated in TKNs via gavage once daily for five days. Starting on day zero, mice receiving DSS were also treated with free TNFα-siRNA/DOTAP complexes (2.3 mg siRNA/kg/day) (TNFα-DOTAP) or PBS. After seven days, the colonic mRNA levels of TNFα and other pro-inflammatory cytokines up-regulated as part of the intestinal inflammatory response, namely IL-6, IL-1, and IFN-γ, were analyzed by real-time PCR (RTPCR). As shown in Figure 3b, mice treated with TNFα-TKNs (2.3 mg siRNA/kg/day) experienced a dramatic ten-fold decrease in colonic TNFα mRNA (p ≤ 0.001). Analysis of colonic IL-6, IL-1, and IFN-γ, mRNA levels show that TNFα-TKNs also inhibited the activation of other pro-inflammatory signaling cascades (Fig. S7).
To determine if the ability of the TKNs to resist both hydrolytic- and enzymatic-mediated release (Fig. S6) improves the efficacy of nanoparticle-mediated oral delivery of siRNA, we also treated mice receiving DSS with a daily gavage of TNFα-siRNA (2.3 mg siRNA/kg) encapsulated in size- and charge-matched PLGA (50:50) nanoparticles (TNFα-PLGA). Figure 3a shows that mice receiving DSS and treated with TNFα-PLGA did not experience a significant decrease in colonic TNFα-mRNA, suggesting that the greater stability of the TKNs over that of PLGA nanoparticles to the GI environment (Fig. S1) is an important factor in their improved efficacy. Although performing these experiments with TNFα-siRNA-loaded nanoparticles formulated from a material similar to PPADT but without the thioketal linkages would provide additional information about the efficacy of the TKNs, the synthetic methodology used to generate PPADT does not allow for the synthesis of such a material.
The high level of colonic TNFα suppression achieved by the TNFα-TKNs at a dose of 2.3 mg siRNA/kg/day motivated us to establish a minimum effective dose for TNFα-TKNs. Starting on day zero, mice receiving DSS were treated with a ten-fold lower dose of TNFα-TKNs (0.23 mg TNFα-siRNA/kg/day ) via a daily oral gavage for five days. Figure 3c illustrates that at a ten-fold lower dose TNFα-TKNs continue to produce a significant inhibition of colonic TNFα mRNA (p ≤ 0.05) in mice receiving DSS. In addition, this approximately three-fold decrease in TNFα mRNA mitigated the activation of other proinflammatory signaling pathways that have been implicated in the development of UC, namely IL-6, IL-1, and IFN-γ (Fig. 3d). These results imply that orally delivered TKNs perform as well as current systemic delivery systems for siRNA that have been used to treat DSS-induced colitis4.
To examine if the ability of the TKNs to localize the delivery of siRNA to inflamed tissues plays a significant role in their ability to treat intestinal inflammation, we compared the efficacy of TNFα-TKNs to (TNFα-siRNA)-loaded β-glucan particles (TNFα-βGPs), which have demonstrated the ability to orally deliver siRNA, but do not target diseased tissues8. Figure 3c shows that mice receiving DSS and treated with TNFα-βGPs (0.23 mg siRNA/kg/day) did not experience a significant decrease in colonic TNFα-mRNA. These results imply that the ability of the TKNs to target inflamed tissues is also an important factor for their in vivo efficacy (see Fig. S5 for characterization of TNFα-βGPs).
