Abstract
Expanding DNA sequence databases and improving methods for comparative analysis are being exploited to identify numerous noncoding RNA elements including riboswitches. Ligands for many riboswitch classes usually can be inferred based on the genomic contexts of representative RNAs, and complex formation or genetic regulation subsequently demonstrated experimentally. However, there are several candidate riboswitches for which ligands have not been identified. In this report, we discuss three of the most compelling riboswitch candidates: the ykkC/yzkD, yybP/ykoY and pfl RNAs. Each of these RNAs is numerous, phylogenetically widespread and carries features that are hallmarks of metabolite-binding riboswitches, such as a well-conserved aptamer-like structure and apparent interactions with gene regulation elements such as ribosome binding sites or intrinsic transcription termination stems. These RNAs likely represent only a small sampling of the challenging motifs that researchers will encounter as new noncoding RNAs are identified.
Key words: riboswitch, purine biosynthesis, alkaline response, formyl-tetrahydrofolate biosynthesis
Introduction
Riboswitches are noncoding RNA (ncRNA) elements that directly bind small molecule metabolites to regulate gene expression. They are characterized by two components, an aptamer domain that interacts with a small molecule ligand, and an expression platform that converts folding changes in the aptamer into changes in gene expression.1,2 Riboswitch aptamers recognize their ligands with high specificity,3 and structural studies have shown that the ligand is often nearly enveloped by the RNA.4,5 Aptamers typically range from ∼35 to 200 nucleotides in length and have relatively complex architectures as compared to many RNA structures that are recognized by proteins.6 The structural complexity of aptamers is due to their need to form binding pockets for ligands while excluding many possible competing compounds. The nucleotides that directly contact the ligand are typically highly conserved. These core nucleotides are held in close proximity by various supporting substructures (stems, pseudoknots and other common structural features) that are formed despite variability in nucleotide sequence.1,3
In contrast to aptamer domains, expression platforms can vary widely and may not always be obvious through inspection of nucleotide sequences.1 The most common expression platforms used by bacteria include stems that block ribosome binding sites7 or intrinsic transcription terminators.8,9 Although the sequences of expression platforms can vary greatly, even within members of the same mechanistic class, the functions of some can be easily predicted based on the hallmarks of the expression platform system. For example, ribosome sequestration involves the formation of a stem encompassing the ribosome binding site, while intrinsic transcription termination involves the formation of a strong stem followed by a run of U residues.
The extensive conservation of aptamer domains makes them readily identifiable through comparative sequence analysis, which has become the predominant method of riboswitch discovery.10–13 Riboswitches identified to date bind a variety of ligands including amino acids, coenzymes and even second messengers such as c-di-GMP.3,14 Ligands are typically inferred from the open reading frames (ORFs) appearing immediately 3′ of candidate riboswitches. Hypothetical ligands are subsequently tested in vitro using direct binding assays15,16 or other functional assays, and in vivo by using genetic reporter constructs to establish expression changes in response to changing ligand concentrations.6
To date, the aptamers of approximately two dozen riboswitch classes have been experimentally validated and matched with their corresponding ligands. However, there remain several ncRNA classes identified by comparative sequence analysis that have characteristics of riboswitch function. These features include an aptamer-like domain with high sequence conservation and structural complexity compared to most RNA domains bound by proteins and a common association with structures that are typical of expression platforms. In many cases these RNAs are phylogenetically widespread, with hundreds of examples appearing in numerous bacterial phyla. However, identifying the ligands for these candidate riboswitches has proven challenging. In this article, we highlight some of the more compelling candidate riboswitches and share the progress that has been made toward demonstrating genetic control and narrowing the scope of possible ligands.
Results
The ykkC/yxkD motif.
A comparative sequence analysis effort conducted using DNA sequences derived from the intergenic regions of Bacillus subtilis and 91 other bacterial organisms revealed numerous structured RNA motifs11 including four that have since been validated as riboswitches. The ykkC/yxkD motif (hereafter called ykkC) is one of several candidate riboswitches from this study that remain to be validated. Over 300 examples have been identified to date among metagenomic sequences and in Firmicutes, Fusobacteria, Dictyoglomi, Proteobacteria (Alpha, Beta, Gamma and Epsilon), Cyanobacteria and Actinobacteria.17
The ykkC motif is not exceeding complex and is predicted to consist of a stem (pairing element 1 or P1) with a large and highly-conserved internal loop, a smaller P2 and a 3′ tail also exhibiting some sequence conservation (Fig. 1A). The conservation of the predicted single-stranded regions is especially striking considering the wide phylogenetic distribution. These regions could form a selective binding pocket for a small molecule, as do numerous other riboswitch aptamers. Examination of sequences flanking several examples of the ykkC RNA suggests that the motif is associated with expression platforms that activate gene expression in response to ligand binding (a genetic ON switch).