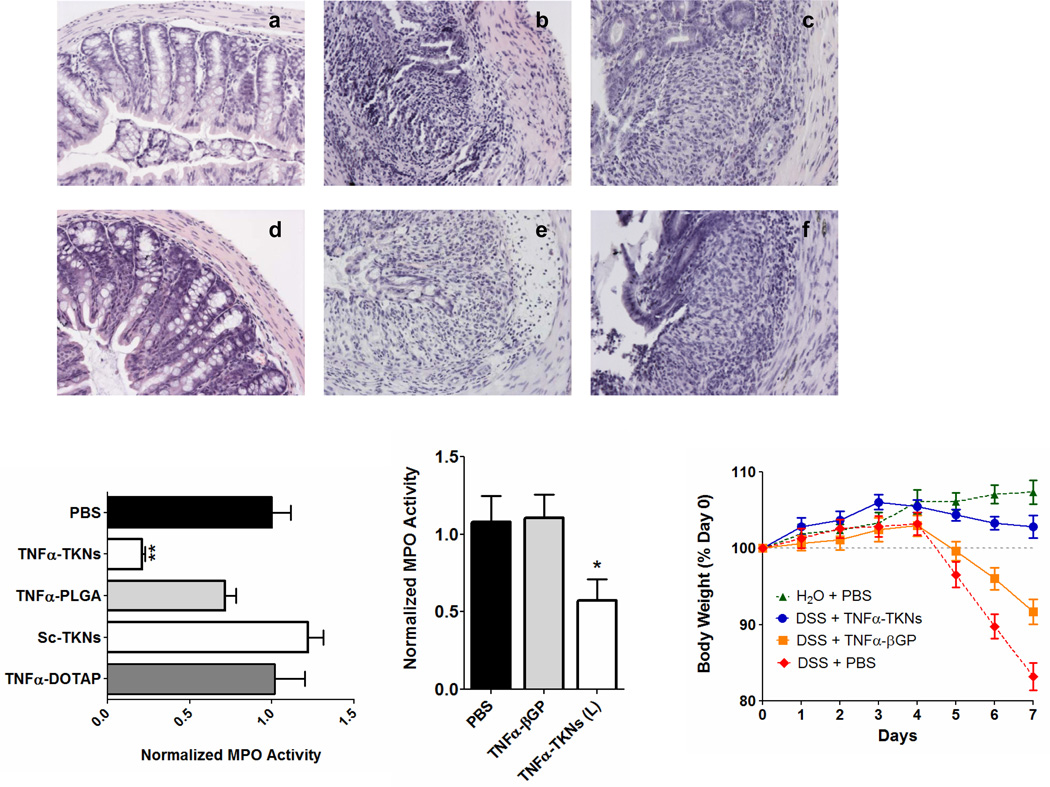
Finally, we investigated if orally delivered TNFα-TKNs could allay the clinical manifestations of DSS-induced UC. Our results demonstrate that TNFα-TKNs protected mice from DSS-induced colitis as assessed by histological analysis, colonic myeloperoxidase (MPO)-activity, and weight loss (Fig. 4, Figs. S9–S11). For example, the colons of mice receiving DSS and treated with TNFα-siRNA (0.23 mg/kg/day) via TNFα-TKNs had intact epitheliums, well defined crypt structures, and relatively low levels of neutrophil invasion (Fig. 4d). Additionally, colonic MPO activity in mice receiving DSS and treated with TNFα-TKNs (0.23 mg siRNA/kg/day) was markedly reduced (Fig. 4g). Finally, as depicted in Figure 4 and Figure S11, mice treated with TNFα-siRNA via TNFα-TKNs were significantly heavier after seven days than mice receiving DSS and other treatments. In contrast, mice receiving DSS and treated with TNFα-PLGA, TNFα-βGPs, Sc-TKNs, TNFα-DOTAP, and PBS showed all of the characteristics of DSS-induced inflammation as measured by histology, high levels of MPO activity, and significant weight loss. These results demonstrate that among the strategies presented here the TKNs are uniquely suited to deliver siRNA and treat inflammatory diseases of the GI tract (Fig. 4 & Figs. S7–S11).
Figure 4. Orally administered TNFα-TKNs protect mice from DSS-induced colitis.
(a) H&E-stained colon section from mice after seven days of receiving normal water and a daily oral gavage of PBS (20X). (b–f) H&E-stained colon sections form DSS-treated mice given a daily gavage of one of the following: (b) PBS, (c) Sc-TKNs (2.3 mg/kg), (d) TNFα-TKNs (0.23 mg/kg), (e) TNFα-PLGA (2.3 mg/kg), or (f) TNFα-βGPs (0.23 mg/kg) (20X). (g) Colonic MPO activity. Results are expressed as mUnits of MPO activity per mg protein and error bars represent ± s.e.m. Statistical significance was calculated using a one-way ANOVA and Bonferroni’s post hoc test (**p ≤ 0.05). (h) Time course of mouse body weight. Mouse body weight was normalized as a percentage of day zero body weight. Body weight depicted as the mean of each treatment group. Error bars represent ± s.e.m. Asterisk represents statistical significance from all other groups and was determined by a one-way ANOVA using Bonferroni’s post hoc test (*p ≤ 0.05).
Oral administration represents the most convenient and cost-effective means to deliver siRNA to diseased intestinal tissues. However, GI fluids, the intestinal mucosa, and cellular barriers to uptake represent significant obstacles for orally delivered siRNA. Here we present evidence that TKNs have the chemical and physical properties needed to overcome these obstacles and provide a therapeutic level of gene silencing in inflamed intestinal tissues. Based on our results, we expect that TKNs will make a significant contribution to the treatment of numerous GI diseases linked to intestinal inflammation, including GI cancers, inflammatory bowel diseases, and viral infections.
METHODS
Unless otherwise noted, all reagents were used as received from Sigma.
PPADT Synthesis and ROS sensitivity assay
Briefly, a two-necked flask was charged with distilled benzene, 1,4-benzenedimethanethiol 1 (1.0 eq) and 2,2-dimethoxypropane 2 (1.0 eq.), then equipped with a metering funnel and distillation head for removal of the methanol by-product. The mixture was then stirred continuously and heated to 95 °C before a catalytic amount of re-crystallized p-toluenesulfonic acid (0.003 eq.) in distilled ethyl acetate was added to start the reaction. After 1 h, a solution of 2,2-dimethoxypropane and distilled benzene was added to the metering funnel and the funnel stopcock was set so that a small amount of 2,2-dimethoxypropane (0.08 eq per h) was add drop-wise over a period of 12 h. The reaction was allowed to stir overnight before the resulting polymer was isolated by precipitation in cold hexanes. The polymer was vacuum-dried and analyzed by 1H-NMR (Burker DMX 400, 400MHz), 13C NMR, and gel permeation chromatography (Shimadzu). 1H-NMR: Per repeating unit, (400MHz, CDCl3,) δ ppm 7.28 (4H), 3.85 (4H), 1.60 (9H). 13C- NMR: (400 MHz, CDCl3) δ ppm 129.54, 77.56, 77.25, 76.93, 35.03, and 31.01. The molecular weight of the resulting polymer was approximately 9 kDa with a polydispersity of 1.8. In order to assess the ROS-sensitivity of PPADT, PPADT was exposed to superoxide according to the procedure described in Reference 19.
Preparation of CMFDA-loaded TKNs
CMFDA (Invitrogen) was encapsulated in TKNs via an oil-in-water single-emulsion procedure. CMFDA (50 µµg) was solubilized in dimethyl sulfoxide (DMSO) and added to a solution of PPADT (100 mg) in dichloromethane (DCM) (500 µl). This organic phase was then added to 10 mL of a 5% solution of polyvinyl alcohol (PVA) in pH 7.4 phosphate buffer saline (PBS) and the biphasic mixture was homogenized (17,500 rpm) for 60 sec. The resulting oil-in-water emulsion was then added to 60 mL of a 1% solution of PVA in PBS and stirred in an open container for 4 h to evaporate the DCM. The resulting particles were isolated by centrifugation and washed three times with PBS to remove any residual PVA. Finally, the particles were frozen at −75°C and lyophilized to obtain a fine powder. Empty TKNs were formulated as described above, but without any dye. Dye encapsulation was determined as described in the supplementary information. Particles contained 27.3 µg of CMFDA per mg of particles. For all particle formulations, particle size was determined by dynamic light scattering [DLS (Brookhaven Instruments Corporation)], and visual evidence of particle formation was obtained via a scanning electron microscope [SEM (Hitachi S-800)] (Fig. S2 & S3).
Preparation of siRNA-loaded TKNs and PLGA nanoparticles
All nanoparticles loaded with siRNA were generated by first preparing siRNA-DOTAP complexes then using the above single-emulsion protocol to encapsulate these complexes into nanoparticles. All siRNA sequences were purchased and used as received from Dharmacon. Either the TNFα-siRNA sequence 5’- CAC AAC CAA CUA GUG GUG CUU-3’, a scrambled non-specific siRNA sequence, or a Cy3-tagged scrambled siRNA sequence were complexed with DOTAP (Avanti Polar Lipids Inc.) by adding 400 µl of a 187.5 µM solution of siRNA in nuclease free water to 400 µl of a 9.45 mM DCM/DOTAP solution. To this two-phase solution was added 880 µl of MeOH and the resulting single-phase solution was vortexed for 60 sec. Next, DCM (400 µl) and nuclease free water (400 µl) were added to the siRNA-DOTAP solution, and the solution was centrifuged (1000 g for 10 min) to separate the two phases. Next, the organic phase (aprox. 800 µl) containing the DOTAP-siRNA complexes was collected and added to 50 mg of PPADT or PLGA. This organic siRNA-DOTAP/PPADT mixture was then used to formulate oil-in-water single-emulsion particles as described above. Small-interfering RNA encapsulation was determined according to the method given in the supplementary information. This protocol produced TNFα-TKNs, Sc-TKNs, Cy3siRNA-TKNs, and TNFα-PLGA that contained 4.7, 4.1, 5.8 and 6.1 µg of siRNA per mg of particles, respectively.