Figure 1.
Consensus sequences and structure models for widespread candidate riboswitches. (A) ykkC/yxkD RNA. (B) yybP/ykoY RNA. (C) pfl RNA. Diagrams were constructed using data from representatives identified in RefSeq38,30 and metagenomic data sets as describes in references 13 and 25. Y = U or C, R = G or A.
The most common genomic context for ykkC RNAs is preceding ORFs whose products are annotated as transporters or permeases, including amino acid transporters, xanthine-uracil permeases, ABC-type bicarbonate transporters and multi-drug resistance efflux pumps. Given that prediction of the precise substrate specificities of transporters remains difficult based on sequence analysis alone,18 the ligand identity of this putative riboswitch is not readily inferred from this genomic context. The RNA motif also precedes many ORFs encoding proteins of unknown function, as well as ORFs encoding enzymes with a variety of functions including Nudix hydrolase, AICAR transformylase (purB), AIR carboxylase (purE), allophanate hydrolase and a protein similar to agmatine ureohydrolase (speB). Some of these enzyme functions suggest the RNA may sense a compound related to purine biosynthesis. If the biological ligand for the ykkC RNA can be identified, then the control and interrelatedness of this large, apparently incongruous set of genes can potentially be understood. Furthermore, understanding the biological triggers for gene expression may also allow natural ligands for many of the transporters to be identified, similarly to the way thiamin was confirmed as the ligand for transporters regulated by TPP riboswitches.19
Two additional features elevate interest in ykkC RNAs. First, several examples have been found in tandem with guanine riboswitches in various species of Firmicutes.20 In these tandem examples, the guanine aptamer and the ykkC putative aptamer appear to share the same expression platform, wherein the ykkC RNA is predicted to be a genetic ON switch. In vitro studies show that the guanine riboswitch aptamer from the tandem system of Moorella thermoacetica indeed is responsive to ligand (data not shown). Identifying a ligand for the ykkC RNA would allow the potential complex genetic logic of a tandem riboswitch architecture containing opposing gene control elements to be elucidated.
Second, additional conserved RNA structures have been identified associated with many of the same genes as the ykkC RNA. The mini-ykkC10 and ykkC-III13 RNAs are structurally distinct from the ykkC RNA and both these RNAs are hypothesized to respond to the same biological conditions or stresses as the ykkC RNA. These structured RNA motifs are positioned as if they serve a common gene control function, which is similar to the multiple riboswitch classes that bind S-adenosylmethionine,21 prequeuosine-1,3 and cyclic-di-GMP.14,22
Our initial attempts to identify a ligand for the ykkC RNA included in vitro assays using a ykkC RNA representative from B. subtilis (RefSeq: NC_000964.3/1376309-1376448) and an extensive collection of compounds chosen based on the genes associated with this RNA class in many organisms (See Table 1). Unfortunately, none of these assays returned a convincing signal of ligand binding. However, genetic reporter assays suggest the RNA functions as a gene control element. For example, the B. subtilis ykkC motif with its native promoter and expression platform (Refseq: NC_000964.3/1376118-1376519) was cloned into plasmid pDG1661,23 and gene expression examined in BGSC strain 1A1. Under both minimal and rich media conditions, low gene expression was observed with the wild-type construct. Furthermore, a series of mutant constructs (Fig. 2A) designed to disrupt ykkC RNA structure (M1 through M3) all yield low gene expression (Fig. 2B). By contrast, a mutant that deletes a portion of the putative expression platform terminator stem (M4) yields high gene expression. These findings could be explained if the ykkC RNA ligand is not present at concentrations sufficient to trigger riboswitch activation under normal growth conditions.
Table 1.
Compounds screened in RNA binding tests with the yykC, yybP and pfl RNAs
| ykkC RNA |
| putrescine; agmatine; spermidine; cadaverine; ornithine; arginine; glutamine; glutamate; histidine; urocanic acid; γ-aminobutyric acid; taurine; adenosine phosphosulfate; phosphoadenosine phosphosulfate; dimethylsulfoxide; tetrahydrofolate (THF); 5-formyl THF; 10-formyl THF; 5-methyl THF; 5, 10-methylene THF; folic acid; adenine; guanine; xanthine; hypoxanthine; uric acid; allantoin; urea; adenosine; uracil; barbituric acid; carbamoyl phosphate; guanosine 5′-monophosphate; adenosine 5′-monophosphate; inosine 5′-monophosphate; 5-phospho-D-ribose 1-diphosphate; glycinamide ribonucleotide; aminoimidazole ribonucleotide; aminoimidazole ribonucleoside; carboxyaminoimidazole ribonucleotide; 5-aminoimidazole-4-(N-succinylocarboxamide) ribonucleotide; 5-aminoimidazole-4-carboxamide ribotide; 5-aminoimidazole-4-carboxamide; hydroxymethylpyrimidine; thiamin pyrophosphate; biotin; pyruvate; ketoglutarate; glyoxalate; acetyl coenzyme A; allophanate; adenosine 3′,5′-cyclic monophosphate; guanosine-3′,5′-bisdiphosphate (ppGpp); cyclic di-guanosine monophosphate; P1,P4-di(adenosine-5′) tetraphosphate (Ap4A) |
| yybP RNA |
| adenosine 3′,5′-cyclic monophosphate; P1,P4-di(adenosine-5′) tetraphosphate (Ap4A); guanosine-3′,5′-bisdiphosphate (ppGpp); cyclic di-guanosine monophosphate; adenosine phosphosulfate; phosphoadenosine phosphosulfate; nicotinamide; nicotinic acid; β-nicotinamide mononucleotide; β-nicotinamide adenine dinucleotide (NAD); β-nicotinamide adenine dinucleotide phosphate (NADP); adenosine 5′-diphosphoribose; cysteine |
| pfl RNA |
| pyruvate; formate; glutamate; glutamine; glycine; serine; aspartate; homocysteine; alanine; histidine; tryptophan; S-adenosylmethionine; acetyl-coenzyme A; coenzyme A; β-nicotinamide adenine dinucleotide (NAD); NADH; β-nicotinamide adenine dinucleotide phosphate (NADP); NADPH; 5-aminoimidazole-4-(N-succinylocarboxamide) ribonucleotide; 5-aminoimidazole-4-carboxamide ribotide; 5-aminoimidazole-4-(N-succinylocarboxamide) ribonucleotide; glycinamide ribonucleotide; inosine 5′-monophosphate; adenine; adenosine 5′-triphosphate; adenosine 5′-diphosphate; adenosine 5′-monophosphate; guanine; guanosine 5′-triphosphate; guanosine 5′-diphosphate; guanosine 5′-monophosphate; uridine 5′-triphosphate; uridine 5′-diphosphate; uridine 5′-monophosphate; uridine, cytidine 5′-triphosphate; hypoxanthine; 2′-deoxyuridine 5′-monophosphate; 2′-deoxycytidine 5′-monophosphate; D-ribose 5′-phosphate; D-ribose; 5-phospho-D-ribose 1-diphosphate; guanosine-3′,5′-bisdiphosphate (ppGpp); adenosine 3′,5′-cyclic monophosphate; hydroxymethylpyrimidine; tetrahydrofolate (THF); 5-formyl THF; 10-formyl THF; 5-methyl THF; 5,10-methylene THF; 5,10-methenyl-THF dihydrofolate. |
Figure 2.
Sequence, secondary structure and activity of a ykkC RNA representative. (A) Bacillus subtilis ykkC RNA construct used for reporter assays with integration vector pDG1661. The reporter plasmid contains a ribosome binding site and start codon that follow ∼50 bp after the inserted riboswitch candidate.23 Mutants M1 and M3 were designed to disrupt base-pairing in P1 and M2 disrupts a highly-conserved region of the RNA. M4 deletes the right shoulder of the putative expression platform terminator stem. (B) β-galactosidase expression levels measured in Miller units using methods described previously in reference 14, for wild-type and the various mutants described in (A) under minimal and rich media (M4 only) as indicated. When present, error bars represent standard deviation among three independent replicates.