Preparation of TNFα siRNA loaded βGPs
βGPs containing TNFα-siRNA were prepared exactly as described in Reference 12.
ROS-responsive release from TKNs in vitro
RAW 264.7 macrophages (ATCC) were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and maintained at 37°C in a humidified 5% CO2 atmosphere. Cells were seeded in 12 well culture plates (107 cells/well) and incubated with 0.2 mg/ml CMFDA-TKNs. After 3 h, the cells were washed with PBS three times, and then treated with either DMEM/FBS, DMEM/FBS spiked with LPS or DMEM/FBS spiked with LPS and TEMPOL (2.5 mM & 5.0 mM). After 20 h, the media was removed and the cells were washed with PBS and suspended in 1.5 ml of PBS containing 1% FBS and 5 mM EDTA. The cells were then passed through a 45 µm nylon mesh before being analyzed via fluorescence-activated cell sorting (FACS) to assess intracellular dye release. FACS analysis was carried out on DB® FACSVantageSE/DiVa® instrumentation, and the results were analyzed with FlowJow® software.
In vitro silencing of TNFα expression with TNFα-TKNs
RAW 264.7 macrophages were cultured as described above, and seeded in 12 well culture plates (107 cells/well). After 12 h, the cell media was replaced with a solution of DMEM/FBS containing one of the following treatments: TNFα-TKNs, Sc-TKNs, TNFα-TKNs, TNFα-βGPs, or siRNA-DOTAP complexes. For treatments containing siRNA cells received 23.0 µg siRNA/ml. After 4 h of treatment, the media was removed, the cells were washed with PBS, and then treated with either DMEM/FBS or DMEM/FBS spiked with LPS (5 µg/ml). After 24 h, 100 µl of media was collected from each sample and analyzed for TNFα via ELISA according to the manufactures protocol (eBioscience).
Induction of colitis and oral siRNA delivery
Animal experiments were performed in female C57BL/6 mice (8wk, 17 – 20 g, Jackson Laboratories). Mice were group housed under a controlled temperature (25 °C), photoperiod (12:12-h light-dark cycle), and allowed unrestricted access to potables and standard mouse chow. They were allowed to acclimate to these conditions for at least seven days before being included in experiments. Colitis was induced by adding 3% (wt./vol) DSS [35,000 Da, (ICN Biochemicals)] to their drinking water. For each of the animal experiments, groups of mice were treated with DSS or regular water for seven days. Mice were observed daily and evaluated for changes in body weight and development of the clinical symptoms of colitis. Starting on day zero, mice receiving DSS were given a daily gavage of PBS (200µl) or a PBS solution (200µl) containing one of the following: TNFα-TKNs, TNFα-PLGA, Sc-TKNs, TNFα-βGPs, or TNFα-DOTAP. Mice receiving siRNA-loaded particles or TNFα-DOTAP were treated with either 2.3 mg/kg or 0.23 mg/kg of TNFα-siRNA or scrambled siRNA per day for six consecutive days (i.e. days 0–5). Mice were sacrificed on day seven, and histological assessment of colonic inflammation was performed by hematoxylin and eosin (H&E) staining of 5 µm-colonic tissue sections and analyzed by microscopy (20X & 10X). All animal experiments were approved by The Animal Care Committee of Emory University, Atlanta (IACUC ID: 156-2008) and were performed in accordance with the guide for the Care and Use of Laboratory Animals, published by the U.S. Public Health Service.