The various reporter constructs described above also were used to assess gene expression under additional growth conditions, including nitrogen starvation and carbon starvation, to determine whether specific stresses may cause build-up of a ligand. Reporter gene expression was also examined in the context of several purine biosynthesis knockout strains [ΔpurM (BGSC 1A251), ΔpurF (BGSC 1A12), ΔpurE (BGSC 1A383, 1A385), ΔpurH (BGSC 1A293)] to examine whether a build-up of purine biosynthetic intermediates triggers a change in gene expression. Again, increased levels of gene expression were not observed in constructs containing the wild-type RNA.
Further attempts to identify the ligand included genetic selections of UV-induced and transposon-induced mutants of a ykkC RNA reporter strain containing both a kanamycin resistance cassette and lacZ reporter. No viable mutants with increased gene expression were identified. However, numerous false-positives were identified that carry mutations that disrupt the stability of the intrinsic transcription terminator in the ykkC expression platform. Again, these observations suggest that the ykkC ligand is not produced in appreciable quantities by B. subtilis under the conditions tested, and that mutations to the genome that cause ligand build-up either cannot readily occur or such mutations may be lethal.
The yybP/ykoY motif.
The yybP/ykoY RNA motif (hereafter called yybP) was identified in the same study as the ykkC RNA. The yybP motif has a much wider distribution and by far the most examples for any candidate riboswitch class.24 Over 1,000 unique examples of yybP RNAs have been identified in many bacterial phyla including Firmicutes, Tenericutes, Actinobacteria, Chloroflexi, Cyanobacteria, Deinococcus-Thermus, as well as in Alpha-, Beta-, Gamma-, Delta- and Epsilon-proteobacteria in addition to numerous examples in various metagenomic datasets.17,25 The RNA consists of a single elongated stem with strong co-variation and a highly conserved internal loop (Fig. 1B). Examining the flanking sequence of some yybP RNA examples suggests that the RNA is a genetic ON switch.11 Representatives predominantly precede ORFs annotated as transporters and permeases and are commonly associated with cation transporters.
These gene associations suggest that yybP RNAs may sense a ligand that is an indicator of pH stress. We also tested a series of candidate ligands (Table 1) for this riboswitch candidate without success. Intriguingly, a recent report26 described the characterization of a pH-sensing RNA element (PRE) in E. coli that precedes the alx gene and partially overlaps the yybP RNA associated with this mRNA. This element is important for gene expression induction under alkaline conditions. However, the portion of the PRE corresponding to the yybP RNA (helices A and B) was not studied in detail, and the structural probing studies performed under different pH conditions revealed structural changes distal from the yybP RNA. In addition, the yybP motif portion of the PRE is much more widely distributed than the PRE itself, which is present only in Gram-negative bacteria. Although it is possible that the yybP RNA is a direct sensor of proton concentration, pH stress could produce some other ligand that is sensed by this RNA class.
The pfl motif.
The pfl RNA motif is a recently discovered riboswitch candidate13 that has a genetic context that provides numerous suggestions for possible ligands. In contrast to ykkC and yybP RNAs, most of the genes associated with pfl RNAs have known biochemical functions. This motif most commonly precedes genes with roles in purine biosynthesis such as AICAR formyltransferase (purH) or in the synthesis of formyltetrahydrofolate (formyl-THF) such as formate-tetrahydrofolate ligase (fhs), pyruvate formate lyase (pfl), glycine hydroxymethyltransferase (glyA) and methenyltetrahydrofolate cyclohydrolase (folD). Most other genes presumably under pfl RNA control encode enzymes that perform other steps in purine synthesis, or catalyze inter-conversions between THF and various single-carbon derivatives.13 In vitro binding assays with a representative of the RNA from Clostridium acetobutylicum13 were conducted using a variety of compounds (Table 1) related to both purine and folate biosynthesis.13 Although these assays did not produce a binding signal for any of the compounds, the analyses confirm the hypothesized secondary structure of the RNA.
As with the other riboswitch candidates described herein, examples of the pfl RNA are widespread, with over 300 examples found in species of Firmicutes, Tenericutes, Actinobacteria and Alpha-, Beta-, Gamma- and Delta-proteobacteria. In addition, the number of unique examples has been nearly doubled by analyzing a human gut metagenome.25 The pfl RNA motif has a complex structure that includes extensive sequence conservation as well as evidence of nucleotide covariation in the predicted base-paired regions P1, P2 and P3 (Fig. 1C). Some instances of the pfl RNA aptamer overlap a transcription terminator stem and thus appear to be genetic ON switches. However, some riboswitch classes have members that activate expression and other that repress expression, and this could be the case with the pfl RNA class. Like the ykkC RNAs, some pfl RNAs are found in tandem with members of a known riboswitch class, in this case the THF riboswitch27 as observed in Clostridium kluyveri DSM 555. Expression platforms in this tandem architecture are not clear from a cursory analysis of the sequences flanking the elements, and therefore the gene control logic of this tandem arrangement remains to be established.
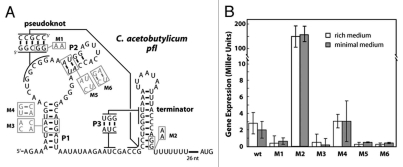
Wild-type and various mutant DNA constructs corresponding to this pfl RNA accompanied by its native expression platform (RefSeq: NC_003030.1/2562148-2562276) (Fig. 3A) were cloned into pDG1661 with a constitutive B. subtilis promoter.14 Only the construct carrying a disrupted terminator stem (M2) exhibits robust gene expression (Fig. 3B). However, the wild-type pfl RNA construct yields higher expression than mutants that disrupt ligand binding such as M1, M3 and M5. One of the mutant constructs designed to restore base-pairing, M4, exhibits expression similar to that of wild type. However, the other restorative mutant, M6, does not reflect wild-type behavior. This may be due to the relative conservation of nucleotides in this paired structure and due to the proximity of these mutations to the conserved core of the RNA.
Figure 3.
Sequence, secondary structure and activity of a pfl RNA representative. (A) C. acetobutylicum pfl RNA construct used in reporter assays with pDG1661 in B. subtilis. Mutants M1, M3, and M5 disrupt P1, pseudoknot and P2 formation, respectively. M4 and M6 restore the disruptions caused by M3 and M5, respectively. M2 disrupts formation of the transcription terminator stem. (B) β-galactosidase expression measured in Miller units as described previously in reference 14, for wild-type and mutant constructs under both minimal and rich media conditions. Error bars represent standard deviation among three independent replicates.
The association of pfl RNA with purine and formyl-THF biosynthesis motivated us to examine whether inhibiting these processes might elicit an increase in reporter gene expression. Unfortunately, the reporter strains showed similar behavior under a variety of media conditions including both glucose minimal medium and rich medium. Furthermore, purine biosynthesis knockout strains (ΔpurM (BGSC 1A251) and ΔpurH (BGSC 1A293, 1A294)), and wild-type cell in which folate biosynthesis is inhibited using the antibiotic trime thoprim,28 showed no significant changes in reporter gene expression (data not shown).
The predicted structure of the pfl RNA from C. acetobutylicum and the reporter gene data derived with this construct suggests that the RNA functions as a genetic ON switch. Although the ligand for the pfl RNA appears to be present under normal growth conditions, its concentration must be far too low to trigger full activation of gene expression. However, since we used a surrogate organism for the genetic studies, we cannot rule out the possibility that the C. acetobutylicum pfl RNA may not have performed as it would in its native organism. In particular, metabolic differences between the predominantly aerobic surrogate and predominantly anaerobic native organism may influence levels of the ligand in ways that are not predicted from our assays.
Conclusions
Riboswitch candidates can be routinely identified through comparative sequence analysis and ligands for many candidates have been relatively easily assigned. However, there are several very compelling riboswitch candidates that appear to control important processes based on bioinformatic data, are present in a large range of organisms, and yet have no assigned ligand. Among the four most common “orphan riboswitch” classes are the yybP, pfl and ykkC RNAs discussed in this report. The second most common candidate riboswitch class is the ydaO/yuaA RNAs11 (hereafter called ydaO), which are commonly associated with genes important for bacterial osmotic stress responses. Details on the search for a ligand for this candidate riboswitch class have been reported in reference 29. Validation of ligand binding and riboswitch function for this collection of RNA motifs would reveal much about the adaptive responses to chemical and metabolic stresses that affect a wide range of bacteria.