Real time RT-PCR
Total RNA was extracted from mouse colons using TRIzol (Invitrogen). A reverse transcription (RT) reaction was performed on 2 µg of each sample and an oligo-dT primer, using a RETROscript® System (Ambion Inc.). Next, 10 ng of reverse-transcribed cDNA, 400 nM of gene-specific primers, and the iQ SYBR Green Suppermix (Biorad) was amplified at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The 36B4 expression levels were used as reference, and fold-induction was calculated by the Ct method as follows: ΔΔCT=(Ct Target − Ct 36B4)DSS+Treatment - (Ct Target − Ct 36B4)DSS+ PBS. Thus producing results normalized against mice receiving DSS and treated with PBS. The final data were then derived from 2−ΔΔCT. The primers used were designed using the Primer Express Program (Applied Biosystems) and were as follows: TNFα sense 5’-AGG CTG CCC CGA CTA CGT-3’ antisense 5’-GAC TTT CTC CTG GTA TGA GAT AGC AAA-3’; IL-1β sense 5’-TCG CTC AGG GTC ACA AGA AA-3’ antisense 5’-CAT CAG AGG CAA GGA GGA AAA C-3’; IL-6 sense 5’-ACA AGT CGG AGG CTT AAT TAC ACA T-3’ antisense 5’-TTG CCA TTG CAC AAC TCT TTT C-3’; IFN-γ sense 5’-CAG CAA CAG CAA GGC GAA A -3’ antisense 5’-CTG GAC CTG TGG GTT GTT GAC -3’.
Myeloperoxidase (MPO) Activity
Neutrophil infiltration into the colon was quantified by measuring MPO activity. Briefly, a portion of the colon was homogenized in 1:20 (w/v) of 50 mM phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethyl ammonium bromide on ice using a homogenizer (Polytron). The homogenate was then sonicated for 10 s, freeze-thawed three times, and centrifuged at 14,000 rpm for 15 min. The supernatant (14 µl) was then added to 1 mg/ml o-dianisidine hydrochloride and 0.0005% hydrogen peroxide, and the change in absorbance at 460 nm was measured. MPO activity was expressed as units per mg of protein, where one unit was defined as the amount that degrades 1 µmol of hydrogen peroxide per minute at 25°C.
In vivo targeting of Cy3-labed siRNA to inflamed tissues using TKNs
Mice receiving DSS or normal water were given a daily gavage of a PBS solution (200µl) containing either empty TKNs or Cy3siRNA-TKNs (3.5 mg siRNA/kg/day) (total of four groups with n =10 per group). After seven days, each mouse receiving DSS had lost at least 10% of their body weight and had elevated fecal blood levels consistent with disease development. Organ samples (~0.6 mg) were removed, washed with cold PBS, patted dry with a paper towel, and then homogenized in 1 ml of PBS (Polytron). Samples were then centrifuged at 20,000 rpm for 30 min, and Cy3 was quantified in 100 µl of the supernatant using a fluorometer (λex/λem = 550/570; Shimadzu). To correct for the background fluorescence resulting from the tissue and residual PPADT, the fluorescence measured from organ samples taken from mice receiving empty TKNs was subtracted from the fluorescence measured in samples taken from animals treated with Cy3siRNA-TKNs. Results are expressed as fluorescent units per gram of tissue.
Supplementary Material
Acknowledgments
This project was funddd in part by the NSF-BES-0546962 CAREER AWARD. D. Scott Wilson is supported by the Center for Drug Design, Development and Delivery at the Georgia Institute of Technology and the NIH Cellular and Tissue Engineering Training Grant T32 GM08433.
Footnotes
Author Contributions
(D.S.W and G.D. contributed equally to this work) D.S.W. synthesized and characterized PPADT; formulated particles; designed, performed and analyzed experiments; and wrote the manuscript. G.D. designed, performed and analyzed experiments; and proof read the manuscript. L.W. performed experiments. S.V.S. supervised the project. D.M. designed experiments and supervised the project. N.M. designed the synthetic strategy used to synthesize PPADT; supervised the project; and contributed to the writing of the manuscript.
Competing Financial Interests
The authors have no competing financial interests.