The putative ligands for ydaO29 and pfl RNAs are present in cells under normal growth conditions, although the level of the pfl ligand appears to be below the concentration required to fully trigger riboswitch activation. In contrast, intracellular ligand levels are not sufficient to elicit even low levels of gene expression with a reporter gene construct incorporating a ykkC RNA element. Considering the prevalence of genetic OFF switches among riboswitches with known ligands, it is striking that all three of these riboswitch candidates mostly occur as genetic ON switches and do not appear to be directly involved in biosynthetic feedback response. This, combined with the diverse sets of genes controlled by these RNAs, implicates recognition of signaling molecules whose identities may be more difficult to ascertain compared to primary metabolites. This possibility is exemplified by c-di-GMP riboswitches,14,22 which control a wide range of genes involved with virulence, competence, motility and other physiological changes of bacterial cells. If this second messenger and its physiological roles had not been as well studied, it would have been difficult to assign a ligand to its two corresponding riboswitch classes.
Given the widespread nature of the riboswitch candidates discussed here, we expect that the compounds they sense are likely to be present in most bacteria. Looking forward, we anticipate that the biological ligands of these ncRNAs will be identified as improvements to comparative transcriptomics, proteomics and metabolomics shed additional light on which genes are co-regulated, how protein levels reflect changes in transcript levels, and how this affects what compounds are present within the cell. As more riboswitch candidates are identified, they will pose similar challenges in ligand identification. However, unlike the motifs discussed in this report, rare riboswitch classes have an increasing likelihood of sensing compounds that are also rare and perhaps unknown to researchers.
Acknowledgements
The authors thank Aaron Hoskins, Joanne Stubbe and Tadhg Begley for compounds used for in vitro ligand screening. We also thank Zasha Weinberg and other members of the Breaker laboratory for helpful comments. M.M.M. is supported by an NIH NRSA (F32GM079974). M.C.H. is supported by a Career Award at the Scientific Interface from B.W.F. This work was also supported by funding to R.R.B. from the NIH (GM022778) and from the Howard Hughes Medical Institute.
Abbreviations
- ncRNA
noncoding RNA
- BGSC
Bacillus Genetic Stock Center
- PRE
pH-sensing RNA element
- THF
tetrahydrofolate
References
- 1.Barrick JE, Breaker RR. The distributions, mechanisms and structures of metabolite-binding riboswitches. Genome Biol. 2007;8:239. doi: 10.1186/gb-2007-8-11-r239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dambach MD, Winkler WC. Expanding roles for metabolite-sensing regulatory RNAs. Curr Opin Microbiol. 2009;12:161–169. doi: 10.1016/j.mib.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth A, Breaker RR. The structural and functional diversity of metabolite-binding riboswitches. Annu Rev Biochem. 2009;78:305–334. doi: 10.1146/annurev.biochem.78.070507.135656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwalbe H, Buck J, Furtig B, Noeske J, Wohnert J. Structures of RNA switches: insight into molecular recognition and tertiary structure. Angew Chem Int Ed Engl. 2007;46:1212–1219. doi: 10.1002/anie.200604163. [DOI] [PubMed] [Google Scholar]
- 5.Serganov A. The long and the short of riboswitches. Curr Opin Struct Biol. 2009;19:251–259. doi: 10.1016/j.sbi.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regulski EE, Moy RH, Weinberg Z, Barrick JE, Yao Z, Ruzzo WL, et al. A widespread riboswitch candidate that controls bacterial genes involved in molybdenum cofactor and tungsten cofactor metabolism. Mol Microbiol. 2008;68:918–932. doi: 10.1111/j.1365-2958.2008.06208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shine J, Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975;254:34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- 8.Gusarov I, Nudler E. The mechanism of intrinsic transcription termination. Mol Cell. 1999;3:495–504. doi: 10.1016/s1097-2765(00)80477-3. [DOI] [PubMed] [Google Scholar]