REFERENCES
- 1.Lih-Brody L, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–2086. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 2.Kountouras J, Chatzopoulos D, Zavos C. Reactive oxygen metabolites and upper gastrointestinal diseases. Hepatogastroenterology. 2001;48:743–751. [PubMed] [Google Scholar]
- 3.Simmonds NJ, et al. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992;103:186–196. doi: 10.1016/0016-5085(92)91112-h. [DOI] [PubMed] [Google Scholar]
- 4.Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe F, Michaud K, Anderson J, Urbansky K. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum. 2004;50:372–379. doi: 10.1002/art.20009. [DOI] [PubMed] [Google Scholar]
- 6.Reddy JG, Loftus EV., Jr Safety of infliximab and other biologic agents in the inflammatory bowel diseases. Gastroenterol Clin North Am. 2006;35:837–855. doi: 10.1016/j.gtc.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Heraganahally SS, et al. Pulmonary toxicity associated with infliximab therapy for ulcerative colitis. Intern Med J. 2009;39:629–630. doi: 10.1111/j.1445-5994.2009.001999.x. [DOI] [PubMed] [Google Scholar]
- 8.Aouadi M, et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180–1184. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pertuit D, et al. 5-amino salicylic acid bound nanoparticles for the therapy of inflammatory bowel disease. J Control Release. 2007;123:211–218. doi: 10.1016/j.jconrel.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka YJ, Leong KW. Engineering strategies to enhance nanoparticle-mediated oral delivery. J Biomater Sci Polym Ed. 2008;19:1549–1570. doi: 10.1163/156856208786440479. [DOI] [PubMed] [Google Scholar]
- 11.Sedghi S, et al. Increased production of luminol enhanced chemiluminescence by the inflamed colonic mucosa in patients with ulcerative colitis. Gut. 1993;34:1191–1197. doi: 10.1136/gut.34.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng YC, et al. Chemiluminescence assay of mucosal reactive oxygen species in gastric cancer, ulcer and antral mucosa. Hepatogastroenterology. 2008;55:770–773. [PubMed] [Google Scholar]
- 13.Shukla AK, Verma M, Singh KN. Superoxide induced deprotection of 1,3-dithiolanes: A convenient method of dedithioacetalization. Indian J Chem B. 2004;43:1748–1752. [Google Scholar]
- 14.Colonna S, Gaggero N, Carrea G, Pasta P. Enantio and diastereoselectivity of cyclohexanone monooxygenase catalyzed oxidation of 1,3-dithioacetals. Tetrahedron-Asymmetr. 1996;7:565–570. [Google Scholar]
- 15.Mahida YR, Wu KC, Jewell DP. Respiratory Burst Activity of Intestinal Macrophages in Normal and Inflammatory Bowel-Disease. Gut. 1989;30:1362–1370. doi: 10.1136/gut.30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 17.Sorensen DR, Leirdal M, Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327:761–766. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- 18.Leirdal M, Sioud M. Gene silencing in mammalian cells by preformed small RNA duplexes. Biochem Biophys Res Commun. 2002;295:744–748. doi: 10.1016/s0006-291x(02)00736-2. [DOI] [PubMed] [Google Scholar]
- 19.Murata N, Takashima Y, Toyoshima K, Yamamoto M, Okada H. Anti-tumor effects of anti-VEGF siRNA encapsulated with PLGA microspheres in mice. J Control Release. 2008;126:246–254. doi: 10.1016/j.jconrel.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Palliser D, et al. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439:89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- 21.Akhtar S, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117:3623–3632. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, Zhao B, Jiang H, Wang B, Ma B. Cationic lipids and polymers mediated vectors for delivery of siRNA. J Control Release. 2007;123:1–10. doi: 10.1016/j.jconrel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Thiele L, et al. Evaluation of particle uptake in human blood monocyte-derived cells in vitro. Does phagocytosis activity of dendritic cells measure up with macrophages? J Control Release. 2001;76:59–71. doi: 10.1016/s0168-3659(01)00412-6. [DOI] [PubMed] [Google Scholar]
- 24.Hariharan S, et al. Design of estradiol loaded PLGA nanoparticulate formulations: a potential oral delivery system for hormone therapy. Pharm Res. 2006;23:184–195. doi: 10.1007/s11095-005-8418-y. [DOI] [PubMed] [Google Scholar]
- 25.Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res. 1996;13:1838–1845. doi: 10.1023/a:1016085108889. [DOI] [PubMed] [Google Scholar]
- 26.Lamprecht A, Schafer U, Lehr CM. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm Res. 2001;18:788–793. doi: 10.1023/a:1011032328064. [DOI] [PubMed] [Google Scholar]
- 27.Yan Y, et al. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One. 2009;4:e6073. doi: 10.1371/journal.pone.0006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.