- 9.Yarnell WS, Roberts JW. Mechanism of intrinsic transcription termination and antitermination. Science. 1999;284:611–615. doi: 10.1126/science.284.5414.611. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg Z, Barrick JE, Yao Z, Roth A, Kim JN, Gore J, et al. Identification of 22 candidate structured RNAs in bacteria using the CMfinder comparative genomics pipeline. Nucleic Acids Res. 2007;35:4809–4819. doi: 10.1093/nar/gkm487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrick JE, Corbino KA, Winkler WC, Nahvi A, Mandal M, Collins J, et al. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proc Natl Acad Sci USA. 2004;101:6421–6426. doi: 10.1073/pnas.0308014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbino KA, Barrick JE, Lim J, Welz R, Tucker BJ, Puskarz I, et al. Evidence for a second class of S-adenosylmethionine riboswitches and other regulatory RNA motifs in alpha-proteobacteria. Genome Biol. 2005;6:70. doi: 10.1186/gb-2005-6-8-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberg Z, Wang JX, Bogue J, Yang J, Corbino K, Moy RH, et al. Comparative genomics reveals 104 candidate structured RNAs from bacteria, archaea and their metagenomes. Genome Biol. 2010;11:31. doi: 10.1186/gb-2010-11-3-r31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, et al. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science. 2008;321:411–413. doi: 10.1126/science.1159519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regulski EE, Breaker RR. In-line probing analysis of riboswitches. Methods Mol Biol. 2008;419:53–67. doi: 10.1007/978-1-59745-033-1_4. [DOI] [PubMed] [Google Scholar]
- 16.Merino EJ, Wilkinson KA, Coughlan JL, Weeks KM. RNA structure analysis at single nucleotide resolution by selective 2′-hydroxyl acylation and primer extension (SHAPE) J Am Chem Soc. 2005;127:4223–4231. doi: 10.1021/ja043822v. [DOI] [PubMed] [Google Scholar]
- 17.Kazanov MD, Vitreschak AG, Gelfand MS. Abundance and functional diversity of riboswitches in microbial communities. BMC Genomics. 2007:8. doi: 10.1186/1471-2164-8-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelfand MS, Rodionov DA. Comparative genomics and functional annotation of bacterial transporters. Physics of Life Reviews. 2008;5:22–49. [Google Scholar]
- 19.Erkens GB, Slotboom DJ. Biochemical characterization of ThiT from Lactococcus lactis: A thiamin transporter with picomolar substrate binding affinity. Biochemistry. 2010;49:3203–3212. doi: 10.1021/bi100154r. [DOI] [PubMed] [Google Scholar]
- 20.Sudarsan N, Hammond MC, Block KF, Welz R, Barrick JE, Roth A, et al. Tandem riboswitch architectures exhibit complex gene control functions. Science. 2006;314:300–304. doi: 10.1126/science.1130716. [DOI] [PubMed] [Google Scholar]
- 21.Wang JX, Breaker RR. Riboswitches that sense S-adenosylmethionine and S-adenosylhomocysteine. Biochem Cell Biol. 2008;86:157–168. doi: 10.1139/O08-008. [DOI] [PubMed] [Google Scholar]
- 22.Lee ER, Baker JL, Weinberg Z, Sudarsan N, Breaker RR. An allosteric self-splicing ribozyme triggered by a bacterial second messenger. Science. 2010;329:845–848. doi: 10.1126/science.1190713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerout-Fleury AM, Frandsen N, Stragier P. Plasmids for ectopic integration in Bacillus subtilis. Gene. 1996;180:57–61. doi: 10.1016/s0378-1119(96)00404-0. [DOI] [PubMed] [Google Scholar]
- 24.Ames TD, Breaker RR. Bacterial riboswitch discovery and analysis. In: Mayer G, editor. The Chemical Biology of Nucleic Acids. West Sussex, UK: Wiley; 2010. pp. 433–455. [Google Scholar]
- 25.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nechooshtan G, Elgrably-Weiss M, Sheaffer A, Westhof E, Altuvia S. A pH-responsive riboregulator. Genes Dev. 2009;23:2650–2662. doi: 10.1101/gad.552209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ames TD, Rodionov DA, Weinberg Z, Breaker RR. A eubacterial riboswitch class that senses the coenzyme tetrahydrofolate. Chem Biol. 2010;17:681–685. doi: 10.1016/j.chembiol.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gready JE. Dihydrofolate reductase: binding of substrates and inhibitors and catalytic mechanism. Adv Pharmacol Chemother. 1980;17:37–102. doi: 10.1016/s1054-3589(08)60007-1. [DOI] [PubMed] [Google Scholar]
- 29.Block KF, Hammond MC, Breaker RR. Evidence for widespread gene control function by the ydaO riboswitch candidate. J Bacteriol. 2010;192:3983–3989. doi: 10.1128/JB.00450-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pruitt KD, Tatusova T, Maglott DR. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2007;35:61–65. doi: 10.1093/nar/gkl842. [DOI] [PMC free article] [PubMed] [Google Scholar]